Abstract
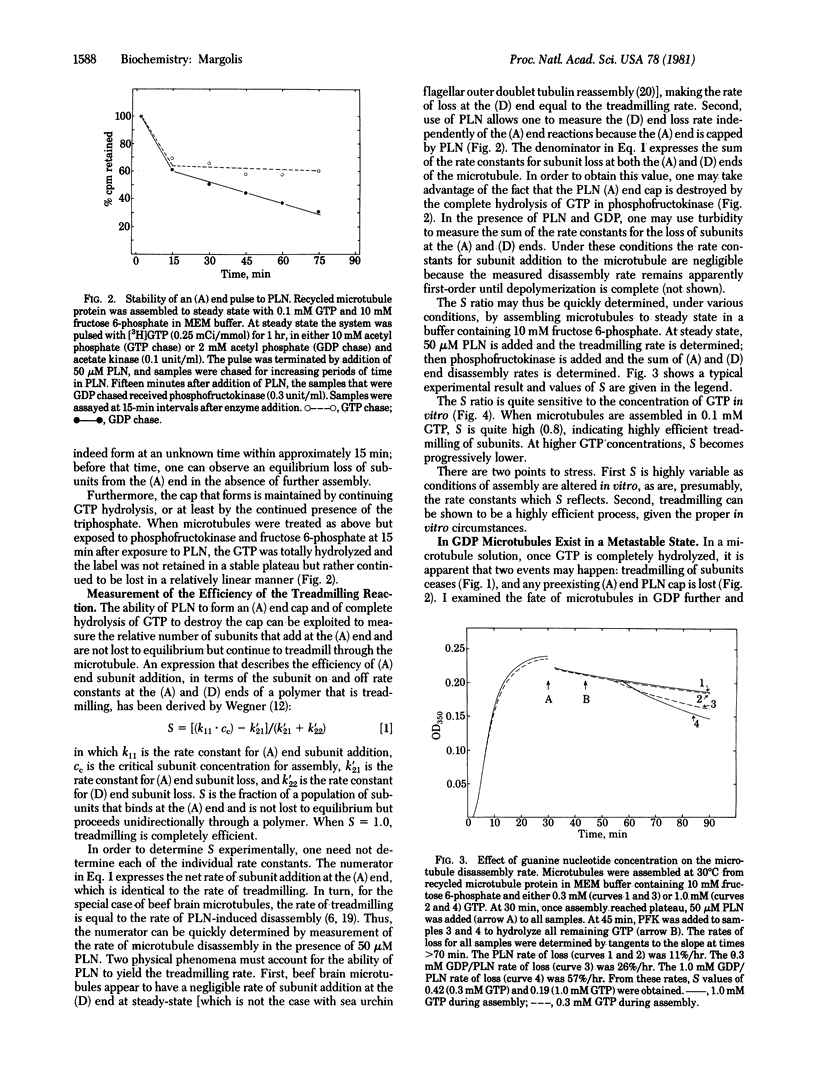
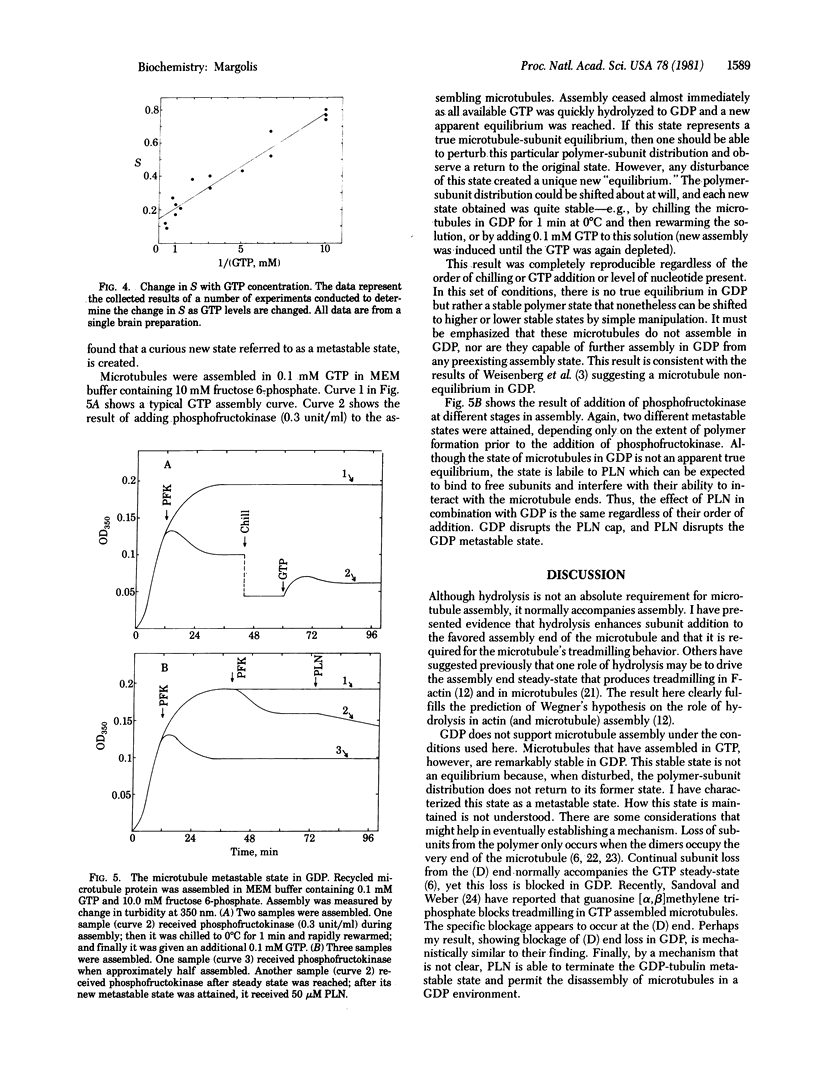
GTP hydrolysis accompanies addition of tubulin to microtubules. I find that hydrolysis is a requirement for the opposite-end assembly/disassembly of microtubules and consequent subunit treadmilling from one end to the other of the polymer. Neither GDP nor guanosine 5'-[beta, gamma-imido]triphosphate allows or participates in the treadmilling reaction. Therefore, there is a requirement for hydrolysis in the addition of subunits to the favored assembly end of the microtubule. Podophyllotoxin, an assembly inhibitory drug, "caps" the microtubule assembly end, preventing subunit loss from that site to equilibrium. Continued hydrolysis of GTP is required to maintain the podophyllotoxin cap. A corollary of this finding is that GTP hydrolysis is required for cap formation. Microtubules assembled in GTP enter a metastable state when all remaining GTP is hydrolyzed. This state is characterized by its ability to maintain indefinitely a subunit/polymer distribution ratio that is arbitrary and that can be altered at will by brief chilling or by addition of small amounts of GTP. This metastable state is labile to podophyllotoxin. Use of podophyllotoxin allows measurement of the microtubule treadmilling rate; use of podophyllotoxin in the absence of GTP allows measurement of the overall rate of dimer dissociation from the microtubule. Measurement of these rates has permitted determination of the efficiency with which adding dimers incorporate into the microtubule treadmill and are not lost to assembly end equilibrium. The efficiency varies with GTP concentration for unknown reasons, being high at 0.1 mM GTP and low at higher GTP concentrations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Kaziro Y. Effect of guanine nucleotides on the assembly of brain microtubles: ability of 5'-guanylyl imidodiphosphate to replace GTB in promoting the polymerization of microtubules in vitro. Biochem Biophys Res Commun. 1976 Mar 22;69(2):369–376. doi: 10.1016/0006-291x(76)90531-3. [DOI] [PubMed] [Google Scholar]
- Arai T., Kaziro Y. Role of GTP in the assembly of microtubules. J Biochem. 1977 Oct;82(4):1063–1071. doi: 10.1093/oxfordjournals.jbchem.a131777. [DOI] [PubMed] [Google Scholar]
- Asnes C. F., Wilson L. Isolation of bovine brain microtubule protein without glycerol: polymerization kinetics change during purification cycles. Anal Biochem. 1979 Sep 15;98(1):64–73. doi: 10.1016/0003-2697(79)90706-1. [DOI] [PubMed] [Google Scholar]
- Bergen L. G., Borisy G. G. Head-to-tail polymerization of microtubules in vitro. Electron microscope analysis of seeded assembly. J Cell Biol. 1980 Jan;84(1):141–150. doi: 10.1083/jcb.84.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Marcum J. M., Olmsted J. B., Murphy D. B., Johnson K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann N Y Acad Sci. 1975 Jun 30;253:107–132. doi: 10.1111/j.1749-6632.1975.tb19196.x. [DOI] [PubMed] [Google Scholar]
- Brenner S. L., Korn E. D. Substoichiometric concentrations of cytochalasin D inhibit actin polymerization. Additional evidence for an F-actin treadmill. J Biol Chem. 1979 Oct 25;254(20):9982–9985. [PubMed] [Google Scholar]
- Bryan J. A quantitative analysis of microtubule elongation. J Cell Biol. 1976 Dec;71(3):749–767. doi: 10.1083/jcb.71.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell K. W., Kassis J. A., Wilson L. Outer doublet tubulin reassembly: evidence for opposite end assembly-disassembly at steady state and a disassembly end equilibrium. Biochemistry. 1979 Jun 12;18(12):2642–2647. doi: 10.1021/bi00579a033. [DOI] [PubMed] [Google Scholar]
- Karr T. L., Podrasky A. E., Purich D. L. Participation of guanine nucleotides in nucleation and elongation steps of microtubule assembly. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5475–5479. doi: 10.1073/pnas.76.11.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr T. L., Purich D. L. A microtubule assembly/disassembly model based on drug effects and depolymerization kinetics after rapid dilution. J Biol Chem. 1979 Nov 10;254(21):10885–10888. [PubMed] [Google Scholar]
- Kobayashi T. Dephosphorylation of tubulin-bound guanosine triphosphate during microtubule assembly. J Biochem. 1975 Jun;77(6):1193–1197. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maccioni R., Seeds N. W. Stoichiometry of GTP hydrolysis and tubulin polymerization. Proc Natl Acad Sci U S A. 1977 Feb;74(2):462–466. doi: 10.1073/pnas.74.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Regulation of the microtubule steady state in vitro by ATP. Cell. 1979 Nov;18(3):673–679. doi: 10.1016/0092-8674(79)90122-3. [DOI] [PubMed] [Google Scholar]
- Penningroth S. M., Kirschner M. W. Nucleotide binding and phosphorylation in microtubule assembly in vitro. J Mol Biol. 1977 Oct 5;115(4):643–673. doi: 10.1016/0022-2836(77)90108-5. [DOI] [PubMed] [Google Scholar]
- Purich D. L., MacNeal R. K. Properties of tubulin treated with alkaline phosphatase to remove guanine nucleotides from the exchangeable binding site. FEBS Lett. 1978 Dec 1;96(1):83–86. doi: 10.1016/0014-5793(78)81067-9. [DOI] [PubMed] [Google Scholar]
- Sandoval I. V., Weber K. Guanasone 5'-(alpha,beta-methylene)triphosphate enhances specifically microtubule nucleation and stops the treadmill of tubulin protomers. J Biol Chem. 1980 Jul 25;255(14):6966–6974. [PubMed] [Google Scholar]
- Sloboda R. D., Rudolph S. A., Rosenbaum J. L., Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975 Jan;72(1):177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976 Nov;108(1):139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Deery W. J., Dickinson P. J. Tubulin-nucleotide interactions during the polymerization and depolymerization of microtubules. Biochemistry. 1976 Sep 21;15(19):4248–4254. doi: 10.1021/bi00664a018. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Deery W. J. Role of nucleotide hydrolysis in microtubule assembly. Nature. 1976 Oct 28;263(5580):792–793. doi: 10.1038/263792a0. [DOI] [PubMed] [Google Scholar]



