Abstract
Rab38 is a rat Hermansky-Pudlak syndrome gene that plays an important role in surfactant homeostasis in alveolar type II (ATII) pneumocytes. We examined Rab38 function in regulating lamellar body (LB) morphology in ATII cells. Quantitative electron microscopy revealed that LBs in ATII cells were ∼77% larger in Rab38-null fawn-hooded hypertension (FHH) than control Sprague-Dawley (SD) rats. Rab38 protein expression was restricted in lung epithelial cells but was not found in primary endothelial cells. In SD ATII cells, Rab38 protein level gradually declined during 5 days in culture. Importantly, endogenous Rab38 was present in LB fractions purified from SD rat lungs, and transiently expressed enhanced green fluorescent protein (EGFP)-tagged Rab38 labeled only the limiting membranes of a subpopulation (∼30%) of LBs in cultured ATII cells. This selective targeting was abolished by point mutations to EGFP-Rab38 and was not shared by Rab7 and Rab4b, which also function in the ATII cells. Using confocal microscopy, we established a method for quantitative evaluation of the enlarged LB phenotype temporally preserved in cultured FHH ATII cells. A direct causal relationship was established when the enlarged LB phenotype was reserved and then rescued by transiently reexpressed EGFP-Rab38 in cultured FHH ATII cells. This rescuing effect was associated with dynamic EGFP-Rab38 targeting to and on LB limiting membranes. We conclude that Rab38 plays an indispensible role in maintaining LB morphology and surfactant homeostasis in ATII pneumocytes.
Keywords: Hermansky-Pudlak syndrome, lysosome-related organelles
in eukaryotic cells, Rab GTPases are master regulators of intracellular membrane trafficking (29, 53). Among the ≥60 identified Rabs, Rab38 expression is highly selective in melanocytes and lung alveolar type II (ATII) epithelial cells and, thus, is hypothesized to carry out specialized trafficking functions in these cells (40, 62). Consistently, in melanocytes, Rab38 functions interchangeably with its closest homolog Rab32 in trafficking melanogenic enzymes to maturing melanosomes (55, 57, 62). Rab38 mutations in Chocolate mice (i.e., Rab38/Gly19Vla) (31) and Long-Evans Cinnamon (LEC) rats (i.e., Rab38/Met1Ile) (38) apparently result in dramatically enlarged lamellar bodies (LBs) in lung ATII cells (39, 41). These pathologically enlarged LBs, also called giant LBs, are characteristic markers of diseased lungs from Hermansky-Pudlak syndrome (HPS) patients.
HPS is a multisystem disorder characterized primarily by tyrosinase-positive oculocutaneous albinism due to immature melanosomes and bleeding diathesis resulting from platelet dense granule deficiency (26). Two major HPS subtypes (i.e., HPS1 and HPS4) additionally develop granulomatous colitis and pulmonary fibrosis, the latter of which is a particularly fatal condition for HPS1 patients in their third to fifth decades (1, 6, 18). For cell biologists, the significance of this rare human disease was revealed by the discoveries of nine HPS-related genes (i.e., HPS1–9) corresponding to nine HPS subtypes; these genes encode subunits of novel multiprotein complexes [i.e., biogenesis of lysosome-related organelle (LRO) complex-1, -2, and -3 and adaptor protein complex-3] that are essential for the biogenesis of lysosomes and LROs (12, 13). LROs are a group of loosely defined cellular organelles that carry out storage and secretory functions in specialized cell types; thus their disruptions are associated with a variety of human diseases (14, 26, 48). Like lysosomes, LROs have acidic lumens and are endowed with various sets of lysosomal proteins and enzymes. A classic LRO is the melanosome in pigment cells. Mutations in human genes functioning in the biogenesis and trafficking of melanosomes result in the tyrosinase-positive oculocutaneous albinism that is characteristic of HPS. Similar mutations in mice result in fur and skin color dilutions from the C57BL/6J black mouse background (30). From these color changes and the additional criterion of platelet dense granule deficiency, ≥16 murine models of HPS have been established. Genetic studies of these animals revealed not only orthologs of human HPS genes, but also additional genes encoding subunits of the homotypic fusion and protein sorting complex (26, 54), the melanophilin-Rab27A-MyoVa complex (22, 33, 36, 64), and the α-subunit of Rab geranylgeranyl transferase (15).
Rab38 was identified as a rat HPS gene in fawn-hooded (FH) and Tester-Moriyama rats, both of which carry the Rab38-null mutation (i.e., Met1/Ile) and develop oculocutaneous albinism and bleeding diathesis (38). It is likely that the FH and Tester-Moriyama rats, together with several substrains of LE rats (including the LEC rat), inherited the homozygous Rab38/Met1Ile mutation on rat chromosome 1 from a common ancestor (38). In contrast, the milder Rab38/Gly19Val mutation prevents Rab38 COOH-terminal prenylation (41) and causes oculocutaneous albinism, but not bleeding diathesis, in the Chocolate mice (31). Nevertheless, both Rab38 mutations are correlated with altered lung alveolar structures and enhanced susceptibilities to lung injuries in Chocolate mice and LEC rats (39, 41). These pathological changes are hypothesized to be caused by disrupted surfactant homeostasis in the lung ATII cells of the Rab38-mutated animals. Lung surfactant is a lipid-protein mixture (i.e., ∼90% lipids and ∼10% proteins) that forms an amphiphilic film along the liquid-air interface of an alveolar space to substantially reduce local surface tension (45). Thus surfactant plays a critical role in maintaining alveolar stability during a lung ventilation cycle (71). In addition, lung surfactant also contributes significantly to the lung's innate immunodefense system (11, 28, 66). ATII epithelial cells are responsible for the synthesis, storage, secretion, and recycling of surfactant lipids and proteins (2, 46). Intracellularly, surfactant is stored in LBs that are the LROs of lung ATII cells. Consistently, single or combined mutations to the mouse HPS genes result in a spectrum of enlarged LB phenotypes (19, 21, 32, 58). Because of the prominent and upstream roles that Rab proteins typically play in regulating intracellular membrane trafficking, we propose that understanding the Rab38-related trafficking mechanism may provide an advantageous entry point to unravel the complex, yet interdependent, relationships among the HPS proteins involved in the biogenesis and trafficking of LROs. Furthermore, LBs are larger (i.e., typically 1–2 μm diameter) than other LROs, and their numbers in individual ATII cells are limited. These are favorable properties for investigating an Rab38-related trafficking mechanism using our previously developed optical imaging techniques (23–25). Finally, the LB is a fascinating cellular organelle that simultaneously assumes multiple trafficking identities, i.e., the receiving site for newly synthesized surfactant lipids and proteins delivered through the synthetic/exocytic pathway, the terminal compartment for recycled surfactant components trafficked from the degradative/endocytic pathway, and the regulated secretory granule transporting surfactant to the alveolar space (2, 63). Thus there are a wealth of trafficking mechanisms to be discovered.
In this study, we examined the hypothesis that Rab38 is a specialized Rab protein regulating LB morphology in lung ATII epithelial cells. Pioneering work in the field has been carried out by Osanai et al. (39–42) in the past decade. Disrupted surfactant homeostasis, enlarged LB phenotypes, and altered alveolar structures and functions have been reported in Chocolate mice and LEC rats (39, 41). However, the molecular mechanism of Rab38-regulated trafficking in lung ATII cells has yet to be elucidated. Particularly, the reported endogenous Rab38 localization on endoplamsic reticulum (ER) membranes requires an indirect mechanism to explain the distal effect of the small GTPase on LB morphology (40, 42). Using a third model of Rab38 mutation, the FH hypertension (FHH) rat, and quantitative transmission electron microscopy (TEM) and confocal microscopy, we confirmed the enlarged LB phenotype on the different genetic background. Importantly, cell fractionation study and imaging of enhanced green fluorescent protein (EGFP)-tagged Rab38 (EGFP-Rab38) transiently expressed in isolated ATII cells conclusively established direct Rab38 targeting to the limiting membranes of a subpopulation of LBs. Using quantitative confocal microscopy, we carried out rescue experiments in cultured FHH ATII cells that confirmed Rab38's causal role in regulating LB morphology. Intriguingly, fluorescence recovery after photobleaching (FRAP) study revealed dynamic Rab38 targeting to and on LB limiting membranes, as well as potential Rab38-positive transporting vesicles emerging from the perinuclear region and “dock” on the LB surface in cultured ATII cells.
MATERIALS AND METHODS
Animals.
FHH rats were initially purchased from PhysioGenix (Milwaukee, WI), and an authorized local colony was subsequently established using breeding pairs obtained from PhysioGenix. FHH and FH low blood pressure rats were established through brother × sister inbreeding of a FH rat stock, with the former strain highly susceptible to development of hypertension and proteinuria (52). For this study, only 5- to 15-wk-old male FHH rats were used; the control age-matched male Sprague-Dawley (SD) rats were purchased from Charles River Breeding Laboratories (Kingston, NY). All protocols for animal use were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Plasmid constructs.
The pEGFP-C1 plasmids containing the mouse wild-type or mutant (i.e., T23N) Rab38 genes were prepared as described previously (57). Plasmid constructs containing the mouse monomeric red fluorescent protein (mRFP1)-tagged rat wild-type Rab38 gene or EGFP-tagged rat mutant (i.e., G19V) Rab38 gene were gifts from Dr. Miguel Seabra's laboratory (Imperial College London) (62). Constructs containing EGFP-tagged Rab4b or Rab5 were obtained from Dr. Michael Czech's laboratory (University of Massachusetts Medical School, Worcester, MA), and constructs containing EGFP-tagged Rab7 or Rab21 were obtained from Dr. David Lambright's laboratory (University of Massachusetts Medical School). All plasmid constructs were amplified in DH5α competent cells (Invitrogen, Carlsbad, CA) and then purified using the HiSpeed Plasmid Maxi kit (Qiagen, Valencia, CA). DNA concentrations were determined using a spectrophotometer (model ND-1000, NanoDrop Technologies, Wilmington, DE), with typical absorbance ratios (A260/A280) >1.8 indicating minimal protein contamination.
Cell isolation, culture, and transfection.
ATII cells were isolated from rat lungs according to the method described previously (4, 16). Briefly, a rat was anesthetized with pentobarbital sodium (50 mg/kg ip; Ovation Pharmaceuticals, Deerfield, IL); then its trachea was cannulated for air ventilation. The animal was killed by cutting the major abdominal vessels, and its lung vasculature was perfused with a Krebs-Ringer bicarbonate buffer [in mM: 118 NaCl, 4.7 KCl, 1.2 MgSO4·7 H2O, 1.3 CaCl2·2 H2O, 1.2 KH2PO4, 10 glucose, and 24.9 NaHCO3 (pH 7.4)] through the cannulated main pulmonary artery. The lung was excised and lavaged and then digested with 90 U of intratracheal elastase (Worthington, Lakewood, NJ) for 30 min at 37°C. The lung lobes were placed in 3 ml of FBS to quench the digestion reaction; then the tissue was minced using a McIlwain chopper. The resulting mixture was diluted in 50 mM Tris buffer (pH 9.5) containing 4 mg/ml DNase (Sigma, St. Louis, MO), and separated lung cells were sequentially filtered through three layers (160-, 37-, and 15-μm mesh size, respectively) of nylon gauze. The filtered cells were collected by centrifugation at 200 g for 10 min and then plated on a petri dish coated with rat IgG (Sigma) for 1 h at 37°C to allow fast macrophage attachment. The unattached ATII cells in the culture medium were collected through centrifugation at 200 g for 10 min and then seeded on fibronectin-coated coverslips in a 24-well plate at a density of 4 × 105 cells/well or on 35-mm culture dishes at a density of 3 × 106 cells/dish. Cells were typically cultured for 1–2 days in MEM (Invitrogen) supplemented with 10% FBS and 1% penicillin-streptomycin.
ATII cell transfection was carried out using Amaxa Nucleofector reagents (i.e., normal human bronchial epithelial cell kit; Lonza, Walkersville, MD). Briefly, 3 × 106 freshly isolated ATII cells were suspended in 100 μl of Nucleofector solution. Cells were then mixed with 5 μg of plasmid DNA and electroporated in an Amaxa Nucleofector II apparatus using the preset program W001. Immediately after electroporation, cells were aspirated in prewarmed MEM containing 10% FBS, incubated at 37°C for 10 min, and then transferred to a 24-well plate at a density of 4 × 105 cells/well. Transfected cells were cultured for 24–48 h before treatment for fluorescence microscopy (see below).
Immortalized lung epithelial cell lines were obtained from the American Type Culture Collection (Manassas, VA). The two murine cell lines MLE-12 and MLE-15 were grown in HITES medium [i.e., 50:50 DMEM-Ham's F-12 medium containing 0.005 mg/ml insulin, 0.01 mg/ml transferrin, 30 nM sodium selenite, 10 nM hydrocortisone, 10 nM β-estradiol, 2 mM l-glutamine, 2% FBS, 1% penicillin-streptomycin, and 10 mM HEPES buffer (pH 7.4)]. H441 cells, derived from a human lung papillary adenocarcinoma, were grown in RPMI 1640 medium containing 10% FBS and 1% antibiotics. A549 cells, derived from a human lung carcinoma, were cultured in MEM supplemented with 10% FBS and 1% antibiotics.
For plasmid transfection, MLE-15 cells were grown to ∼70% confluence on glass coverslips in a 24-well plate. For each plasmid construct to be delivered, the cells in 0.5 ml of HITES medium were transfected with 0.5 μg of purified DNA and 1.5 μl of Lipofectamine LTX reagent per well. Culture medium was replaced after 4 h to remove residual transfection reagents and cultured at least overnight before further experimentation.
Immunofluorescence labeling and fluorescence microscopy.
Cells growing on glass coverslips were rinsed with PBS (Invitrogen), fixed with 4% formaldehyde for 15 min, and then permeabilized with 1% Triton X-100 for 15 min. The cells were then blocked for 2 h at room temperature (RT) in PBS containing 5% normal goat serum and 2% BSA. Monoclonal antibody (i.e., 3C9) (35, 69) against LB marker protein ABCA3 was developed at the Institute for Environmental Medicine (IFEM) and applied to the fixed and permeabilized cells at 1:1,000 dilution for 2 h at RT. The coverslips were washed five times (5 min each) with PBS containing 0.2% Tween 20 and then incubated with 1:500 diluted Alexa 488- or Alexa 594-conjugated rabbit anti-mouse IgG (Invitrogen) for 1 h at RT. Alternatively, 50 nM LysoTracker Red (LTR; Invitrogen) was added to live ATII or MLE-15 cells for 30 min at RT before fixation to reveal their acidic lysosomal compartments. Surfactant lipids were stained by addition of 5 μM Nile Red to the cell culture, which was incubated at room temperature for 15 min. The cells were then washed twice with PBS and fixed with 4% formaldehyde for confocal microscopy. Cell nuclei were stained with 1 μg/ml Hoechst 33342 for 5 min at RT.
Confocal and multiphoton microscopy were performed using a state-of-the-art laser scanning microscope (Meta510, Zeiss) equipped with a Plan-apochromat ×63 1.4-numerical aperture oil objective. An argon-krypton laser and a helium-neon laser provide excitation light sources for the following fluorophores, with resulting fluorescence detected using corresponding emission filters as follows: excitation = 488 nm and emission = 500–550 nm band pass for EGFP, excitation = 594 nm and emission = 615 nm long pass for Alexa 594, excitation = 543 nm and emission = 560 nm long pass for mRFP1, and excitation = 543 nm and emission = 560 nm long pass for LTR. Two-photon excitation of the nuclear stain Hoechst 33342 was accomplished using mode-locked (i.e., wavelength center = 750 nm and width ∼10 nm) femtosecond laser pulses generated by a Chameleon Ultra solid-state laser (Coherent, Santa Clara, CA), with resulting fluorescence detected by a 390- to 465-nm band-pass filter. Confocal microscopy of one-photon-excited fluorescence was carried out with the pin-hole size of 1 optical unit, while the two-photon-excited fluorescence was detected with the confocal pin-hole opened to its maximum aperture.
FRAP experiments were carried out using the confocal microscope. Briefly, SD ATII cells transiently overexpressing the EGFP-Rab38 fusion protein were cultured in 35-mm-diameter dishes. After 2 days of culture, the culture medium in each dish was switched to 2 ml of Krebs-Ringer-HEPES buffer [i.e., 125 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 1.2 mM MgSO4, 20 mM d-glucose, 25 mM HEPES (pH 7.4), and 0.2% (wt/vol) BSA]. A dish was transferred to a temperature controller (model TC-202A, Harvard Apparatus, Holliston, MA) operating at 37°C, and the cells were imaged using a Zeiss plan-Aprochromat ×63 1.0 NA water-immersion objective. The bleaching area was precisely defined by a scanning 488-nm laser beam controlled by an acoustic-optical shutter with response time in milliseconds. Photobleaching used 100% of the 488-nm laser power, and regular image acquisition used <5% of the laser power. A basal image was acquired immediately before photobleaching, and an image was obtained immediately after photobleaching. Subsequent fluorescence recovery images were taken every 10 s for a total of 480 s.
Images were processed and analyzed using ImageJ (http://rsbweb.nih.gov/ij/). Briefly, eight-bit red-green-blue color images were contrast-rescaled separately to achieve similar visual brightness among the color channels. The green images are further translated, relative to the red image, in the left and bottom directions for one pixel each to correct for the chromatic effect of the imaging system. Quantitative image analysis was carried out using ImageJ (see Supplemental Fig. S1 in Supplemental Material for this article, available online at the Journal website).
Lung homogenization and LB purification.
LBs were purified from rat lungs by upward flotation on a discrete sucrose gradient, as described previously (10). Briefly, a perfused and lavaged lung was minced and homogenized in 1 M sucrose. The homogenate was filtered through one layer of gauze and then layered to the bottom of a discontinuous 0.9–0.2 M sucrose gradient, with sucrose concentrations decreasing incrementally by 0.1 M from bottom to top. After centrifugation at 100,000 g for 3 h at 4°C, the LB fraction was collected at the 0.4–0.5 M sucrose interface. Sucrose concentration in the collected LB fraction was diluted to 0.2 M determined by a refractometer. The purified LBs were then centrifuged at 20,000 g for 15 min, and resulting pellets were rinsed with 0.9% (wt/vol) NaCl and stored in a freezer at −80°C. Protein concentrations of purified LB fractions were measured by the Bradford method using the RC-DC protein assay kit (Bio-Rad, Hercules, CA).
Western immunoblotting.
Cells were detached from culture dishes with 0.25% trypsin and collected by centrifugation at 500 g for 5 min. Resulting cell pellets were lysed in an ice-cold RIPA buffer [150 mM NaCl, 1.0% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris·HCl (pH 8.0)] containing a protease inhibitor cocktail (Sigma) for 5 min on ice. Cell lysates were spun at 12,000 g for 15 min, and resulting pellets and supernatants were stored in a freezer at −20°C. Protein concentrations were determined using the RC-DC protein assay kit before Western blot analysis. Macrophages were obtained by centrifugation of rat lung lavage fluid at 500 g for 10 min, and the resulting cell pellets were lysed in the ice-cold RIPA buffer. Human pulmonary microvascular endothelial cells (59) and rat pulmonary microvascular endothelial cells (34), obtained from the IFEM Cell Culture Core, were lysed according to the procedure described above. Human umbilical vein endothelial cell (Lonza) lysate was a gift from the Muzykantov laboratory at IFEM. Lung homogenate was obtained as follows: perfused lung was isolated, cleared of blood, suspended in 0.9% (wt/vol) NaCl, rinsed with ice-cold 50 mM Tris·HCl buffer (pH 7.6) containing 0.1 mM EDTA and the protease inhibitor cocktail, scissor-minced into small pieces, rinsed with ethanol, and then homogenized in 5–10 ml of the Tris-EDTA buffer using a motor-driven homogenizer. The crude homogenate was centrifuged at 1,000 g for 5 min, and the resulting supernatant was further sonicated and centrifuged at 1,500 g to produce the final supernatant used for Western blot analysis.
Equivalent amounts (i.e., 40 μg protein per lane) of the cell lysates or lung homogenates were loaded onto a 12% Bis-Tris SDS-polyacrylamide gel (Invitrogen). Electrophoresis was carried out using a MOPS running buffer under reducing conditions (NuPAGE sample reducing reagent, Invitrogen), and separated protein bands were transferred to a nitrocellulose membrane using an XCell II Blot Module (Invitrogen). Fluorescence of the transferred protein bands was detected using an Odyssey Imager (LI-COR Biosciences, Lincoln, NE). Briefly, the nitrocellulose membrane was blocked with the Odyssey blocking buffer at RT for 2 h on a shaking platform and incubated overnight at 4°C with a primary antibody in the Odyssey blocking buffer. After the membrane was washed three times with PBS containing 0.2% Tween 20 (i.e., PBST buffer), it was incubated with a secondary antibody conjugated with near-infrared dye (IRDye800 or IRDye700) at 1:10,000 dilution for 1 h at RT. The membrane was subsequently rinsed three times in PBST buffer and once in PBS buffer and then scanned using the Odyssey infrared scanner. The membrane was further stripped using the Odyssey stripping buffer and reprobed using another primary antibody. Monoclonal antibodies against Rab38 and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA) were applied to the Western blot at 1:1,000 dilution. Monoclonal anti-actin antibody was purchased from Sigma and applied to the Western blot at 1:2,000 dilution. Rabbit polyclonal anti-surfactant protein (SP)-A antibody (a gift from Dr. Sandra Bates' laboratory at IFEM) was used at 1:1,500 dilution. Rabbit polyclonal anti-calnexin antibody (Enzo Life Sciences, Ann Arbor, MI) was used at 1:1,000 dilution.
Electron microscopy.
Lungs from the FHH and SD rats were perfused through the main pulmonary arteries with 0.1 M sodium cacodylate buffer until the lungs became white, indicating that they were clear of blood. The lungs were fixed using 5% glutaraldehyde delivered through the main pulmonary artery and trachea. The trachea was then ligated, and the whole lungs were kept in the fixative for >4 h. Subsequently, the left lungs were chopped into 1-mm3 blocks, treated with 2% osmium for 2 h, dehydrated with graded acetone, embedded in EPON, and polymerized at 60°C for 48 h. Ultrathin (80-nm-thick) lung sections were prepared with a Leica Ultracut UCT ultramicrotome and then stained with 2% uranyl acetate and 0.5% lead citrate. The tissue sections were examined using a TEM (model 100-CX, Jeol).
RESULTS
Enlarged LBs in lung ATII cells of the Rab38-null FHH rat.
Previous studies demonstrated the enlarged LB phenotypes in lung ATII cells of Rab38-mutated Chocolate mice and LEC rats (39, 41), although a direct causal relationship has not been established. For example, besides the Rab38-null mutation, the LEC rat additionally carries a mutation on the ATP7p copper-transporting ATPase (67). While the latter genetic mutation makes the LEC rat a widely used animal model for human Wilson disease, it may complicate phenotypic analysis attributed to the Rab38-null mutation in this rat. Therefore, using TEM, we quantitatively evaluated the enlarged LB phenotype associated with the Rab38-null mutation on the different genetic background of the FHH rat.
The FHH rat likely inherited the Rab38/Met1Ile mutation from a substrain of the LE rat (38). Consistently, Western immunoblotting of lung homogenates obtained from the FHH rats revealed no Rab38 protein expression (Fig. 1A). In contrast, abundant Rab38 expression was detected in lungs of control SD rats (Fig. 1A). The monoclonal anti-Rab38 antibody used here revealed one unambiguous band migrating besides the 25,000-molecular weight (MW) marker (Fig. 1A). This result agrees well with the predicted 24,000 MW for the rat Rab38 protein (40), and the slightly higher apparent MW may reflect its COOH-terminal prenylation (41).
Fig. 1.
Fawn-hooded hypertension (FHH) rat lungs were deficient in Rab38 protein expression, with significantly enlarged lamellar bodies (LBs) in their alveolar type II (ATII) epithelial cells. A and B: equal amounts (40 μg protein/lane) of lung homogenates (LHs) obtained from 2 pairs of age-matched (10-wk-old) FHH and Sprague-Dawley (SD) rats were subjected to SDS-PAGE and Western blot analysis using the anti-Rab38 and anti-actin antibodies. C and D: representative transmission electron microscopy images of LB cross sections in ATII cells of ultrathin (∼80-nm-thick) lung slices obtained from a pair of 10-wk-old SD and FHH rats. E–G: long axes of LB cross sections (i.e., “diameter”) in transmission electron microscope images were measured (red lines in C and D), and their numbers were counted in individual ATII cells, producing normalized (by total LB number) frequency distributions of measured LB diameters, average LB diameters, and average LB numbers per cell. Error bars, SE. *Statistically significantly different (P < 0.001) as evaluated by the Excel function of 2-tailed t-test with 2 unpaired samples of unequal variances.
To examine LB morphology under physiologically relevant conditions, ATII cells embedded in ultrathin (∼80-nm-thick) lung slices obtained from the SD or FHH rats were imaged using TEM. LBs are prominent organelles in lung ATII cells, and they were clearly enlarged in these cells from lungs of FHH rats (Fig. 1, C and D). To quantify relative change in LB size, we measured long axes of LB cross sections (hereafter, LB “diameters”) in the TEM images (red lines in Fig. 1, C and D). A total of 221 and 269 LBs were measured from 24 SD and 33 FHH ATII cells, respectively. Resulting frequency distributions of these LB diameters, normalized to total LB numbers, revealed a global shift toward larger sizes in the FHH cells (Fig. 1E). This shift appeared to be asymmetric, with a subpopulation of FHH ATII cells containing only a few dramatically enlarged giant LBs (Supplemental Fig. S1). The intact lamellar structures in these giant LBs suggest that SPs (e.g., SP-B) critical for such structures were not diminished by the Rab38-null mutation. On average, LB diameter was ∼77% larger in a FHH ATII cell than in a SD ATII cell (Fig. 1F). There was a slight decrease (∼12%) in the average LB number per FHH ATII cell, but this decrease was not statistically significant based on our current sample populations (Fig. 1G). Using these average numbers, we can calculate that the average total LB volume is nearly five times larger in a FHH ATII cell than in a SD ATII cell. This result is consistent with the almost threefold increase in LB phosphatidylcholine content in the Rab38-null LEC ATII cells (39) and confirms the association between enlarged LBs and the Rab38-null mutation in the FHH rat.
Restricted Rab38 expression in epithelial, but not endothelial, cells.
The dramatically enlarged LBs in the Rab38-null FHH ATII cells strongly suggest that Rab38 plays a special trafficking role in these specialized lung epithelial cells. Thus, using Western immunoblot assay, we compared Rab38 protein expressions in several types of epithelial vs. endothelial cells (Fig. 2, A and B). In addition to the freshly (day 0) isolated SD ATII cells, abundant Rab38 expression was found in two epithelial cell lines derived from mouse lung tumors (i.e., MLE-12 and MLE-15; Fig. 2A). Two human lung tumor epithelial cell lines (i.e., H441 and A549) also expressed Rab38 protein, with the protein level in the former comparable to that in the mouse/rat cells (Fig. 2A). In contrast, no Rab38 protein was detected in three types of primary endothelial cells (i.e., human and rat pulmonary microvascular endothelial cells and human umbilical vein endothelial cells; Fig. 2A). It has been reported that Rab38 mRNA and protein are absent in rat alveolar macrophages (40). However, with use of the same method to isolate alveolar macrophages from lung lavage, our Western immunoblot revealed a trace amount of Rab38 protein in these cells. This minor discrepancy may be due to the different antibodies used in these studies or to potential leukocyte contamination in our macrophage preparation (5). After normalization of Rab38 expressions against their respective actin or GAPDH protein levels in the above-mentioned cells, the MLE-15 cell apparently had the second-highest Rab38 content, behind the freshly isolated SD ATII cells (Fig. 2B). Consistently, TEM images revealed cellular organelles in some MLE-15 cells that morphologically resembled the LBs in ATII cells (Supplemental Fig. S2B). These morphological LBs are further consistent with the recent study demonstrating that the MLE-15 cell expresses most of the marker genes associated with a primary lung ATII cell (9). Taken together, these results indicate restricted Rab38 expression in lung epithelium, and the MLE-15 cell can be an alternative system for investigating Rab38-regulated trafficking mechanisms.
Fig. 2.
Rab38 protein expression was restricted in lung epithelial cells. A: Rab38 protein expression (Exp) was analyzed using Western immunoblotting in epithelial cells, including ATII cells freshly isolated (day 0) from SD rat lungs, human lung epithelial cell lines H441 and A549, and mouse lung epithelial cell lines MLE-12 and MLE-15, in endothelial cells, including human pulmonary microvascular endothelial cells (HPMVEC), rat pulmonary microvascular endothelial cells (RPMVEC), and human umbilical vein endothelial cells (HUVEC), and in macrophages isolated from SD lung lavage. An equal amount (i.e., 40 μg protein/lane) of cell lysates was loaded onto a 12% Bis-Tris gel, and separated protein bands were analyzed using the anti-Rab38, anti-actin, and anti-GAPDH antibodies. B: densitometry quantification of Western blot in A. Rab38 protein levels were normalized (Nor) against corresponding actin or GAPDH levels in cells listed in A. C: Western blot analysis of Rab38 protein levels in isolated SD ATII cells cultured consecutively for 5 days. An equal amount (40 μg protein/lane) of cell lysates was subjected to SDS-PAGE and probed with anti-Rab38 and anti-GAPDH antibodies. D: densitometry quantification of Rab38 expressions, normalized against corresponding GAPDH levels, as a function of days in culture. Error bars, SE of 4 experiments.
Consistent with a specialized trafficking role for Rab38 in lung ATII cells, we found a gradual decline in Rab38 protein level during 5 days of culture of isolated SD ATII cells (Fig. 2, C and D). In contrast, Rab38 mRNA level has been reported to decrease precipitously after overnight culture of isolated rat ATII cells (42). If both data are correct, then the Rab38 protein turnover rate is relatively slow. Our result was obtained after normalization of Rab38 expressions against the respective GAPDH protein levels and after the Western immunoblot experiment was repeated four times (Fig. 2D). The protein levels varied significantly during the day 1 of culture (Fig. 2D), which likely reflected variation of qualities of the isolated ATII cells from different preparations and the subsequent variation in efficiency of attaching to culture dishes. Since it has been well established that cultured ATII cells gradually lose their characteristic gene expression (9), the accompanying decline in Rab38 protein expression (Fig. 2, C and D) supports a specialized role for Rab38 in regulating intracellular surfactant homeostasis and LB morphology in lung ATII cells.
Presence of endogenous Rab38 in LBs purified from SD rat lungs.
Although the morphological and physiological consequences of Rab38 deficiencies in mouse/rat lungs are well documented (Fig. 1) (39, 41), the precise subcellular Rab38 localization in ATII cells has yet to be firmly established. Particularly, endogenous Rab38 has been reported to be primarily associated with ER membranes of ATII cells but was absent on purified LB fractions (40). Consequently, an indirect mechanism (i.e., away from the LB compartment) seems to be necessary to explain the dramatically enlarged LBs in the Rab38-mutated ATII cells (Fig. 1) (39, 41). Therefore, we reexamined endogenous Rab38 presence in LBs purified from SD rat lungs.
Following the previously established method of equilibrium centrifugation of homogenized lungs (10), LB fractions were purified from FHH and SD rats and subjected to Western blot analysis (Fig. 3A). In contrast to an previous report (40), we found Rab38 not only in the lung homogenates (LHs), but also in the LB fractions, obtained from SD rats (Fig. 3A). In the control FHH samples, such Rab38 bands were not detected in the Western immunoblot (Fig. 3A). The constitutive LB protein SP-A was dramatically concentrated (i.e., 14–20×) from its levels detected in the LHs (Fig. 3A), indicating an effective purification procedure. Interestingly, as percentage of loaded protein samples (i.e., 20 and 30 μg/lane for LBs and LHs, respectively, in Fig. 3A), SP-A appeared at higher concentrations in LB fractions purified from FHH lungs. On the other hand, Rab38 was ∼42% less abundant in the LBs than in the LHs (Fig. 3A). This result may be explained by previous reports that a substantial fraction of endogenous Rab38 is present in ATII cell cytosol (40, 41). Importantly, our purified LB fractions were free of cytosol contamination, as indicated by the absence of GAPDH, an abundant cytosolic enzyme, in the LB fractions (Fig. 3A). Nevertheless, Rab38 content in the purified LB fraction appeared to be highly variable. In a follow-up experiment (Fig. 3B), we found that, as percentage of loaded SD protein samples (i.e., 20 μg/lane for LB and 40 μg/lane for ATII and LH), Rab38 was enriched by nearly three- and ninefold in the ATII cell lysate and LB fraction, respectively, compared with the LH. Consistent with the earlier experiment (Fig. 3A), SP-A was concentrated by ∼1× and ∼15× in the ATII cell lysate and LB fraction, respectively (Fig. 3B). The Rab38 concentration in this LB sample was not due to cytosol or ER contamination, as indicated by the almost complete absence of the marker proteins GAPDH and calnexin in the sample, respectively (Fig. 3B). Thus, Rab38 content in purified LBs appeared to be sensitive to the purification procedure, which may explain the previous negative result (40). Furthermore, Rab38 membrane association in ATII cells shall also be tightly regulated (see below and discussion), which may also contribute to the varying results. Collectively, the above-described results establish endogenous Rab38 presence in LB fractions purified from SD rat lungs.
Fig. 3.
Endogenous Rab38 in LB fractions purified from SD rat lungs. A: purified LBs (20 μg protein/lane) and LHs (30 μg protein/lane) derived from 2 pairs of age-matched FHH and SD rats were loaded onto a 12% Bis-Tris gel, and separated protein bands were probed using anti-Rab38, anti-surfactant protein (SP)-A, and anti-GAPDH antibodies. B: purified LBs (20 μg protein/lane), lysate of freshly isolated ATII cells (40 μg protein/lane), and LHs (40 μg protein/lane), all obtained from SD rats, were subjected to SDS-PAGE and Western blot analysis with anti-calnexin antibody in addition to antibodies used in A.
Rab38 selectively targets to a subpopulation of LBs in lung ATII cells.
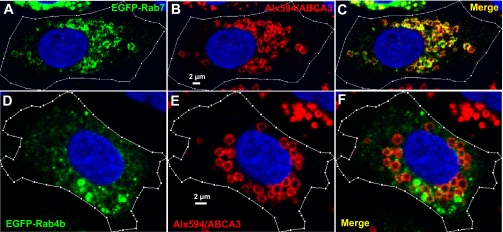
To further probe precise Rab38 localization among and within the LBs, EGFP-Rab38 fusion protein was transiently reexpressed in the Rab38-null FHH ATII cells (Fig. 4; Supplemental Fig. S4). The electroporation procedure introducing the EGFP-Rab38 plasmid into the freshly isolated ATII cells is a stochastic process, resulting in protein expression levels varying over several orders of magnitude in individual cells cultured for 2 days following transfection (Fig. 4D; Supplemental Fig. S4). These cells were fixed, and the limiting membranes of their LBs were immunofluorescently labeled using the previously characterized monoclonal antibody against the LB membrane protein ABCA3, a lipid transporter required for LB biogenesis (35, 69). Confocal and two-photon (for imaging the nuclear stain Hoechst 33342) microscopy produced optical slices (∼0.8 μm thick) of these cells containing LB cross sections with clearly visible lumens (Fig. 4B; Supplemental Fig. S3). Careful normalization of the EGFP fluorescence within a confocal cell section to the section area and image-acquisition conditions (Supplemental Fig. S3) results in the normalized fluorescence intensity (NI), which reflects EGFP-Rab38 protein level in a transfected ATII cell (Fig. 4A). Low laser power and the ultrasensitive detectors of our confocal/multiphoton microscope resulted in EGFP fluorescence that was highly photostable (i.e., essentially unchanged for >30 min), so that our quantitative analysis was not distorted by the photobleaching effect. This approach showed that, at an expression level (<IEGFP>) barely above background signals (<Ibackground>; Fig. 4A), only a few of the LBs revealed by the anti-ABCA3/Alexa 594 immunofluorescence (i.e., the primary anti-ABCA3 antibody combined with the secondary Alexa 594-conjugated antibody; Fig. 4B) were targeted by the EGFP-Rab38 fusion protein in the FHH ATII cell (Fig. 4C). At this low expression level, EGFP-Rab38 was also visible in the cytosol (Fig. 5A), consistent with previous reports (40, 41). Importantly, the fusion protein revealed, for the first time, Rab38 localization to the limiting membranes of LBs. Surprisingly, we found that EGFP-Rab38 consistently labeled ∼30% ABCA3-positive LBs in individual FHH ATII cells, with fusion protein expression levels ranging from barely above background to two orders of magnitude higher (Fig. 4D; Supplemental Fig. S4). This result indicates that the subcellular localization revealed by the EGFP-Rab38 fusion protein is unlikely due to nonspecific targeting but reflects a specific mechanism recruiting the small GTPase to a subpopulation of the seemingly homogeneous LBs. Transiently overexpressed EGFP-Rab38 in 1-day-cultured ATII cells isolated from SD lungs revealed the same selective targeting to a subpopulation of the ABCA3-positive LBs (Fig. 4, E–G). These results indicate that the Rab38-recruiting mechanism is preserved in the SD and FHH ATII cells. Interestingly, in some SD ATII cells, the EGFP-Rab38 protein, overexpressed on top of the endogenous protein, revealed perinuclear localization (Fig. 4E), which is consistent with the previous reports of ER targeting for the endogenous Rab38 (40, 42).
Fig. 4.
Enhanced green fluorescent protein (EGFP)-Rab38 selectively targets to a subpopulation of LBs in cultured ATII cells. A–D: ATII cells freshly isolated from FHH rat lungs were electroporated in the presence of a plasmid construct containing the EGFP-tagged wild-type mouse Rab38 gene (EGFP-Rab38; green). Cells were cultured for 2 days to allow recovery and then fixed and immunofluorescently stained with the primary anti-ABCA3 antibody and the secondary Alexa 594-conjugated antibody (ABCA3/Alx594; red). Cell nuclei were stained with Hoechst 33342 (blue). An FHH ATII cell with EGFP-Rab38 reexpressed at a minimal level (A) and with its LB limiting membranes immunofluorescently labeled with anti-ABCA3/Alexa 594 antibodies (B) was imaged using confocal and multiphoton (for exciting Hoechst 33342 dye) microscopy, revealing only a few of the ABCA3-positive LBs labeled by the EGFP-Rab38 fusion protein (C). EGFP (<IEGFP>) and background (<Ibackground>; e.g., dark current, scattered light, autofluorescence) signals were normalized to the cell area (white outline) and imaging conditions (Supplemental Fig. 3) and, thus, were expressed as normalized intensity (NI) per square micrometer of confocal cell section. D: percentage of LBs targeted by EGFP-Rab38 vs. corresponding fusion protein expression level in individual FHH ATII cells. Cells near the y-axis had very low, but above background, EGFP-Rab38 intensities. Data point associated with images (A–C) is shown in green; average data are shown in purple. Error bars, SE. E–J: EGFP-Rab38 was transiently overexpressed in SD ATII cells cultured for 1 day after electroporation. LBs were labeled using the anti-ABCA3/Alexa 594 antibodies (E–G) or LysoTracker Red (LTR) dye (H–J). N1 and N2 represent 2 unstained nuclei.
Fig. 5.
Point mutations abolished EGFP-Rab38 targeting to ABCA3-positive LBs. SD ATII cells were transfected with EGFP-Rab38 plasmid and immunofluorescently labeled using the anti-ABCA3/Alexa 594 antibodies after 1 day of culture. A–C: transiently overexpressed EGFP-Rab38/G19V mutant (green), lack of its COOH-terminal prenylation, did not specifically target to the ABCA3-positive LBs (red) in a SD ATII cell. D–F: GTP-binding-deficient EGFP-Rab38/T23N mutant (green), when transiently overexpressed in a SD ATII cell, did not colocalize with the ABCA3-positive LBs (red) but appeared to cause cell stress and death.
To test whether the EGFP-Rab38-targeted cellular organelles are LBs, LTR was used to stain the acidic LB lumen (Fig. 4I). It was thus shown that the EGFP-Rab38-targeted organelles preserved the acidic luminal environment that was indistinguishable from that found in the untargeted LBs (Fig. 4, H–J). Similarly, the EGFP-Rab38-targeted organelles also contained abundant LB protein SP-A (Supplemental Fig. S5, A–C) and surfactant lipids stained by the lipophilic dye Nile Red (Supplemental Fig. S5, D–F). Thus the LB markers ABCA3, LTR, SP-A, and Nile Red collectively confirmed that the EGFP-Rab38-targeted organelles were a subpopulation of LBs in lung ATII cells.
Specific Rab38 targeting to LBs was further confirmed using Rab38 mutant proteins transiently overexpressed in 1-day-cultured SD ATII cells (Fig. 5). It has been previously established that the Rab38/Gly19Val mutant, found in the Chocolate mice, loses its COOH-terminal lipidation that is essential for Rab38 membrane association (43). Consistently, overexpressed EGFP-Rab38/Gly19Val mutant was predominantly found in the cytosol and nuclear space of the SD ATII cells (Fig. 5A). Abundant ABCA3-positive LBs were found in these cells (Fig. 5B) and did not specifically colocalize with the EGFP-Rab38/Gly19Val fusion protein (Fig. 5C). These apparently normal LBs suggest that the Rab38 mutant does not interfere with the function of the endogenous Rab38 protein in the SD ATII cell. In contrast, overexpression of the dominant-negative Rab38/Thr23Asn mutant was toxic to the SD ATII cells; very few cells expressed the EGFP-tagged mutant protein, and these cells, without exception, rounded up and contained defragmented nuclei (Fig. 5, D–F). As a result, no Rab38/Thr23Asn-expressing cells were found after day 2 of culture. This result suggests that the dominant-negative Rab38 mutant is able to interfere with the endogenous Rab38 function, which is critical for survival of SD ATII cells.
Finally, we demonstrated that selective LB targeting is a unique property of Rab38, but not the other Rab proteins also functioning in ATII cells. Rab7 plays a critical role in mediating late endosome-lysosome fusion and has been previously used as a LB marker (65). Consistently, transiently overexpressed EGFP-tagged Rab7 was targeted to the limiting membranes of all ABCA3-positive LBs (Fig. 6, A–C). In contrast, Rab4 has been proposed to regulate recycling of endocytosed SP-A back toward the plasma membrane, before the endocytosed protein reaches the LBs (65). Consistently, there was minimal colocalization between the transiently overexpressed EGFP-tagged Rab4b fusion protein and the ABCA3-positive LBs in cultured SD ATII cells (Fig. 6, D–F). These results suggest that selective Rab38 targeting to a subpopulation of LBs is required for its specific trafficking function in lung ATII cells.
Fig. 6.
Rab7 and Rab4b were not selectively targeted to ABCA3-positive LBs. A–C: transiently overexpressed EGFP-Rab7 (green) labeled essentially all ABCA3-positive LBs (red) in a SD ATII cell. D–F: transiently overexpressed EGFP-Rab4b (green) was minimally colocalized with ABCA3-positive LBs (red) in a SD ATII cell.
Reexpressing Rab38 in FHH ATII cells rescues the enlarged LB phenotype.
The enlarged LB phenotypes observed in the three types of Rab38-mutated rodents (i.e., Chocolate mice and LEC and FHH rats) strongly suggest that Rab38 is involved in intracellular surfactant homeostasis reflected in LB volume. To establish a causal relationship between Rab38 mutation and altered LB morphology, we first developed a confocal microscopy method for quantifying relative change in LB size using cultured ATII cells (Fig. 7; Supplemental Fig. S3). ATII cells were isolated from 7-wk-old SD and FHH rats and cultured overnight on glass coverslips to allow the cell attachment and spreading that were necessary for revealing the intracellular LBs. After anti-ABCA3/Alexa 488 immunofluorescence labeling, confocal microscopy revealed the enlarged LB phenotype preserved in the cultured FHH ATII cells (Fig. 7, A and B). To avoid analysis of nonspecific immunofluorescence stains, only LB cross sections with visible lumens were measured for their long axes (i.e., LB “diameters”; Supplemental Fig. S3). A total of 617 and 503 LBs were measured in 28 SD and 29 FHH cells, respectively. Resulting normalized frequency distributions of these LB diameters revealed a global shift toward larger sizes in the FHH rat cells (Fig. 7C), consistent with the similar shift derived from the TEM images (Fig. 1E). The average LB diameter was ∼44% larger in the cultured FHH than in the SD rat cells (Fig. 1D) compared with the corresponding ∼77% increase observed using the TEM images (Fig. 1F). Furthermore, the confocal analysis detected, on average, a ∼21% decrease in LBs in an FHH ATII cell (statistically significant; Fig. 7E) compared with the corresponding ∼12% decrease revealed by TEM (not statistically significant; Fig. 1G). These quantitatively, but not qualitatively, different results are expected from the two entirely different imaging techniques and could be mostly explained by the different imaging resolutions associated with the microscopy methods (see discussion). Important for the following rescue experiments, the cultured FHH ATII cells temporally preserved its enlarged LB phenotype, which can be reliably evaluated using quantitative confocal microscopy.
Fig. 7.
Enlarged LB phenotype in cultured FHH ATII cells quantitatively evaluated using confocal microscopy. A and B: representative confocal/multiphoton images of LBs in overnight-cultured ATII cells isolated from a pair of 7-wk-old SD and FHH rats. Limiting membranes of LBs were immunofluorescently labeled using anti-ABCA3 antibody and secondary Alexa 488 (Alx488)-conjugated (green) rabbit anti-mouse antibody. Cellular nuclei were stained with Hoechst 33342 dye (blue). C–E: LB “diameters” and numbers in individual cells were measured and counted as described by Supplemental Fig. S3, producing normalized frequency distributions of measured LB diameters, average LB diameters, and average LB numbers per cell. Error bars, SE. *Statistically significantly different (P < 0.001) as evaluated by the Excel function of 2-tailed t-test with 2 unpaired samples of unequal variances.
Using MLE-15 cells, we further established that the EGFP-Rab38 fusion protein preserved its reported molecular function (Supplemental Figs. S6 and S7). Rab38 and Rab32 have been reported to be upstream regulators of Rab21, but not Rab5, through interactions with their shared downstream effector, the VPS9-ankyrin-repeat protein (Varp) (8, 57, 61, 70). Consistently, cotransfecting MLE-15 cells with EGFP-tagged Rab21 and mRFP1-tagged Rab38 resulted in extensive colocalization of these two Rabs on the perinuclear membranes (Supplemental Fig. S6). In contrast, cotransfected EGFP-Rab5 and mRFP1-Rab38 did not colocalize on the peripheral Rab5-positive early endosomes or on the perinuclear membranes (Supplemental Fig. S7). These results establish the validity of the EGFP-Rab38 fusion protein as a suitable reagent for the prior subcellular localization studies and for the following rescue experiments.
Next, ATII cells freshly isolated from 12-wk-old SD and FHH rats were electroporated with plasmids containing the EGFP-Rab38 fusion gene or EGFP alone, with SD and FHH rat ATII cells undergoing mock transfections used as additional controls. Compared with the unelectroporated ATII cells shown in Fig. 7, A and B, the electroporated ATII cells displayed higher degrees of heterogeneity in LB morphology that may be attributed to various degrees of cell injuries inflicted by the electroporation procedure. Therefore, the cells were cultured for 2 days after electroporation to allow sufficient recovery. Batches of the electroporated cells after each day of culture were then immunostained using the anti-ABCA3/Alexa 594 antibodies for quantitative confocal microscopy. After 1 day of culture (Fig. 8, A–C), the EGFP protein was expressed nearly four times more efficiently than the EGFP-Rab38 fusion protein in the transfected FHH cells (Fig. 8A), as indicated by the respective average normalized EGFP fluorescence intensities (Supplemental Fig. S3). This finding is consistent with EGFP being an alien protein to the ATII cells, while EGFP-Rab38 shall be subjected to intrinsic mechanisms controlling its protein level. Nevertheless, the significantly higher EGFP expression had no statistically discernable effect on the average LB size in the FHH cells (Fig. 8B). In contrast, the reexpressed EGFP-Rab38 reduced the average LB diameter in the FHH cells by ∼20%, eliminating approximately half of the average LB diameter gap between the cultured SD and FHH ATII cells (Fig. 8B). Consistently, the normalized frequency distribution for LB diameters measured in the EGFP-Rab38-expressing FHH cells shifted about halfway toward that derived from the mock-transfected SD cells (Fig. 8C). The average LB diameters derived from the electroporated and 1-day-cultured SD and FHH ATII cells were 1.08 and 1.61 μm, respectively (Fig. 8B). In comparison, the average LB diameters for the nonelectroporated and 1-day-cultured SD and FHH ATII cells were 1.22 and 1.75 μm, respectively (Fig. 7D). Therefore, the electroporation procedure resulted in small and similar reductions in the average LB diameters for the cultured SD and FHH rat cells. Importantly, after electroporation and 1 day of culture, LBs were still, on average, ∼49% larger in the FHH than SD cells (Fig. 8B), essentially unchanged from the ∼44% gap observed between the nonelectroporated and 1-day-cultured cells (Fig. 7D). After 2 days of culture, the transiently expressed EGFP and EGFP-Rab38 protein levels were reduced by ∼41% and ∼94%, respectively (Fig. 8D). This dramatic reduction in the EGFP-Rab38 protein level not only reflects the transient nature of the exogenous protein expression but also the gradual decline of endogenous Rab38 protein level during a 5-day culture of isolated SD ATII cells (Fig. 2, C and D). It has been reported that cultured ATII cells gradually transdifferentiate into type I-like alveolar epithelial cells, accompanied by gradual loss of ATII cell-specific gene expressions (17). Thus the EGFP-Rab38 fusion protein could be regulated by the intrinsic mechanisms governing the transdifferentiation process. Such a process might also contribute to the reduced, but still statistically significant, gap (∼12%) between the average LB diameters derived from the mock-transfected and 2-day-cultured SD and FHH cells (Fig. 8E). Again, EGFP alone had no effect on the average LB diameter in 2-day-cultured FHH cells, while the dramatically reduced EGFP-Rab38, after 2 days of intracellular presence and function, was able to completely eliminate the average LB diameter gap between the mock-transfected SD and FHH cells (Fig. 8E). Consistently, the normalized frequency distribution of LB diameters measured in the EGFP-Rab38-transfected FHH cells overlapped with that in the mock-transfected SD cells (Fig. 8F). Interestingly, we found that the average LB diameters were larger in the 2- than in the 1-day-cultured ATII cells (Fig. 8, B and E). This was probably because freshly isolated ATII cells were round objects with thin cytoplasm layers surrounding large nuclei (Supplemental Fig. S8). During subsequent culture, the cells attached to the coverslips and started to flatten and spread. After 2 days of culture, the average confocal cell sections were ∼40% larger than corresponding cells cultured for 1 day (Supplemental Fig. S9). Nevertheless, under the same experimental conditions and within the same culture durations, the reexpressed EGFP-Rab38 fusion protein was able to significantly reverse and then completely eliminate the enlarged LB phenotype in the cultured FHH ATII cells.
Fig. 8.
Reexpressed EGFP-Rab38 reversed and rescued the enlarged LB phenotype in cultured FHH ATII cells. FHH and SD ATII cells underwent mock transfection or were transfected with EGFP-Rab38 or EGFP plasmid and were immunofluorescence-labeled with the anti-ABCA3/Alexa 594 antibodies. Exogenous protein expression levels and LB “diameters” were measured as described in Supplemental Fig. S3. A–C: exogenous protein expression levels, average LB diameters, and normalized frequency distributions of measured LB diameters derived from ATII cells cultured for 1 day after electroporation. D–F: protein expression levels, average LB diameters, and normalized frequency distributions of measured LB diameters derived from ATII cells cultured for 2 days after electroporation. For clarity, diameter distribution of LBs in ATII cells transfected with the EGFP construct is not shown in C and F. Error bars, SE. *Statistically significantly different (P < 0.001) as evaluated by the Excel function of 2-tailed t-test with 2 unpaired samples of unequal variances.
Dynamic Rab38 targeting to and on the LB limiting membrane.
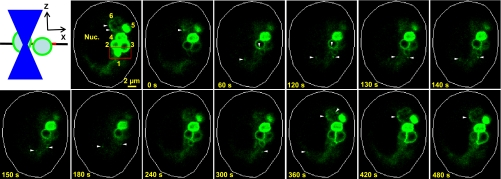
The ability of the EGFP-Rab38 fusion protein to normalize the enlarged LBs in cultured FHH ATII cells (Fig. 8) while targeting to a subpopulation of LBs in fixed cells (Fig. 4) suggests that Rab38-LB association is dynamic. Thus we carried out FRAP experiments using our confocal microscope to probe EGFP-Rab38 dynamics in cultured SD ATII cells (Fig. 9; Supplemental Movie 1). The diagram of figure 9 illustrates the confocal photobleaching geometry in the xz plane. In the confocal image taken immediately before photobleaching, there were six EGFP-Rab38-labeled LBs (i.e., LB 1–6), with LB 6 only weakly labeled by the fusion protein. A limited area in the focal plane, enclosing completely LB 1 and partially LBs 2 and 3, was photobleached by the 488-nm laser beam focused to a diffraction-limited spot (∼260 nm diameter). In the confocal setup, fluorescence above and below the focal plane was also bleached by the focused laser beam [blue triangles in diagram (left) in Fig. 9]. In contrast, areas adjacent to the bleaching box in the focal plane were not affected. Consistently, in the first image taken immediately after photobleaching (i.e., 0 s), LB 1 was almost completely bleached, while LBs 2 and 3 were partially bleached, with the remaining EGFP-Rab38 fluorescence approximately equally distributed along their limiting membranes. Fluorescence on LB 4, immediately adjacent to LBs 2 and 3, was not affected. These results indicate that the EGFP-Rab38 molecules associated with the LB limiting membranes diffuse rapidly and reequilibrate within the ∼1.3 s required for single image acquisition. Fluorescence recovery was monitored every 10 s (Supplemental Movie 1), with the recovery images potentially complicated by LB movements into and out of the focal plane (see LBs 5 and 6) during the 480-s experimental duration (selective recovery images shown in Fig. 9). Nevertheless, it was clear that EGFP-Rab38 fluorescence was substantially recovered on LBs 1, 2, and 3 after 240 s. Fluorescence on LB 1 seemed to disappear later in the recovery process (420–480 s), which could be due to 1) vesicle movements, 2) subsequent loss of EGFP-Rab38 binding, and/or 3) fusion with LB 3. Nevertheless, these heterogeneous recovery processes on LBs 1–3 clearly indicate an active mechanism recruiting EGFP-Rab38 to the LB limiting membranes. These newly recruited EGFP-Rab38 proteins could not come from the adjacent LB 4, since its fluorescence was relatively unchanged throughout the photobleaching experiment. Intriguingly, we observed punctate EGFP-Rab38 structures near the LBs or on their limiting membranes (arrowheads in Fig. 9). Some of the structures emerged from the perinuclear region with diffusive EGFP-Rab38 presence that was typical when the fusion protein was transiently overexpressed over the endogenous Rab38 (Fig. 4E). These structures were dynamic (see 120–150 s in Fig. 9; Supplemental Movie 1) and could represent Rab38-positive vesicles derived from perinuclear membranes (e.g., ER, Golgi, and perinuclear endosomes). Furthermore, relatively static (i.e., >10-s) EGFP-Rab38 puncta were also observed on the limiting membranes of LBs 6 and 2, and they were visible only when the existing fluorescence was weak or photobleached. Since membrane-associated EGFP-Rab38 diffuses rapidly (see above), these immobile structures imply docked Rab38-positive vesicles on the LB surfaces. We could not rule out these structures as Rab38-positive membranes ready to pinch off the LB surfaces. However, since they were observed on LB 2 during its fluorescence recovery process, they more likely represented docked vesicles. Although we did not observe new LB labeled by EGFP-Rab38 during the 480-s FRAP experiment, LB 6 was a good candidate to become a fully Rab38-targeted LB. Collectively, these intriguing observations lead to our hypothesis that Rab38 functions in trafficking LB-targeted cargo(s) from perinuclear membranes to the LRO in lung ATII cells.
Fig. 9.
Dynamic EGFP-Rab38 targeting to and on LB limiting membrane. SD ATII cells transiently overexpressing EGFP-Rab38 fusion protein were cultured for 2 days after electroporation, before fluorescence recovery after photobleaching experiment. Left: confocal photobleaching geometry in the xz plane. First image (i.e., the focal plane; black line in diagram at left) was obtained immediately before photobleaching, with red box depicting limited photobleaching area (red line in diagram at left). There were 6 EGFP-Rab38-labeled LBs (1–6) in the focal plane and a region with diffusive EGFP-Rab38 presence surrounding the nucleus (Nuc). Fluorescence recovery images were obtained immediately after photobleaching (0 s) and every 10 s for a total of 480 s (Supplemental Movie 1).
DISCUSSION
Among ≥60 Rab proteins, Rab38 is unique, in that its mRNA and protein expressions are highly restricted in melanocytes and lung ATII epithelial cells (27, 40, 62). This observation leads to the hypothesis that Rab38 plays cell/tissue-specific roles in the biogenesis and trafficking of LROs such as melanosomes and LBs. Indeed, Rab38, together with Rab32 and their downstream effector Varp, has been shown to function in trafficking melanogenic enzymes to maturing melanosomes (55, 57, 62). In contrast, the precise functions and molecular mechanisms of Rab38 in lung ATII cells are less well established. Rab38 mutations in the Chocolate mice and LEC rats are associated with enlarged LB phenotypes in lung ATII cells (39, 41), an observation further confirmed in this study using the Rab38-null FHH rat (Figs. 1 and 7). Disrupted surfactant homeostasis and enhanced susceptibility to lung injuries are reported using the previous two animal models and are suspected to cause lung disease similar to that found in HPS patients. However, while the Chocolate mice and LEC rats have been used to study oculocutaneous albinism and Wilson disease, respectively, until recently, they have not been employed to study lung disease (39, 41). In contrast, the FHH rat has long been used to investigate the disease mechanisms of pulmonary hypertension and hypertension-associated renal failure (3, 7). Therefore, there is a possibility that disrupted surfactant homeostasis in the FHH ATII cells, combined with effects of other genetic defects in the FHH rats (see below), could lead to a form of interstitial lung disease resulting in pulmonary hypertension. Importantly, the similar enlarged LB phenotypes observed in the three animal models of diverse genetic backgrounds strongly support the hypothesis that Rab38 is an indispensible regulator of surfactant homeostasis and LB morphology in lung ATII cells.
The reported expression patterns of Rab38 lead us to hypothesize that this small GTPase functions in epithelial, but not endothelial, cells. Indeed, Rab38 protein was expressed in every type of lung epithelial cell we examined, but not in the three types of primary endothelial cells (Fig. 2, A and B). This apparent expression dichotomy may have significant implications for the disease models represented by these Rab38-mutated mice and rats. The FH rat, in particular, is a widely studied animal model for a variety of human disorders, including systolic and pulmonary hypertension, hypertension-associated renal failure, depression, and alcoholism (49, 50, 68). For the FHH rat strain, extensive genetic studies have identified five quantitative trait loci [i.e., renal failure (Rf) 1–5] associated with the multiple syndromes of proteinuria, albuminuria, and focal glomerulosclerosis associated with end-stage renal failure (7, 51). Among all the quantitative trait loci, only Rab38 within the Rf-2 region is identified to be mutated (i.e., Rab38/Met1Ile) (47). Thus, Rab38 is proposed to be the strongest candidate accounting for ∼40% proteinuria and ∼60% albuminuria associated with end-stage renal failure in FHH rats (47). Importantly, the Rab38 mutation apparently does not affect glomerular filtration but does affect protein reabsorption by proximal tubular epithelial cells (47). This result further supports our hypothesis that Rab38 is specialized in regulating membrane trafficking in epithelial cells.
One major finding of this study is that Rab38 is selectively targeted to the limiting membranes of ∼30% of LBs in the lung ATII cells (Fig. 4; Supplemental Figs. S4 and S5). In contrast to an earlier report (40), we found that endogenous Rab38 was present in LB fractions purified from homogenized SD lungs (Fig. 3). The effectiveness of our purification procedure was controlled by dramatic concentration of the constitutive LB protein SP-A and almost complete absence of the cytosol and ER marker proteins GAPDH and calnexin in the LB samples (Fig. 3). However, Rab38 content in the LB fractions appeared to be highly sensitive to the purification procedure, which may explain the previous report that Rab38 is absent in purified LBs (40). Furthermore, Rab membrane association is dependent on its GTP-loading status, which is regulated by a variety of Rab-associated proteins (e.g., guanine nucleotide exchange factor and GTPase-activating protein) (53). In addition, Rab38 contains a COOH-terminal CaaX motif, which suggests a reversible palmitoylation mechanism regulating its membrane attachment, rather than the irreversible geranylgeranylation modification required for typical Rab membrane association (27, 41). Therefore, future studies shall determine the precise Rab38 membrane-cytosol distribution in lung ATII cells and the mechanisms regulating such distribution.
The previous conclusion of Rab38 targeting to ER membranes was obtained through immunofluorescence staining of endogenous Rab38 in rat ATII cells and through cell fractionation analysis of rat liver cells (40, 42). However, using two anti-Rab38 antibodies, i.e., monoclonal anti-human Rab38 (Santa Cruz Biotechnology) and polyclonal rabbit anti-mouse Rab38 (Fukuda Laboratory) (57), we were not able to obtain satisfactory endogenous Rab38 immunofluorescence other than the mostly diffusive staining attributable to cytosolic Rab38 (41) and occasional punctate patterns that could represent Rab38 localization to LBs (Supplemental Fig. S8). Our current Rab38 immunofluorescence images are not much different from those published previously that show partial colocalization between endogenous Rab38 and the LB marker protein SP-C (40). However, we recognized that the prevailing presence of background/diffusive Rab38 immunofluorescence in those images made precise colocalization analysis difficult. Furthermore, there is the possibility that the anti-Rab38 antibodies, although suitable for Western immunoblotting, may not be optimal for immunofluorescence staining. Therefore, we carried out additional subcellular localization studies using EGFP-tagged Rab38 reexpressed in the Rab38-null FHH ATII cells.
These reexpressing studies revealed the striking result that EGFP-Rab38 was only targeted to the limiting membranes of a subpopulation of LBs in the FHH and SD ATII cells (Fig. 4; Supplemental Figs. S4 and S5). The EGFP-Rab38-targeted cellular organelles contained the LB marker proteins ABCA3 and SP-A, as well as acidic lumen enriched with lipids (Fig. 4; Supplemental Fig. S5); thus they are true LBs with a unique mechanism recruiting Rab38. Importantly, selective Rab38 targeting was not affected by the fusion protein expression levels (Fig. 4D; Supplemental Fig. S4), was abolished by point mutations to the EGFP-Rab38 protein (Fig. 5), and was not shared by Rab7 and Rab4b also functioning in the ATII cells (Fig. 6). These results significantly strengthened our conclusion that endogenous Rab38 was directly targeted to LBs (Fig. 3) and revealed that the seemingly homogeneous LBs were, in fact, different in their molecular compositions. Consistently, it has been reported that ∼24% of ABCA3-positive LBs are selectively targeted by endogenous Rab3D in ATII cells isolated from Wistar rats and are hypothesized to represent a subpopulation of fusion-ready LBs (60). Furthermore, Rab14 partially colocalizes with ABCA3 on LB limiting membranes in SD ATII cells, and Rab14 short hairpin RNA knockdown inhibits surfactant secretion (20). These results, together with our finding, suggest that Rab proteins bestow special trafficking identities to LBs that intercept the synthetic, degradative, and regulated secretory pathways in lung ATII cells (see introduction).
The enlarged LB phenotype, previously observed in human, mouse, and rat HPS (19, 21, 32, 37, 58), was evaluated using quantitative TEM and confocal microscopy (Figs. 1 and 7; Supplemental Fig. S3). Unlike the enlarged LB phenotype observed in the pale ear/pearl (ep/pe) mice (mutations to HSP1 and Ap3b1 genes) (21), Rab38 deficiency in FHH rats resulted in enlarged LBs but no increase in LB numbers in the ATII cells. In contrast, the numbers and sizes of LBs in the ep/pe ATII cells appear to be greatly increased, an observation attributed to decreased basal and stimulated surfactant secretion in these mice (21). It has been reported that LB number increased in the ATII cells of Chocolate mice with the Rab38/Gly19Val mutation, but the quantification procedure was not described (41). On the other hand, no increase in LB number in the ATII cells of the Rab38-null LEC rats was reported (39), consistent with our finding (Figs. 1G and 7E). Furthermore, basal lipid secretion is modestly inhibited but stimulated lipid secretion is dramatically enhanced for cultured LEC ATII cells (39). Since it is difficult to relate “basal” ATII cells cultured on a hard plastic surface to the physiological ATII cells in the dynamic alveolar space, we infer from these results that the LB secretion mechanism is most likely unaltered in the Rab38-null ATII cells. This hypothesis is consistent with the unchanged or even reduced LB numbers in the FHH ATII cells (Figs. 1G and 7E). Therefore, the varying enlarged LB phenotypes observed in different rodent models of HPS indicate distinct yet concerted functions of HPS proteins in maintaining surfactant homeostasis in lung ATII cells, with Rab38 most likely functioning in trafficking events involved in intracellular membrane compartments (see below).
Compared with the TEM quantification (Fig. 1, E–G), the confocal method was a robust method for quantifying the enlarged LB phenotype that was temporarily preserved in cultured FHH ATII cells (Figs. 7 and 8). There were quantitative differences between results derived from these two imaging methods; for example, the average LB diameter gap between SD and FHH ATII cells measured by TEM is ∼77% (Fig. 1F) compared with the corresponding ∼44% gap measured by confocal microscopy (Fig. 7D). This discrepancy may be partially attributed to the different samples used for these two imaging techniques, i.e., ATII cells embedded in dehydrated ultrathin lung slices vs. isolated and 1-day-cultured ATII cells fixed for immunofluorescence labeling. However, the lateral resolution difference between confocal microscopy (∼260 nm) and TEM (∼1 nm) could also account for the difference. Thus the luminal space of small LBs, clearly resolvable by TEM (Fig. 1C), might not be visible in the confocal optical sections. Such small LBs were thus eliminated from confocal image analysis (Supplemental Fig. S3), producing average LB diameters (Fig. 7D) that were substantially larger than those derived from the TEM images (Fig. 1F). Since more small LBs in the SD ATII cells were eliminated from confocal analysis (Fig. 1E), there was a larger increase in the average LB diameter for the SD ATII cells [i.e., 0.64 (TEM) vs. 1.22 (confocal) μm; a ∼85% increase] than for the FHH ATII cells [i.e., 1.13 (TEM) vs. 1.76 (confocal) μm; a ∼56% increase]. Correction of this bias introduced by the confocal method (e.g., with the assumption that overestimation in LB diameter by the confocal method is the same ∼56% for the SD and FHH cells) would result in a ∼76% larger average LB diameter in the FHH cells using the confocal analysis, the same enlargement (∼77%; Fig. 1F) derived using the TEM method. Similarly, the sampling plane for LB cross sections was much thicker for the confocal optical section (∼0.8 μm) than the ultrathin (∼80-nm-thick) lung slice used for TEM (a factor also contributing to less LB cross sections without clear lumens in the confocal images), resulting in more than twofold more LBs counted using the former method (Figs. 7E and 1G). The precise quantitative relationship between sampling plane thickness and counted LB is more complex, involving considerations of the sampling plane thickness, LB diameter and LB density, as well as the lateral imaging resolution discussed above. Nevertheless, a statistically significant decrease (∼21%) in the average LB number per FHH cell was derived using the confocal method (Fig. 7E) compared with the statistically insignificant ∼12% decrease estimated using the TEM method (Fig. 1G). This discrepancy was due to the same effect of eliminating small LBs from confocal analysis, so that the subpopulation of FHH ATII cells containing only a few giant LBs (Fig. 1E; Supplemental Fig. S1) became statistically significant in the confocal sample population. Overall, the above-mentioned argument demonstrates that the confocal method produces data that are qualitatively consistent with those derived from the TEM approach, and the quantitative differences may be mostly accounted for by the resolution differences (lateral and axial) of the two imaging techniques. Both methods reliably evaluate relative changes in LB morphology, while the confocal approach provides extra experimental flexibility using cultured ATII cells.
Taking advantage of the quantitative confocal microscopy (Supplemental Fig. S3), we were able to carry out rescue experiments that established a causal role for Rab38 in normalizing LB size in ATII cells (Fig. 8; Supplemental Fig. S9). Our results are tentatively supported by a report that intratracheally delivered adenoviral vector containing the Rab38 gene qualitatively rescued the enlarged LB phenotype in the ATII cells of LEC rats (K. Osanai et al., unpublished observations). Although indirect evidence obtained from previous and current studies strongly supports such a causal relationship (39, 41), direct confirmation is important, so that further research of the Rab38-regulated trafficking mechanisms can be carried out on a solid foundation. Additional questions were raised by the rescue experiments. For example, we observed that the EGFP-Rab38-targeted LBs were slightly larger than the nontargeted LBs, but this difference was statistically insignificant based on our current sample populations. Thus we hypothesized that dynamic Rab38 targeting to LB limiting membrane was required to allow management of the whole LB population over an extended period of time. Our FRAP experiment established fast EGFP-Rab38 diffusion on LB limiting membranes (Fig. 9; Supplemental Movie 1). This was in contrast to the punctate and long-lasting (>10 s) EGFP-Rab38 structures found on the limiting membranes, suggesting docked vesicles prior to membrane fusion. These vesicles emerged from the perinuclear region with diffusive EGFP-Rab38 presence, suggesting transporting vesicles derived from ER, Golgi, or perinuclear endosomes. Together, these potential Rab38-positive vesicles suggest that this small GTPase may function in trafficking cargos to LBs, like its identified function in melanocytes (55, 57, 62). However, SP-A was further concentrated in LBs purified from the Rab38-null FHH lungs (Fig. 3A). SP-B and phospholipid levels are further increased in LBs derived from the Rab38-null LEC lungs (39). These results suggest that Rab38 may not be involved in trafficking the constitutive LB proteins and lipids but may be responsible for delivering lysosomal enzymes to LB, the LRO of ATII cells. Lack of degradative enzymes could lead to the enlarged LBs, similar to enlarged lysosomes found in a variety of lysosomal storage diseases (44). In melanocytes, Rab38 recruits a member of soluble N-ethylmaleimide-sensitive protein attachment protein receptor (SNARE) fusion machinery (i.e., vesicle-associated membrane protein 7) through its downstream effector Varp (56). Thus, Rab38 may similarly play a role in regulating membrane fusion with LBs in ATII cells, a hypothesis that is consistent with our observation of the potentially docked vesicles on LB limiting membranes (Fig. 9). Our short (480-s) FRAP experiment, however, did not demonstrate formation of new Rab38-labeled LBs, although LB 6 in Fig. 9 may be in the process of doing so. This and other unresolved issues, e.g., the Rab38-regulated trafficking routes and cargos, as well as molecular partners (Varp and possibly VAMP7) required for carrying out Rab38 functions, remain to be investigated in our future studies.
GRANTS
This research is supported by National Heart, Lung, and Blood Institute Grants P01 HL-019737-34 and T32 HL-007748-17 and by the IFEM Shared Research Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1. Anderson PD, Huizing M, Claassen DA, White J, Gahl WA. Hermansky-Pudlak syndrome type 4 (HPS-4): clinical and molecular characteristics. Hum Genet 113: 10–17, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 293: L259–L271, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol 294: H570–H578, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bates SR, Dodia C, Fisher AB. Surfactant protein A regulates uptake of pulmonary surfactant by lung type II cells on microporous membranes. Am J Physiol Lung Cell Mol Physiol 267: L753–L760, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Biernacki MA, Marina O, Zhang W, Liu F, Bruns I, Cai A, Neuberg D, Canning CM, Alyea EP, Soiffer RJ, Brusic V, Ritz J, Wu CJ. Efficacious immune therapy in chronic myelogenous leukemia (CML) recognizes antigens that are expressed on CML progenitor cells. Cancer Res 70: 906–915, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brantly M, Avila NA, Shotelersuk V, Lucero C, Huizing M, Gahl WA. Pulmonary function and high-resolution CT findings in patients with an inherited form of pulmonary fibrosis, Hermansky-Pudlak syndrome, due to mutations in HPS-1. Chest 117: 129–136, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Brown DM, Provoost AP, Daly MJ, Lander ES, Jacob HJ. Renal disease susceptibility and hypertension are under independent genetic control in the fawn-hooded rat. Nat Genet 12: 44–51, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Burgo A, Sotirakis E, Simmler MC, Verraes A, Chamot C, Simpson JC, Lanzetti L, Proux-Gillardeaux V, Galli T. Role of Varp, a Rab21 exchange factor and TI-VAMP/VAMP7 partner, in neurite growth. EMBO Rep 10: 1117–1124, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao Y, Vo T, Millien G, Tagne JB, Kotton D, Mason RJ, Williams MC, Ramirez MI. Epigenetic mechanisms modulate thyroid transcription factor 1-mediated transcription of the surfactant protein B gene. J Biol Chem 285: 2152–2164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chander A, Dodia CR, Gil J, Fisher AB. Isolation of lamellar bodies from rat granular pneumocytes in primary culture. Biochim Biophys Acta 753: 119–129, 1983 [DOI] [PubMed] [Google Scholar]
- 11. Chroneos ZC, Sever-Chroneos Z, Shepherd VL. Pulmonary surfactant: an immunological perspective. Cell Physiol Biochem 25: 13–26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cullinane AR, Curry JA, Carmona-Rivera C, Summers CG, Ciccone C, Cardillo ND, Dorward H, Hess RA, White JG, Adams D, Huizing M, Gahl WA. A BLOC-1 mutation screen reveals that PLDN is mutated in Hermansky-Pudlak syndrome type 9. Am J Hum Genet 88: 778–787, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Dell'Angelica EC. The building BLOC(k)s of lysosomes and related organelles. Curr Opin Cell Biol 16: 458–464, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Dell'Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J 14: 1265–1278, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Detter JC, Zhang Q, Mules EH, Novak EK, Mishra VS, Li W, McMurtrie EB, Tchernev VT, Wallace MR, Seabra MC, Swank RT, Kingsmore SF. Rab geranylgeranyl transferase-α mutation in the gunmetal mouse reduces Rab prenylation and platelet synthesis. Proc Natl Acad Sci USA 97: 4144–4149, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 134: 141–145, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Foster CD, Varghese LS, Skalina RB, Gonzales LW, Guttentag SH. In vitro transdifferentiation of human fetal type II cells toward a type I-like cell. Pediatr Res 61: 404–409, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gahl WA, Brantly M, Kaiser-Kupfer MI, Iwata F, Hazelwood S, Shotelersuk V, Duffy LF, Kuehl EM, Troendle J, Bernardini I. Genetic defects and clinical characteristics of patients with a form of oculocutaneous albinism (Hermansky-Pudlak syndrome). N Engl J Med 338: 1258–1264, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Gautam R, Novak EK, Tan J, Wakamatsu K, Ito S, Swank RT. Interaction of Hermansky-Pudlak syndrome genes in the regulation of lysosome-related organelles. Traffic 7: 779–792, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Gou D, Mishra A, Weng T, Su L, Chintagari NR, Wang Z, Zhang H, Gao L, Wang P, Stricker HM, Liu L. Annexin A2 interactions with Rab14 in alveolar type II cells. J Biol Chem 283: 13156–13164, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guttentag SH, Akhtar A, Tao JQ, Atochina E, Rusiniak ME, Swank RT, Bates SR. Defective surfactant secretion in a mouse model of Hermansky-Pudlak syndrome. Am J Respir Cell Mol Biol 33: 14–21, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang JD, Cope MJ, Mermall V, Strobel MC, Kendrick-Jones J, Russell LB, Mooseker MS, Copeland NG, Jenkins NA. Molecular genetic dissection of mouse unconventional myosin-VA: head region mutations. Genetics 148: 1951–1961, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 5: 237–252, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Huang S, Lifshitz L, Patki-Kamath V, Tuft R, Fogarty K, Czech MP. Phosphatidylinositol-4,5-bisphosphate-rich plasma membrane patches organize active zones of endocytosis and ruffling in cultured adipocytes. Mol Cell Biol 24: 9102–9123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang S, Lifshitz LM, Jones C, Bellve KD, Standley C, Fonseca S, Corvera S, Fogarty KE, Czech MP. Insulin stimulates membrane fusion and GLUT4 accumulation in clathrin coats on adipocyte plasma membranes. Mol Cell Biol 27: 3456–3469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu Rev Genomics Hum Genet 9: 359–386, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jager D, Stockert E, Jager E, Gure AO, Scanlan MJ, Knuth A, Old LJ, Chen YT. Serological cloning of a melanocyte Rab guanosine 5′-triphosphate-binding protein and a chromosome condensation protein from a melanoma complementary DNA library. Cancer Res 60: 3584–3591, 2000 [PubMed] [Google Scholar]
- 28. Kingma PS, Whitsett JA. In defense of the lung: surfactant protein A and surfactant protein D. Curr Opin Pharmacol 6: 277–283, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Lee MT, Mishra A, Lambright DG. Structural mechanisms for regulation of membrane traffic by Rab GTPases. Traffic 10: 1377–1389, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li W, Rusiniak ME, Chintala S, Gautam R, Novak EK, Swank RT. Murine Hermansky-Pudlak syndrome genes: regulators of lysosome-related organelles. Bioessays 26: 616–628, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Loftus SK, Larson DM, Baxter LL, Antonellis A, Chen Y, Wu X, Jiang Y, Bittner M, Hammer JA, 3rd, Pavan WJ. Mutation of melanosome protein RAB38 in Chocolate mice. Proc Natl Acad Sci USA 99: 4471–4476, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lyerla TA, Rusiniak ME, Borchers M, Jahreis G, Tan J, Ohtake P, Novak EK, Swank RT. Aberrant lung structure, composition, and function in a murine model of Hermansky-Pudlak syndrome. Am J Physiol Lung Cell Mol Physiol 285: L643–L653, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Matesic LE, Yip R, Reuss AE, Swing DA, O'Sullivan TN, Fletcher CF, Copeland NG, Jenkins NA. Mutations in Mlph, encoding a member of the Rab effector family, cause the melanosome transport defects observed in leaden mice. Proc Natl Acad Sci USA 98: 10238–10243, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Milovanova T, Chatterjee S, Hawkins BJ, Hong N, Sorokina EM, Debolt K, Moore JS, Madesh M, Fisher AB. Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta 1783: 1866–1875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem 277: 22147–22155, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Nagashima K, Torii S, Yi Z, Igarashi M, Okamoto K, Takeuchi T, Izumi T. Melanophilin directly links Rab27a and myosin Va through its distinct coiled-coil regions. FEBS Lett 517: 233–238, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Nakatani Y, Nakamura N, Sano J, Inayama Y, Kawano N, Yamanaka S, Miyagi Y, Nagashima Y, Ohbayashi C, Mizushima M, Manabe T, Kuroda M, Yokoi T, Matsubara O. Interstitial pneumonia in Hermansky-Pudlak syndrome: significance of florid foamy swelling/degeneration (giant lamellar body degeneration) of type-2 pneumocytes. Virchows Arch 437: 304–313, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Oiso N, Riddle SR, Serikawa T, Kuramoto T, Spritz RA. The rat Ruby (R) locus is Rab38: identical mutations in fawn-hooded and Tester-Moriyama rats derived from an ancestral Long Evans rat sub-strain. Mamm Genome 15: 307–314, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Osanai K, Higuchi J, Oikawa R, Kobayashi M, Tsuchihara K, Iguchi M, Huang J, Voelker DR, Toga H. Altered lung surfactant system in a Rab38-deficient rat model of Hermansky-Pudlak syndrome. Am J Physiol Lung Cell Mol Physiol 298: L243–L251, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Osanai K, Iguchi M, Takahashi K, Nambu Y, Sakuma T, Toga H, Ohya N, Shimizu H, Fisher JH, Voelker DR. Expression and localization of a novel Rab small G protein (Rab38) in the rat lung. Am J Pathol 158: 1665–1675, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Osanai K, Oikawa R, Higuchi J, Kobayashi M, Tsuchihara K, Iguchi M, Jongsu H, Toga H, Voelker DR. A mutation in Rab38 small GTPase causes abnormal lung surfactant homeostasis and aberrant alveolar structure in mice. Am J Pathol 173: 1265–1274, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Osanai K, Takahashi K, Nakamura K, Takahashi M, Ishigaki M, Sakuma T, Toga H, Suzuki T, Voelker DR. Expression and characterization of Rab38, a new member of the Rab small G protein family. Biol Chem 386: 143–153, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Osanai K, Voelker DR. Analysis and expression of Rab38 in oculocutaneous lung disease. Methods Enzymol 438: 203–215, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Parkinson-Lawrence EJ, Shandala T, Prodoehl M, Plew R, Borlace GN, Brooks DA. Lysosomal storage disease: revealing lysosomal function and physiology. Physiology (Bethesda) 25: 102–115, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Perez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions. Biochim Biophys Acta 1778: 1676–1695, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Perez-Gil J, Weaver TE. Pulmonary surfactant pathophysiology: current models and open questions. Physiology (Bethesda) 25: 132–141, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Rangel-Filho A, Sharma M, Datta YH, Moreno C, Roman RJ, Iwamoto Y, Provoost AP, Lazar J, Jacob HJ. RF-2 gene modulates proteinuria and albuminuria independently of changes in glomerular permeability in the fawn-hooded hypertensive rat. J Am Soc Nephrol 16: 852–856, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr Opin Cell Biol 19: 394–401, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rapp JP. Genetic analysis of inherited hypertension in the rat. Physiol Rev 80: 135–172, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Rezvani AH, Parsian A, Overstreet DH. The fawn-hooded (FH/Wjd) rat: a genetic animal model of comorbid depression and alcoholism. Psychiatr Genet 12: 1–16, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Shiozawa M, Provoost AP, van Dokkum RP, Majewski RR, Jacob HJ. Evidence of gene-gene interactions in the genetic susceptibility to renal impairment after unilateral nephrectomy. J Am Soc Nephrol 11: 2068–2078, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Simons JL, Provoost AP, Anderson S, Troy JL, Rennke HG, Sandstrom DJ, Brenner BM. Pathogenesis of glomerular injury in the fawn-hooded rat: early glomerular capillary hypertension predicts glomerular sclerosis. J Am Soc Nephrol 3: 1775–1782, 1993 [DOI] [PubMed] [Google Scholar]
- 53. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Suzuki T, Oiso N, Gautam R, Novak EK, Panthier JJ, Suprabha PG, Vida T, Swank RT, Spritz RA. The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proc Natl Acad Sci USA 100: 1146–1150, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tamura K, Ohbayashi N, Ishibashi K, Fukuda M. Structure-function analysis of VPS9-ankyrin-repeat protein (Varp) in the trafficking of tyrosinase-related protein 1 in melanocytes. J Biol Chem 286: 7507–7521, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tamura K, Ohbayashi N, Ishibashi K, Fukuda M. Structure-function analysis of VPS9-ankyrin-repeat protein (Varp) in the trafficking of tyrosinase-related protein 1 in melanocytes. J Biol Chem 286: 7507–7521, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tamura K, Ohbayashi N, Maruta Y, Kanno E, Itoh T, Fukuda M. Varp is a novel Rab32/38-binding protein that regulates Tyrp1 trafficking in melanocytes. Mol Biol Cell 20: 2900–2908, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tang X, Yamanaka S, Miyagi Y, Nagashima Y, Nakatani Y. Lung pathology of pale ear mouse (model of Hermansky-Pudlak syndrome 1) and beige mouse (model of Chediak-Higashi syndrome): severity of giant lamellar body degeneration of type II pneumocytes correlates with interstitial inflammation. Pathol Int 55: 137–143, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Unger RE, Krump-Konvalinkova V, Peters K, Kirkpatrick CJ. In vitro expression of the endothelial phenotype: comparative study of primary isolated cells and cell lines, including the novel cell line HPMEC-ST1.6R. Microvasc Res 64: 384–397, 2002 [DOI] [PubMed] [Google Scholar]
- 60. van Weeren L, de Graaff AM, Jamieson JD, Batenburg JJ, Valentijn JA. Rab3D and actin reveal distinct lamellar body subpopulations in alveolar epithelial type II cells. Am J Respir Cell Mol Biol 30: 288–295, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Wang F, Zhang H, Zhang X, Wang Y, Ren F, Zhai Y, Chang Z. Varp interacts with Rab38 and functions as its potential effector. Biochem Biophys Res Commun 372: 162–167, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J Cell Biol 175: 271–281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Weaver TE, Na CL, Stahlman M. Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Semin Cell Dev Biol 13: 263–270, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Wilson SM, Yip R, Swing DA, O'Sullivan TN, Zhang Y, Novak EK, Swank RT, Russell LB, Copeland NG, Jenkins NA. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci USA 97: 7933–7938, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wissel H, Lehfeldt A, Klein P, Muller T, Stevens PA. Endocytosed SP-A and surfactant lipids are sorted to different organelles in rat type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 281: L345–L360, 2001 [DOI] [PubMed] [Google Scholar]
- 66. Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5: 58–68, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Wu J, Forbes JR, Chen HS, Cox DW. The LEC rat has a deletion in the copper transporting ATPase gene homologous to the Wilson disease gene. Nat Genet 7: 541–545, 1994 [DOI] [PubMed] [Google Scholar]
- 68. Yagil Y, Yagil C. Genomic research in rat models of kidney disease. Methods Mol Biol 597: 427–444, 2010 [DOI] [PubMed] [Google Scholar]
- 69. Zen K, Notarfrancesco K, Oorschot V, Slot JW, Fisher AB, Shuman H. Generation and characterization of monoclonal antibodies to alveolar type II cell lamellar body membrane. Am J Physiol Lung Cell Mol Physiol 275: L172–L183, 1998 [DOI] [PubMed] [Google Scholar]
- 70. Zhang X, He X, Fu XY, Chang Z. Varp is a Rab21 guanine nucleotide exchange factor and regulates endosome dynamics. J Cell Sci 119: 1053–1062, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Zuo YY, Veldhuizen RA, Neumann AW, Petersen NO, Possmayer F. Current perspectives in pulmonary surfactant—inhibition, enhancement and evaluation. Biochim Biophys Acta 1778: 1947–1977, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.