Abstract
In decerebrate rats, we reported previously that the exercise pressor reflex arising from a limb whose femoral artery was occluded for 72 h before the experiment was significantly higher than the exercise pressor reflex arising from a contralateral freely perfused limb. These findings prompted us to examine whether reactive oxygen species contributed to the augmented pressor reflex in rats with femoral artery occlusion. We found that the pressor reflex arising from the limb whose femoral artery was occluded for 72 h before the experiment (31 ± 5 mmHg) was attenuated by tempol (10 mg), a superoxide dismutase (SOD) mimetic (18 ± 5 mmHg, n = 9, P < 0.05), that was injected into the arterial supply of the hindlimb. In contrast, the pressor reflex arising from a freely perfused hindlimb (20 ± 3 mmHg) was not attenuated by tempol (17 ± 4 mmHg, n = 10, P = 0.49). Nevertheless, we found no difference in the increase in 8-isoprostaglandin F2α levels, an index of reactive oxygen species, in response to contraction between freely perfused (3.76 ± 0.82 pg/ml, n = 19) and 72-h occluded (3.51 ± 0.92 pg/ml, n = 22, P = 0.90) hindlimbs. Moreover, tempol did not reduce the 8-isoprostaglandin F2α levels during contraction in either group (P > 0.30). A second SOD mimetic, tiron (200 mg/kg), had no effect on the exercise pressor reflex in either the rats with freely perfused hindlimbs or in those with occluded femoral arteries. These findings suggest that tempol attenuated the exercise pressor reflex in the femoral artery-occluded hindlimb by a mechanism that was independent of its ability to scavenge reactive oxygen species.
Keywords: static contraction, thin fiber muscle afferents, 8-isoprostanes, peripheral artery disease
the exercise pressor reflex (27) is evoked by muscle contraction and results in increased arterial pressure, heart rate, and ventilation (5) (25). The sensory arm of the reflex is comprised of group III and IV muscle afferents (6, 25). The former are thinly myelinated and are thought to be primarily mechanically sensitive whereas the latter are unmyelinated and thought to be primarily metabolically sensitive (19, 20, 26). Even though several by-products of contraction are thought to be involved in stimulating and/or sensitizing group III and IV muscle afferents, the exact metabolite or combination of metabolites that evoke the exercise pressor reflex has not been elucidated.
In humans with peripheral arterial disease, the magnitude of the exercise pressor reflex is larger than it is in humans with normally perfused muscles (1). In rats, a model of peripheral arterial disease has been developed (44), an important feature of which is ligation of the femoral artery for 72 h before the start of the experiment (34, 49). This model accurately simulates the blood flow patterns seen in a limb of humans with peripheral artery disease, but it does not simulate the atherosclerotic mechanism that often impairs blood flow to exercising muscles. Using this model, Tsuchimochi et al. (41, 42) have found that the exercise pressor reflex is larger in rats whose femoral arteries were ligated than it was in rats whose femoral arteries were patent. Nevertheless, the mechanism(s) of the augmented exercise pressor reflex in rats with ligated femoral arteries remains to be determined.
The term reactive oxygen species (ROS) refers to cascade of free oxygen radicals that begins with the superoxide anion, which in turn is neutralized by superoxide dismutase (36). ROS may play a role in augmenting the exercise pressor reflex seen in peripheral arterial disease. In fact, ROS have been shown to stimulate group IV muscle afferents, an effect which was attenuated by a superoxide dismutase mimetic (8). Moreover, ROS production was found to increase when skeletal muscles were contracted (8, 35). These findings prompted us to determine if ROS play a role in evoking the exercise pressor reflex in rats whose hindlimbs were freely perfused and in rats whose femoral arteries were ligated 72 h before the start of the experiment. We tested the hypothesis that ROS played a role in evoking the pressor response to contraction in rats whose femoral arteries were ligated, but did not play a role in evoking the reflex in rats whose femoral arteries were patent.
METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University, Hershey Medical Center. Adult male rats (Sprague-Dawley, n = 73 weighing between 345 and 510 g) were used in this study. The rats were housed in a temperature-controlled room (24 ± 1°C) with a 12:12-h light-dark cycle. Rats were fed a standard diet and tap water ad libitum. Seventy-two hours before an experiment, 37 of 73 rats underwent surgery to induce unilateral femoral artery occlusion according to the procedure described previously (34, 49). Briefly, rats were anesthetized with a mixture of 4% isoflurane balanced with oxygen; one femoral artery was isolated and then tightly ligated with 5–0 silk suture just distal to the inguinal ligament. Using radiolabeled microspheres, it has been shown that this femoral artery ligation procedure reduced blood flow reserve capacity to ∼10–20% of normal but allowed sufficient blood flow to meet resting requirements (50). The rats recovered for 72 h before the experiments were started. Femoral artery occlusion has been reported to have no effect on normal cage activity (39).
Surgical Preparation
On the day of the experiment, rats were anesthetized with a mixture of 4% isoflurane and 100% oxygen. The right jugular vein and common carotid artery were cannulated for the delivery of drugs and fluids and the measurement of arterial blood pressure, respectively. The carotid arterial catheter was connected to a pressure transducer (model P23 XL, Statham). Heart rate was calculated beat to beat from the arterial pressure pulse (Gould Biotach). The trachea was cannulated, and the lungs were ventilated mechanically (Harvard Apparatus). Arterial blood gases and pH were measured by an automated blood-gas analyzer (model ABL-700, Radiometer). Pco2 and arterial pH were maintained within normal range by either adjusting ventilation or by intravenous administration of sodium bicarbonate (8.5%). A rectal temperature probe was inserted and the core body temperature of the animal was maintained at 37–38°C by a heating lamp.
We cannulated (PE-10, polyethylene tubing) the right femoral artery in a retrograde direction and advanced the tip to the bifurcation of the abdominal aorta. This allowed us to administer drugs into the arterial supply of the left hindlimb. A reversible vascular occluder was placed around the abdominal aorta and the inferior vena cava just above the aortic bifurcation. When tightened this occluder helped to keep the injectate within the circulation of the left hindlimb.
The rat was placed in a Kopf stereotaxic frame. Dexamethasone (0.2 mg) was injected intravenously just before the decerebration procedure to minimize brain stem edema. The left common carotid artery was tied off and a precollicular decerebration was performed. The plane of section was <1 mm anterior to the superior colliculi. All neural tissue rostral to the section was removed and the cranial cavity was packed with cotton.
A laminectomy exposing the lower lumbar and sacral portions of the spinal cord (L1-L5) was performed. The rat was then secured in a customized spinal frame by clamps placed on rostral lumbar vertebrae and the pelvis. Using the skin on the back, we formed a pool that was filled with warm (37°C) mineral oil. The dura was cut and reflected allowing visual identification of the spinal roots. The left L4 and L5 ventral roots were identified and cut close to their exits from the spinal cord. The calcaneal bone of a left hindlimb was severed, and the triceps surae muscles were isolated. Once the surgeries were completed, the anesthesia was withdrawn, and the lungs were ventilated with room air.
Experimental Protocols
Reflex protocol.
The peripheral cut ends of the L4 and L5 ventral roots were placed on shielded stimulating electrodes. Each calcaneal tendon was attached to a force transducer (model FT 10, Grass), which in turn was attached to a rack-and-pinion. The tendon was stretched so that baseline tension was set between 80 and 110 g. Static contraction was evoked by electrically stimulating (40 Hz, 0.1 ms, >2 times motor threshold) the cut peripheral ends of the L4 and L5 ventral roots. A muscle mechanoreceptor reflex was evoked by stretching the triceps surae muscles by manually turning the rack-and-pinion that was attached to the calcaneal tendon (38). Baseline tension was set between 80 and 110 g. Both muscle contraction and tendon stretch lasted for 30 s. The order of presentation of the two stimuli was varied randomly.
In 19 rats, we injected tempol (10 mg; Sigma-Aldrich), a compound that mimics the enzymatic activity of superoxide dismutase, retrogradely into contralateral femoral artery catheter. In these rats, the vascular occluder was inflated before injection of tempol to trap the injectate in the circulation of the limb to be contracted. The inflation was maintained for 5 min, after which it was deflated and the hindlimb was reperfused. The dose of tempol used in our experiments was identical to that used by Koba et al. (22) and about twice that used by Wang et al. (43). Tempol tended to decrease arterial pressure below baseline levels. Arterial pressure would then increase back to baseline within 10–15 min. Thus we waited for a steady arterial pressure before evoking static contraction and tendon stretch again.
In 12 other rats, we injected tiron (0.2 g/kg; Sigma-Aldrich), another compound that mimics the enzymatic activity of superoxide dismutase, retrogradely into the contralateral femoral artery catheter. Like the rats given tempol, the vascular occluder was inflated before injection of tiron to trap the injectate in the circulation of the limb to be contracted. The inflation was maintained for 5 min, after which it was deflated and the hindlimb was reperfused. The dose of tiron used was based on previous experiments in which this agent was infused intravascularly in a dose of 1 g/kg per hour (11, 40, 46). Because we injected this agent intra-arterially into one hindlimb, we used one-fifth of the dose used in these previous studies, which allowed tiron to travel throughout the entire body.
Intravenous control.
In 12 rats, tempol (10 mg) was injected into the jugular vein to test whether our findings with intra-arterial injection of tempol could be explained by its circulation to the spinal cord. Static contraction and tendon stretch were evoked at least 15 min after the injection. All contractions lasted for 30 s.
Microdialysis protocol.
Microdialysis can be used to introduce and remove ions, molecules, and drugs of interest to, or from, the interstitial space of skeletal muscle (12). We manufactured microdialysis probes by gluing both ends of a 2-cm length of capillary microdialysis membrane (0.20 mm in diameter, with a 13-kDa molecular cutoff) into nylon tubing. The nylon tubing was attached to a Luer tip adapter stub that connected the probe and the perfusate-filled syringe. Each rat had four microdialysis fibers placed in each triceps surae muscles, for a total of eight fibers; the fibers were separated by ∼0.25 cm. The probes were inserted into the muscles via a 20-gauge cannula inserted parallel to muscle fiber orientation. The insertion and exit points in and out of the muscle were ∼3 cm apart. The microdialysis probe was threaded through the internal lumen of the needle. The needle was withdrawn, leaving the membrane in place. The luer tip adapter stub was attached to a syringe for administration of saline through a perfusion pump (model 402, CMA) at 20 μl/min. After insertion of the eight microdialysis probes, we waited 2 h for equilibration.
The tibial nerve was placed on shielded stimulating electrodes. Each calcaneal tendon was attached to a force transducer (model FT 10, Grass), which in turn was attached to a rack-and-pinion. The tendon was stretched so that baseline tension was set between 80 and 110 g. Static contraction was evoked by electrically stimulating (40 Hz, 0.025 ms, >2 times motor threshold) the tibial nerve. Muscle contraction lasted for 60 s.
In 41 rats, we measured interstitial concentrations of 8-isoprostaglandin F2α (also known as 8-isoprostane, 8-epiprostaglandin F2α, iPF2α-III or 15-F2t-IsoP), an index of oxidative stress, immediately before and during a 60-s static contraction. In 20 rats, we measured the increase in interstitial concentrations of 8-isoprostaglandin F2α in response to a 60-s static contraction before and after administering tempol (200 mg/kg iv). Tempol was injected over a 10-min period and caused a marked attenuation in baseline blood pressure. We waited until mean arterial pressure increased as close as possible to baseline blood pressure before initiating contraction again, usually 10–20 min after the start of the tempol injection. Muscle microdialysate samples were taken during both the baseline and contraction periods before and after injecting tempol. Dialysate samples were stored at −80°C until analysis with a commercially available enzyme immunoassay kit (8-isoprostaglandin F2α, Cayman Chemical, Ann Arbor, MI). 8-Isoprostaglandin F2α concentrations are expressed in picograms per milliliter. We did not measure interstitial concentration of 8-isoprostaglandin F2α in response to tendon stretch.
To collect enough muscle interstitial fluid to run the enzyme immunoassay kit in triplicate for each sample, we needed eight probes inserted between the two triceps surae muscles and a collection period of 60 s. Because both hindlimbs would be in need of drug injection, we were not able to insert a femoral artery catheter as we did in the reflex protocol (see above). Therefore, to inject tempol into the circulation of both hindlimbs we injected this substance through the jugular vein catheter. In five rats, we injected blue dye into the jugular vein catheter and both triceps surae muscles turned blue, suggesting that the intravenous tempol injection reached the two hindlimbs. We did not determine the effect on tiron on the increase in muscle interstitial concentrations of 8-isoprostaglandin F2α that was evoked by contraction.
Data Analysis
Arterial blood pressure, heart rate, and muscle tension were recorded with a Spike 2 data acquisition system (CED, Cambridge). Mean arterial pressure (MAP) is expressed in millimeters Hg and heart rate (HR) in beats per minute (bpm). The initial 30-s values were used to compare the differences between baseline and the response to each maneuver. The tension-time index (TTI) was calculated by integrating the area between the tension trace and the baseline level (Spike 2) and is expressed in kilogram seconds (kg·s). All values are expressed as means ± standard error of the mean (SE). Statistical comparisons were performed with either one-way repeated-measures ANOVA, two-way repeated-measures ANOVA, or a linear mixed-effects model ANOVA. Then post hoc tests were performed with the Tukey test between individual means. The criterion for statistical significance was set at P < 0.05.
RESULTS
Effect of Tempol on Responses to Static Contraction
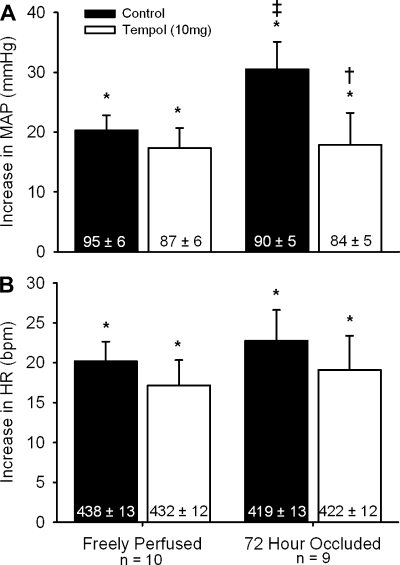
In 10 rats whose hindlimbs were freely perfused, static contraction increased mean arterial pressure and heart rate above baseline levels both before and after tempol (P < 0.05; Fig. 1A). The magnitudes of the pressor and cardioaccelerator responses to contraction were not attenuated by tempol (P > 0.46; Fig. 1B). The static contraction tension-time index was not different before and after tempol (P = 0.48; Table 1).
Fig. 1.
The pressor (A) and cardioaccelerator (B) responses to static contraction before (filled bars) and after intra-arterial injection of 10 mg of tempol (open bars) in rats whose hindlimbs were freely perfused and whose femoral arteries were ligated. Baseline mean arterial pressure and heart rate values are shown within vertical bars. Means ± SE. *Significant difference (P < 0.05) between baseline and peak. †Significant difference (P < 0.05) between before and after tempol. ‡Significant difference (P < 0.05) between freely perfused and 72-h ligated.
Table 1.
The tension-time indexes for static contraction and tendon stretch in rats whose hindlimbs were freely perfused and in rats whose femoral artery had been ligated 72 h before the start of the experiment before and after intra-arterial tempol (10 mg) or tiron (0.2 g/kg) injection
| TTI, kg · s |
|||
|---|---|---|---|
| n | Before | After | |
| Tempol | |||
| Freely perfused group | |||
| Static contraction | 10 | 18.4 ± 2.8 | 17.4 ± 3.0 |
| Tendon stretch | 8 | 17.7 ± 0.4 | 18.9 ± 0.5 |
| Ligated (72 h) group | |||
| Static contraction | 9 | 16.7 ± 2.9 | 13.9 ± 2.7 |
| Tendon stretch | 8 | 18.1 ± 0.5 | 19.4 ± 0.4* |
| Tiron | |||
| Freely perfused group | |||
| Static contraction | 6 | 20.1 ± 1.0 | 21.5 ± 1.0 |
| Tendon stretch | 6 | 23.5 ± 1.3 | 25.0 ± 1.4 |
| Ligated (72 h) group | |||
| Static contraction | 6 | 17.0 ± 3.5 | 18.3 ± 3.4 |
| Tendon stretch | 6 | 21.6 ± 4.8 | 24.8 ± 4.0 |
Values are means ± SE, n; no. of rats. TTI, tension-time index.
P < 0.05, after vs. before tempol.
We found that the pressor response to static contraction was greater in rats whose femoral artery had been ligated 72 h before the start of the experiment (n = 9) than that in rats whose hindlimbs were freely perfused (P < 0.05; Fig. 1A). There was not a difference in the cardioaccelerator response to contraction between the two rat groups (P = 0.55; Fig. 1B). In the rats whose femoral artery was ligated, static contraction increased mean arterial pressure above baseline levels both before and after tempol (P < 0.05; Fig. 1A). However, the magnitude of the pressor response to contraction was attenuated by tempol (P < 0.05; Fig. 1A). The magnitude of the cardioaccelerator response to contraction was not attenuated by tempol (P = 0.25; Fig. 1B). The static contraction tension-time index was not different before and after tempol (P = 0.20; Table 1).
Effect of Tempol on Responses to Stretch
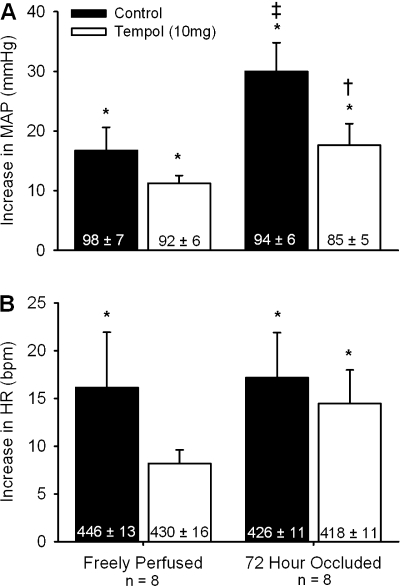
In eight rats whose hindlimbs were freely perfused, tendon stretch increased mean arterial pressure above baseline levels both before and after tempol (P < 0.05; Fig. 2A). The magnitude of the pressor response to tendon stretch was not attenuated by tempol (P = 0.14; Fig. 2A). Tendon stretch increased heart rate above baseline levels before tempol (P < 0.05) but not after tempol (P = 0.07; Fig. 2B). The tendon stretch tension-time index was not different before and after tempol (P = 0.18; Table 1).
Fig. 2.
The pressor (A) and cardioaccelerator (B) responses to tendon stretch before (filled bars) and after intra-arterial injection of 10 mg of tempol (open bars) in rats whose hindlimbs were freely perfused and whose femoral arteries were ligated. Baseline mean arterial pressure and heart rate values are shown within vertical bars. Means ± SE. *Significant difference (P < 0.05) between baseline and peak. †Significant difference (P < 0.05) between before and after tempol. ‡Significant difference (P < 0.05) between freely perfused and 72-h ligated.
We found that the pressor response to tendon stretch was greater in rats whose femoral artery had been ligated 72 h before the start of the experiment (n = 8) than that in rats whose hindlimbs were freely perfused (P < 0.05; Fig. 2A). There was no difference in the cardioaccelerator response to stretch between the two groups (P = 0.89; Fig. 2B). In the rats whose femoral artery was ligated, stretch increased mean arterial pressure above baseline levels both before and after tempol (P < 0.05; Fig. 2A). However, the magnitude of the pressor response to stretch was attenuated by tempol (P < 0.05; Fig. 2B). The magnitude of the cardioaccelerator response to stretch was not attenuated by tempol (P = 0.51; Fig. 2B). The tendon stretch tension-time index was slightly higher after tempol (P < 0.05; Table 1).
Intravenous Control for Tempol
We found that intravenous injection of tempol had no effect on the pressor responses to static contraction in rats whose hindlimbs were freely perfused (n = 6) or in rats whose hindlimbs had been ligated 72 h before the start of the experiment (n = 6) (P > 0.74; Table 2). In two of the six freely perfused rats, intravenous injection of tempol attenuated the pressor response to contraction.
Table 2.
Baseline and peak responses in MAP, HR, and TTI for static contraction in rats whose hindlimbs were freely perfused and in rats whose femoral artery had been ligated 72 h before the start of the experiment before and after intravenous tempol injection
| MAP, mmHg |
HR, beats/min |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline | Peak | Δ | Baseline | Peak | Δ | TTI, kg · s | |
| Freely perfused group | ||||||||
| Control | 6 | 85 ± 4 | 102 ± 5* | 17 ± 2 | 414 ± 18 | 433 ± 17* | 19 ± 3 | 21.8 ± 2.5 |
| Tempol (10 mg iv) | 6 | 77 ± 5 | 93 ± 7* | 16 ± 2 | 388 ± 14 | 409 ± 14* | 21 ± 4 | 22.8 ± 2.4 |
| Ligated (72 h) group | ||||||||
| Control | 6 | 92 ± 5 | 129 ± 11* | 37 ± 7† | 396 ± 17 | 424 ± 13* | 27 ± 8 | 19.8 ± 1.7 |
| Tempol (10 mg iv) | 6 | 95 ± 4 | 131 ± 6* | 36 ± 5† | 348 ± 55 | 368 ± 56* | 20 ± 5 | 18.5 ± 1.6 |
Values are means ± SE, n, no. of rats. MAP, mean arterial pressure; HR, heart rate.
P < 0.05, peak vs. baseline;
P < 0.05, 72 h ligated vs. freely perfused.
In addition, we found that intravenous injection of tempol had no effect on the pressor responses to tendon stretch in rats whose hindlimbs were freely perfused (n = 6) or in rats whose hindlimbs had been ligated 72 h before the start of the experiment (n = 5) (P > 0.12 ; Table 3). In two of the six freely perfused rats and in one of the five ligated rats, intravenous injection of tempol attenuated the pressor response to stretch. In the rats whose hindlimbs were freely perfused, tempol decreased baseline heart rate and the peak cardioaccelerator and pressor responses to stretch (P < 0.05; Table 3).
Table 3.
Baseline and peak responses in MAP, HR, and TTI for tendon stretch in rats whose hindlimbs were freely perfused and in rats whose femoral artery had been ligated 72 h before the start of the experiment before and after intravenous tempol injection
| MAP, mmHg |
HR, beats/min |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline | Peak | Δ | Baseline | Peak | Δ | TTI, kg · s | |
| Freely perfused group | ||||||||
| Control | 6 | 87 ± 3 | 102 ± 4* | 15 ± 2 | 433 ± 20 | 448 ± 22* | 15 ± 5 | 18.4 ± 0.3 |
| Tempol (10 mg iv) | 6 | 77 ± 7 | 89 ± 8*† | 12 ± 1 | 414 ± 21† | 425 ± 21*† | 11 ± 4 | 18.9 ± 0.7 |
| Ligated (72 h) group | ||||||||
| Control | 5 | 96 ± 8 | 119 ± 14* | 23 ± 7 | 415 ± 14 | 434 ± 11* | 19 ± 4 | 19.2 ± 0.5 |
| Tempol (10 mg iv) | 5 | 93 ± 5 | 113 ± 9* | 20 ± 7 | 411 ± 17 | 424 ± 15* | 13 ± 3 | 19.3 ± 0.4 |
Values are means ± SE; n, no. of rats.
P < 0.05, peak vs. baseline;
P < 0.05, control vs. intravenous tempol within same time point.
Effect of Tiron on Responses to Contraction
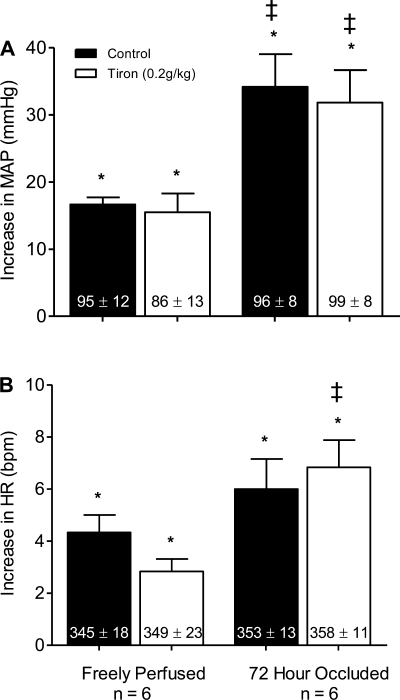
In six rats whose hindlimbs were freely perfused, static contraction increased mean arterial pressure and heart rate above baseline levels both before and after tiron (P < 0.05; Fig. 3A). The magnitudes of the pressor and cardioaccelerator responses to contraction were not attenuated by tiron (P > 0.19; Fig. 3). The static contraction tension-time index was not different before and after tiron (P = 0.48; Table 1).
Fig. 3.
The pressor (A) and cardioaccelerator (B) responses to static contraction before (filled bars) and after intra-arterial injection of 0.2 g/kg of tiron (open bars) in rats whose hindlimbs were freely perfused and whose femoral arteries were ligated. Baseline mean arterial pressure and heart rate values are shown within vertical bars. Means ± SE. *Significant difference (P < 0.05) between baseline and peak. ‡Significant difference (P < 0.05) between freely perfused and 72-h ligated rats.
We found that the pressor response to static contraction was greater in rats whose femoral artery had been ligated 72 h before the start of the experiment (n = 6) than that in rats whose hindlimbs were freely perfused (P < 0.05; Fig. 3A). There was no difference in the cardioaccelerator response to contraction between the two groups (P = 0.55; Fig. 3B). In the rats whose femoral artery was ligated, static contraction increased mean arterial pressure above baseline levels both before and after tiron (P < 0.05; Fig. 3A). Unlike tempol, however, the magnitude of the pressor response to contraction was not attenuated by tiron (P = 0.74; Fig. 3A). The magnitude of the cardioaccelerator response to contraction was not attenuated by tiron (P = 0.55; Fig. 3B). The static contraction tension-time index was not different before and after tiron (P = 0.52; Table 1).
Effect of Tiron on the Responses to Stretch
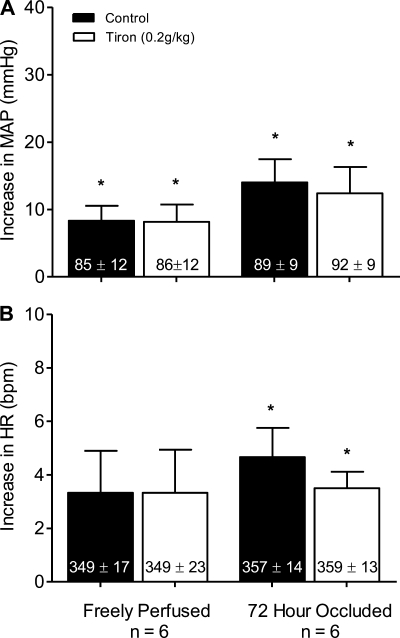
In six rats whose hindlimbs were freely perfused, tendon stretch increased mean arterial pressure and heart rate above baseline levels both before and after tiron (P < 0.05; Fig. 4). The magnitudes of the pressor (P = 0.74) and cardioaccelerator (P = 1.00) responses to tendon stretch were not attenuated by tiron (Fig. 4). The tendon stretch tension-time index was not different before and after tiron (P = 0.08; Table 1).
Fig. 4.
The pressor (A) and cardioaccelerator (B) responses to tendon stretch before (filled bars) and after intra-arterial injection of 0.2 g/kg of tiron (open bars) in rats whose hindlimbs were freely perfused and whose femoral arteries were ligated. Baseline mean arterial pressure and heart rate values are shown within vertical bars. Means ± SE. *Significant difference (P < 0.05) between baseline and peak.
We found that the pressor response to tendon stretch was greater in rats whose femoral artery was ligated 72 h before the start of the experiment (n = 6) than it was in the rats (n = 6) whose hindlimbs were freely perfused (P < 0.05; Fig. 4A). There was no difference in the cardioaccelerator response to stretch between the two groups (P = 0.50; Fig. 4B). In the rats whose femoral artery was ligated, stretch increased mean arterial pressure above baseline levels both before and after tiron (P < 0.05; Fig. 4A). Unlike the effect of tempol, the magnitude of the pressor response to stretch was not attenuated by tiron (P = 0.74; Fig. 4A). Likewise, the magnitude of the cardioaccelerator response to stretch was not attenuated by tiron (P = 0.16; Fig. 4B). The tendon stretch tension-time index was no different before and after tiron (P = 0.16; Table 1).
8-Isoprostane Concentrations
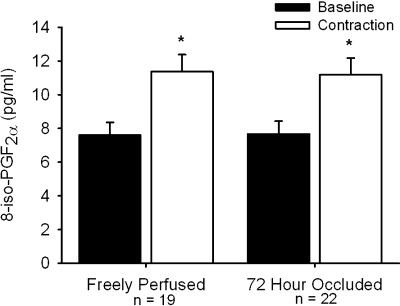
In 19 rats whose hindlimbs were freely perfused, static contraction increased the concentration of 8-isoprostaglandin F2α in muscle interstitial fluid above its baseline level (P < 0.05; Fig. 5). Likewise, in 22 rats whose femoral artery had been ligated 72 h before the start of the experiment, static contraction increased the concentration of 8-isoprostaglandin F2α in muscle interstitial fluid above its baseline level (P < 0.05; Fig. 5). However, there was not a difference in the magnitude of the increase between the two groups of rats (P = 0.89; Fig. 5). The tension-time index was not different between freely perfused (12.6 ± 1.1 kg·s) and 72-h-ligated (11.0 ± 0.8 kg·s; P = 0.14) groups.
Fig. 5.
8-Isoprostaglandin F2α concentrations (pg/ml) measured in muscle interstitial fluid during a baseline period and in response to static contraction in rats whose hindlimbs are freely perfused and whose femoral artery had been ligated 72 h prior. Means ± SE. *Significant difference (P < 0.05) between baseline and peak.
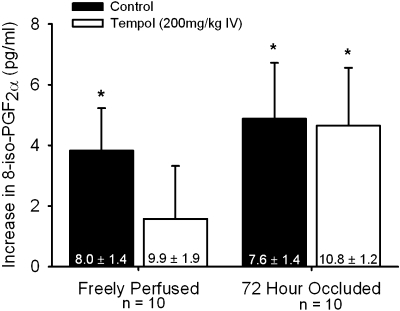
Ten of the 19 freely perfused and 10 of the 22 ligated rats were further tested with static contraction after tempol had been administered. Tempol did not attenuate the magnitude of 8-isoprostaglandin F2α increase evoked by contraction in either the freely perfused or the ligated hindlimbs (P > 0.30; Fig. 6). The tension-time indexes were not different before and after tempol in either group (freely perfused: before 13.8 ± 1.2, tempol 13.4 ± 0.9 kg·s; 72 h ligated: before 11.4 ± 1.1, tempol 11.9 ± 1.0 kg·s; P > 0.45).
Fig. 6.
The increase in 8-isoprostaglandin F2α in muscle interstitial fluid during contraction before (filled bars) and after 200 mg/kg tempol intra-venous injection (open bars) in rats whose hindlimbs are freely perfused and whose femoral artery had been ligated 72 h prior. Baseline 8-isoprostaglandin F2α concentrations are within vertical bars. Means ± SE. *Significant difference (P < 0.05) between baseline and peak.
DISCUSSION
We tested the hypothesis that oxidative stress played a greater role in evoking the exercise pressor reflex in rats whose femoral arteries were ligated 72 h before the start of the experiment than it did in evoking the reflex in rats whose hindlimb muscles were freely perfused. We attempted to reduce oxidative stress by injecting either tempol or tiron, both of which are superoxide dismutase mimetics, into the arterial circulation of the muscles being contracted. We found that tempol, but not tiron, attenuated the exercise pressor reflex in the rats whose femoral arteries were ligated; neither of the two mimetics attenuated the reflex in rats whose hindlimbs were freely perfused. Despite the fact that tempol attenuated the exercise pressor reflex in rats whose femoral arteries were occluded, this superoxide dismutase mimetic did not significantly reduce muscle interstitial levels of 8-isoprostaglandin F2α, an index of oxidative stress, during rest or contraction in either group of rats. Taken together, these findings suggest that tempol attenuated the exercise pressor reflex in the rats whose femoral arteries were ligated by a mechanism other than that which reduced oxidative stress.
The mechanism by which tempol attenuated the exercise pressor reflex in rats whose femoral arteries were ligated may involve potassium channels. Specifically, tempol has been found to open calcium-activated potassium (BK) (45, 48) and ATP-sensitive potassium (KATP) channels (4), both of which when activated hyperpolarize cells and in turn make them less excitable (3, 31, 45, 47, 48). This mechanism may have been responsible for the observation that tempol, applied topically, decreased renal sympathetic nerve activity (37). Likewise, this mechanism may have been responsible for the vasodilation and subsequent decrease in arterial blood pressure caused by tempol (4, 45, 47). In our experiments, this mechanism may have been responsible for the attenuation of the exercise pressor reflex induced by tempol in rats with occluded femoral arteries. Moreover, this mechanism is consistent with our finding that tiron, a superoxide dismutase mimetic that does not open potassium channels (48) and that does not relax vascular smooth muscle (45), had no effect on the exercise pressor reflex in the rats whose femoral arteries were occluded.
We can only speculate as to why tempol in our experiments attenuated the exercise pressor reflex in rats with femoral artery ligation, but had no effect on the reflex in rats with freely perfused hindlimbs. In our experiments, femoral arterial ligation might have increased the number of BK and/or KATP channels. Our speculation is consistent with two other observations. First KRN4884, a compound that opens KATP channels, increased blood flow to the gastrocnemius muscles in rats whose femoral arteries were ligated, but had no effect on blood flow to these muscles in rats whose femoral arteries were patent (51). Second, BK channels were found to be upregulated in DOCA salt-sensitive rats, but were not upregulated in normotensive rats (45, 48). Whether or not BK or KATP channels are upregulated in the rats whose femoral arteries are ligated for 72 h before the experiment remains to be determined.
Our finding that tempol had no effect on the exercise pressor reflex in rats with patent femoral arteries confirms a previous finding by Koba et al. (22) but contrasts with that reported by Wang et al. (43). In each of the three studies tempol was injected intra-arterially, but in the studies by Koba et al. and ours, tempol was injected into a trapped circulation over a 1-min period, and then trapped for another 4 min. Subsequently, the occluder was released, and was followed by a 10-min period of inactivity. In contrast, Wang et al. (43) continuously infused tempol into the femoral artery and made no attempt to trap it in the vasculature of the hindlimb. In our study, the circulation was occluded only during the 5-min injection period, a duration that was far too short to produce an ischemia-induced increase in superoxide radicals, which in turn might reduce the effectiveness of tempol. Indeed, when the resting anterior tibialis muscles of rats were rendered ischemic for 4 h, no increase in superoxide radicals was found in the interstitial fluid taken from this muscle during either the ischemic or the subsequent reperfusion periods (32). Even more importantly, the continuous intra-arterial infusion of tempol, such as that done by Wang et al. (43), might have, by opening potassium channels, hyperpolarized group III and IV muscle afferents to a far greater extent than did tempol infusion in either our experiments or those of Koba et al. (22). Consequently, these thin fiber muscle afferents may have been less responsive to contraction in the experiments reported by Wang et al. (43) than they were in either ours or those of Koba et al. (22). Clearly, decreasing the excitability of the afferents would result in an attenuated exercise pressor reflex.
Exercise is well known to increase production of reactive oxygen species within skeletal muscle (9, 18, 24, 30, 35), a finding which was confirmed in our experiments. Nevertheless, we found that ligating the femoral artery had no effect on the contraction-induced production of 8-isoprostaglandin F2α, our index of oxidative stress. This latter finding might appear at first glance to conflict with that reported by Judge et al. who reported that contraction of the gastrocnemius and soleus muscles in rats with ligated femoral arteries increased oxidative stress to a greater extent than did contraction of these muscles in rats whose femoral arteries were patent (14, 15). Judge et al,, however, contracted the muscles forcefully and measured oxidative stress 1 h after their 30-min intermittent contraction protocol ended, their purpose being to damage the muscles. In contrast, we contracted the muscles for only 30 s, our purpose being to determine if oxidative stress played a role in the exaggeration of the exercise pressor reflex that was caused by ligating the femoral artery. This exaggeration could only occur during the 30-s contraction period. What happened 1 h afterward was not relevant to our purpose.
8-Isoprostaglandin F2α can be generated by free radical-induced peroxidation of arachidonic acid, a reaction that is believed to be independent of cyclooxygenase, whose activity is blocked by the administration of nonsteroidal anti-inflammatory drugs such as indomethacin or meclofenamate (7, 23, 29). Although 8-isoprostaglandin F2α can be generated by a cyclooxygenase pathway, the magnitude of effect is small and in some assays nonexistent (2, 17, 21, 33). Moreover, nonsteroidals, such as indomethacin and meclofenamate, appear to have antioxidant effects that are independent of their ability to block cyclooxygenase (17). 8-Isoprostaglandin F2α concentrations have been found to be increased in numerous instances of oxidative stress and are generally accepted to be a good index of oxidative stress (13, 16, 28, 29). Specifically, the Biomarkers and Oxidative Stress Study concluded that “the lipid degradation products, such as 8-isoprostaglandin F2α, primarily constitute markers of oxidative stress” (17).
Any interpretation of our findings must be viewed with three limitations in mind. First, even though the interval between the start of either tempol or tiron injection and contraction was only 15 min, the possibility exists that the antioxidant effect of the two superoxide dismutase mimetics was either not present or reduced when we contracted the hindlimb muscles. Nevertheless, tempol in our experiments was still capable of attenuating the exercise pressor reflex in the rats whose femoral arteries were ligated, a finding that might be interpreted as evidence that tempol was still active, although the effect might have been on potassium channels rather on superoxide ions. Second, our measurement of oxidative stress was taken for the entire contraction period of 60 s and as result did not allow us to determine the time course of the effect. Third, although tempol reduces superoxide radicals, it also increases hydrogen peroxide, which in turn can form hydroxyl radicals by Fenton's reaction. Therefore we cannot rule out the possibility that tempol's inability to reduce 8-isoprostaglandin F2α concentrations in our experiments was caused by a compensatory increase in hydroxyl radicals.
In summary, even though ROS are known to stimulate unmyelinated afferents innervating either hindlimb muscle or skin (8, 10), they do not appear to be responsible for the exaggerated exercise pressor reflex evoked by static contraction of hindlimb muscles with a restricted arterial blood supply. We come to this conclusion because static contraction in our experiments increased muscle interstitial concentrations of 8-isoprostaglandin F2α, an index of ROS, to the same extent in rats whose femoral arteries were ligated as it did in rats whose femoral arteries were patent. Perhaps the search for the cause of the exaggerated exercise pressor reflex in this preparation should shift from ROS to a change in number or types of receptors on the endings of the group III and IV muscle afferents whose stimulation by contraction comprise the afferent arm of the exercise pressor reflex.
GRANTS
This work was supported by National Institutes of Health Grants HL-096570 and AR-059397.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Sarah Simmonds for technical assistance and data collection.
REFERENCES
- 1. Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Bachi A, Brambilla R, Fanelli R, Bianchi R, Zuccato E, Chiabrando C. Reduction of urinary 8-epi-prostaglandin F2 alpha during cyclo-oxygenase inhibition in rats but not in man. Br J Pharmacol 121: 1770–1774, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burgdorf C, Dendorfer A, Kurz T, Schomig E, Stolting I, Schutte F, Richardt G. Role of neuronal KATP channels and extraneuronal monoamine transporter on norepinephrine overflow in a model of myocardial low flow ischemia. J Pharmacol Exp Ther 309: 42–48, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Chen X, Patel K, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Acute antihypertensive action of Tempol in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 293: H3246–H3253, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coote JH, Pérez-González JF. The response of some sympathetic neurones to volleys in various afferent nerves. J Physiol 208: 261–278, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cracowski JL, Durand T. Cardiovascular pharmacology and physiology of the isoprostanes. Fundam Clin Pharmacol 20: 417–427, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Delliaux S, Brerro-Saby C, Steinberg JG, Jammes Y. Reactive oxygen species activate the group IV muscle afferents in resting and exercising muscle in rats. Pflugers Arch 459: 143–150, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Diaz PT, She ZW, Davis WB, Clanton TL. Hydroxylation of salicylate by the in vitro diaphragm: evidence for hydroxyl radical production during fatigue. J Appl Physiol 75: 540–545, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Evans AR, Junger H, Southall MD, Nicol GD, Sorkin LS, Broome JT, Bailey TW, Vasko MR. Isoprostanes, novel eicosanoids that produce nociception and sensitize rat sensory neurons. J Pharmacol Exp Ther 293: 912–920, 2000 [PubMed] [Google Scholar]
- 11. Gutierrez JA, Clark SG, Giulumian AD, Fuchs LC. Superoxide anions contribute to impaired regulation of blood pressure by nitric oxide during the development of cardiomyopathy. J Pharmacol Exp Ther 282: 1643–1649, 1997 [PubMed] [Google Scholar]
- 12. Hickner RC. Applications of microdialysis in studies of exercise. Exerc Sport Sci Rev 28: 117–122, 2000 [PubMed] [Google Scholar]
- 13. Ishii Y, Sakamoto T, Ito R, Yanaga K. F2-isoprostanes and 2-arachidonylglycerol as biomarkers of lipid peroxidation in pigs with hepatic ischemia/reperfusion injury. J Surg Res 161: 139–145, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Judge AR, Dodd SL. Oxidative damage to skeletal muscle following an acute bout of contractile claudication. Atherosclerosis 171: 219–224, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Judge AR, Selsby JT, Dodd SL. Antioxidants attenuate oxidative damage in rat skeletal muscle during mild ischaemia. Exp Physiol 93: 479–485, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, FitzGerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med 38: 698–710, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Kadiiska MB, Gladen BC, Baird DD, Graham LB, Parker CE, Ames BN, Basu S, FitzGerald GA, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, Rokach J, Shigenaga MK, Sun J, Walter PB, Tomer KB, Barrett JC, Mason RP. Biomarkers of oxidative stress study. III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radic Biol Med 38: 711–718, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Karamouzis I, Christoulas K, Grekas D, Giannoulis K, Vamvakoudis E, Mandroukas K. The response of muscle interstitial F2-isoprostane (8-ISO-PGF2alpha) during dynamic muscle contractions in humans. Prostaglandins Leukot Essent Fatty Acids 71: 87–90, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 20. Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 21. Klein T, Reutter F, Schweer H, Seyberth HW, Nusing RM. Generation of the isoprostane 8-epi-prostaglandin F2alpha in vitro and in vivo via the cyclooxygenases. J Pharmacol Exp Ther 282: 1658–1665, 1997 [PubMed] [Google Scholar]
- 22. Koba S, Gao Z, Sinoway LI. Oxidative stress and the muscle reflex in heart failure. J Physiol 587: 5227- 5237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lawson JA, Rokach J, FitzGerald GA. Isoprostanes: formation, analysis and use as indices of lipid peroxidation in vivo. J Biol Chem 274: 24441–24444, 1999 [DOI] [PubMed] [Google Scholar]
- 24. McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 280: C621–C627, 2001 [DOI] [PubMed] [Google Scholar]
- 25. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mense S, Stahnke M. Responses in muscle afferent fibers of slow conduction velocity to contractions and ischemia in the cat. J Physiol 342: 383–397, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol 45: 229–242, 1983 [DOI] [PubMed] [Google Scholar]
- 28. Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. FASEB J 18: 1791–1800, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res 36: 1–21, 1997 [DOI] [PubMed] [Google Scholar]
- 30. O'Neill CA, Stebbins CL, Bonigut S, Halliwell B, Longhurst JC. Production of hydroxyl radicals in contracting skeletal muscle of cats. J Appl Physiol 81: 1197–1206, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Oe K, Sperlagh B, Santha E, Matko I, Nagashima H, Foldes FF, Vizi ES. Modulation of norepinephrine release by ATP-dependent K+-channel activators and inhibitors in guinea-pig and human isolated right atrium. Cardiovasc Res 43: 125–134, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Pattwell D, McArdle A, Griffiths RD, Jackson MJ. Measurement of free radical production by in vivo microdialysis during ischemia/reperfusion injury to skeletal muscle. Free Radic Biol Med 30: 979–985, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Pratico D, Lawson JA, FitzGerald GA. Cyclooxygenase-dependent formation of the isoprostane, 8-epi prostaglandin F2 alpha. J Biol Chem 270: 9800–9808, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Reid MB, Durham WJ. Generation of reactive oxygen and nitrogen species in contracting skeletal muscle: potential impact on aging. Ann NY Acad Sci 959: 108–116, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Salvemini D, Muscoli C, Riley DP, Cuzzocrea S. Superoxide dismutase mimetics. Pulm Pharmacol Ther 15: 439–447, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Shokoji T, Nishiyama A, Fujisawa Y, Hitomi H, Kiyomoto H, Takahashi N, Kimura S, Kohno M, Abe Y. Renal sympathetic nerve responses to tempol in spontaneously hypertensive rats. Hypertension 41: 266–273, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol 65: 1539–1547, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Taylor JC, Li Z, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic inhibition increases collateral circuit conductance in rats following acute occlusion of the femoral artery. J Physiol 586: 1649–1667, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomas GD, Zhang W, Victor RG. Impaired modulation of sympathetic vasoconstriction in contracting skeletal muscle of rats with chronic myocardial infarctions: role of oxidative stress. Circ Res 88: 816–823, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsuchimochi H, McCord JL, Kaufman MP. Peripheral mu-opioid receptors attenuate the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 299: H557–H565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang HJ, Pan YX, Wang WZ, Zucker IH, Wang W. NADPH oxidase-derived reactive oxygen species in skeletal muscle modulates the exercise pressor reflex. J Appl Physiol 107: 450–459, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol 97: 773–780, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Xu H, Bian X, Watts SW, Hlavacova A. Activation of vascular BK channel by tempol in DOCA-salt hypertensive rats. Hypertension 46: 1154–1162, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Xu H, Fink GD, Galligan JJ. Nitric oxide-independent effects of tempol on sympathetic nerve activity and blood pressure in DOCA-salt rats. Am J Physiol Heart Circ Physiol 283: H885–H892, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Xu H, Fink GD, Galligan JJ. Tempol lowers blood pressure and sympathetic nerve activity but not vascular O2- in DOCA-salt rats. Hypertension 43: 329–334, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Xu H, Jackson WF, Fink GD, Galligan JJ. Activation of potassium channels by tempol in arterial smooth muscle cells from normotensive and deoxycorticosterone acetate-salt hypertensive rats. Hypertension 48: 1080–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Yang HT, Terjung RL. Angiotensin-converting enzyme inhibition increases collateral-dependent muscle blood flow. J Appl Physiol 75: 452–457, 1993 [DOI] [PubMed] [Google Scholar]
- 51. Yoshino T, Ohta H, Jinno Y, Torii Y, Ogawa N, Izawa T, Okada Y. Protective effect of the K+ channel opener KRN4884 on peripheral occlusive arterial disease in rats. Gen Pharmacol 31: 59–62, 1998 [DOI] [PubMed] [Google Scholar]