Abstract
Extraocular muscle (EOM) myofibers do not fit the traditional fiber typing classifications normally used in noncranial skeletal muscle, in part, due to the complexity of their individual myofibers. With single skinned myofibers isolated from rectus muscles of normal adult rabbits, force and shortening velocity were determined for 220 fibers. Each fiber was examined for myosin heavy chain (MyHC) isoform composition by densitometric analysis of electrophoresis gels. Rectus muscle serial sections were examined for coexpression of eight MyHC isoforms. A continuum was seen in single myofiber shortening velocities as well as force generation, both in absolute force (g) and specific tension (kN/m2). Shortening velocity correlated with MyHCIIB, IIA, and I content, the more abundant MyHC isoforms expressed within individual myofibers. Importantly, single fibers with similar or identical shortening velocities expressed significantly different ratios of MyHC isoforms. The vast majority of myofibers in both the orbital and global layers expressed more than one MyHC isoform, with up to six isoforms in single fiber segments. MyHC expression varied significantly and unpredictably along the length of single myofibers. Thus EOM myofibers represent a continuum in their histological and physiological characteristics. This continuum would facilitate fine motor control of eye position, speed, and direction of movement in all positions of gaze and with all types of eye movements—from slow vergence movements to fast saccades. To fully understand how the brain controls eye position and movements, it is critical that this significant EOM myofiber heterogeneity be integrated into hypotheses of oculomotor control.
Keywords: extraocular muscles, myosin heavy chain isoforms, muscle force
muscle contractile characteristics are a function of the array of fiber types within each muscle. These characteristics depend, in part, on the expression patterns of myosin heavy chain (MyHC) isoforms within any given muscle. These MyHC isoforms exist as part of a multigene family (90). Classically, limb skeletal muscles are considered to be composed of four main fiber types: three fast fiber types containing MyHC isoforms IIA, IIX, IIB, and one slow fiber type, containing MyHCI. While the majority of myofibers within limb skeletal muscles contains only one MyHC isoform (e.g., slow myofibers in the soleus), others may show significant MyHC polymorphism (79), containing more than one of these MyHC isoforms. This MyHC coexpression pattern is most common in physiologically fast muscles, such as diaphragm and plantaris muscles, where IIA, IIB, IIX, and I can all be coexpressed (15). The contractile properties of hybrid myofibers reflect the mixture of MyHC isoforms and other fiber type specific proteins, and these hybrid myofibers are thought to play a role in refining the control of shortening velocity (53).
The extraocular muscles (EOM) have a unique embryological origin from nonsegmented head mesoderm compared with skeletal muscles, which are derived from somites (85). The EOM retain a number of characteristics that are downregulated in somite-derived skeletal muscle (58), including myofibers with multineuronal (49, 69) and polyneuronal innervation (22), expression of N-CAM (60), as well as expression of the immature isoform of the nicotinic acetylcholine receptor (40). In contrast to the four MyHC isoforms found in trunk and limb skeletal muscles, gene and protein expression analyses show that nine different isoforms exist in EOM, including an EOM-specific isoform (8, 10, 13, 45, 66, 87, 91). The nine, and possibly more, MyHC isoforms expressed in the EOM are: MyHCI (MYH7, slow beta-cardiac), MyHCIIA (MYH2), MyHCIIB (MYH4), MyHCIIX/D (MYH1), MyHCembryonic (developmental, MYH3), MyHCperinatal (neonatal, MYH8), MyHCalpha-cardiac (MYH6), MyHCEOM-specific (MYH13), and MyHCslow-tonic (MYH14/7b) (73). A recent report indicates MYH15 is also expressed in the EOM (73). The differential patterns and proportions of MyHC isoform expression within the orbital and global layers combine with this diversity of MyHC isoforms to result in increased overall muscle fiber complexity (10, 43, 69, 96).
There are distinct variations of MyHC isoform expression longitudinally in individual EOM, with the most striking differences in the middle region (midbelly) of the EOM compared with the tendon ends (13, 59, 74). Single EOM myofibers also coexpress multiple isoforms of the MyHC family, which are distributed differentially along the length of the myofibers (42, 45, 74), adding to the already complex three-dimensional pattern of MyHC expression in different regions of the whole muscle.
There is increasing evidence for coexpression of multiple MyHC within single myofibers in all mammalian EOM thus far investigated, from mouse to humans (13, 42, 45, 47, 48). The EOM can, within short time periods, exhibit a wide array of movements, from steady fixation, slow and fast saccades, smooth pursuit, as well as slow vergence, in an infinite number and wide range of eye and gaze positions. On the basis of these studies, we hypothesize that the individual myofibers within EOM represent a continuum, in terms of MyHC isoform expression patterns, force generation, and shortening velocities. To support this hypothesis, 220 single skinned myofibers from adult rabbit EOM were analyzed individually to determine force generation and shortening velocity. The MyHC isoform composition for each single identified myofiber was determined with gel electrophoresis (SDS-PAGE) followed by densitometric determination of the relative abundance of each distinct MyHC isoform. For EOM there was an extremely wide continuum of both shortening velocity and contractile force and practically all myofibers analyzed contained more than one MyHC isoform.
MATERIALS AND METHODS
New Zealand White rabbits obtained from Bakkom Rabbitry were housed with Research Animal Resources at the University of Minnesota in an AAALAC approved facility. All research conformed to the guidelines of the NIH for the Use of Animals in Research and was approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Normal adult rabbits were euthanized with an overdose of barbiturate anesthesia and muscles were prepared for single fiber analysis. The individual rectus muscles were dissected free and removed, taking care not to stretch the muscles during removal. The muscles were placed on ice in relaxing solution at pH 7.0 consisting of (in mM) 7 EGTA, 0.016 CaCl2, 5.6 MgCl2, 80 KCl, 20 imidazole, 14.5 creatine phosphate, and 4.8 ATP, with a pCa of 9.0. Bundles of myofibers, ∼8 by 1 mm, were tied to capillary tubes and stored in permeabilization buffer for up to 1 wk at −20°C. Permeabilization buffer contains (in mM) 125 K proprionate, 2 EGTA, 4 ATP, 1 MgCl, 20 imidazole, 0.1% leupeptin, pH 7.0, plus 50% glycerol (vol/vol).
For contractile analysis, individual myofiber segments, ∼2–4 mm long, were randomly isolated from the fiber bundles and placed in an experimental chamber mounted to an inverted microscope. The chamber allowed the fiber to be transferred to wells containing activating or relaxing solutions, and the solutions were maintained at 20°C. One end of the fiber was attached to a force transducer (Cambridge Technology/Aurora Scientific) and the other end to a length ergometer using microtweezers (Cambridge Technology/Aurora Scientific). Sarcomere length was visually measured using a microscope and calibrated eyepiece micrometer. Sarcomere length along an isolated single muscle segment was adjusted to a sarcomere spacing of 2.5 μm. Each fiber segment was transferred from relaxing solution to activating solution to determine force generation. Activating solution was the same as relaxing solution except calcium concentration was brought to a pCa of 4.5 and KCl to 180 mM. Fiber cross-sectional areas were determined at three locations along the fiber segment. The slack test method was used to determine the maximal unloaded shortening velocity (Vo). Briefly, the myofiber was maximally activated and then rapidly shortened by 7–18% of the fiber length such that force was reduced to zero. The time between zero force and initiation of force redevelopment was measured, and this procedure was repeated at various fiber lengths, graphed, and Vo determined by the slope of the linear regression line divided by fiber length as previously described (86). A total of 220 single skinned fibers from 10 rabbits was examined.
After determining force and Vo, each fiber was removed from the experimental chamber and solubilized in sample buffer containing 6 mg/ml EDTA, 0.06 M Tris, 1% SDS, 2 mg/ml bromphenol blue, 15% glycerol, and 5% β-mercaptoethanol. Aliquots were removed for separation of single fiber MyHC isoforms using SDS-PAGE and visualized with silver staining. Stacking gels were composed of 4% acrylamide:N-N′-methylenebisacrylamide (bis) (50:1), 0.5 M Tris (pH 6.8), 0.1 M EDTA (pH 7.0), 10% SDS, 80% glycerol, 10% ammonium persulfate (APS), and 0.5% tetramethylethylenediamine (TEMED). Separating gels were composed of 5% acrylamide:bis (50:1), 1.5 M Tris (pH 8.8), 1.0 M glycine, 10% SDS, 80% glycerol, 10% APS, and 0.5% TEMED. Samples were loaded on an electrophoresis system (Hoefer SE600, GE Healthcare) and run at 250 V for 24 h. Water baths maintained the temperature at 12–15°C. Following electrophoresis the gels were silver-stained for MyHC identification (30). MyHC isoform mobility was assessed by comparing each fiber to standards prepared from EOM and leg samples. Densitometry was performed on each lane/gel by scanning them with a molecular multi-imaging system (GS-800, Bio-Rad). The relative expression of the MyHC isoforms was determined from the optical density using a densitometry image analysis software program (Quantity One). Data were graphed based on Vo and MyHC isoform.
A second group of four adult rabbits was used for histological examination of coexpression patterns of MyHC in serial sections. Individual rectus muscles were removed after the rabbits were euthanized with an overdose of barbiturate anesthesia. The specimens were frozen in 2-methylbutane chilled to a slurry on liquid nitrogen. In a cryostat, 12 μm serial frozen sections were prepared and mounted on glass microslides. Serial sections were immunostained with antibodies specific for the following MyHC isoforms using standard methods (59): pan-fast (specific for MyHCIIA and B, 1:40), slow (MyHCI, 1:40), and neonatal (MyHCneo, 1:20; all Vector Labs, Burlingame, CA) and embryonic (MyHCemb, F1.652; 1:20), EOM-specific (MyHCeom, 4A6; 1:10), slow tonic (MyHCslowtonic, S46; 1:20), fast IIA (MyHCIIASC-71; 1:20), fast IIB (MyHCIIB, BF-F3; straight), and fast IIX (MyHCIIX, 6H1; 1:20) MyHC isoforms (Hybridoma Bank, University of Iowa, Ames, IA). Sections were selected for analysis midway between the tendon end and the midbelly region of the rectus muscles. After incubation in blocking serum, the sections were incubated in primary antibody for 1 h. The sections were rinsed and incubated using the reagents of the Vectastain Elite mouse ABC peroxidase kit (Vector Labs). The reacted tissue sections were processed for visualization of the bound secondary antibodies using the heavy metal intensified diaminobenzidine procedure. Specificity of antibody binding was verified by immunostaining sections in the absence of primary antibody. The immunostained serial sections were examined microscopically and photographed for identification of coexpression of MyHC isoforms in individual myofibers. Reconstructions of individual myofibers positive for neonatal MyHC were performed by tracing individual myofibers in serial sections using the Bioquant NovaPrime software (Bioquant, Nashville, TN). Three-dimensional reconstructions were then built using the Topographer software (Bioquant; 17).
Statistical analysis.
Data are presented as means ± SE. Pearson's correlation coefficients were calculated for the data and, where significant, are indicated with both r and P values. Data were deemed significant if P ≤ 0.05. All statistical analyses were performed using Prism software (Graphpad, San Diego, CA).
RESULTS
Single myofiber diameter, force, and shortening velocity.
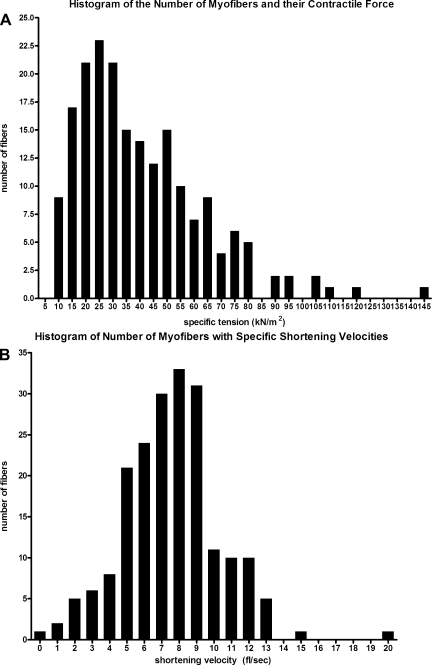
Contractility (force and Vo) was determined on 220 myofibers. Histograms representing the number of myofibers for both specific tension and Vo are shown in Fig. 1. Notably, individual myofibers from the EOM showed a wide range of contractile values compared with limb skeletal muscle myofibers. Specific tension (in kN/m2) ranged significantly among the fibers examined, with a low of 9.99 to a high of 143, over a 14-fold difference. Specific tension (±SE) for the 220 individual fibers was 40.24 ± 1.66 kN/m2. Vo in single myofibers ranged significantly too, with a low of 0.439 fl/s to a high of 19.8 fl/s, almost a 50-fold difference. Mean Vo (±SE) was 7.569 ± 0.196 fl/s. Mean myofiber diameter was 53.4 ± 0.623 μm, with a range from 31.2 to 80.8 μm. It should be noted that there is an ∼10–30% swelling of skinned fibers (31); however, there is minimal change to contractile proteins (23). These are similar to previously published fiber diameters (54), and their values ranged from 22 to 83 μm, with the highest value of 82.55 μm in rabbit superior rectus muscle.
Fig. 1.
Histograms of contractility for the 220 myofibers. A: specific tension in kN/m2 with a mean of 40.24. B: shortening velocity in fl/s with a mean of 7.569. Shortening velocity was determined by the slack method.
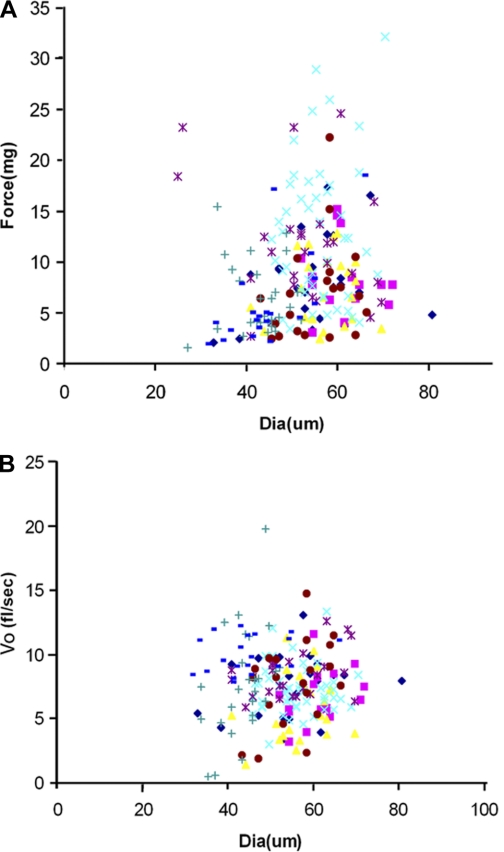
Contractile parameters of individual fibers from limb skeletal muscles are traditionally graphed diameter vs. force (mg) because the size of the individual fiber predicts the force-generating capacity. Figure 2 represents the contractile parameters of the 220 myofibers from the EOM plotted in this traditional manner. When the diameter and force properties of individual myofibers were plotted, contrary to what is seen in limb skeletal muscle, there was no correlation between myofiber diameter and force generation (Fig. 2A). When individual myofiber diameter and Vo (fiber lengths/s) were graphed, as was expected and similar to limb skeletal muscle, there was no correlation between these two parameters (Fig. 2B).
Fig. 2.
Contractility correlations. A: graph of fiber diameter of single myofibers plotted against force (n = 220). B: graph of fiber diameter of single fibers plotted against shortening velocity (Vo) in fl/s determined by the slack method. Each colored set of symbols for each indicated series represents representative values for single fibers (n = 220).
Myofiber force, Vo, and MyHC isoform composition.
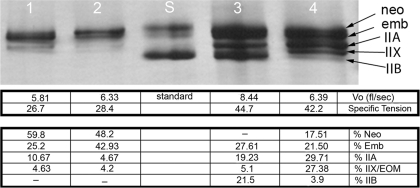
After force and Vo were determined for individual fiber segments, their MyHC isoform composition was determined by electrophoresis. The electrophoresis method used in the initial analyses allowed separation of only five of the nine MyHC isoforms found in adult EOM (Fig. 3). Almost 50% of single myofiber segments examined expressed three MyHC isoforms (Table 1), while another 37.8% coexpressed two MyHC isoforms. Only ∼5% expressed one MyHC based on mobility in these electrophoresis gels.
Fig. 3.
Representative silver-stained electrophoresis gel showing separation of myosin heavy chain (MyHC) isoforms obtained from single myofibers after force measurements had been obtained. The corresponding Vo, specific tension (mN/cm2), and percent composition of MyHC isoforms in each fiber are beneath each gel lane.
Table 1.
Percentage of fibers coexpressing individual MyHC
| Number of Myosin Bands | Percent of Total Fibers Analyzed |
|---|---|
| 1 | 5.17 |
| 2 | 37.76 |
| 3 | 48.98 |
| 4 | 6.12 |
| 5 | 1.53 |
| 6 | 0.51 |
MyHC, myosin heavy chain.
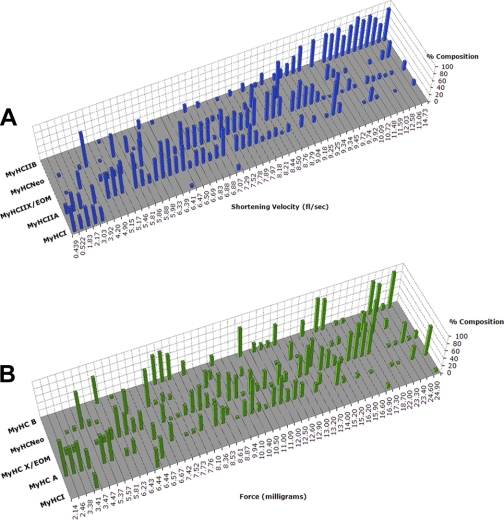
A proportion of the 220 myofibers analyzed was graphed in increasing order of Vo (in fl/s) on the x-axis, the percent content of five MyHC isoforms (MyHCIIA, IIX, neonatal, IIB, and I) on the y-axis (Fig. 4A, Table 1), and the individual MyHC types on the z-axis. Certain trends were immediately apparent. First, as expected, the fiber segments with the slowest shortening velocities contained the largest proportion of MyHCI, and the fiber segments with the fastest shortening velocities contained a large proportion of MyHCIIB. However, it was readily apparent that there was a continuum of single myofiber shortening velocities, and this was associated with a significant variation in percent expression of the five MyHC isoforms typically visualized in the silver-stained electrophoresis gels. There was a highly significant and positive correlation between Vo and MyHCIIB (r2 = 0.82, P = 0.00001) and between Vo and MyHCI (r2 = 0.99, P = 0.028). There was also a statistically significant correlation between Vo and expression of MyHCIIA (r2 = 0.54, P = 0.00028).
Fig. 4.
Continuum of contractile properties across the MyHC isoforms. A: graph of shortening velocity plotted against myosin heavy chain isoform composition based on densitometric analysis of silver-stained electrophoresis gels of a representative sample (48) of individual fiber segments analyzed. Velocity determined by the slack method. B: graph of force in milligrams plotted against MyHC isoform composition.
A subset of randomly selected myofibers out of the total 220 analyzed were graphed in increasing order of contractile specific force (in mg) on the x-axis, the percent content of five MyHC isoforms (MyHCIIA, IIX, neonatal, IIB, and I) on the y-axis, with individual MyHCs depicted on the z-axis (Fig. 4B). No clear relationship was seen between force and MyHC isoform content, and statistical analysis confirmed the lack of correlation between force and MyHC isoform expression (r2 varied between 0.003 and 0.017; P varied between 0.07 to 0.998). This analysis may be colored by the fact that only five MyHC isoforms were visualized.
Patterns of MyHC coexpression in the orbital layer.
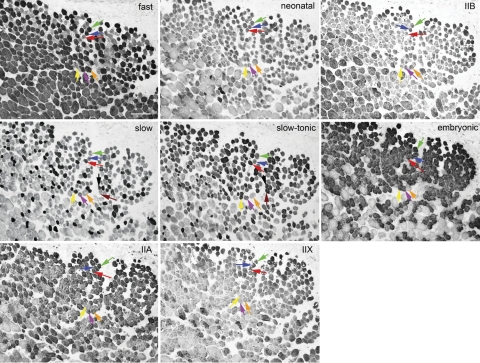
We further investigated the occurrence of coexpression of MyHC isoforms in single identified myofibers in serial histological sections of the orbital layer immunostained for fast, neonatal, IIB, slow, slow-tonic, IIA, and IIX (Fig. 5) to confirm the SDS-PAGE data. While the vast majority of the orbital layer fibers in this region of the muscle express embryonic and IIA MyHC isoforms, they are differentially positive for other isoforms. Six fibers are followed in all eight sections to serve as examples (Fig. 5). Looking at the three fibers indicated in the most superficial part of the orbital layer (Fig. 5, green, blue, and red arrows), if they are followed in all eight sections, the fibers indicated by the green and blue arrows show positive immunostaining for pan-fast, embryonic, and IIA; slightly positive for neonatal; and negative for IIB, slow, slow-tonic, and IIX. The fiber indicated by the red arrow shows positive immunostaining for slow, slow-tonic, embryonic, and IIA; slightly positive for neonatal; and negative for the pan-fast, IIB, and IIX isoforms. In another group of three fibers (Fig. 5, yellow, purple, and orange arrows), the orange arrow points to a fiber that immunostains positive for slow, slow-tonic, and embryonic MyHCs and negative for pan-fast, neonatal, IIB, IIA, and IIX MyHCs. The two fibers indicated by the purple and orange arrows are both positive for the pan-fast and IIA; negative for neonatal, IIB, and IIX; and differentially positive for slow, slow-tonic, and embryonic MyHC isoforms. An additional two fibers are indicated by the black and maroon arrows in the slow and slow-tonic photomicrographs. While both are positive for slow tonic, one is lightly positive for slow MyHC and the other negative (Fig. 5, black and maroon arrows). Other fibers with equally complex coexpression patterns can easily be seen in these serially sectioned and immunostained tissues. This complex MyHC coexpression patterning in single myofibers has been described for orbital layer fibers in the EOM of other mammalian EOM as well as in humans, although not with eight isoforms simultaneously (10, 44).
Fig. 5.
Photomicrograph of serial sections of orbital layer of a superior rectus muscle from normal adult rabbit immunostained for each of the following MyHC isoforms: fast, neonatal, MyHCI (slow) (from Vector) and type IIB (BF-F3), slow-tonic (S46), embryonic (F1.652), type IIA (SC-71), and type IIX (6H1) (from Hybridoma Bank). Six individual myofibers are followed in the 8 serial sections, each identified with a single colored arrow (green, blue, red, yellow, purple, and orange). Black and maroon arrows indicate fibers that are differentially positive for slow and slow-tonic antibody staining. Bar represents 20 μm.
Patterns of MyHC coexpression in the global layer.
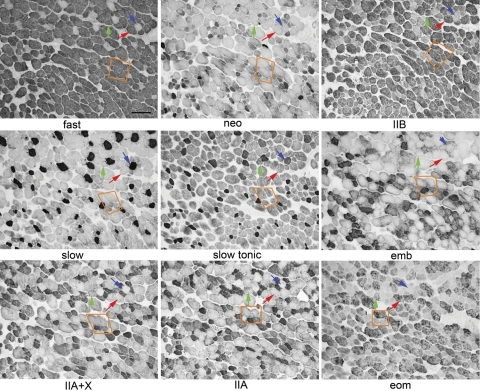
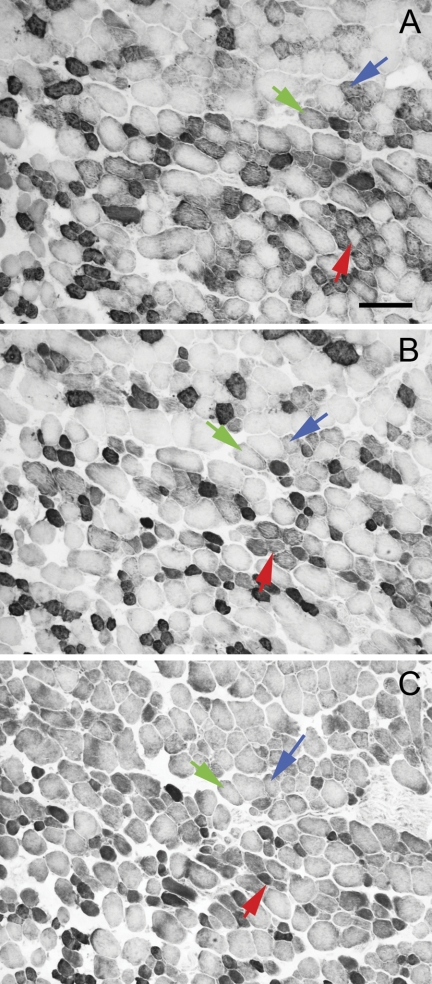
The coexpression of MyHC isoforms in single identified EOM myofibers was examined histologically in serial sections of the global layers (Fig. 6). The majority of the myofibers in this region midway between the tendon and the midbelly region of the global layer expressed fast IIB MyHC (Fig. 6). While the myofibers in the global layer did not show as complex a coexpression pattern as seen in the orbital layer, most myofibers expressed more than one MyHC isoform. Three fibers are followed in all nine cross-sections (Fig. 6, green, red, and blue arrows). The fibers indicated by the green and red arrows are relatively similar throughout the distances examined here (∼216 μm). These fibers are immunopositive for the pan-fast, IIB, and EOM-specific MyHC isoforms; negative for neonatal, slow, slow-tonic, embryonic, and IIA MyHCs; but one fiber (green arrow) is lightly stained with the IIX/A antibody while the other is negative. The fiber indicated by the blue arrow is positive for slow and slow tonic MyHCs. The orange box (Fig. 6) is another example of the myofiber complexity within the EOM; these four myofibers can be seen to change their intrafascicular and interfascicular position in this portion of the muscle. Additionally these four myofibers again demonstrate coexpression of multiple MyHCs within single fibers and significantly different patterns of expression among the four indicated fibers. When the percent of myofibers that coexpress slow and slow tonic is assessed, in the global layer it varied from 70 to 96% (mean 77.25 ± 6.65%) and in the orbital layer it varied from 47 to 59% (mean 53.33 ± 3.48%). It should be noted that these percentages will vary significantly when the muscle is examined in the midregion or in the tendon region of the EOM (56). In three sections immunostained for embryonic myosin, the change in expression within single fibers along even a small part of their length can be seen (Fig. 7). The total distance from the section in Fig. 7A to the section in Fig. 7C is 396 μm. All three identified myofibers change their expression for embryonic MyHC isoform in this distance. While immunohistochemistry is not quantitative, these serial sections demonstrate the complex MyHC expression patterns of individual myofibers, as well as the differential presence of detectable protein along the length of single myofibers.
Fig. 6.
Photomicrograph of serial sections of global layer of a superior rectus muscle from a normal adult rabbit immunostained for each of the following MyHC isoforms:, fast, neonatal (neo), slow (I) (from Vector) and type IIB (BF-F3), slow tonic (S46), embryonic (emb, F1.652), type IIA/X (6H1), type IIA (SC-71), embryonic (F1.652), EOM-specific (4A6) (from Hybridoma Bank). Three myofibers are followed in all 9 panels identified by blue, red, and green arrows. An additional 4 fibers are enclosed in an orange box. The most common fiber type in the global layer in this region of the muscle is positive for MyHCIIB, with some light immunostaining for MyHCIIA. Blue arrows indicate a myofiber immunopositive for slow and slow-tonic, lightly positive for embryonic, and negative for all other MyHC isoforms stained. Green arrows point to a myofiber positive for pan-fast, MyHCIIB, and EOM-specific and lightly positive for IIX. Red arrows indicate a myofiber positive for pan-fast, IIB, and EOM-specific. Bar represents 20 μm.
Fig. 7.
Three cross-sections of an inferior rectus muscle immunostained for embryonic MyHC. There are 216 μm between A and B and 180 μm between B and C. Three fibers are identified in all 3 sections with blue, green, and red arrows. In each identified fiber the expression of embryonic MyHC changes along the length. Bar is 100 μm.
Segmental changes in MyHC in single myofibers.
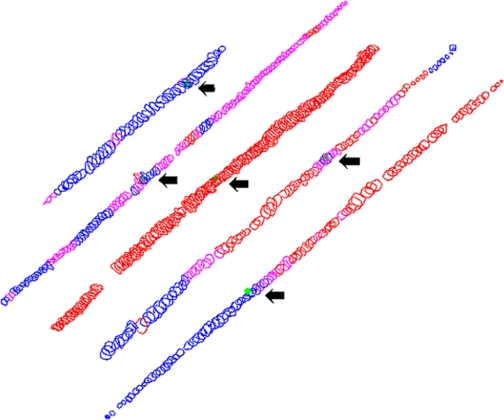
To further demonstrate the variation in expression of MyHC along individual myofibers, five myofibers were reconstructed from serial sections of an EOM where every other section was immunostained for neonatal MyHC (Fig. 8). The arrow indicates where the reconstructions were begun, and the entire length of each randomly selected myofiber was reconstructed. Note that one myofiber was uniformly positive, one basically negative, and three other fibers whose expression of neonatal MyHC substantially changed in different fiber segments.
Fig. 8.
Reconstruction of 5 myofibers from serial sections immunostained for neonatal MyHC (Vector). Blue indicates regions that were negative, pink indicates regions that were stained at an intermediate level, and red indicates regions that were strongly positive. The green dot represents the section where the reconstructions were started. The reconstructed myofibers averaged 1.296 to 2.45 mm in length.
DISCUSSION
The traditional description of EOM fiber types stems from early studies where mitochondrial density and patterns of innervation were the major criteria used (56, 82). These authors described two fiber types in the orbital layer: 1) a fast, fatigue-resistant singly innervated fiber type with large numbers of mitochondria and 2) a less common multiply innervated slow-tonic fiber type. Four fiber types were described in the global layer, also based on innervation and concentration of mitochondria: 1) a fast, fatigue-resistant fiber type with large numbers of mitochondria, 2) a fast fiber type with intermediate level of contraction speed and fatigue resistance, and 3) a fast fatigable type with few mitochondria. These are singly innervated. A fourth global layer fiber type is multiply innervated, has very few mitochondria, with slow tonic contractile properties. As our study and others have shown, when additional biochemical and physiological characteristics are considered, this classification scheme becomes inadequate (7, 44, 45).
Single fiber physiology.
Analysis of the 220 skinned myofibers in this study demonstrated several important properties of EOM myofibers that differ from those seen in limb musculature. First, there was no correlation between fiber diameter and force (in mg). Second, when single fiber shortening velocity and contractile force are examined, very wide ranges of values were seen in the fiber segments. Jointly the single fibers spanned a full spectrum of velocity and force measurements. It should be noted that when motor nerves in the EOM are directly stimulated, motor unit and whole muscle forces are not intrinsically weaker than limb muscles; in fact EOM and limb muscles can generate the same force and specific tension (26, 32). However, normal eye movements produce only small amounts of muscle force, and the force produced is less than predicted from direct motor unit measurements (32, 33). This loss in muscle force in contracting EOM is hypothesized to be due to myofiber branching and short myofibers that do not run tendon-to-tendon within individual EOM (32, 33, 36, 81). EOM have the fastest shortening velocities of any skeletal muscle (6) and are extremely fatigue resistant (7). Despite the fact that 85–90% of the myofibers within the EOM are “fast,” this unusual combination supports the view that they represent a distinct allotype compared with limb and body skeletal muscle. This is based on their complex coexpression patterns of the many molecules responsible for these physiological properties (39).
As stated, the shortening velocity of the EOM is faster than all other muscles that have been examined (6), while additionally demonstrating a higher fusion frequency and greater fatigue resistance (27, 28) than all other fast twitch muscles. While the extraocular muscles as a whole normally operate at less than their maximal force potential, they can generate significant maximal tetanic tension (26, 33). However, the single myofibers in the present study generally had lower specific tensions than those from single limb fibers (86). However, eye movements can use very few of the thousands of EOM myofibers in any given muscle to effect movement. Estimates suggest that as few as two motor units appear to be sufficient to move the eye by one degree, indicating a precise control of eye position by a small number of myofibers (32). Indeed, experimental removal of a large portion of the lateral rectus muscle did not alter force generation or eye movement accuracy (33). No change in eye movement accuracy was seen after a loss of 22% of the normal motoneuron pool in monkeys after chronic electrophysiological recordings in eye movement studies, suggesting a large amount of myofiber and neuronal redundancy as well as polyneuronal innervation (22, 57).
Despite attempting to fit their data into the traditional EOM fiber typing classification scheme based on innervation and mitochondrial content (82), a number of studies have data where the range in values in EOM myofibers suggests a continuum, as well as far exceeding ranges seen in myofibers derived from limb muscles. For example, in a study of cross-bridge kinetics that measured myofiber dynamic stiffness, EOM single fibers had a greatly increased mechanical kinetic range compared with limb fibers (52). In a study of Ca2+ and Sr2+ activation, EOM myofibers not only had a wider range of values than limb muscle fibers, but over one-third of the myofibers exhibited mixed fast- and slow-twitch contractile properties within single contracting units (54). Twitch contraction time, fusion frequency, and fatigability in motor units of cat lateral rectus muscles showed a wide range of measured values (81), and the individual motoneurons and myofibers showed a continuum along the range of values for each of the physiological parameters examined (81). In contrast to limb skeletal muscle, their data show overlapping ranges when parsed into the “traditional” fiber type groups. It should be noted that none of these studies examined the MyHC composition in relation to shortening velocity or force.
Colocalization of MyHC isoforms in single myofibers.
We showed that the vast majority of the myofibers within the rabbit EOM expresses more than one MyHC isoform, and in fact, serial sections demonstrate that single fibers can coexpress up to six isoforms simultaneously in the same region of the myofiber. The coexpression of various isoforms of MyHC in single myofibers is somewhat common in skeletal muscle (12, 68, 77, 80). In plantaris muscle, for example, over 50% of the myofibers examined coexpressed at least two fast MyHC isoforms (12). The same was shown for diaphragm muscle; polymorphic fibers constituted the majority of myofibers examined (15). A number of studies demonstrated coexpression of multiple MyHC isoforms in single EOM myofibers in a wide range of animal species (10, 13, 42, 47, 59, 74–76, 89, 91, 96) including humans (45, 48, 89). While none examined the number of MyHC isoforms evaluated in the present study and species differences exist, hybrid fibers with multiple MyHC isoforms are the rule not the exception. EOM muscles appear to represent the far end of the allotypic continuum, where the majority of the myofibers are hybrid (48, 93).
A direct correlation has been demonstrated between twitch contraction time and MyHC composition (11, 50, 67). The complex spectrum of coexpression of MyHC isoforms in EOM myofibers allows the continuous spectrum of contraction shortening velocities and force generation found. When limb skeletal muscle fibers were assessed physiologically, the contractile velocities spanned an overlapping continuum ranging from 0.35 to 2.84 fl/s, approximately a 10-fold difference (11). In contrast, the values for EOM in this study ranged from a low of 0.439 fl/s to a high of 19.8 fl/s, almost a 50-fold difference.
We clearly showed the presence of single myofibers coexpressing fast and slow MyHC isoforms in rabbit EOM, and this phenomenon has been seen in limb muscle also. These were described in diaphragm muscle (15, 51), plantaris and soleus (12), masseter (14), and several other muscles (84). These distinct myofibrils intermingle within any given cross-section (29). In aging muscle, 20% of the myofibers in limb muscle coexpressed fast and slow MyHC (5). However, in a comprehensive study of the MyHC composition of human EOMs, there were no fibers coexpressing MyHC slow and fast (45).
Heterogeneity in MyHC composition along single myofiber length.
The reconstructed myofibers expressing neonatal MyHC in the current study demonstrate the significant heterogeneity along individual myofiber segments. This also suggests that the diversity of MyHC isoforms within single myofibers is probably greater than can be gleaned from fiber segments with a limited pool of protein, as performed using skinned fibers. Despite the traditionally described six fiber types for EOM, as early as 1984 variability of the histochemical composition in single myofibers in EOM was described (64). Limb skeletal muscles also can have nonuniform MyHC expression in different regions of single fibers along their length (76, 77, 83, 95). For example, immature MyHC isoforms were observed at the tapered ends of single myofibers, but not within the entire fiber length (72). Functionally, can this longitudinal variation in MyHC isoforms within single fibers affect muscle contractile characteristics? This type of heterogeneity of MyHC expression in segment lengths within single fibers occurs in frog muscle and was shown to result in measurable differences in maximum shortening velocity along the fiber length (1, 25). When examined physiologically not only did fiber segments from these frog muscles display different shortening velocities, but often strained in opposite directions for up to one-third of the contraction cycle. This supports the hypothesis that these MyHC variations along a fiber's length must play a functional role in EOM contractile function.
Another example of variation along fiber length is EOM-specific MyHC, which was localized to the innervation zone in both the orbital and global layers, colocalizing with IIB and less commonly with IIX (13). In another study only the myofiber ends were shown to express neonatal MyHC (75). Functionally, in rat single orbital layer EOM myofibers, the end-plate region expressing fast MyHC displayed fast-twitch contractile properties, while the fiber ends expressing slow tonic MyHC showed graded tonic contractions (41, 42).
Individual skeletal muscle myofibers are long multinucleated cells that develop by fusion of many precursor cells. Each myonucleus regulates protein expression in what is called its myonuclear domain, the fiber segment in which it is contained (2, 65). It was elegantly shown that gene regulation in individual myonuclei is independently regulated, and activation of any given nucleus within a myofiber is dynamic and occurs in pulses (62). Thus the machinery that controls gene expression functions to regulate protein content independently within each fiber segment; this would include MyHC isoforms. Parsing this continuously changing continuum into six fiber types overly simplifies the physiology of these complex muscles.
Other support for the continuum hypothesis.
In addition to the considerable complexity in MyHC coexpression patterns in single myofibers in EOM, other proteins also show coexpression patterns that do not correspond to the “historically” defined fiber types. EOM, as is true in limb muscle, express MyHC binding protein C (MBPC). In contrast to limb muscle, where MBPC-fast is associated with fast-expressing MyHC and MBPC-slow with slow myofibers, in EOM MBPC-slow is expressed in all myofibers with a complete absence of MBPC-fast expression (44). This is despite the fact that 80–90% of the myofibers express a fast MyHC isoform (45). EOM fast-twitch myofibers express both SERCA1 and SERCA2 (43), again in contrast to limb skeletal muscle where fast fibers express only SERCA1 (92). An examination of succinate dehydrogenase (SDH) and α-glycerophosphate dehydrogenase (GPDH) expression patterns in EOM showed that the fast myofibers formed a continuum relative to coexistence of these two metabolic pathways (7). When expression of SDH and GPDH was graphed as scatterplots, there was significant overlap of all the fast fibers; only the slow tonic fibers formed a distinct group. Even the first publication parsing the EOM muscle fibers into fiber types using innervation and mitochondrial content describe the mitochondrial density as a “spectrum” (56). Examined in aggregate, the overwhelming evidence leads to the conclusion that, with the exception of the slow tonic myofibers, the twitch myofibers form a continuum with significantly overlapping metabolic and functional properties. As the technology for protein separation improves, we hypothesize that single fibers will be shown to contain more MyHC isoforms than can currently be demonstrated.
Functional consequences.
This complexity must play an important role in the fine control of EOM contraction velocities, force, and specific tension generation, with the resultant ability to control eye position with great accuracy, regardless of movement (e.g., fixation, slow vergence movements, or fast saccades). How do these complex coexpression patterns of MyHC affect contractile behavior? This has been partly explained in an interesting series of experiments where activation characteristics of single fast and slow myofibers tied together either in series or in parallel were examined (55). When fast and slow myofibers were attached in parallel, the contraction was directly related to the known proportion of fast and slow fiber components. However, when tied in series, the contractile characteristics did not match the ratio of fast and slow fiber components, but instead resulted in “mixed” contractile behaviors that generally behaved more similar to fast-twitch fibers. In other words, the fast MyHC isoforms were “dominant” (55). This directly mirrors the mixed contractile properties that would be expected of the majority of EOM myofibers examined in the current study.
Directions of movement controlled by limb skeletal muscles are generally constrained by their bony attachment sites and the weight of the moving limb. The EOM do not insert into bone, but rather into the sclera of the globe. The eye in the bony orbit is angled at 25° off the midline (94). To maintain eye position in primary gaze, e.g., straight ahead and fixating on an object in the far horizon, the EOM must maintain continuous muscle tension of the medial rectus muscles to keep the direction of gaze parallel to the direction of the head. The EOM also must be able to hold the eye in a single position of gaze during fixation, move the eyes slowly during vergence, as well as move the eyes quickly when making saccades. A number of the unusual properties of extraocular motoneurons and associated muscle fibers, i.e., the motor unit, can be partly explained by myofibers with a continuum of MyHC isoform expression. Despite the wide array of movement speeds performed by the eye, ocular motor units do not have specialized roles (81). This is presumably the functional correlate of having very small motor units with diverse MyHC isoforms, allowing different motor units to produce distinct fiber tensions and shortening velocities. In fact, a continuum of fusion frequencies within EOM motor units was demonstrated electrophysiologically (61). Also, no correlations were seen between the mechanical parameters of single abducens motoneurons and recruitment order (81). In a series of studies in cat and monkey, stimulation rate and resultant muscle tension were nonlinear. Based on predicted force vs. actual force in whole abducens nerve stimulations, it was determined that as few as two or three motor units could move the eye 1° (32, 33). There is also evidence that unlike adult limb and trunk musculature, the adult EOM maintain a number of myofibers with polyneuronal innervation (22). As the pattern of motor nerve stimulation plays an important role in determination of MyHC isoform composition (34), single fibers receiving “instructions” from more than one motoneuron would be predicted to respond with increased MyHC isoform diversity.
The cranial motoneurons that form the oculomotor, trochlear, and abducens nerves have properties that are different from those of spinal motoneurons and different from each other. Comparative studies of spinal vs. extraocular cranial motoneurons suggest several mechanisms that might explain these differences. In a study of long-term cultures of EOM primordia with either spinal or cranial motoneurons, the EOM survived long term only in the presence of oculomotor neurons (70). Normal adult extraocular motoneurons continue to express a number of neurotrophic factors and their receptors that are downregulated in spinal motoneurons, including tyrosine receptor kinase type 1 (TrkA; 9) and growth factors such as fibroblast growth factor isoforms (35, 37) and insulin growth factors I and II (4, 38). Growth factors such as brain-derived neurotrophic factor and neurotrophin-3 differentially alter extraocular motoneuron firing patterns (19). Extraocular motoneurons also significantly differ from spinal motoneurons in their differential survival rates in spinal motor degenerative diseases (63). These intrinsic differences extend to the EOM themselves. Pax3, the gene responsible for the formation of somite-derived skeletal muscle, does not play a role in EOM formation (85); instead formation of the EOM depends on the gene Pitx2 (21). Thus the variability of MyHC isoforms within individual EOM myofibers is just one manifestation of a large array of molecular and genetic differences both at the muscle and motoneuron levels.
From a functional point of view, there is accumulating evidence that the motoneurons that control EOM contractile characteristics also represent a continuum in terms of their firing rates, eye position sensitivity, and velocity sensitivity (20). Abducens motoneurons control both initial eye position, by a burst of firing followed by maintenance of lateral rectus muscle tension by tonic firing during the “slide phase” for any given duration of eye position. Even in the presence of a rigorous eye movement fatigue protocol, where eye velocity kinematics decrease, the EOM compensate and maintain the accuracy of final eye position (71). In other words, despite a decreased neuronal firing velocity after rapid and repetitive eye saccades, no evidence of change in EOM function or eye position was seen. Presumably, because populations of muscle fibers contract after any given stimulation, the summed EOM dynamics do not change. The ability of extraocular motoneurons to draw on a myriad of “fiber types” supports the findings of these studies. The existence of a myofiber continuum relative to shortening velocity and force and specific tension generation will hopefully inform future electrophysiological experiments, allowing a more accurate modeling of the eye plant relative to oculomotor control of eye movements.
Conclusions.
The complexity of patterns of coexpression of MyHC isoforms in EOM results in a wider spectrum of shortening velocities and contractile forces than is seen in single fibers from limb skeletal muscle and forms a continuum. Single fibers with the same apparent shortening velocity can have significantly different MyHC isoform ratios. This complexity is compounded by differential MyHC isoform expression along the length of single EOM myofibers. This may play a role in increasing the range of power output of an individual muscle. For example, if initial shortening velocity is reduced, force development is accelerated when the fiber is activated (24). How this myosin isoform complexity affects control of eye position and eye movements is unknown; however, motor unit and whole muscle responses to whole nerve stimulation also show a continuum of properties. In addition it is known that these properties rapidly adapt to changes in stretch, innervation, hormone status, drug treatments, and the like (3, 16–18, 88). Future studies are needed to understand how these complex coexpression patterns of MyHC isoform are controlled. In addition, it is critical to consider the implications of this heterogeneity when investigating how the oculomotor control system is able to accurately and reproducibly move the eye in a wide range of velocities and gaze directions.
GRANTS
This work was supported by Grants EY15313 and EY11375 from the National Eye Institute, the Minnesota Medical Foundation, the Minnesota Lions and Lionesses, Swedish Research Council (K2010-62x20399-04-02, FPD), Research to Prevent Blindness Lew Wasserman Mid-Career Development Award (to L. K. McLoon), and an unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Ahn AN, Monti RJ, Biewener AA. In vivo and in vitro heterogeneity of segment length changes in the semimembranosus muscle of the toad. J Physiol 549: 877–888, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22: 1350–1360, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Anderson BC, Christiansen SP, McLoon LK. Myogenic growth factors can decrease extraocular muscle force generation: a potential biological approach to the treatment of strabismus. Invest Ophthalmol Vis Sci 49: 221–229, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson BC, Christiansen SP, Grandt S, Grange RW, McLoon LK. Increased extraocular muscle strength with direct injection of insulin-like growth factor-1. Invest Ophthalmol Vis Sci 47: 2461–2467, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson JL, Terzis G, Kryger A. Increase in the degree of co-expression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve 22: 449–454, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Asmussen G, Gaunitz U. Mechanical properties of the isolated inferior oblique muscle of the rabbit. Pflügers Arch 392: 183–190, 1981 [DOI] [PubMed] [Google Scholar]
- 7. Asmussen G, Punkt K, Bartsch B, Soukup T. Specific metabolic properties of rat oculorotatory extraocular muscles can be linked to their low force requirements. Invest Ophthalmol Vis Sci 49: 4865–4871, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Asmussen G, Traube I, Pette D. Electrophoretic analysis of myosin heavy chain isoform patterns in extraocular muscles of the rat. FEBS Lett 335: 243–245, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Benitez-Temino B, Morcuende S, Mentis GZ, de la Cruz RR, Pastor AM. Expression of Trk receptors in the oculomotor system of the adult cat. J Comp Neurol 473: 538–552, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Bicer S, Reiser PJ. Myosin isoform expression in dog rectus muscles: patterns in global and orbital layers and among single fibers. Invest Ophthalmol Vis Sci 50: 157–167, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Bottinelli R, Schiaffino S, Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol 437: 65–672, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bottinelli R, Betto R, Schiaffino S, Reggiani C. Maximum shortening velocity and coexistence of myosin heavy chain isoforms in single skinned fast fibres of rat skeletal muscle. J Muscle Res Cell Motil 15: 413–419, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Briggs MM, Schachat F. The superfast extraocular myosin (MYH13) is localized to the innervation zone in both the global and orbital layers of rabbit extraocular muscle. J Exp Biol 205: 3133–3142, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Butler-Browne GS, Eriksson PO, Laurent C, Thornell LE. Adult human masseter muscle fibers express myosin isozymes characteristic of development. Muscle Nerve 11: 610–620, 1988 [DOI] [PubMed] [Google Scholar]
- 15. Caiozzo VJ, Baker MJ, Huang K, Chou H, Wu YZ, Baldwin KM. Single-fiber myosin heavy chain polymorphism: how many patterns and what proportions? Am J Physiol Regul Integr Comp Physiol 285: R570–R580, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Christiansen SP, Antunes-Foschini R, McLoon LK. Effect of recession versus tenotomy surgery without recession in adult rabbit extraocular muscle. Invest Ophthalmol Vis Sci 51: 5646–5656, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Christiansen SP, McLoon LK. The effect of resection on satellite cell activity in rabbit extraocular muscle. Invest Ophthalmol Vis Sci 47: 605–613, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Croes SA, Baryshnikova LM, Kaluskar SS, von Bartheld CS. Acute and long-term effects of botulinum neurotoxin on the function and structure of developing extraocular muscles. Neurobiol Dis 25: 649–664, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis-Lopez de Carrizosa MA, Morado-Diaz CJ, Tena JJ, Benitez-Temino B, Pecero ML, Morcuende SR, de la Cruz RR, Pastor AM. Complementary actions of BDNF and neurotrophin-3 on the firing patterns and synaptic composition of motoneurons. J Neurosci 29: 575–587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davis-Lopez de Carrizosa MA, Morado-Diaz CJ, Miller JM, de la Cruz RR, Pastor AM. Dual encoding of muscle tension and eye position by abducens motoneurons. J Neurosci 31: 2271–2279, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diehl AG, Zareparsi S, Qian M, Khanna R, Angeles R, Gage PJ. Extraocular muscle morphogenesis and gene expression are regulated by Pitx2 gene dose. Invest Ophthalmol Vis Sci 47: 1785–1793, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Dimitrova DM, Allman BL, Shall MS, Goldberg SJ. Polyneuronal innervation of single muscle fibers in cat eye muscles: inferior oblique. J Neurophysiol 101: 2815–2821, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eastwood AB, Wood DS, Bock KL, Sorenson MM. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell 11: 553–566, 1979 [DOI] [PubMed] [Google Scholar]
- 24. Edman KA, Reggiani C. Length-tension-velocity relationships studies in short consecutive segments of intact muscle fibres of the frog. Adv Exp Med Biol 170: 495–509, 1984 [DOI] [PubMed] [Google Scholar]
- 25. Edman KA, Reggiani C, Kronnie GT. Differences in maximum velocity of shortening along single muscle fibers of the frog. J Physiol 365: 147–163, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frueh BR, Gregorevic P, Williams DA, Lynch GS. Specific force of the rat extraocular muscles, levator and superior rectus, measured in situ. J Neurophysiol 85: 1027–1032, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Frueh BR, Hayes A, Lynch GS, Williams DA. Contractile properties and temperature sensitivity of the extraocular muscles, the levator and superior rectus, of the rabbit. J Physiol 475: 327–336, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fuchs AF, Binder MD. Fatigue resistance of human extraocular muscles. J Neurophysiol 49: 28–34, 1983 [DOI] [PubMed] [Google Scholar]
- 29. Gauthier GF. Differential distribution of myosin isoforms among the myofibrils of individual developing muscle fibers. J Cell Biol 110: 693–701, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guilian GG, Moss RL, Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver stained gels. Anal Biochem 129: 277–287, 1983 [DOI] [PubMed] [Google Scholar]
- 31. Godt RE, Maughan DW. Swelling of skinned muscle fibers of the frog: experimental observations. Biophys J 19: 103–116, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goldberg SJ, Meredith MA, Shall MS. Extraocular motor unit and whole-muscle responses in the lateral rectus muscle of the squirrel monkey. J Neurosci 18: 10629–10639, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldberg SJ, Wilson KE, Shall MS. Summation of extraocular motor unit tensions in the lateral rectus of the cat. Muscle Nerve 20: 1229–1235, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Goldspink G, Scutt A, Martindale J, Jaenicke T, Turay L, Gerlach GF. Stretch and force generation induce rapid hypertrophy and myosin isoform gene switching in adult skeletal muscle. Biochem Soc Trans 19: 368–373, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res 701: 201–226, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Harrison AR, Anderson BC, Thompson LV, McLoon LK. Myofiber length and three-dimensional localization of NMJs in normal and botulinum toxin treated adult extraocular muscles. Invest Ophthalmol Vis Sci 48: 3594–3601, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hattori Y, Yamasaki M, Konishi M, Itoh N. Spatially restricted expression of fibroblast growth factor-10 mRNA in the rat brain. Brain Res Mol Brain Res 47: 139–146, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Hedlund E, Karlsson M, Osborn T, Ludwig W, Isacson O. Global gene expression profiling of somatic motor neuron populations with different vulnerability identify molecules and pathways of degeneration and protection. Brain 133: 2313–2330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoh JF, Hughes S, Hoy JF. Myogenic and neurogenic regulation of myosin gene expression in cat jaw-closing muscles regenerating in fast and slow limb muscle beds. J Muscle Res Cell Motil 9: 59–72, 1988 [DOI] [PubMed] [Google Scholar]
- 40. Horton RM, Manfredi AA, Conti-Tronconi BM. The ‘embryonic’ gamma subunit of the nicotinic acetylcholine receptor is expressed in adult extraocular muscle. Neurology 43: 983–986, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Jacoby J, Chiarandini DJ, Stefani E. Electrical properties and innervation of fibers in the orbital layer of rat extraocular muscles. J Neurophysiol 61: 116–125, 1989 [DOI] [PubMed] [Google Scholar]
- 42. Jacoby J, Ko K, Weiss C, Rushbrook JI. Systematic variation in myosin expression along extraocular muscle fibres of the adult rat. J Muscle Res Cell Motil 11: 25–40, 1990 [DOI] [PubMed] [Google Scholar]
- 43. Kjellgren D, Ryan M, Ohlendieck K, Thornell LE, Pedrosa-Domellöf F. Sarco(endo)plasmic reticulum Ca2+ ATPases (SERCA1 and -2) in human extraocular muscles. Invest Ophthalmol Vis Sci 44: 5057–5062, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Kjellgren D, Stal P, Larsson L, Furst D, Pedrosa-Domellöf F. Uncoordinated expression of myosin heavy chains and myosin-binding protein C isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci 47: 4188–4193, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Kjellgren D, Thornell LE, Andersen J, Pedrosa-Domellöf F. Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci 44: 1419–1425, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Aging alters the myosin heavy chain composition of single fibers from human skeletal muscle. Acta Physiol Scand 140: 55–62, 1990 [DOI] [PubMed] [Google Scholar]
- 47. Kranjc BS, Sketelj J, D'Albis A, Ambroz M, Erzen I. Fibre types and myosin heavy chain expression in the ocular medial rectus muscle of the adult rat. J Muscle Res Cell Motil 21: 753–761, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Kranjc BS, Smerdu V, Erzen I. Histochemical and immunohistochemical profile of human and rat ocular medial rectus muscles. Graefes Arch Clin Exp Ophthalmol 247: 1505–1515, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kupfer C. Motor innervation of extraocular muscle. J Physiol 153: 522–526, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kwa SHS, Weijs WA, Juch PJW. Contraction characteristics and myosin heavy chain composition of rabbit masseter motor units. J Neurophysiol 73: 538–549, 1995 [DOI] [PubMed] [Google Scholar]
- 51. LaFramboise WA, Daood MJ, Guthrie RD, Schiaffino S, Moretti P, Brozanski B, Ontell MP, Butler-Browne GS, Whalen RG, Ontell M. Emergence of the mature myosin phenotype in the rat diaphragm muscle. Dev Biol 144: 1–15, 1991 [DOI] [PubMed] [Google Scholar]
- 52. Li ZB, Rossmanith GH, Hoh JFY. Cross-bridge kinetics of rabbit single extraocular and limb muscle fibers. Invest Ophthalmol Vis Sci 41: 3770–3774, 2000 [PubMed] [Google Scholar]
- 53. Lowey S, Waller GS, Trybus KM. Function of skeletal muscle myosin heavy and light chain isoforms by an in vitro motility assay. J Biol Chem 268: 20414–40418, 1993 [PubMed] [Google Scholar]
- 54. Lynch GS, Frueh BR, Williams DA. Contractile properties of single skinned fibres from the extraocular muscles, the levator and superior rectus, of the rabbit. J Physiol 475: 337–346, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lynch GS, Stephenson DG, Williams DA. Analysis of Ca2+ and Sr2+ activation characteristics in skinned muscle fibre preparations with different proportions of myofibrillar isoforms. J Muscle Res Cell Motil 16: 65–78, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Mayr R. Structure and distribution of fibre types in the external eye muscles of the rat. Tissue Cell 3: 433–462, 1971 [DOI] [PubMed] [Google Scholar]
- 57. McClung JR, Cullen KE, Shall MS, Dimitrova DM, Goldberg SJ. Effects of electrode penetrations into the abducens nucleus of the monkey: eye movement recordings and histopathological evaluation of the nuclei and lateral rectus muscle. Exp Brain Res 158: 180–188, 2004 [DOI] [PubMed] [Google Scholar]
- 58. McLoon LK. The extraocular muscles. In: Adler's Physiology of the Eye, Chapter 8 Kaufman P, Alm A, Levin LA, Nilsson S, Ver Hoeve J, Wu SM. (Eds.). New York: Mosby, 2011, p. 182–207 [Google Scholar]
- 59. McLoon LK, Rios L, Wirtschafter JD. Complex three-dimensional patterns of myosin isoform expression: differences between and within specific extraocular muscles. J Muscle Res Cell Motil 20: 771–783, 1999 [DOI] [PubMed] [Google Scholar]
- 60. McLoon LK, Wirtschafter JD. N-CAM is expressed in mature extraocular muscles in a pattern conserved between species. Invest Ophthalmol Vis Sci 37: 318–327, 1996 [PubMed] [Google Scholar]
- 61. Nelson JS, Goldberg SJ, McClung JR. Motoneuron electrophysiological and muscle contractile properties of superior oblique motor units in cat. J Neurophysiol 55: 715–726, 1986 [DOI] [PubMed] [Google Scholar]
- 62. Newlands S, Levitt LK, Robinson CS, Karpf ABC, Hodgson VRM, Wade RP, Hardeman EC. Transcription occurs in pulses in muscle fibers. Genes Dev 12: 2748–2758, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nimchinsky EA, Young WG, Yeung G, Shah RA, Gordon JW, Bloom FE, Morrison JH, Hof PR. Differential vulnerability of oculomotor, facial, and hypoglossal nuclei in G86R superoxide dismutase transgenic mice. J Comp Neurol 416: 112–125, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Pachter BR. Rat extraocular muscle: Histochemical variability along the length of multiply-innervated fibers of the orbital surface layer. Histochemistry 80: 535–538, 1984 [PubMed] [Google Scholar]
- 65. Pavlath GK, Rich K, Webster SG, Blau HM. Localization of muscle gene products in nuclear domains. Nature 337: 570–573, 1989 [DOI] [PubMed] [Google Scholar]
- 66. Pedrosa-Domellöf F, Holmgren Y, Lucas CA, Hoh JF, Thornell LE. Human extraocular muscles: unique pattern of myosin heavy chain expression during myotube formation. Invest Ophthalmol Vis Sci 41: 1608–1616, 2000 [PubMed] [Google Scholar]
- 67. Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol 116: 1–76, 1990 [DOI] [PubMed] [Google Scholar]
- 68. Peuker H, Pette D. Quantitative analysis of myosin heavy-chain mRNA and protein isoforms in single fibers reveals a pronounced fiber heterogeneity in normal rabbit muscles. Eur J Biochem 247: 30–36, 1997 [DOI] [PubMed] [Google Scholar]
- 69. Pilar G, Hess A. Differences in internal structure and nerve terminals of the slow and twitch muscle fibers in the cat superior oblique. Anat Rec 154: 243–252, 1966 [DOI] [PubMed] [Google Scholar]
- 70. Porter JD, Hauser KF. Survival of extraocular muscle in long-term organotypic culture: differential influence of appropriate and inappropriate motoneurons. Dev Biol 160: 39–50, 1993 [DOI] [PubMed] [Google Scholar]
- 71. Prsa M, Dicke PW, Thier P. The absence of eye muscle fatigue indicates that the nervous system compensates for non-motor disturbances of oculomotor function. J Neurosci 30: 15834–15842, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rosser BWC, Waldbillig DM, Lovo SD, Armstrong JC, Bandman E. Myosin heavy chain expression within the tapered ends of skeletal muscle fibers. Anat Rec 242: 462–470, 1995 [DOI] [PubMed] [Google Scholar]
- 73. Rossi AC, Mammucari C, Argentini C, Reggiani C, Schiaffino S. Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles. J Physiol 588: 353–364, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rubinstein N, Hoh JF. The distribution of myosin heavy chain isoforms among rat extraocular muscle fiber types. Invest Ophthalmol Vis Sci 41: 3391–3398, 2000 [PubMed] [Google Scholar]
- 75. Rushbrook JI, Weiss C, Ko K, Feuerman MH, Carleton S, Ing A, Jacoby J. Identification of alpha-cardiac myosin heavy chain mRNA and protein in extraocular muscle of the adult rabbit. J Muscle Res Cell Motil 15: 505–515, 1994 [DOI] [PubMed] [Google Scholar]
- 76. Sakuma K, Yamaguchi A, Ohmori H, Katsuta S. Nonuniform changes in fiber types in the soleus muscle of the developing rat. Eur J Appl Physiol 70: 132–137, 1995 [DOI] [PubMed] [Google Scholar]
- 77. Sant'ana Pereira JA, Wessels A, Nijtmans L, Moorman AFM, Sargeant AJ. New method for the accurate characterization of single human skeletal muscle fibers demonstrates a relation between mATPase and MyHC expression in pure and hybrid fiber types. J Muscle Res Cell Motil 16: 21–34, 1995 [DOI] [PubMed] [Google Scholar]
- 78. Sartore S, Mascarello F, Rowlerson A, Gorza L, Ausoni S, Vianello M, Schiaffino S. Fibre types in extraocular muscles: a new myosin isoform in the fast fibres. J Muscle Res Cell Motil 8: 161–172, 1987 [DOI] [PubMed] [Google Scholar]
- 79. Schiaffino S. Fibre types in skeletal muscle: a personal account. Acta Physiol (Oxf) 199: 451–463, 2010 [DOI] [PubMed] [Google Scholar]
- 80. Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76: 371–423, 1996 [DOI] [PubMed] [Google Scholar]
- 81. Shall MS, Goldberg SJ. Extraocular motor units: classification and motoneuron stimulation frequency-muscle unit force relationships. Brain Res 587: 291–300, 1992 [DOI] [PubMed] [Google Scholar]
- 82. Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog Brain Res 151: 43–80, 2006 [DOI] [PubMed] [Google Scholar]
- 83. Staron RS, Pette D. Nonuniform myosin expression along single fibers of chronically stimulated and contralateral rabbit tibialis anterior muscles. Pflügers Arch 409: 67–73, 1987 [DOI] [PubMed] [Google Scholar]
- 84. Stephenson GMM. Hybrid skeletal muscle fibres: a rare or common phenomenon? Clin Exp Pharmacol Physiol 28: 692–702, 2001 [DOI] [PubMed] [Google Scholar]
- 85. Tajbakhsh S, Rocancout D, Cossu G, Buckingham M. R Redefining the genetic hierarchies controlling skeletal myogenesis: Pax3 and Myf-5 act upstream of MyoD. Cell 89: 127–138, 1997 [DOI] [PubMed] [Google Scholar]
- 86. Thompson LV, Shoeman JA. Contractile function of single muscle fibers after hindlimb unweighting in aged rats. J Appl Physiol 84: 229–235, 1998 [DOI] [PubMed] [Google Scholar]
- 87. Toniolo L, Maccatrozzo L, Patruno M, Caliaro F, Mascarello F, Reggiani C. Expression of eight distinct MHC isoforms in bovine striated muscles: evidence for MHC-2B presence only in extraocular muscles. J Exp Biol 208: 4243–4253, 2005 [DOI] [PubMed] [Google Scholar]
- 88. Ugalde I, Christiansen SP, McLoon LK. Botulinum toxin treatment of extraocular muscles in rabbits results in increased myofiber remodeling. Invest Ophthalmol Vis Sci 46: 4114–4120, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wasicky R, Ziya-Ghazvini F, Blumer R, Lukas JR, Mayr R. Muscle fiber types of human extraocular muscles: a histochemical and immunohistochemical study. Invest Ophthalmol Vis Sci 41: 980–990, 2000 [PubMed] [Google Scholar]
- 90. Weiss A, Leinwand LA. The mammalian myosin heavy chain gene family. Annu Rev Cell Dev Biol 12: 417–439, 1996 [DOI] [PubMed] [Google Scholar]
- 91. Wieczorek DF, Periasamy M, Butler-Browne GS, Whalen RG, Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular muscles. J Cell Biol 101: 618–629, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wu KD, Lytton J. Molecular cloning and quantification of sarcoplasmic reticulum Ca(2+)-ATPase isoforms in rat muscles. J Physiol 264: C333–341, 1993 [DOI] [PubMed] [Google Scholar]
- 93. Wu YZ, Baker MJ, Crumley RL, Blanks RH, Caiozzo VJ. A new concept in laryngeal muscle: multiple myosin isoform types in single muscle fibers of the lateral cricoarytenoid. Otolaryngol Head Neck Surg 118: 86–94, 1998 [DOI] [PubMed] [Google Scholar]
- 94. Zaldivar RA, Lee MS, Harrison AR. Orbital bony anatomy and orbital fractures. In: Encyclopedia of the Eye, Vol. 3 Oxford: Academic, 2010, p. 210–218 [Google Scholar]
- 95. Zhang MY, Zhang WJ, Medler S. The continuum of hybrid IIX/IIB fibers in normal mouse muscles: MHC isoform proportions and spatial distribution within single fibers. Am J Physiol Regul Integr Comp Physiol 299: R1582–R1591, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhou Y, Liu D, Kaminski HJ. Myosin heavy chain expression in mouse extraocular muscle: more complex than expected. Invest Ophthalmol Vis Sci 51: 6355–6363, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]