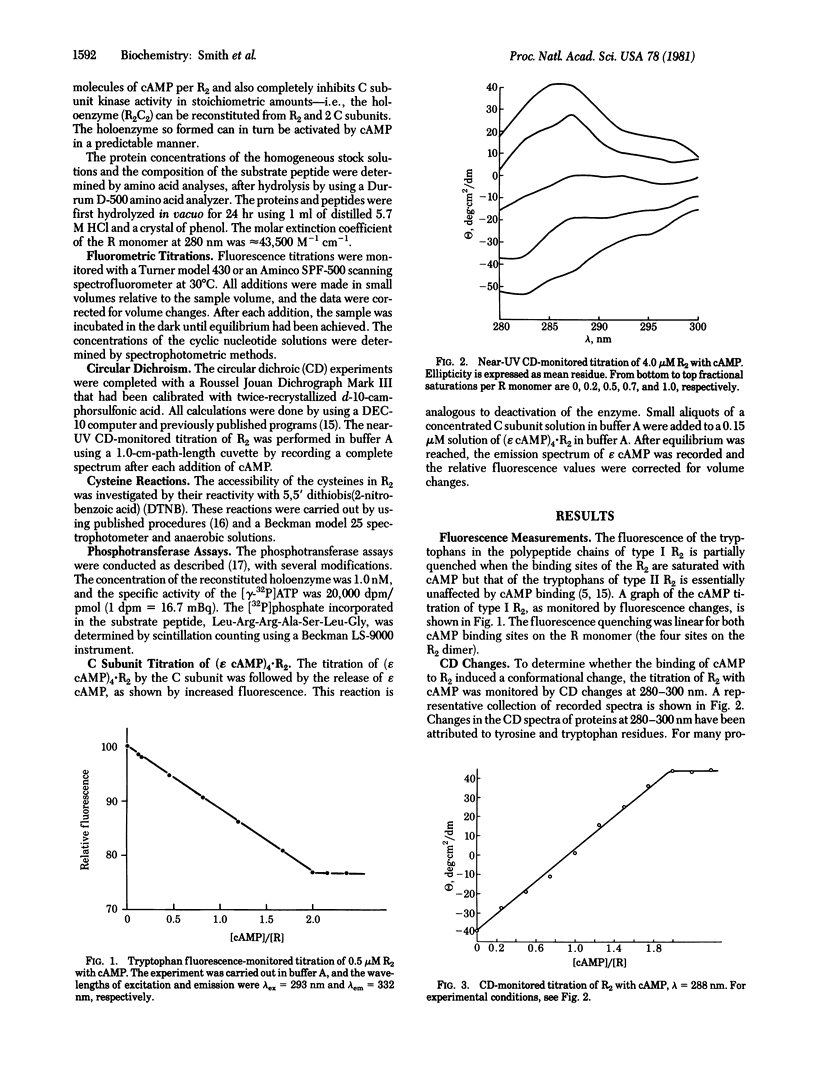
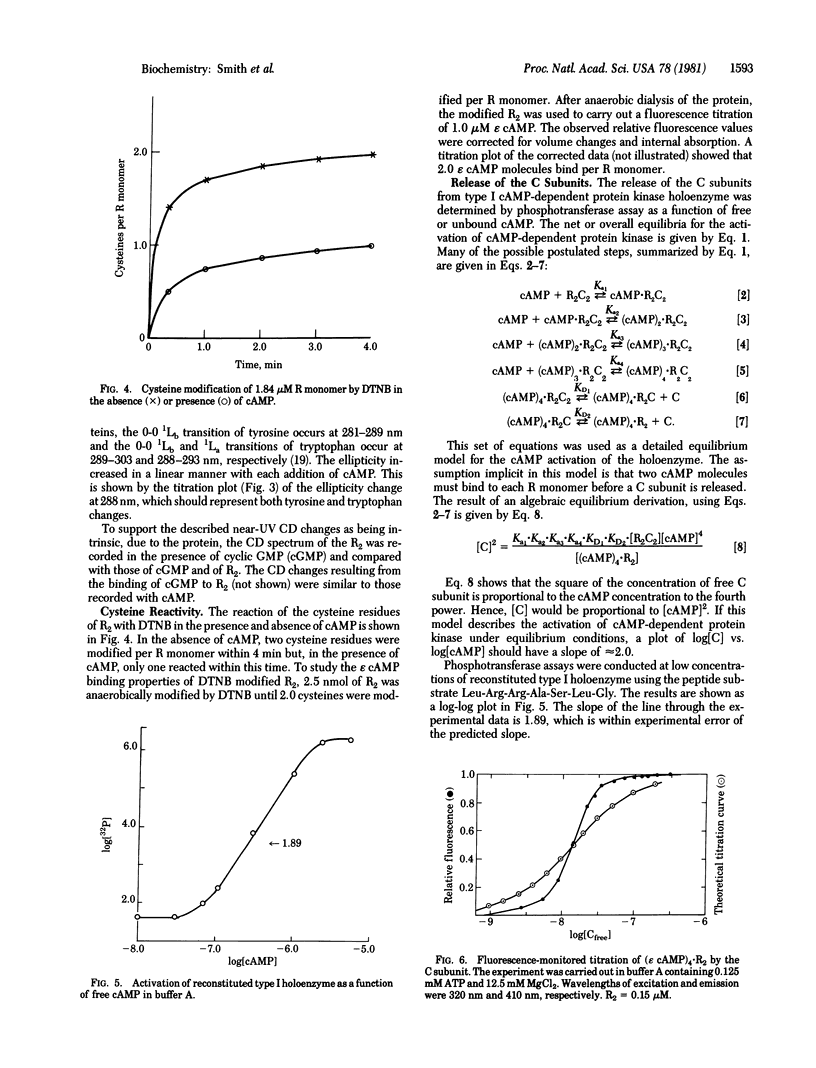
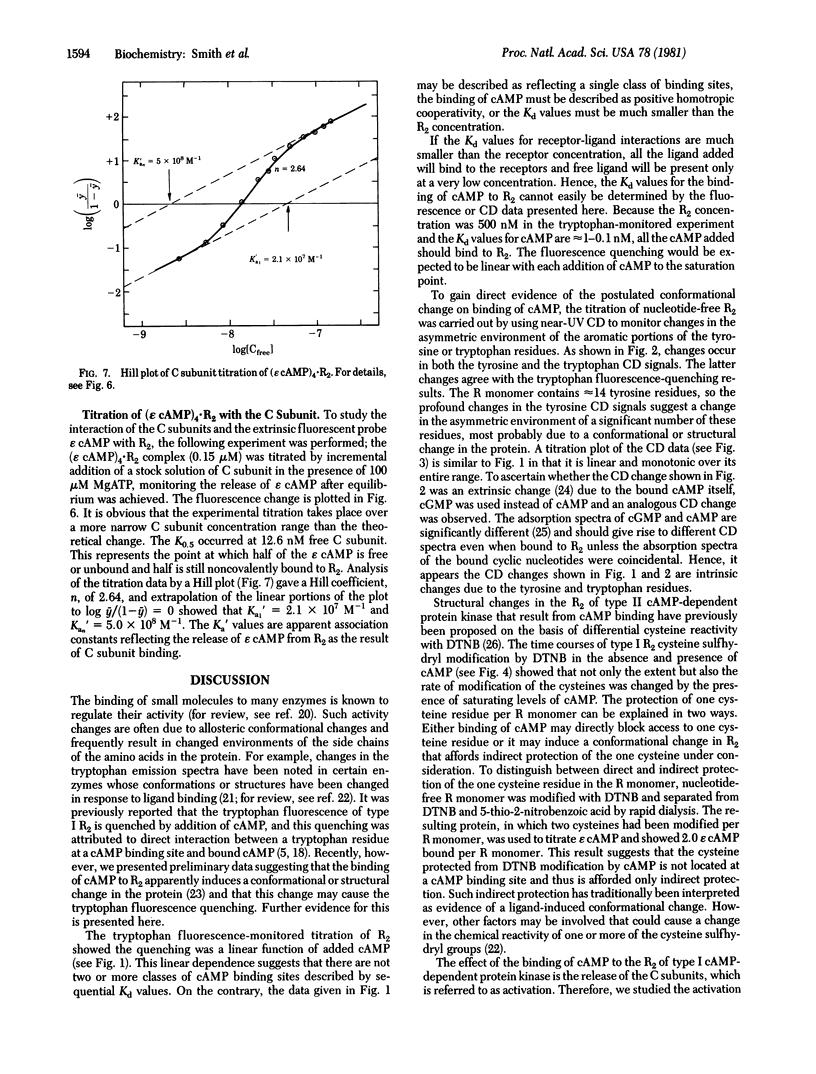
Abstract
Type I cyclic AMP (cAMP)-dependent protein kinase is composed of a dimeric regulatory subunit (R2) and two catalytic subunits (C subunits). The R2 dimer binds four cAMP molecules to release the two C subunits. To characterize the cAMP binding sites and elucidate their role in the release of the C subunits, the R2 dimer has been studied by equilibrium methods. The cAMP titration of R2 was monitored by endogenous tryptophan fluorescence, and the results suggest one class of binding sites. The titration plot is monotonic for saturation of four sites per R2. A similar titration monitored by near-UV circular dichroic changes exhibited profound changes in the region of the 1Lb tyrosine and 1La and 1Lb tryptophan transitions; a plot of these data also showed a linear monotonic response. Thus, the fluorescence and circular dichroic changes show that cAMP binding to R2 induces a conformational or structural change. The one apparent class of binding sites implies that all binding sites are characterized by similar Kd values or by Kd values much less than the receptor concentration. The reactivity of the cysteine sulfhydryl groups with 5,5′-dithiobis(2-nitrobenzoic acid) showed that saturation with cAMP indirectly protects one sulfhydryl group per R monomer. Analysis of cAMP activation of the holoenzyme, detected by phosphotransferase assays, showed that saturation of both cAMP binding sites per R monomer is necessary to effect the release of the C subunit. By using a fluorescent analog of cAMP, 1,N6-etheno-cyclic AMP (ε cAMP), the (ε cAMP)4·R2 complex was titrated with C subunit, causing the release of ε cAMP. The titration showed that the release of ε cAMP was a positive cooperative process; its Hill plot had a slope of 2.6 and the Ka1 and Kan values obtained by extrapolation were 2.1 × 107 M-1 and 5.0 × 108 M-1, respectively. The calculated ΔΔG for first and last site coupling was 1.9 kcal/mol (1 cal = 4.18 J) of holoenzyme.
Keywords: activation, deactivation, fluorescence, circular dichroism
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. N., Kaiser E. T. Sulfhydryl group reactivity of adenosine 3',5'-monophosphate dependent protein kinase from bovine heart: a probe of holoenzyme structure. Biochemistry. 1978 Jul 11;17(14):2840–2845. doi: 10.1021/bi00607a022. [DOI] [PubMed] [Google Scholar]
- Bechtel P. J., Beavo J. A., Krebs E. G. Purification and characterization of catalytic subunit of skeletal muscle adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Apr 25;252(8):2691–2697. [PubMed] [Google Scholar]
- Brostrom C. O., Corbin J. D., King C. A., Krebs E. G. Interaction of the subunits of adenosine 3':5'-cyclic monophosphate-dependent protein kinase of muscle. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2444–2447. doi: 10.1073/pnas.68.10.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Builder S. E., Beavo J. A., Krebs E. G. Stoichiometry of cAMP and 1,N6-etheno-cAMP binding to protein kinase. J Biol Chem. 1980 Mar 25;255(6):2350–2354. [PubMed] [Google Scholar]
- Chau V., Huang L. C., Romero G., Biltonen R. L., Huang C. Kinetic studies on the dissociation of adenosine cyclic 3',5'-monophosphate from the regulatory subunit of protein kinase from rabbit skeletal muscle. Biochemistry. 1980 Mar 4;19(5):924–928. doi: 10.1021/bi00546a016. [DOI] [PubMed] [Google Scholar]
- Citri N. Conformational adaptability in enzymes. Adv Enzymol Relat Areas Mol Biol. 1973;37:397–648. doi: 10.1002/9780470122822.ch7. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Koshland D. E., Jr Diagnostic uses of the Hill (Logit and Nernst) plots. J Mol Biol. 1975 Jun 25;95(2):201–212. doi: 10.1016/0022-2836(75)90390-3. [DOI] [PubMed] [Google Scholar]
- Dills W. L., Goodwin C. D., Lincoln T. M., Beavo J. A., Bechtel P. J., Corbin J. D., Krebs E. G. Purification of cyclic nucleotide receptor proteins by cyclic nucleotide affinity chromatography. Adv Cyclic Nucleotide Res. 1979;10:199–217. [PubMed] [Google Scholar]
- Glass D. B., Krebs E. G. Protein phosphorylation catalyzed by cyclic AMP-dependent and cyclic GMP-dependent protein kinases. Annu Rev Pharmacol Toxicol. 1980;20:363–388. doi: 10.1146/annurev.pa.20.040180.002051. [DOI] [PubMed] [Google Scholar]
- Glass D. B., Masaracchia R. A., Feramisco J. R., Kemp B. E. Isolation of phosphorylated peptides and proteins on ion exchange papers. Anal Biochem. 1978 Jul 1;87(2):566–575. doi: 10.1016/0003-2697(78)90707-8. [DOI] [PubMed] [Google Scholar]
- Hoppe J., Lawaczeck R., Rieke E., Wagner K. G. Mechanism of activation of protein kinase I from rabbit skeletal muscle. The equilibrium parameters of ligand interaction and protein dissociation. Eur J Biochem. 1978 Oct 16;90(3):585–593. doi: 10.1111/j.1432-1033.1978.tb12639.x. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Builder S. E., Storm D. R. Spectroscopic studies of the cAMP binding sites of the regulatory subunits of types I and II protein kinase. J Biol Chem. 1980 Mar 25;255(6):2343–2349. [PubMed] [Google Scholar]
- Lincoln T. M., Corbin J. D. Adenosine 3':5'-cyclic monophosphate- and guanosine 3':5'-cyclic monophosphate-dependent protein kinases: possible homologous proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3239–3243. doi: 10.1073/pnas.74.8.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. B., Steinmetz W. E., Long G. L. A computer-assisted model for estimating protein secondary structure from circular dichroic spectra: comparison of animal lactate dehydrogenases. Anal Biochem. 1980 May 1;104(1):160–167. doi: 10.1016/0003-2697(80)90292-4. [DOI] [PubMed] [Google Scholar]
- Ulmer D. D., Vallee B. L. Extrinsic cotton effects and the mechanism of enzyme action. Adv Enzymol Relat Areas Mol Biol. 1965;27:37–104. doi: 10.1002/9780470122723.ch2. [DOI] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Weber W., Hilz H. Stoichiometry of cAMP binding and limited proteolysis of protein kinase regulatory subunits R I and R II. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1074–1081. doi: 10.1016/0006-291x(79)91935-1. [DOI] [PubMed] [Google Scholar]


