Abstract
Diethylcarbamazine (DEC), first introduced in 1947, was shown to have strong efficacy and safety for treatment of human lymphatic filariasis, which is caused mostly by a species Wuchereria bancrofti. Many studies to optimize the dosage and treatment schedule of DEC followed, and, based on the results, control programs with various regimens were implemented in different endemic areas/countries. By the mid 1970s, with endorsement by the WHO Expert Committee on Filariasis (3rd report, 1974), the standard DEC regimen for W. bancrofti infection in mass treatment had been established in principle: a total dose of 72 mg/kg of body weight given in 12 divided doses, once weekly or monthly, at 6 mg/kg each. Not long after the committee report, the efficacy of annual single-dose treatment at 6 mg/kg, which is only one twelfth of the WHO-recommended dose in a year, was reported effective in French Polynesia (study period: 1973-78), and later in Samoa (study period: 1979-81). These results were published between 1978 and 1985 in the Bulletin of WHO but received little attention. In the mid 1980s, the efficacy of ivermectin, the first-choice drug for onchocerciasis, against lymphatic filariae came to light. Since the effect at a single dose was remarkable, and often better than DEC, it was predicted that the newly introduced drug would replace DEC. Treatment experiments with ivermectin increased quickly in number. Meanwhile, annual single-dose mass drug administration (MDA) with DEC at 6 mg/kg was under scrutiny in Samoa and Fiji. In the early 1990s, the Samoan study, which covered the entire population of 160,000 with 3 annual MDAs, reported a significant reduction in microfilaria (mf) prevalence and mean mf density, while in Fiji, the efficacy of 5 rounds of annual MDA (total dose, 30 mg/kg) was shown to be as effective as 28 multi-dose MDA spread over 2 years (6 weekly plus 22 monthly treatments at 5 mg/kg; total dose, 140 mg/kg). Several additional studies carried out in Samoa in relation to the annual single-dose MDAs revealed that low density mf carriers, who have a very low mf count of 1-20/ml of venous blood, could not play a significant role in filariasis transmission.
From around 1990, studies on spaced low-dose DEC treatments and various types of combination chemotherapy with DEC and ivermectin increased. Albendazole, a well-known anti-intestinal helminths agent, was later added to the combination. The main findings of these studies with W. bancrofti are: (i) a single dose of DEC at 6 mg/kg reduced mean mf density by ca. 90% 1 year after treatment; (ii) the same dose could damage/kill adult worms; (iii) a single dose of ivermectin at ca. 400 µg/kg was more effective than DEC in reducing mf density during the first year and was similarly or less effective in the second year; (iv) ivermectin probably could not kill adult worms; (v) a single combined dose of albendazole (400 mg) and DEC (6 mg/kg) was effective to reduce mf density by 85 to nearly 100% 12-24 months after treatment; and (vi) ivermectin or albendazole included in the combination chemotherapy produced “beyond-filariasis” benefits: clearance/reduction of intestinal helminths, and, additionally, in the case of ivermectin, skin-dwelling ectoparasites.
The Global Programme to Eliminate Lymphatic Filariasis (GPELF) started its worldwide activities in 2000, with the target of elimination by 2020. The basic strategy is to conduct annual single-dose MDAs for 4-6 years. In 2000-2007, a minimum of 570 million individuals were treated in 48 of 83 endemic countries. The drugs used are DEC 6 mg/kg plus albendazole 400 mg in most countries, or ivermectin 200-400 µg/kg plus albendazole 400 mg particularly in onchocerciasis endemic countries in Africa. (MDAs with DEC alone had been used in India.)
The GPELF achieved impressive results in terms of parasitological cure/improvement, clinical benefits, social and economic impacts, etc. However, the most impressive result of all was the programme’s success in mobilizing hundreds of millions of local people, who not only took drugs but many of them actively supported MDAs as drug distributors and volunteers. Beyond filariasis, the role people can play in supplementing rural health services is now a topic of discussion and a source of hope for a new sustainable system.
Keywords: Lymphatic filariasis, global programme for elimination, diethylcarbamazine, albendazole, ivermectin, annual single-dose, mass drug administration, Samoa, Fiji
A. Introduction
a-1. Parasite and disease
Human lymphatic filariae, which are characterized by the parasitism of their adult worms in the lymphatic system, include 3 species, Wuchereria bancrofti, Brugia malayi and Brugia timori. Female adults reproduce offspring or microfilariae (mf) which are carried into blood circulation by the lymph flow and accumulate in the lungs. Mf are released from the lungs into the circulation at night (nocturnal periodicity of mf), synchronizing with the circadian biting cycle of mosquitoes that transmit the parasite between humans. In some Pacific islands, where Aedes mosquitoes bite humans in the daytime, the release from the lungs is mainly in the afternoon (diurnally subperiodicity). The detection of mf in blood is a basic method of diagnosis. After being ingested by mosquito vectors, mf develop to the infective stage of larvae in about 10-14 days. When mosquitoes with infective larvae bite humans, filarial parasites have an opportunity to enter the host. Infective larvae, males and females, penetrate the human skin, migrate in the body and reach the lymphatic system where they mature, mate and reproduce mf in about 6-12 months after skin invasion. Adult worms will damage and dilate lymphatic vessels, and cause lymphostasis often in the lower limb and around the testes and kidney. This is the basic pathology of chronic filariasis characterized by lymphedema, hydrocele, and chyluria. Lymphedema often triggers secondary bacterial infections resulting in acute fever attacks (acute dermatolymphangioadenitis [1]), which aggravate lymphedema/inflammation. In some people, the edematous skin gradually thickens, hardens and may grow to wart-like lesions. The word ‘elephantiasis’ refers to this condition with the often serious deformities that have caused enormous suffering among affected people worldwide for thousands of years (Fig. 1) [2].
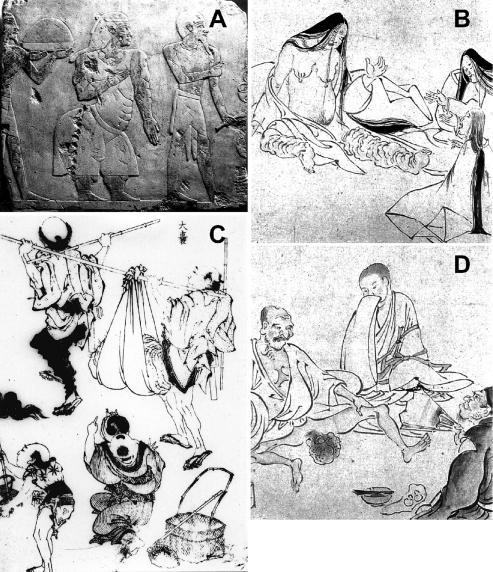
Fig. 1.
Chronic symptoms of lymphatic filariasis
A: An Egyptian relief from Queen Hatshepsut’s temple, Luxor, depicting the Princess of Punt, a possible elephantiasis case. (The Queen’s reign: 1503-1482 B.C.); B and D: Elephantiasis of the legs and scrotum, described some 1,000 years ago in “Strange Diseases Picture Scroll.” (Kyoto National Museum, Japan); C: Huge elephantiasis of the scrotum, painted by Hokusai Katsushika (1760-1849). Manga in the Edo era of Japan. {From Ref. [2], courtesy of Prof.Yoshihito Otsuji}
a-2. Epidemiology and global efforts to eliminate lymphatic filariasis
The total number of lymphatic filariasis cases in the world, as estimated in 1996 [3], was 120 million, about 90% of which were caused by W. bancrofti. The total figure includes 16 million lymphedema (including elephantiasis) and 27 million hydrocele cases, the rest being cases with microfilaremia only. The infection was more prevalent among males, and adults. By region/country, India and sub-Saharan Africa had more than 40 million cases each, followed by other Asia and Islands (ca. 20 mil.) and China (ca. 10 mil.). With the swollen leg and/or scrotum, lymphatic filariasis was ranked as the 4th leading cause of permanent and long-term disability [4]. Most of the patients have been neglected and suffer from mental distress, social isolation, and economic misery due to the stigma of the disfiguring disease [5]. The estimated disability-adjusted life years (DALYs) lost in 1999 was 4.92 million [6]. As for economic loss, in India alone, the cost of treatment for acute fever attacks and chronic symptoms reached an estimated US$ 31.1 million per year, and the loss of productivity US$ 811 million per year [7].
These gloomy statistics have changed rapidly for the better since the Global Programme to Eliminate Lymphatic Filariasis (GPELF) started in 2000 (details in Section F). The Global Alliance to Eliminate Lymphatic Filariasis (GAELF) was formed the same year to support the unprecedented global program. The alliance includes health ministries of endemic countries, UN agencies (especially WHO as the secretariat), the private sector, NGDOs, academia, and government bodies (including JICA). Particularly noteworthy are the contributions of two pharmaceutical companies: GlaxoSmithKline donates albendazole free of charge and Merck & C., Inc. ivermectin. Both drugs are essential for the Mass Drug Administration (MDA) carried out annually in endemic countries. In 2007, 48 out of 81 endemic countries conducted MDAs, and 546 million people in the world were treated for lymphatic filariasis. The same year, China declared the elimination of filariasis, which was followed by Korea’s declaration in 2008 [8].
B. The “new” anti-filarial drug diethylcarbamazine (DEC): Early studies to find the optimal dosage
b-1. Trials with multi-dose treatment
The anti-filarial effect of 1-diethylcarbamyl-4-methylpiperazine hydrochloride (DEC hydrochloride) was first reported in 1947 by Hewitt et al. [9] using naturally acquired filarial parasites in cotton rats (Litomosoides carinii) and dogs (Dirofilaria immitis). The same drug was tried for human bancroftian filariasis and its microfilaricidal and possible adulticidal effects were confirmed the following year [10]. A series of experimental treatments was conducted using DEC citrate for DEC hydrochloride to determine suitable dosage and treatment schedule. Many important studies were carried out in the South Pacific islands, where more than 10,000 American soldiers suffered clinical filariasis due to diurnally subperiodic W. bancrofti during World War II [11].
In American Samoa, 5 different multi-dose trials with DEC (3-9 mg/kg of body weight per day for 7-30 days, total dosage 21-270 mg/kg) confirmed rapid microfilaricidal effects, but the treatments could not prevent the reappearance of mf in 2-year follow-up studies (reported in 1953 [12]). Using 111-175 mf positives, Mahoney & Kessel [13] reported in 1971 that DEC given at 6 mg/kg daily for 6 days resulted in 32% persistence (rate of mf positive 1 week after treatment) and that the recurrence rate was 24% (rate of mf reappearance within 1 year of treatment). In this study, the diagnosis of infection was made by the detection of mf in 20 µl capillary blood obtained by the finger-prick (F-P) method.
In Fiji, Manson-Bahr (1952) [14] conducted treatment experiments with Hetrazan (DEC) at 100-300 mg daily for 15 to over 70 days and concluded that treatment must be continuous for at least two months. This conclusion was based on a finding that, even after multi-dose treatments cleared mf in 20 µl of F-P blood, mf were still positive when 1 ml of venous blood was examined by Knott’s method. Burnet & Mataika (1961) [15] administered 6 weekly doses of DEC at 400 mg (ca. 5-8 mg/kg) each and repeated the same regimen half a year later (total dosage 4,800 mg). The treatment reduced mf rate, determined with 60 µl of F-P blood, from 12.2% to 2.7%. However, they noted that over 40% of mf negatives (who had been positive before treatment) were in fact positive by Knott’s method using 1 ml of venous blood.
In French Polynesia, Kessel (1957) [16] compared 3 different dosages and reported that 6 mg/kg once a month for 24 months showed the best result in terms of mf prevalence and density (mf/20 µl of blood) reductions. In Japan, among various dosage schemes tested by different workers, Sasa (1976) [17] stressed the importance of the size of total dosage given, rather than the schedule of daily, weekly or monthly treatment, and recommended a total dosage of 72 mg/kg at 6 mg/kg daily for 12 days.
The WHO Expert Committee on Filariasis analyzed the accumulated data on DEC dosage and reported in 1967 [18] that “an adequate amount seems to be a total dose of about 72 mg of diethylcarbamazine citrate per kg body weight”. Spaced doses of 6 mg/kg once a week or once a month (12 times) were preferred to daily doses to reduce adverse reactions. The next WHO Expert Committee Report (1974) [19] confirmed the same total dose of 72 mg/kg for W. bancrofti infections and 30-40 mg/kg for B. malayi infections. Daily treatment was considered impractical for mass treatment. In 1984, the 4th Committee Report [20] reiterated the same total dosage for W. bancrofti.
b-2. Effect of low-dose treatments: results from a “minority” group
Several studies with W. bancrofti reported the remarkable effectiveness of DEC at low dosages. Rachou & Scaff (1958) in Brazil (quoted by Hawking, 1962 [21]) reported that only one dose at 6 mg/kg reduced the mf prevalence rate from 100% (pretreatment level) to 62%, and mf density by 91.4% when assessed 12 months after treatment. The authors recommended annual or biannual mass treatments without prior blood tests for mf. In Gambia, McGregor & Gilles (1960) [22] observed that a total dose of 12.5 mg/kg (2.5 mg/kg daily for 5 days) reduced microfilarial load by 90-98% 43 months after treatments and left the noteworthy comment that “mass-treatment campaigns aimed at dosing all inhabitants at spaced intervals (2-4 years) might in the long run prove to be the most effective and economical way.” Nearly 2 decades later, in French Polynesia, Laigret et al. (1978) [23] reported that DEC 6 mg/kg (400 mg for males and 300 mg for females) given once per year for 3 years successfully reduced the mf rate from 100% (pretreatment level) to 12% and the average mf density from 15 per 20 µl of blood to 0.3. The annual single-dose treatment was applied to ca. 50,000 people for 4 years and succeeded in reducing the mf prevalence from 4.4% to 1.9%. In practice, not all of the people took 4 doses; the result was obtained with the average of 2.76 doses in 4 years [24].
It is surprising to find that the dosages recommended for DEC treatment differed so widely in range. Understandably, researchers seemed to focus more on the cure of infection in the early stage of dosing trials. Due to the reappearance of mf after treatments, adulticidal effect was considered a key issue in judging the efficacy of a drug. Thus, the necessity of multi-dose treatment with a high total dosage must be stressed. On the other hand, it seems that a small number of researchers, especially those working in less-developed settings, paid more attention to the applicability of a treatment scheme. Also, the experience of difficulty in conducting multi-dose treatments and the recognition of adverse effects in such treatments must have convinced researchers to accept regimens not ideally effective but operationally feasible. The realization that very light infections had been missed previously when conventional blood tests (with 20-60 µl of F-P blood) were used for diagnosis called for a more suitable means of large-scale mass treatment. In 1984, the WHO Expert Committee on Filariasis, for the first time, mentioned the effectiveness of yearly treatments at 6 mg/kg [20].
C. Filariasis control in Samoa and Fiji with annual single-dose MDA using diethylcarbamazine
c-1. Countries and their filariasis situations
Samoa, an independent country in the South Pacific, had a population of 160,000 (1990) in the 2 main islands of Upolu and Savaii. Diurnally subperiodic W. bancrofti, transmitted by Aedes polynesiensis and Aedes samoanus, is endemic. The prevalence study in 1965 revealed a mf rate of 19.1% (n = 10,129) by the 20 µl F-P blood smear method. The first nationwide MDA in 1965/66 using DEC (5 mg/kg once a week for 6 weeks, followed by the same dosage once a month for 12 months) reduced the mf rate to 1.63% (n = 42,697) in 1967, and the second MDA in 1971 (6 mg/kg once a month for 12 months) further reduced the rate ― assessed by the 60 µl F-P method in 1972 ― to 0.24% (n = 6,361). Despite continuing treatment of known mf positives, the prevalence increased gradually, and in 1979, reached 3.8% (n = 8,385) by 60 µl blood smear and 4.5% (n = 8,385) by the nuclepore filtration method using 1 ml of venous blood (1 ml NP method). The situation was alarming, because mf rate (by 1 ml NP method) of adult males aged ≧30 years had already reached the 20% level [25].
Fiji is the largest island country in the region, with a total population of 726,400 (1990) scattered over 100 islands. The two main islands are Viti Levu and Vanua Levu. Diurnally subperiodic type W. bancrofti, transmitted by Ae. polynesiensis, Ae. pseudoscutellaris and several other mosquito species, is endemic. In 1958, the mf prevalence determined by 60 µl F-P method in the delta area of the Rewa River, Viti Levu, was 12.2% (n = 1,200), which decreased to 2.7% (n = 1,123) in 1959 after 2 rounds of MDA with DEC (400 mg once, 6 times weekly). However, by 1963, the rate increased to a level of 5% [26]. The 1968-69 studies in Taveuni and Koro islands, and Vanua Levu revealed a mf prevalence of 23% (n = 947) and 13% (n = 3,538) by the 60 µl F-P method, respectively (computed from the data by Mataika et al., 1971 [27]). A nationwide MDA campaign was commenced in 1969 using DEC at 5 mg/kg weekly for 6 weeks, followed by 22 monthly treatments (totally 28 doses, 140 mg/kg). The whole country was covered in 5 stages, and the campaign, which reached completion in 1975, successfully reduced the mf prevalence to 1% or less, but subsequent blood surveys suggested a gradual increase in infection [28]. The surveys in 1983-84 in the 2 remote islands of Lau and Rotuma revealed mf rates of 7.9% (n = 2,329) and 21.2% (n = 1,689) by the 60 µl F-P method, respectively [29]. In a 1985 survey in Kadavu island, the mf rate was 6.9% by the same method (n = 4,686) [30].
c-2. WHO/Samoa Filariasis Research Project: Confirmation of efficacy of annual single-dose treatment
In 1976, when the WHO/Samoa Filariasis Research Project started, it had become standard practice in the treatment of bancroftian filariasis to give a total DEC dose of 72 mg/kg in 12 treatments at 6 mg/kg each. However, difficulties in multi-dose treatment had been encountered in many endemic countries. Especially in Samoa, where a year-long multi-dose MDA was conducted twice in 1965/66 and 1971 utilizing limited health resources, the government was reluctant to repeat the procedure. In the midst of this situation, a report from French Polynesia that annually spaced single-dose DEC at 6 mg/kg was effective in reducing mf rate and density [23] brought encouraging news. Although the regimen was not popular in those days, the Research Project in Samoa decided to evaluate the efficacy of DEC single dose 12 months after treatment.
In the study in 1979-81, a single DEC dose of 4 mg/kg, 6 mg/kg or 8 mg/kg was administered to mf positive persons and the change in mf was assessed at 12 months by the 1 ml NP method (Table 1). The cure rate (% mf negative after treatment) was 29.4%, 53.7% and 40.0%, and the % decrease in geometric mean mf count was 81.5%, 94.4% and 93.5%, respectively for 4 mg/kg, 6 mg/kg and 8 mg/kg regimens. There was no significant difference among the cure rates, but the % decrease obtained with 4 mg/kg regimen was less than that of the 6 mg/kg or 8 mg/kg regimen (P < 0.01). Side reactions were studied by questioning people between 5 and 15 days after treatment. The occurrence of reactions (all types combined) was significantly higher in the 8 mg/kg regimen (77.9%) than in the other regimens (57-59%). It was concluded that annual single-dose DEC treatment at 6 mg/kg was suitable for the nationwide treatment for filariasis [31]. In 1981, upon completion of the study, the government of Samoa in collaboration with the Western Pacific Regional Office of WHO decided to implement a national MDA program based on this treatment.
Table 1.
Comparison of the effects of 3 different DEC dosages given as a single dose and assessed 12 months after treatment
| Dosages | |||
|---|---|---|---|
| 4 mg/kg | 6 mg/kg | 8 mg/kg | |
| No. examined (mf carriers) | 51 | 41 | 45 |
| No. mf negative after treatment (% cure rate) | 15 (29.4) | 22 (53.7) | 18 (40.0) |
| Decrease in mf count, expressed as mean of log (mf +1) | |||
| Pre-treatment (A) | 2.117 | 2.003 | 2.198 |
| Post-treatment (B) | 1.384 | 0.751 | 1.010 |
| Change | 0.733 | 1.251 | 1.188 |
| % decrease* | 81.5 | 94.4 | 93.5 |
* Calculated as 100 x [antilog (A) - antilog (B)]/antilog (A). {Source: Partially adopted from Ref. [31]}
c-3. Nationwide MDA in Samoa with once-a-year treatment: Long-term efficacy
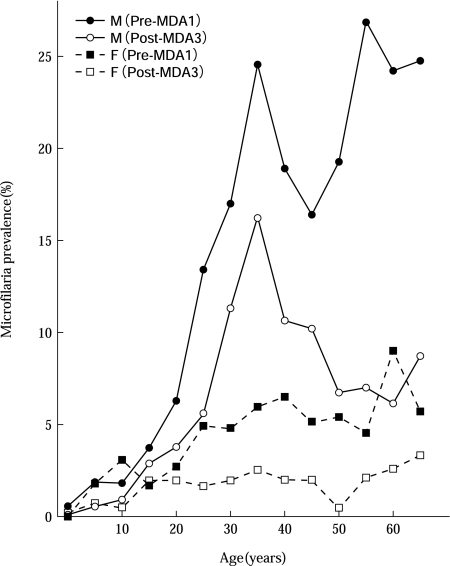
A national MDA using a single dose of DEC at 6 mg/kg was started in 1981. All Samoans, except infants under the age of 1 year, pregnant women, sick people, and the very old, were the targets. After completion of the census in every village and town in the country with assistance of village Women’s Committees, 3 MDAs were carried out under the supervision of medical staff by members of Women’s Committees in 1982, 1983, and 1986, with a treatment coverage of 86.3%, 83.8% and 82.6%, respectively. The total population in 1986 was 159,199. The evaluation blood surveys were carried out 4 times, before and after each MDA, using 60 µl blood smears from some 9,600-13,700 people in 26-34 villages on each occasion. The MDAs reduced the mf prevalence gradually from 8.0% to 3.8% (52% reduction) in males, and from 3.2% to 1.3% (59% reduction) in females. The mf densities (geometric mean of positive counts per 60 µl) decreased from 23.1 to 9.1 (61% reduction) in males and from 14.6 to 9.4 (36% reduction) in females. The change in mf prevalence is summarized in Fig. 2, before and after 3 MDAs according to sex and age [32].
Fig. 2.
Change in microfilaria prevalence before and after 3 annual single-dose MDAs with DEC at 6 mg/kg, analyzed by sex and age group (study in Samoa) {Figure redrawn from Ref. [32]}
The transmission potential or infectivity index (%) of total population (IIT), which is an estimated mosquito infection rate [33], was reduced from 2.18 before MDA to 0.67 (70% reduction) after 3 MDAs. Entomological studies were also conducted at Vailu’utai village on Upolu Island. A total of 1,758 Ae. polynesiensis were dissected before MDA and 5,206 after the 2nd MDA. The results revealed a decrease in mosquito infection rate from 0.97% to 0.06% and the infective rate (% of mosquitoes having the infective stage of larvae) from 0.28% to 0.02% [32].
These findings indicated the remarkable long-term efficacy of annually spaced single-dose MDAs, given in fact 3 times in 6 years, and at the same time, the feasibility of a nationwide control program in which people are the major players.
c-4. Fiji study for confirmation of efficacy of 5 rounds of annual single-dose treatment with DEC: comparison with 28 multi-dose MDA
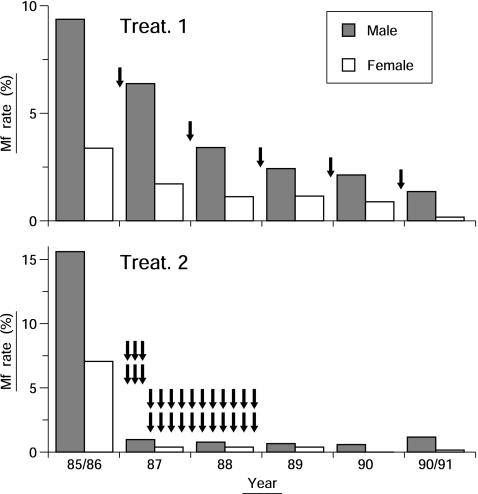
Mataika et al. (1993) [34] carried out an extensive study comparing DEC efficacy between 5 annual single-dose MDAs at 6 mg/kg (total 30 mg/kg) and very intensive 28-dose MDA (5 mg/kg once a week for 6 weeks, then monthly for 22 months; total 140 mg/kg). The results are shown in Fig. 3 [35]. The annual scheme reduced the mf rate year by year from 6.5% before treatment (average of males and females) to 0.9% after 5 treatments (87% reduction), while in the multi-dose scheme, the rate dropped sharply from 11.6% to 0.8% in 1987, and to 0.9% at 5 years (93% reduction). However, without treatment for more than 2 years after completion of the intensive 2-year regimen, a slight but significant increase in mf rate was observed in 1990/91 compared with the previous year. This indicates the advantage of continued annual doses rather than a concentrated multi-dose treatment. This study reconfirmed the effectiveness of annual single-dose MDAs. It was obvious that the annual scheme was much easier and more practical than the multi-dose scheme.
Fig. 3.
Comparison of 2 MDA schemes with DEC: 5 rounds of annual single-dose treatment at 6 mg/kg (Treat. 1) and 28-dose treatment at 5 mg/kg given weekly for 6 weeks and monthly for 22 months (Treat. 2). Each arrow indicates a single treatment. {Figure redrawn from Ref. [35]}
D. Low-density microfilaremia in Samoa and its significance in filarial transmission
d-1. What is low-density microfilaremia?
There are mf densities that are too low to be detected by conventional blood smears with 20-60 µl of F-P blood. The employment of nuclepore/millipore filtration of 1 ml venous blood has facilitated the detection of low density mf carriers. Low mf density was defined variously by researchers. A mf range of 1-10 mf/ml was cited but often proved inconvenient for analytical studies as not many cases fell into this category. Comparing the 60 µl F-P method and 1 ml nuclepore filtration method, 1-20 mf/ml was defined as low-density microfilaremia (l.d.m.) for studies in Samoa [36]. The prevalence of l.d.m. in all mf positives was 23.6% (90/381), which led to an estimate of 1,700 low-density mf carriers in 1979 throughout the country. L.d.m was proportionally more frequent in villages with lower mf prevalences and in people <20 years of age. By sex, there was no difference.
Since low-density mf carriers were recognized to occupy a substantial proportion of all mf carriers in Samoa, and Ae. polynesiensis were known to ingest more mf than estimated (up to 4.7 times) while having a blood meal on low-density mf carriers [37], the significance of l.d.m. as a source of transmission was seriously discussed, especially because it was suspected that annual single-dose DEC would not exert a sufficient adulticidal effect and therefore produce more low-density carriers than multi-dose treatment.
d-2. Significance of low-density mf carriers in filariasis transmission: Quantitative assessment
Based on the mosquito “infectivity index” concept proposed by Sasa [33], the significance of l.d.m. in transmission can be determined by estimating the proportion of mosquito infectivity produced by the l.d.m. group as compared to the total mosquito infectivity produced by all levels of mf carriers in an endemic community. Assuming that all people are exposed evenly to mosquito bites, the latter can be computed as follows:
Total mosquito infectivity = ∑ (infection rate when mosquitoes feed on a carrier with mf density of k) x (No. of carriers with mf density of k in a community)
where k is from 1 to the maximum mf density (/1 ml of venous blood) observed in the community. Mosquito infectivity produced by low-density mf carriers will be obtained using the same formula with k value from 1 to 20.
To study the rate of mosquito infection, Samarawickrema et al. (1985) [37] conducted a detailed transmission experiment using Ae. polynesiensis, a main vector in Samoa, and 14 mf carriers with different mf densities ranging from 0 to 5,290 mf/ml. The results showed that the percentage of mosquitoes infected and the average number of larvae found in each infected mosquito were directly proportional to the mf densities in the carrier at the time of feeding. Based on the data from this transmission experiment, it was possible to obtain theoretical mosquito infection rates when mf carriers with different mf densities were blood sucked by mosquitoes [36]. To estimated the numbers of mf carriers with certain mf densities, a negative binomial distribution was fitted following the method of Pichon et al. (1980) [38] to the 1979 mf data from Samoa (n = 358 mf positives) [25].
The computation of mosquito infectivity is shown in Table 2. The infectivity produced by l.d.m. was 251.2 (B) and that by all levels of mf density was 11645.8 (A), and the contribution of l.d.m. to the total (B)/(A) was 0.0216 or only 2.16%. This would suggest a minor role of l.d.m. in the transmission of filariasis in Samoa [36].
Table 2.
Mosquito infectivity produced by the low-density microfilaria (mf) carriers (B) and all levels of mf carriers (A)
| Microfilaria density (mf/ml) | Theoretical % of infected fed mosquitoes (I)* | Theoretical No. of mf positive persons in each mf density group (II) | (I) x (II) |
|---|---|---|---|
| 1 | 0.492 | 13.214 | 6.5 |
| 2 | 1.152 | 8.584 | 9.9 |
| −4 | 2.102 | 11.999 | 25.2 |
| −6 | 2.842 | 8.774 | 24.9 |
| −8 | 3.473 | 7.074 | 24.6 |
| −10 | 4.032 | 6.000 | 24.2 |
| −20 | 6.254 | 21.729 | 135.9 |
| Subtotal 251.2 ...(B) | |||
| −30 | 7.985 | 15.076 | 120.4 |
| −40 | 9.458 | 11.854 | 112.1 |
| −50 | 10.766 | 9.892 | 106.5 |
| −60 | 11.954 | 8.550 | 102.2 |
| −70 | 13.052 | 7.565 | 98.7 |
| −80 | 14.078 | 6.805 | 95.8 |
| −90 | 15.045 | 6.198 | 93.2 |
| −100 | 15.963 | 5.700 | 91.0 |
| −200 | 23.456 | 41.077 | 963.5 |
| −300 | 29.292 | 26.579 | 778.6 |
| −400 | 34.259 | 19.642 | 672.9 |
| −500 | 38.666 | 15.443 | 597.1 |
| −600 | 42.673 | 12.589 | 537.2 |
| −700 | 46.374 | 10.511 | 487.4 |
| −800 | 49.834 | 8.925 | 444.8 |
| −900 | 53.094 | 7.675 | 407.5 |
| −1000 | 56.187 | 6.666 | 374.5 |
| −2000 | 81.449 | 36.479 | 2971.2 |
| −3000 | 100 (101.123) | 13.242 | 1324.2 |
| −4000 | 100 (117.869) | 5.524 | 552.4 |
| ≥4001 | 100 (--) | 4.634 | 463.4 |
| Subtotal 11394.6 | |||
| Total | 358.000 | 11645.8 ...(A) | |
* Estimated from log (Y + 1) = 0.5278 log X + 0.1739, where Y = % infected, X = mf density of a carrier. {Source: Adopted from Ref. [36]}
E. Re-evaluation of low-dose DEC treatment, introduction of new drugs and their combination therapy
e-1. New evidences supporting the efficacy of low-dose DEC treatments
After around 1990, a variety of low-dose DEC treatments were tested in various countries where W. bancrofti is endemic. In Tahiti, a single dose of 3 mg/kg was reported effective in reducing geometric mean mf count by 95% when assessed 180 days after treatment [39]; in Papua New Guinea, 2 annual single doses at 6 mg/kg reduced the mf rate from 41% to 17%, and mf density from 71 mf/20 µl to 20 [40]; in Brazil, the efficacy of 6 mg/kg given only once was reported equally effective to 12 daily doses at 6 mg/kg when measured 12 months after treatment, although the single dose was significantly less effective than the multi doses during the first 6 months [41]; and in Tanzania, two 6-monthly treatments at 6 mg/kg reduced the geometric mean mf intensity by 92.2%, while 12 daily doses at 6 mg/kg reduced it by 98.6%, when assessed 1 year after the start of treatment. The former regimen was considered more suitable for MDAs than the standard 12-dose treatment [42]. The single dose treatment with DEC at 6 mg/kg was also applied to Brugia malayi infections. In Kerala, India, 2 annual mass treatments reduced the mf prevalence from 4.9% to 1.2%, and the mean mf count by 81%. In addition, clinical benefits such as a reduction in acute manifestations and recent edema cases were reported [43].
A single dose DEC at 6 mg/kg reduced not only the mf level by 90.5% but circulating filarial antigen by 39.7% 18 months after treatment, suggesting that the treatment was effective against adult parasites [44]. Brazilian researchers successfully studied the adulticidal effect of low-dose DEC treatment by direct observation of live adults using ultrasonography. The adults live in a dilated lymph vessel in the scrotal area, making a “nest” and moving actively. Amaral et al. (1994) [45] named the movement “filaria dance sign” ([46] for video image). Norões et al. (1997) [47] reported that within a week after treatment at 6 mg/kg single dose, the dance sign became undetectable in 7 of 14 nests, and scrotal nodules became palpable at each site of the 7 nests. Biopsy specimens from these nodules revealed “nests” of degenerating adults, confirming the adulticidal effect. A separate histopathological study with DEC-induced nodules reported that even a single dose of 1 mg/kg could damage adult worms. However, it should be noted that, even after repeated high dose DEC treatments, some worms in the same nest remained intact [47, 48].
Fortunately, no drug resistance has been reported so far with DEC.
e-2. Ivermectin as a new drug against lymphatic filariae
On the other hand, ivermectin, the drug of choice for onchocerciasis that is also effective against intestinal helminths and ectoparasites such as lice, was reported in 1988 [49] to be effective against W. bancrofti. Since it is effective with a single oral dose at 25-200 µg/kg, ivermectin was cited as a candidate to replace DEC. The efficacy was further confirmed: when assessed at 6 months, a single dose at 21.3 µg/kg and 126 µg/kg reduced mf to 18.3% and 19.5% of the original levels, respectively, while 13 daily DEC treatments (one 3 mg/kg dose, followed by 12 daily doses at 6 mg/kg) reduced the level to 6.0% [50]. With a higher dosage of 420 µg/kg (20 µg/kg at day 1 plus 400 µg/kg at day 5), the geometric mean mf density was reduced to 0.9% of the pretreatment level 1 year after treatment, while DEC at 6 or 7 mg/kg reduced the mean to 9.3% (P < 0.006) [51]. Another study with 420 µg/kg of ivermectin reported a mean mf reduction of 86.3% after 18 months, while it was 90.5% with a single dose of DEC at 6 mg/kg (P > 0.05). In this study, the efficacy of ivermectin was much stronger than DEC in the first 30 days after treatment, but by 18 months, the latter took over the lead and resulted in a slightly higher reduction [44]. The stronger effect of DEC as opposed to ivermectin in the second year was also reported in Brazil [52]. To study adulticidal effect of ivermectin, Dreyer et al. (1995) [53] treated 15 W. bancrofti-infected Brazilian men with a single dose of ivermectin at 400 µg/kg and observed the filaria dance sign by ultrasound for 3-9 months. Contrary to all expectations, there was no observable change in the dance sign, and it was concluded that ivermectin had no effect on adult worms.
e-3. Combination chemotherapies
Having two potent anti-filarial drugs, DEC and ivermectin, researchers tested the efficacy of their use in combination. More recently, the effectiveness of albendazole, an established antiparasitic agent, against filarial parasites was reported, and various combinations of these 3 drugs have been evaluated.
A possible additive or synergistic effect of DEC and ivermectin was reported in Haiti based on a finding that 20 µg/kg ivermectin given as a clearing dose at day 1, followed by a single 6 mg/kg dose of DEC at day 5 resulted in higher efficacy in reducing mf density than DEC alone 1 year after treatment [50]. The same regimen tested in Brazil produced the best results among different combinations of the 2 drugs: reduction of microfilaremia to 2.4% of the pretreatment level (100%) at 2 years [51]. In Tahiti, 2 annual single dose MDAs were conducted using 4 different regimens: (i) ivermectin 400 µg/kg plus DEC 6 mg/kg, (ii) ivermectin 400 µg/kg alone, (iii) DEC 6 mg/kg alone, and (iv) ivermectin 400 µg/kg plus DEC 3 mg/kg [54]. After 1 year, regimens (i) and (iv) resulted in the same 32% reduction in mf prevalence, while the reduction was only 11-14% using regimens (ii) and (iii). As for mf density, the former 2 regimens brought about a 95-96% reduction from the pretreatment level, and the latter 2 regimens 80-82%. To clarify the effect of combination therapy on adult worms, a single dose of DEC at 6 mg/kg was co-administered with either 200 µg/ kg or 400 µg/kg of ivermectin, and filaria dance sign was observed with ultrasonography [55]. Interestingly, the dance sign was evident in all of the 30 nests studied, suggesting that the co-administration interfered with the already established adulticidal effect of DEC.
Other studies have looked at the combination of albendazole and DEC or ivermectin. A review by Ottesen et al. (1999) [56] summarized the role of albendazole for lymphatic filariasis elimination. Ismail et al. (1998) [57], working with W. bancrofti, showed more reduction in geometric mean mf density with a combined single dose of albendazole (600 mg) plus ivermectin (400 µg/kg) than with a combination of albendazole (600 mg) plus DEC (6 mg/kg) up to 12 months after treatment. At 15 months, however, there was no significant difference between the two (reduction of >98% in both regimens). As for circulating filarial antigen, the latter combination resulted in significantly more reduction (77%) than the former at 15 months. A combined single dose of albendazole (400 mg) plus DEC (6 mg/kg) reduced mean mf density by 85.7-99.6% 12-24 months after treatment [58-61]. On the other hand, several studies could not confirm the benefit of albendazole in various combinations with ivermectin/DEC [62-65]. Dreyer et al. (2006) [66] compared the adulticidal effect of DEC (6 mg/kg) alone and DEC (6 mg/kg) plus albendazole (400 mg), and reported that the combination resulted in a much lower effect on filaria dance sign. The authors concluded that coadministration appeared to reduce the adulticidal effect of DEC. Further studies are necessary to confirm or refute anti-filarial effects of albendazole in combined use with the other anti-filarials [67].
The role of albendazole in the global program for filariasis elimination has to be emphasized for its “beyond-filariasis” benefits [55]. The drug is very effective against intestinal helminths such as Ascaris, Trichuris and hookworm, which have inflicted a tremendous burden on the health of poor people in developing countries. In Haiti, 2 annual single-dose MDAs with DEC (6 mg/kg) and albendazole (400 mg) reduced Ascaris, Trichuris and hookworm prevalences from 20.9% to 14.1%, 34.0% to 14.6%, and 11.2% to 2.0%, respectively, 9 months after the second MDA [68]. In India, the same treatment reduced the prevalence of Ascaris by 83%, Trichuris by 63% and hookworm by 69%, 11 months after the second MDA [69]. These “beyond-filariasis” benefits will not only give the anti-filariasis campaign a broader public health significance but also help to improve compliance among people and sustain the elimination program. The combination of drugs is also said to be effective in preventing the acquirement of drug resistance by filarial parasites.
F. Global Programme to Eliminate Lymphatic Filariasis (GPELF)
f-1. Strategies/activities
The World Health Assembly made a resolution in 1997 to eliminate lymphatic filariasis from the world as a public health problem by 2020. To execute the resolution, the GPELF was organized with a main strategy of conducting annual single-dose MDA for 4-6 years. Under MDA, all people living in endemic areas with or without filarial infection are expected to be treated, meaning that the number in 83 endemic countries will reach some 13 billion people. The drugs used for MDA are the combination of DEC (6 mg/kg) and albendazole (400 mg) in onchocerciasis-free areas, and ivermectin (200-400 µg/kg) and albendazole (400 mg) in onchocerciasis-endemic areas of Africa. The reason for the use of 2 separate regimens is that DEC could cause severe reactions if administered to Onchocerca-infected individuals. The biggest barrier to financing the drug supply was removed by the donation of albendazole and ivermectin by the 2 pharmaceutical giants. The GPELF has also put particular emphasis on the care of existing clinical cases of lymphedema/elephantiasis and hydrocele. Simple procedures for lymphedema management have been established [70], in which the cure and prevention of bacterial/fungal infections by maintaining good hygiene of the affected skin is the basic concept. Daily washing with soap and water, together with exercise and elevation of the affected limb to drain accumulated lymph fluid, is the most essential part of the “care” for which family members and volunteers have been trained [71].
In the beginning, many researchers/clinicians were suspicious about the success of such a huge program. Some rejected the idea outright because they considered filariasis a low-priority disease. However, a tectonic shift was already underway: the idea of DALY had changed disease priority in favor of filariasis that produces permanent or long-lasting disability, and non-profit activities for the underprivileged by a variety of voluntary groups/citizens had matured. People suffering from lymphatic filariasis, one of the world’s most neglected diseases, have gained global attention for the first time in history.
f-2. Achievements
Ottesen et al. (2008) [72] described in detail the results of 8 years of global effort (2000-2007). More than 740 million albendazole tablets and 590 million ivermectin tablets were donated by the partner drug companies of the GAELF, while 4.7 billion DEC tablets were purchased by endemic countries. A minimum of 570 million individuals were treated in 48 of the 83 endemic countries. In 68 pre-fixed sentinel sites to monitor treatment effects, 5 rounds of MDA reduced mf prevalence by ca. 85% and cleared mf in 63% of the sites. The WHO report in 2008 [8] listed 5 countries which no longer have active transmission foci, and 2 countries (China and Korea) where the elimination of filariasis was declared.
The benefits of the MDAs conducted in 2000-2007 include the following: 6.6 million newborns were protected from filarial infection, of whom 1.4 million and 0.8 million individuals will escape hydrocele and lymphedema, respectively, in their lifetimes; 9.5 million asymptomatic parasite carriers were protected from developing hydrocele (6.0 mil.) or lymphedema (3.5 mil.). The DALYs averted in 8 years were estimated to reach 32 million, for which US$ 190 million was spent to cover MDA-related costs. Thus, the cost per DALY averted was US$ 5.90 (excluding donated drugs), which is one of the most cost effective programs in the world [73].
In addition to the benefits of the 2000-07 MDAs relating to filariasis, 56.6 million children and 44.5 million women of childbearing age were treated with albendazole for intestinal parasitic infections, and in onchocerciasis endemic countries of Africa, more than 45 million were treated with ivermectin for various skin diseases caused by Onchocerca volvulus, scabies mites and lice, although DALYs averted by these treatments were not quantified. It can readily be said that the global filariasis program has already established new precedents: collaboration in combating neglected diseases, single-dose treatment for different diseases, and confidence of local people in maintaining a public health program, all on a global scale.
f-3. The future
The progress made by the GPELF has been remarkable so far. However, the elimination program is not necessarily proceeding satisfactorily in all endemic countries. Problems arise when compliance to MDAs is not sufficient, pretreatment endemicity levels are high, the species of vector mosquito is an efficient transmitter, MDA drug dosage is not sufficient (particularly with ivermectin), etc. [74]. A more serious question will be the endpoint for the elimination program in each endemic country. The variability of biological, human-behavioral and socio-economic factors make it difficult to clarify a threshold at which filariasis transmission disappears spontaneously. With strong continuous global cooperation as a precondition, each endemic country needs to carry out well-organized and effective MDAs. The drug administration may have to be repeated if necessary. Vector control measures may become an essential part of the program in some areas [75]. And it is expected that mathematical models will play a more important role in planning future operations [76].
G. Expansion of intervention activities by people: Community-directed treatment (ComDT)
In many endemic areas where health manpower is running short, it is difficult to carry out a large scale MDA. In Okinawa, Japan, in the 1960s, senior high school graduates living in the endemic areas were trained as “ad hoc” laboratory technicians [77]. In Sri Lanka, the MDA in 2003 achieved 80% drug coverage of 9.8 million endemic population, and more than half of the drug coverage (55.2%) was executed by volunteers making door-to-door visits [78].
In West Africa, when the Onchocerciasis Control Programme started employing annual mass treatment with ivermectin, they faced the same problem of manpower shortage. Then in 1995, WHO/TDR conducted a landmark study in 5 African countries to clarify how well local people can plan and execute the distribution of ivermectin by themselves [79]. In the study, 2 different treatment schemes were compared: community-designed treatment and program-designed treatment. In the former, after a minimal essential health education/information session, the community was invited to decide who in the community would be drug distributers and when and how the drugs would be distributed, while in the latter, experienced program staff pre-designed the criteria for selecting drug distributers and detailed procedures for drug distribution. The results were rather unexpected: the former achieved as good a treatment coverage as the latter. This was a clear indication that local people can be a reliable player in public health activities. A similar study with lymphatic filariasis followed in Ghana and Kenya [80], where community-directed treatment with some input from health services (ComDT/HS) was compared with the treatment planned and executed by the regular care system (HST). The results: ComDT/HS achieved a high treatment coverage of 75.7%-88.0%, whereas HST obtained only 43.6%-46.5%.
The African Programme for Onchocerciasis Control (APOC), which was set up in 1995 covering 16 countries, adopted the idea of community-designed treatment from the above 1995 TDR study and implemented the community-directed treatment with ivermectin (CDTI). The program was so successful in reducing the burden of onchocerciasis that, in the year 2007, close to 1 million DALYs could be averted [81]. For CDTI, “community drug distributers” (CDDs) play an essential role. After training, they take a census, distribute drugs, monitor adverse reactions and keep records. In 2006, there were 429,385 trained CDDs in APOC countries. These batteries of manpower with health care knowledge have become involved as a matter of course in other intervention programs. In Nigeria, successful integration of insecticide-treated bed net distribution for malaria control with lymphatic filariasis/onchocerciasis MDA was reported [82]. WHO/TDR, in 2005, launched a study to investigate whether the concept and experience of CDTI can be applied to other interventions such as delivering vitamin A supplement, insecticide treated nets, DOTS treatment for tuberculosis, and home management of malaria. The results showed that all 5 interventions (including CDTI) could be done simultaneously by the community [83].
Community-directed treatment, which was invented as a measure necessary to conduct a large scale MDA for onchocerciasis in areas where health infrastructures were poor, has been transformed into a new sustainable way of delivering therapeutic and preventive measures for rural people suffering from a variety of neglected diseases. The MDAs for lymphatic filariasis, which have been conducted side by side with APOC, can be expected to strengthen the new development synergistically.
Acknowledgment
In compiling this paper, I remembered many people: mentors, colleagues, friends, and even village people, to whom I owe a great deal. They gave me the lessons and experiences that shaped me as a parasitologist. Particularly in relation to this paper, I must mention with hearty thanks several names who guided me when I worked in Samoa and Fiji as an inexperienced WHO Medical Officer: Dr. L. Penaia, Mr. P.F. Sone, Dr. S. Pelenatu, Dr. S.T. Faaiuaso (Filariasis Office, Ministry of Health, Samoa); Dr. J.U. Mataika, Dr. J. Koroivueta, Dr. M.V. Mataitoga (Former Wellcome Virus Laboratory, Fiji); Dr. W.A. Samarawickrema, Dr. K.I. Singh (WPRO/WHO, Samoa); Dr. G.F.S. Spears (WHO Consultant, University of Otago, New Zealand); and Dr. L.S. Self, and Dr. B.C. Dazo (WPRO/WHO, Philippines).
References
- 1.Dreyer G, Piessens WF. In: Nutman T.B. (editor). Lymphatic Filariasis London: Imperial College Press; 2000: 245-264.
- 2.Otsuji Y. In: Kimura E, Rim H-J, Sun D, Weerasooriya MV (editors). Asian Parasitology Vol. III Filariasis in Asia and Western Pacific Islands Chiba: AAA Committee/Federation of Asian Parasitologists; 2005: 81-92.
- 3.Michael E, Bundy DAP, Grenfell BT. Re-assessing the global prevalence and distribution of lymphatic filariasis. Parasitology 1996; 112: 409-428 [DOI] [PubMed] [Google Scholar]
- 4.WHO. World Health Report 1998—Life in the 21st century: A vision for all.
- 5.Perera M, Whitehead M, Molyneux D, Weerasooriya M, Gunatilleka G. Neglected patients with a neglected disease? A qualitative study of lymphatic filariasis. PLoS Negl Trop Dis 2007; 1: 2 (e128). [DOI] [PMC free article] [PubMed]
- 6.WHO. World Health Report 2000—Health systems: improving performance. [PubMed]
- 7.Ramaiah KD, Das PK, Michael E, Guyatt H. The economic burden of lymphatic filariasis in India. Parasitol Today 2000; 16: 251-253 [DOI] [PubMed] [Google Scholar]
- 8.WHO. Weekly Epidemiological Record 2008; 83: 333-347 [Google Scholar]
- 9.Hewitt RI, Kushner S, Stewart HW, White E, Wallace WS, SubbaRow Y. Experimental chemotherapy of filariasis. III. Effect of 1-diethylcarbamyl-4-methylpiperazine hydrochloride against naturally acquired filarial infections in cotton rats and dogs. J Lab Clin Med 1947; 32: 1314-1329 [PubMed] [Google Scholar]
- 10.Santiago-Stevenson D, Oliver-Gonzalez J, Hewitt RI. The treatment of filariasis bancrofti with 1-diethylcarbamyl-4-methylpiperazine hydrochloride (Hetrazan). Ann N Y Acad Sci 1948; 50: 161-170 [DOI] [PubMed] [Google Scholar]
- 11.Trent SC. Reevaluation of World War II veterans with filariasis acquired in the South Pacific. Am J Trop Med Hyg 1963; 12: 877-887 [DOI] [PubMed] [Google Scholar]
- 12.Otto GF, Jachowski Jr LA, Wharton JD. Filariasis in American Samoa III. Studies on chemotherapy against the nonperiodic form of Wuchereria bancrofti. Am J Trop Med Hyg 1953; 2: 495-516 [PubMed] [Google Scholar]
- 13.Mahoney LE, Kessel JF. Treatment failure in filariasis mass treatment programmes. Bull World Health Organ 1971; 45: 35-42 [PMC free article] [PubMed] [Google Scholar]
- 14.Manson-Bahr P. The action of hetrazan in Pacific filariasis. J Trop Med Hyg 1952; 55: 169-173 [PubMed] [Google Scholar]
- 15.Burnett GF, Mataika JU. Mass-administration of diethylcarbamazine citrate in preventing transmission of aperiodic human filariasis. Trans Roy Soc Trop Med Hyg 1961; 55: 178-187 [DOI] [PubMed] [Google Scholar]
- 16.Kessel JF. An effective programme for the control of filariasis in Tahiti. Bull World Health Organ 1957; 16: 633-664 [PMC free article] [PubMed] [Google Scholar]
- 17.Sasa M. Human filariasis—a global survey of epidemiology and control. Tokyo: University of Tokyo Press; 1976: 500-501.
- 18.WHO. WHO Expert Committee on Filariasis (Wuchereria and Brugia Infections): Second Report 1967. [PubMed]
- 19.WHO. WHO Expert Committee on Filariasis: Third Report 1974. [PubMed]
- 20.WHO. Lymphatic Filariasis: Fourth report of the WHO Expert Committee on Filariasis 1984. [PubMed]
- 21.Hawking F. A review of progress in the chemotherapy and control of filariasis since 1955. Bull World Health Organ 1962; 27: 555-568 [PMC free article] [PubMed] [Google Scholar]
- 22.McGregor IA, Gilles HM. Further studies on the control of bancroftian filariasis in West Africa by means of diethylcarbamazine. Ann Trop Med Parasitol 1960; 54: 415-418 [Google Scholar]
- 23.Laigret J, Fagneaux G, Tuira E. Progrès dans l’emploi de la diéthylcarbamazine en chimiothérapie de la filariose lymphatique à Wuchereria bancrofti var. pacifica: la méthode des doses espacées. Bull World Health Organ 1978; 56: 985-990 [PMC free article] [PubMed] [Google Scholar]
- 24.Laigret J, Fagneaux G, Tuira E. Chimiothérapie de masse par la diéthylcarbamazine en doses espacées: effets obtenus à Tahiti sur la microfilarémie à Wuchereria bancrofti, var. pacifica. Bull World Health Organ 1980; 58: 779-783 [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura E, Penaia L, Spears GFS. Epidemiology of subperiodic bancroftian filariasis in Samoa 8 years after control by mass treatment with diethylcarbamazine. Bull World Health Organ 1985; 63: 869-880 [PMC free article] [PubMed] [Google Scholar]
- 26.Burnett GF, Mataika JU. Mass administration of diethylcarbamazine citrate in preventing transmission of aperiodic human filariasis II. Results of a blood survey made four years after drug administration. Trans Roy Soc Trop Med Hyg 1964; 58: 545-551 [DOI] [PubMed] [Google Scholar]
- 27.Mataika JU, Dando BC, Spears GFS, MacNamara FN. Mosquito-borne infections in Fiji I. Filariasis in northern Fiji: epidemiological evidence regarding factors influencing the prevalence of microfilaraemia of Wuchereria bancrofti infections. J Hyg Camb 1971; 69: 273-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasa M. Human filariasis—a global survey of epidemiology and control Tokyo: University of Tokyo Press; 1976: 536-540.
- 29.Mataika JU, Mataitoga MV, Kimura E. Recent situation of filariasis in Lau and Rotuma provinces in Fiji. Fiji Med J 1985; 13: 211-214 [Google Scholar]
- 30.Mataika JU, Kimura E, Koroivueta J, Shimada M. Efficacy of five annual single doses of diethylcarbamazine for treatment of lymphatic filariasis in Fiji. Bull World Health Organ 1998; 76: 575-579 [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura E, Penaia L, Spears GFS. The efficacy of annual single-dose treatment with diethylcarbamazine citrate against diurnally subperiodic bancroftian filariasis in Samoa. Bull World Health Organ 1985; 63: 1097-1106 [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura E, Spears GFS, Singh KI, Samarawickrema WA, Penaia L, Sone PF, Pelenatu S, Faaiuaso ST, Self LS, Dazo BC. Long-term efficacy of single-dose mass treatment with diethylcarbamazine citrate against diurnally subperiodic Wuchereria bancrofti: eight years’ experience in Samoa. Bull World Health Organ 1992; 70: 769-776 [PMC free article] [PubMed] [Google Scholar]
- 33.Sasa M. Human filariasis―a global survey of epidemiology and control Tokyo: University of Tokyo Press; 1976: 700-705.
- 34.Mataika J, Kimura E, Koroivueta J, Kaisuva JN, Brown M, Tuivaga J, Bikai S, Govind SR. Comparison of the efficacy of diethylcarbamazine between 5 rounds of annual single-dose treatment and an intensive 28-dose treatment spread over 2 years against diurnally subperiodic Wuchereria bancrofti in Fiji. Fiji Med J 1993; 19: 2-6 [Google Scholar]
- 35.Kimura E, Mataika JU. Control of lymphatic filariasis by annual single-dose diethylcarbamazine treatments. Parasitol Today 1996; 12: 240-244 [DOI] [PubMed] [Google Scholar]
- 36.Kimura E, Penaia L, Samarawickrema WA, Spears GFS. Low-density microfilaremia in subperiodic bancroftian filariasis in Samoa. Bull World Health Organ 1985; 63: 1089-1096 [PMC free article] [PubMed] [Google Scholar]
- 37.Samarawickrema WA, Spears GFS, Sone F, Ichimori K, Cummings RF. Filariasis transmission in Samoa I. Relation between density of microfilariae and larval density in laboratory-bred and wild-caught Aedes (Stegomyia) polynesiensis (Marks) and wild-caught Aedes (Finlaya) samoanus (Gruenberg). Ann Trop Med Parasitol 1985; 79: 89-100 [PubMed] [Google Scholar]
- 38.Pichon G, Merlin M, Fagneaux G, Riviére F, Laigret J. Etude de la distribution des numérations microfilariennes dans les foyer de filariose lymphatique. Tropenmed Parasitol 1980; 31: 165-180 [PubMed] [Google Scholar]
- 39.Cartel JL, Celerier P, Spiegel A, Burucoa C, Roux JF. A single diethylcarbamazine dose for treatment of Wuchereria bancrofti carriers in French Polynesia: efficacy and side effects. Southeast Asian J Trop Med Public Health 1990; 21: 465-470 [PubMed] [Google Scholar]
- 40.Schuurkamp GJ, Kereu RK, Bulungol PK, Kawereng A, Spicer PE. Diethylcarbamazine in the control of bancroftian filariasis in the Ok Tedi area of Papua New Guinea: Phase 2―annual single-dose treatment. PNG Med J 1994; 37: 65-81 [PubMed] [Google Scholar]
- 41.Andrade LD, Medeiros Z, Pires ML, Pimentel A, Rocha A, Figueredo-Silva J, Coutinho A, Dreyer G. Comparative efficacy of three different diethylcarbamazine regimens in lymphatic filariasis. Trans Roy Soc Trop Med Hyg 1995; 89: 319-321 [DOI] [PubMed] [Google Scholar]
- 42.Meyrowitsch DW, Simonsen PE, Makunde WH. Mass diethylcarbamazine chemotherapy for control of bancroftian filariasis: comparative efficacy of standard treatment and two semi-annual single-dose treatments. Trans Roy Soc Trop Med Hyg 1996; 90: 69-73 [DOI] [PubMed] [Google Scholar]
- 43.Panicker KN, Krishnamoorthy K, Sabesan S, Prathiba J, Abidha Comparison of effects of mass annual and biannual single dose therapy with diethylcarbamazine for the control of Malayan filariasis. Southeast Asian J Trop Med Public Health 1991; 22: 402-411 [PubMed] [Google Scholar]
- 44.Kazura J, Greenberg J, Perry R, Weil G, Day K, Alpers M. Comparison of single-dose diethylcarbamazine and ivermectin for treatment of bancroftian filariasis in Papua New Guinea. Am J Trop Med Hyg 1993; 49: 804-811 [DOI] [PubMed] [Google Scholar]
- 45.Amaral F, Dreyer G, Figueredo-Silva J, Norões J, Cavalcanti A, Samico SC, Santos A, Coutinho A. Live adult worms detected by ultrasonography in human bancroftian filariasis. Am J Trop Med Hyg 1994; 50: 753-757 [DOI] [PubMed] [Google Scholar]
- 46.Mand S, Marfo-Debrekyei Y, Dittrich M, Fischer K, Adjei O, Hoerauf A. Animated documentation of the filaria dance sign (FDS) in bancroftian filariasis. Filaria J 2003; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norões J, Dreyer G, Santos A, Mendes VG, Medeiros Z, Addiss D. Assessment of the efficacy of diethylcarbamazine on adult Wuchereria bancrofti in vivo. Trans Roy Soc Trop Med Hyg 1997; 91: 78-81 [DOI] [PubMed] [Google Scholar]
- 48.Figueredo-Silva J, Jungmann P, Norões J, Piessens WF, Coutinho A, Brito C, Rocha A, Dreyer G. Histological evidence for adulticidal effect of low doses of diethylcarbamazine in bancroftian filariasis. Trans Roy Soc Trop Med Hyg 1996; 90: 192-194 [DOI] [PubMed] [Google Scholar]
- 49.Kumaraswami V, Ottesen EA, Vijayasekaran V, Devi U, Swaminathan M, Aziz MA, Sarma GR, Prabhakar R, Tripathy SP. Ivermectin for the treatment of Wuchereria bancrofti filariasis. Efficacy and adverse reactions. JAMA 1988; 259: 3150-3153 [PubMed] [Google Scholar]
- 50.Ottesen EA, Vijayasekaran V, Kumaraswami V, Perumal Pillai SV, Sadanandam A, Frederick S, Prabhakar R, Tripathy SP. A controlled trial of ivermectin and diethylcarbamazine in lymphatic filariasis. New Eng J Med 1990; 322: 1113-1117 [DOI] [PubMed] [Google Scholar]
- 51.Addiss DG, Eberhard ML, Lammie PJ, McNeeley MB, Lee SH, McNeeley DF, Spencer HC. Comparative efficacy of clearing-dose and single high-dose ivermectin and diethylcarbamazine against Wuchereria bancrofti microfilaremia. Am J Trop Med Hyg 1993; 48: 178-185 [DOI] [PubMed] [Google Scholar]
- 52.Dreyer G, Coutinho A, Miranda D, Norões J, Rizzo JA, Galdino E, Rocha A, Medeiros Z, Andrade LD, Santos A, Figueredo-Silva J, Ottesen EA. Treatment of bancroftian filariasis in Recife, Brazil: a two-year comparative study of the efficacy of single treatments with ivermectin or diethylcarbamazine. Trans Roy Soc Trop Med Hyg 1995; 89: 98-102 [DOI] [PubMed] [Google Scholar]
- 53.Dreyer G, Norões J, Amaral F, Nen A, Medeiros Z, Coutinho A, Addiss D. Direct assessment of the adulticidal efficacy of a single dose of ivermectin in bancroftian filariasis. Trans Roy Soc Trop Med Hyg 1995; 89: 441-443 [DOI] [PubMed] [Google Scholar]
- 54.Moulia-Pelat JP, Nguyen LN, Hascoët H, Luquiaud P, Nicolas L. Advantages of an annual single dose of ivermectin 400 micrograms/kg plus diethylcarbamazine for community treatment of bancroftian filariasis. Trans Roy Soc Trop Med Hyg 1995; 89: 682-685 [DOI] [PubMed] [Google Scholar]
- 55.Dreyer G, Addis D, Santos A, Figueredo-Silva J, Norões J. Direct assessment in vivo of the efficacy of combined single-dose ivermectin and diethylcarbamazine against adult Wuchereria bancrofti. Trans Roy Soc Trop Med Hyg 1998; 92: 219-222 [DOI] [PubMed] [Google Scholar]
- 56.Ottesen EA, Ismail MM, Horton J. The role of albendazole in programmes to eliminate lymphatic filariasis. Parasitol Today 1999; 15: 382-386 [DOI] [PubMed] [Google Scholar]
- 57.Ismail MM, Jayakody RL, Weil GJ, Nirmalan N, Jayasinghe KSA, Abeyewickrema W, Rezvi Sheriff MH, Rajaratnam HN, Amarasekera N, de Silva DCL, Michalski ML, Dissanaike AS. Efficacy of single dose combinations of albendazole, ivermectin and diethylcarbamazine for the treatment of bancroftian filariasis. Trans Roy Soc Trop Med Hyg 1998; 92: 94-97 [DOI] [PubMed] [Google Scholar]
- 58.Ismail MM, Jayakody RL, Weil GJ, Fernand D, de Silva MSG, de Silva GAC, Balasooriya WK. Long-term efficacy of single-dose combinations of albendazole, ivermectin and diethylcarbamazine for the treatment of bancroftian filariasis. Trans Roy Soc Trop Med Hyg 2001; 95: 332-335 [DOI] [PubMed] [Google Scholar]
- 59.El Setouhy M, Ramzy RMR, Ahmed ES, Kandil AM, Hussain O, Farid HA, Helmy H, Weil GJ. A randomized clinical trial comparing single- and multi-dose combination therapy with diethylcarbamazine and albendazole for the treatment of bancroftian filariasis. Am J Trop Med Hyg 2004; 70: 191-196 [PubMed] [Google Scholar]
- 60.Gyapong JO, Kumaraswami V, Biswas G, Ottesen EA. Treatment strategies underpinning the global programme to eliminate lymphatic filariasis. Expert Opin Pharmacother 2005; 6: 179-200 [DOI] [PubMed] [Google Scholar]
- 61.Sunish IP, Rajendran R, Mani TR, Munirathinam A, Reuben R, Dash AP. Impact of single dose of diethylcarbamazine and other antifilarial drug combinations on bancroftian filarial infection variables: assessment after 2 years. Parasitol Int 2006; 55: 233-236 [DOI] [PubMed] [Google Scholar]
- 62.Dunyo SK, Nkrumah FK, Simonsen PE. A randomized double-blind placebo-controlled field trial of ivermectin and albendazole alone and in combination for the treatment of lymphatic filariasis in Ghana. Trans Roy Soc Trop Med Hyg 2000; 94: 205-211 [DOI] [PubMed] [Google Scholar]
- 63.Dunyo SK, Nkrumah FK, Simonsen PE. Single-dose treatment of Wuchereria bancrofti infections with ivermectin and albendazole alone or in combination: evaluation of the potential for control at 12 months after treatment. Trans Roy Soc Trop Med Hyg 2000; 94: 437-443 [DOI] [PubMed] [Google Scholar]
- 64.Pani SP, Reddy GS, Das LK, Vanamail P, Hoti SL, Ramesh J, Das PK. Tolerability and efficacy of single dose albendazole, diethylcarbamazine citrate (DEC) or coadministration of albendazole with DEC in the clearance of Wuchereria bancrofti in asymptomatic microfilaraemic volunteers in Pondicherry, South India: a hospital-based study. Filaria J 2002; 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rizzo JA, Belo C, Lins R, Dreyer G. Children and adolescents infected with Wuchereria bancrofti in Greater Recife, Brazil: a randomized, year-long clinical trial of single treatments with diethylcarbamazine or diethylcarbamazinealbendazole. Ann Trop Med Parasitol 2007; 101: 423-433 [DOI] [PubMed] [Google Scholar]
- 66.Dreyer G, Addiss D, Williamson J, Norões J. Efficacy of co-administered diethylcarbamazine and albendazole against adult Wuchereria bancrofti. Trans Roy Soc Trop Med Hyg 2006; 100: 1118-1125 [DOI] [PubMed] [Google Scholar]
- 67.Addiss D, Gamble CL, Garner P, Gelband H, Ejere HOD, Critchley JA, International Filariasis Review Group. Albendazole for lymphatic filariasis. Cochrane Database Syst Rev 2005; (4): CD003753. [DOI] [PubMed]
- 68.Beau de Rochars M, Direny AN, Roberts JM, Addiss DG, Radday J, Beach MJ, Streit TG, Dardith D, Lafontant JG, Lammie PJ. Community-wide reduction in prevalence and intensity of intestinal helminths as a collateral benefit of lymphatic filariasis elimination programs. Am J Trop Med Hyg 2004; 71: 466-470 [PubMed] [Google Scholar]
- 69.Mani TR, Rajendran R, Sunish IP, Munirathinam A, Arunachalam N, Satyanarayana K, Dash AP. Effectiveness of two annual, single-dose mass drug administrations of diethylcarbamazine alone or in combination with albendazole on soil-transmitted helminthiasis in filariasis elimination programme. Trop Med Int Health 2004; 9: 1030-1035 [DOI] [PubMed] [Google Scholar]
- 70.Dreyer G, Addiss D, Dreyer P, Norões J. Basic Lymphoedema Management: Treatment and prevention of problems associated with lymphatic filariasis Hollis: Hollis Publishing Company; 2002: 1-112.
- 71.Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: a review of the scientific literature. Filaria J 2007; 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ottesen EA, Hooper PJ, Bradley M, Biswas G. The global programme to eliminate lymphatic filariasis: Health impact after 8 years. PLoS Negl Trop Dis 2008; 2: 10 (e317). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laxminarayan R, Mills AJ, Breman JG, Measham AR, Alleyne G, Claeson M, Jha P, Musgrove P, Chow J, Shahid-Salles S, Jamison DT. Advancement of global health: key messages from the Disease Control Priorities Project. Lancet 2006; 367: 1193-1208 [DOI] [PubMed] [Google Scholar]
- 74.Kyelem D, Biswas G, Bockarie MJ, Bradley MH, El-Setouhy M, Fischer PU, Henderson RH, Kazura JW, Lammie PJ, Njenga SM, Ottesen EA, Ramaiah KD, Richards FO, Weil GJ, Williams SA. Determinants of success in national programs to eliminate lymphatic filariasis: A perspective identifying essential elements and research needs. Am J Trop Med Hyg 2008; 79: 480-484 [PMC free article] [PubMed] [Google Scholar]
- 75.Sunish IP, Rajendran R, Mani TR, Munirathinam A, Dash AP, Tyagi BK. Vector control complements mass drug administration against bancroftian filariasis in Tirukoilur, India. Bull World Health Organ 2007; 85: 138-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gambhir M, Michael E. Complex ecological dynamics and eradicability of the vector borne macroparasitic disease, lymphatic filariasis. PLoS ONE 2008; 3: 8 (e2874). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoshida C. In: Kimura E, Rim H-J, Sun D, Weerasooriya MV (editors). Asian Parasitology Vol. III Filariasis in Asia and Western Pacific Islands Chiba: AAA Committee/Federation of Asian Parasitologists; 2005: 109-118.
- 78.Weerasooriya MV, Yahathugoda CT, Wickremasinghe D, Gunawardena KN, Dharmadasa RA, Vidanapathirana KK, Weerasekara SH, Samarawickrema WA. Social mobilisation, drug coverage and compliance and adverse reactions in a Mass Drug Administration (MDA) Programme for the Elimination of Lymphatic Filariasis in Sri Lanka. Filaria J 2007; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.WHO/TDR. Community directed treatment with ivermectin: Report of a multi-country study (TDR/AFR/RP/96.1), 1996.
- 80.WHO/TDR. Community-directed treatment of lymphatic filariasis in Africa: Report of a multi-centre study (TDR/ IDE/RP/CDTI/00.2), 2000.
- 81.WHO/APOC. Revitalising health care delivery in sub-Saharan Africa: The potential of community-directed interventions to strengthen health systems, 2007.
- 82.Blackburn BG, Eigege A, Gotau H, Gerlong G, Miri E, Hawley WA, Mathieu E, Richards F. Successful integration of insecticide-treated bet net distribution with mass drug administration in Central Nigeria. Am J Trop Med Hyg 2006; 75: 650-655 [PubMed] [Google Scholar]
- 83.WHO/TDR. Community-directed interventions for major health problems in Africa: A multi-country study. Final Report, 2008.