Abstract
Infusion of the angiotensin-converting enzyme inhibitor enalaprilat into fetal sheep caused a profound arterial hypotension within days. Five fetal lambs were infused with enalaprilat for 8 days starting at day 128 of gestation. Total accumulated dose was 0.30 ± 0.11 mg/kg. Arterial pressure decreased from 43.6 to 25.6 mmHg; venous pressure did not change. Biventricular output was not statistically significantly changed; placental blood flow decreased almost in proportion to the decrease in pressure but the increase in somatic flow was not statistically significant. There were no significant changes in pressure 30 min after the initial 50-μg loading dose of enalaprilat. However, the arterial pressure responses to test doses of ANG I were largely abolished. After 1 day, however, there was a significant decrease in somatic vascular resistance, which became stronger with time, but almost no decrease in the placental resistance. We conclude that the fetal somatic circulation exhibits a slow but strong decrease in resistance but that the response to hypotension is weak or absent in the fetal placenta, possibly because it is already fully relaxed.
Keywords: enalaprilat, somatic blood flow, vascular resistance, plasma renin activity, blood gases, growth
short-term studies of the fetal placental circulation of the sheep found no evidence of blood flow control; flow was essentially proportional to the driving pressure (3, 14, 15, 17). By contrast, long-term studies from our laboratory with elevated arterial pressures did elicit increases in fetal placental resistance both in the presence of elevated angiotensin concentrations (7) and in the presence of reduced angiotensin concentrations (9). These responses, however, took days to develop. In the latter study we also found an increase in the resistance of the fetal somatic circulation.
The purpose of the present study was to determine if the reverse is also true so that decreases in fetal arterial pressure lead to vasodilation in the fetal placental circulation. Since fetal oxygenation is a function of flow rather than pressure, such a response would significantly ameliorate the deleterious effects of fetal hypotension. While studying the effects of a reduced systolic load on the development of the fetal heart in a previous study (14), we found that intravenous infusion of enalaprilat into the fetal sheep caused a sustained fall in fetal arterial pressure. We, therefore, used this approach in the present study to investigate the long-term effect of fetal arterial hypotension on placental flow and resistance.
This study was facilitated by the fact that the fetal circulation in the sheep differs from that in the human fetus in that the single brachiocephalic artery is, after the coronary arteries, the only vessel to branch from the aortic arch before the aorta is joined by the ductus arteriosus. It is thus possible to measure the fetal biventricular cardiac output by placing flow sensors on the brachiocephalic artery and on the postductal aorta. The sum of these flows is the biventricular output, except only for the pulmonary flow, which is ∼7% of the output (15) and the coronary flow, ∼3% (5). By placing a third flow sensor on the common umbilical artery (a continuation of the aorta beyond the iliac bifurcation in fetal sheep), we could separate the thoracic flows into the somatic (except coronary) and placental components and calculate the resistances of both.
METHODS
Surgical procedures.
All surgical and experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC). Five time-bred ewes of mixed Western breeds, carrying singletons, were obtained from a commercial source. At 123 days of gestational age (range 121–125 days) the ewes were premedicated with 7.5 mg atropine (im) and given 10 mg diazepam and 400 mg ketamine (iv) to induce anesthesia. They were then intubated and mechanically ventilated with a mixture of 2,000 ml/min oxygen and 700 ml/min nitrous oxide with isoflurane at 1–2%, as required for a surgical level of anesthesia in both the ewe and the fetus.
Using strictly sterile surgical technique, we placed indwelling catheters in a fetal carotid artery and jugular vein. The carotid artery catheters were advanced only a few centimeters into the vessel so as not to interfere with the subsequently placed flow sensor on the brachiocephalic artery. We accessed the thoracic vessels through an incision in the fourth left intercostal space and placed flow sensors (Transonic, Ithaca, NY) on the brachiocephalic artery and the postductal aorta without incising the pericardium. We then approached the left flank of the fetus through a separate uterine incision and placed a third flow sensor on the common umbilical artery. Catheters sewn to the fetal skin served to later record amniotic fluid pressure. All incisions were closed in layers, the catheters were filled with heparinized saline; 1 million units of penicillin-G and 2 mg of ciprofloxacin were instilled into the amniotic fluid. The free ends of all cables and catheters were tunneled under the skin of the ewe and exteriorized at the flank where they were kept in a pouch attached to her flank. Typical duration of surgery was three and a half hours. Postoperatively, the ewes received 0.6 mg bupremorphine twice a day for 2 days.
Animal laboratory procedures.
Three to 6 days after surgery, the ewes were placed in stanchions in the laboratory. The animals were watered and fed ad libitum. The experiments were begun on day 128 or 129 of gestation. We obtained control measurements for 3 days, which we labeled days −2, −1, and 0 in the figures. The reason for a long control period is that, in our experience, chronically instrumented fetuses are not quite unstressed immediately after their catheters have been opened and flushed. It can be seen, for instance, in Figs. 3 and 4 in results that there were increases in biventricular output and Po2 during the period from day −2 to day 0. At the end of the control period, the fetuses were given intravenous test doses of ANG I (Bachem, Torrance, CA) to determine the pressure responses before blockade. We then started fetal infusions of enalaprilat (Novaplus Vasotec) with a loading dose of 50 μg (iv); this dose in a 3-kg fetus corresponded to the maximally effective dose in human patients with hypertension (18), and a continuous infusion was initiated by means of a roller pump with an enalaprilat concentration of 2.5 or 5 μg/ml of lactated Ringer's solution. We tested the adequacy of the loading dose in two fetuses with test doses of 7.5 μg of ANG I. Since there was large interfetus variability in the sensitivity of arterial pressure to enalaprilat, we adjusted the infusion rate to obtain an arterial blood pressure of between 25 and 30 mmHg during the final 4 days of the infusion. As a result, the total amounts of enalaprilat given to each fetus in the course of the experiments varied from 0.27 to 2.42 mg (0.30 ± 0.11 mg/kg autopsy body wt). Eight days after the start of the enalaprilat infusions, the experiments were terminated. Test doses of ANG I again established the adequacy of the converting enzyme block, after which the animals were euthanized, as approved by the IACUC. All fetuses were autopsied to verify the placement of the flow sensors and the catheters.
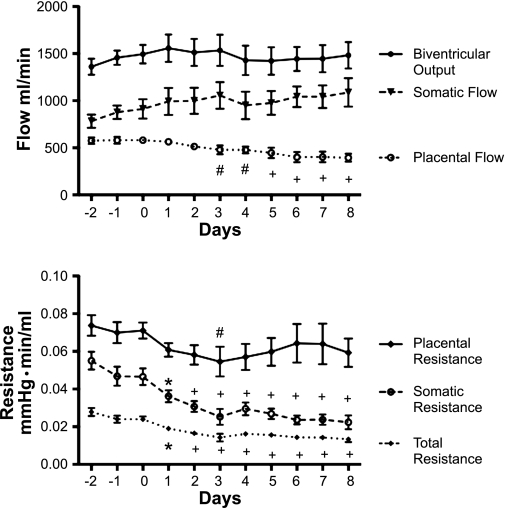
Fig. 3.
Top: biventricular output, somatic blood flow, and placental blood flow. Bottom: placental, somatic, and total vascular resistances 3 days before and during 8 days of enalaprilat infusion. #P < 0.05, *P < 0.01, +P < 0.001 compared with day 0.
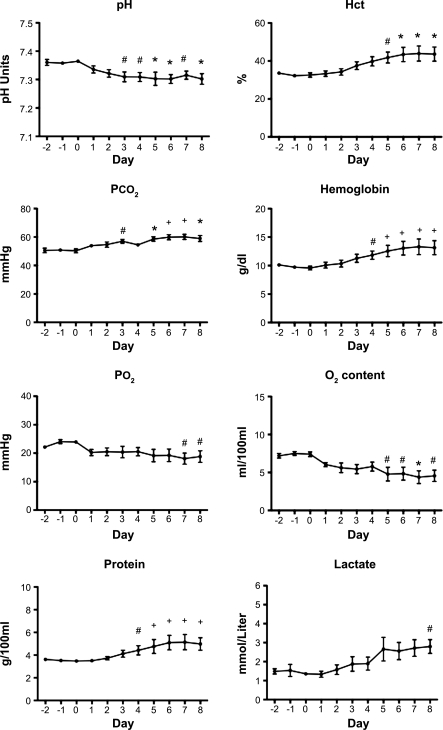
Fig. 4.
Fetal arterial blood pH, hematocrit (Hct), Pco2, hemoglobin concentration, Po2, oxygen content, plasma protein concentration, and lactate concentration before and during 8 days of enalaprilat infusion. #P < 0.05, *P < 0.01, +P < 0.001 compared with day 0.
Measurements.
Fetal arterial, venous, and amniotic fluid pressures as well as all flows were continuously recorded for the duration of the experiments. Pressures were recorded by means of Abbott Transpac transducers (Abbott Park, IL). To maintain catheter patency, we infused lactated Ringer's solution with 2,000 units of heparin per liter through the transducers and catheters at a slow rate that represented a total fetal fluid load of <70 ml/day. The slow infusion rates made no differences in the recorded pressures. Transducer zeros were checked each day, after which we measured pressures and flows for 1 h for statistical analysis. Pressures were accurate to ±0.5 mmHg. Intravascular pressures will be reported with amniotic fluid pressure being considered zero. Flows were recorded by means of synchronized Transonic flowmeters, and all data were processed through AD Instruments (Colorado Springs, CO) electronics and an Apple computer (Cupertino, CA) and stored on disk. Daily samples of fetal arterial blood were analyzed for pH, Pco2, Po2, hemoglobin concentration, hematocrit, oxygen content, and lactate concentration with an ABL 700 blood gas analyzer (Radiometer America, Westlake, OH), and total plasma protein concentration was measured by refractometry. In four of the five animals, plasma samples anticoagulated with EDTA were analyzed for plasma renin activity (Diasorin, Stillwater, MN), as previously described (9).
Statistical methods.
Statistical analyses of the data were performed with Prism-4 software (GraphPad Software, San Diego CA). Repeated-measures ANOVA with Dunnett's multiple-comparison test was used to determine if, and when, the daily measurement differed from the day 0 preinfusion value. Results are presented as means ± 1 SE.
RESULTS
Adequacy of converting enzyme block.
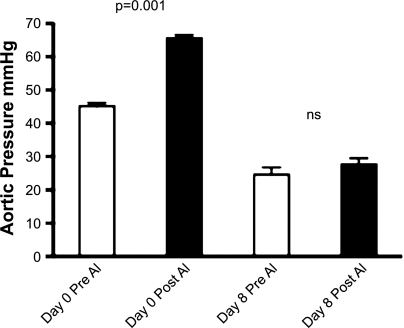
Figure 1 shows that at a maximum dose of 20 ± 3 μg ANG I, there was an average increase in arterial pressure of 21 mmHg (P < 0.001) before the administration of the angiotensin-converting enzyme (ACE) inhibitor. At the end of the enalaprilat infusion period of 8 days, the far larger dose of 64 ± 14 μg of ANG I produced only a very small increase of 3 mmHg in arterial pressure that was not statistically significant. In the two fetuses in which the adequacy of the loading dose was tested by comparing the responses to 7.5 μg of ANG I before and after the loading dose, arterial pressure increased 14 mmHg before the loading dose and 4 mmHg after in the first fetus and 19 mmHg before and 3 mmHg after in the second. We considered this a sufficient block for the purpose at hand.
Fig. 1.
Aortic blood pressures just before (Pre) and just after (Post) the maximal test doses of ANG I (AI) administered on day 0 before infusion (20 ± 3 μg ANG I) and after 8 days of enalaprilat infusion (64 ± 14 μg ANG I). ns, not significant.
Circulatory effects of enalaprilat.
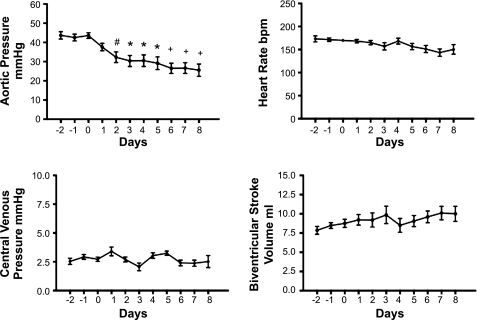
Figure 2 shows the changes in fetal arterial blood pressure, venous blood pressure, biventricular stroke volume, and heart rate. Mean arterial pressure was reduced by the second day of infusion, and this decrease was sustained for the duration of the enalaprilat infusion. Mean fetal arterial pressure on day 0 was 43.6 ± 1.8 mmHg as opposed to 25.6 ± 3.2 mmHg on the eighth and last day of infusion. Fetal central venous pressure was not statistically significantly affected at any time, being 2.5 ± 0.3 and 2.5 ± 0.5 mmHg on days 0 and 8, respectively. Biventricular stroke volume did not change significantly during the infusion period. Heart rate showed a moderate decrease from 170 ± 3 to 150 ± 1 beats/min on days 0 and 8, respectively, and the decline over time was statistically significant at P < 0.03, consistent with the normal gestational decrease in fetal heart rate over time.
Fig. 2.
Blood pressures, heart rate, and biventricular stroke volume 3 days before and during 8 days of enalaprilat infusion. #P < 0.05, *P < 0.01, +P < 0.001 compared with day 0.
Figure 3 illustrates the changes in biventricular output, somatic flow, placental flow, total resistance, somatic resistance, and placental resistance. In contrast to the steep decline in arterial blood pressure, biventricular output was almost unaffected, although the normal slight increase with fetal gestational age appeared to be absent after day 3. Figure 3 does show, however, that the near constancy of biventricular output derived from the fact that while placental blood flow was statistically significantly decreased on day 3 and thereafter, this decrease was balanced by a statistically nonsignificant increase in somatic flow.
A near constant biventricular output in the face of a steep decline in arterial pressure signified a decrease in total peripheral resistance. The decrease in total peripheral resistance was already statistically significant after 1 day and remained significant through the remainder of the experiment.
Somatic resistance measured 10 min before the loading dose of enalaprilat was still unchanged 30 min later; it actually increased by 0.0005 ± 0.0040 mmHg·min/ml (not significant). However, the large decrease in somatic resistance shown in Fig. 3 was already significant 24 h later and intensified during the course of the experiment. Placental resistance measured 10 min before and 30 min after the loading dose decreased insignificantly by 0.0006 ± 0.0020 mmHg·min/ml and was significantly reduced later only on the third day of the infusion (Fig. 3). It follows that the large decrease in the somatic vascular resistance accounted for almost the entire decrease in total resistance.
In addition to the total somatic resistance, mentioned above, we also analyzed the resistance of that part of the somatic vascular bed that was supplied by the brachiocephalic artery because its flow depended on only one flow sensor, as opposed to the three needed for the calculation of the total somatic resistance. Brachiocephalic resistance was, and remained, significantly decreased after 2 days of infusion (P < 0.05).
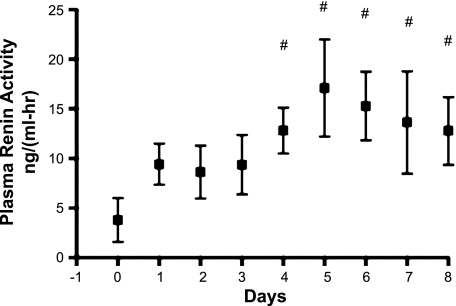
Figure 4 shows the changes in fetal arterial blood in pH, hematocrit, Pco2, hemoglobin concentration, Po2, oxygen content, plasma protein concentration, and lactate concentration before and during 8 days of enalaprilat infusion. Figure 5 shows the plasma renin activities recorded in four of the five fetuses.
Fig. 5.
Plasma renin activity in 4 of the 5 fetuses. #P < 0.05 compared with day 0.
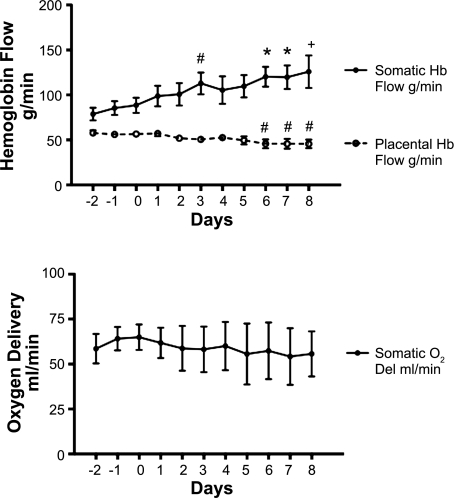
Due to the increase in the concentration of hemoglobin (Fig. 4), the product of the placental blood flow and hemoglobin concentration (which constitutes the placental hemoglobin flow in g of hemoglobin/min) was only marginally decreased early and was statistically significantly, but still minimally, decreased on days 6–8. However, somatic hemoglobin flow actually increased (Fig. 6). As a result somatic oxygen delivery was maintained throughout the infusion period.
Fig. 6.
Top: somatic and placental hemoglobin (Hb) flows before and during 8 days of enalaprilat infusion. Bottom: somatic oxygen delivery during the same period. #P < 0.05, *P < 0.01, +P < 0.001 compared with day 0.
Autopsy.
The placements of all catheters and flow probes were confirmed at autopsy and found to be correct. The fetuses were grossly normal. However, the fetal kidneys were small, partly hemorrhagic, and softer than normal. Average fetal body weight was 4.4 ± 0.5 kg. Average heart weight was 24.4 ± 5.3 g. Fetal heart weight-to-body weight ratio was 5.2 ± 0.6 g/kg. Amniotic and allantoic fluid volumes were not measured but appeared smaller than normal.
DISCUSSION
The major findings of this study are that enalaprilat infusion is associated with a deep and sustained decrease in fetal arterial pressure, little change in fetal biventricular output, a substantial decrease in somatic resistance, and little change in placental resistance. These changes, in concert with the elevations in hematocrit and hemoglobin concentration, increased somatic hemoglobin flow and maintained somatic oxygen delivery. These beneficial responses accounted for the surprisingly mild deleterious effects of arterial hypotension on fetal tissue oxygenation.
Variability in enalaprilat sensitivity.
In the adult, enalaprilat is normally eliminated almost entirely by renal excretion (11). In the fetus this is excretion into the amniotic and allantoic fluids. Depending on the balance between the rates of excretion into these fluids, and fetal reabsorption from those fluids, the concentrations of enalaprilat may be very different in the extrafetal fluids and fetal intravascular water. Without analysis of these fluids and fetal plasma this possibility cannot be further explored.
Effects of fetal arterial hypotension on fetal somatic and cardiac growth.
The average fetal body weight was 4.4 ± 0.5 kg. The mean body weights of the group of control fetuses of similar gestational age in a previous study (14), who received only lactated Ringer's solution, was 3.9 ± 0.4 kg. The mean body weight of the present fetuses was essentially the same. In this previous study also, the body weights of the control group and the hypotensive group were the same. The maintenance of body growth in the hypotensive fetuses is consistent with the observed biventricular outputs and hemoglobin flows. However, as previously shown (14) the result of arterial hypotension was a reduction in the ratio of heart weight to body weight. Normally that ratio is about 7.4 g/kg in singletons (12) or 7.0 g/kg in a mixed group of singletons and twins (14). In the present study, the fetal hearts were smaller as indicated by the fetal heart weight-to-body weight ratio of 5.2 ± 0.6 g/kg. This heart weight-to-body weight ratio is similar to our previous finding in hypotensive fetuses where the ratio was 5.6 ± 0.5 g/kg after 8 days of enalaprilat-induced fetal arterial hypotension (14).
Effects of enalaprilat-induced hypotension on fetal cardiac function and resistances.
The slow decrease in arterial pressure may in part reflect a slow rate of fluid loss to the maternal circulation in the placenta by the mechanism first proposed by Adamson and coworkers (1). These investigators reasoned that an increase in the precapillary resistance of the fetal placenta mediated by angiotensin would decrease fetal placental capillary pressure and thereby promote fluid transfer from mother to fetus. Inhibiting the production of angiotensin, therefore, would do the opposite. This would be consistent with the increases in hemoglobin concentration, hematocrit, and plasma protein in Fig. 4. The ability of the heart to maintain output in the face of a decreased vascular filling may well have been due to the large decrease in afterload. With the biventricular output almost unchanged, most, perhaps all, of the reduction in arterial pressure reflects a decrease in resistance. However, the results were more complex than anticipated because the changes in somatic and placental resistances were very different.
The near absence of a change in placental resistance came as a surprise. A physiologically plausible hypothesis is that angiotensin not only vasoconstricts the fetal placental precapillary resistance but also dilates the fetal placental postcapillary resistance. This would make fetal renal control of fetal fluid volume feasible without causing the significant changes in fetal placental resistance that would follow if only the precapillary resistance was modulated. Resolution of this aspect of fluid control awaits further study. It must be remembered that vascular resistance depends not only on the vascular geometry but also on the viscosity of blood. The increases in the concentrations of hemoglobin and plasma protein may have masked a small decrease in placental geometric resistance and thus a weak form of fetal placental dilation. For the same reason, the response of the somatic circulation may have been stronger than is apparent from our data. The most direct explanation for the presence of an increase in resistance in the placenta when arterial pressure is increased (9) but a near absence of a decrease when pressure is reduced may well be that the fetal placental circulation is already almost completely dilated. A previous study in which fetal placental flow was reduced for long periods of time by mechanical means also failed to show vasodilation (2).
Potential shortcomings.
Although the ultimate effects observed during this study likely arose from the block of ACE demonstrated on day 8 of the experiment, unknown effects of ACE inhibition cannot be excluded. However, the fact that the effects of losartan are similar to those of captopril led other investigators to conclude that the results of converting enzyme inhibition are independent of any direct effects on bradykinin or prostaglandin levels (16).
The fetal nephrotoxicity of ACE inhibitors is well documented (4, 10). It should be noted, however, that the total dose of enalaprilat accumulated over the 8 days of infusion was only 0.30 ± 0.11 mg/kg fetus autopsy weight compared with, for instance, a total of 18.5 mg/kg administered previously over a period of only 3 days (8).
Conclusions.
We conclude that the resulting combination of a fetal arterial hypotension and a strong vasodilation of the somatic circulation is beneficial in the sense of protecting somatic hemoglobin flow and oxygen delivery. However, any response of the fetal placental circulation is weak, if it exists at all.
We further conclude that the fetal somatic circulation is uniquely suited to the study of the control of flow. Fetal arterial blood pressure can be modulated up or down over the necessary long period of time in a manner that would be nearly impossible to duplicate in the adult circulation. This is due to the fact that fetal arterial pressure is extremely sensitive to intravascular volume (6) and that intravascular volume can be easily modulated by a variety of experimental interventions that invoke the mechanism proposed by Adamson et al. (1), such as those exemplified in other studies (6, 9, 14).
GRANTS
This research was funded by a National Institutes of Health Program Project Grant (P01-HD-34430), the Department of Veterans Affairs, and the M. Lowell Edwards Endowment.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Robert Webber and Loni Socha for excellent assistance.
REFERENCES
- 1. Adamson SL, Morrow RJ, Bull SB, Langille BL. Vasomotor responses of the umbilical circulation in fetal sheep. Am J Physiol Regul Integr Comp Physiol 256: R1056–R1062, 1989 [DOI] [PubMed] [Google Scholar]
- 2. Anderson DF, Faber JJ. Regulation of fetal placental blood flow in the lamb. Am J Physiol Regul Integr Comp Physiol 247: R567–R574, 1984 [DOI] [PubMed] [Google Scholar]
- 3. Berman W, Jr, Goodlin RC, Heymann MA, Rudolph AM. Relationships between pressure, and flow in the umbilical and uterine circulations in sheep. Circ Res 38: 262–266, 1976 [DOI] [PubMed] [Google Scholar]
- 4. Brent TL, Beckman DA. Angiotensin converting enzyme inhibitors, an embryopathic class of drugs with unique properties: information for clinical teratology councilors. Teratology 43: 543–546, 1991 [DOI] [PubMed] [Google Scholar]
- 5. Davis LE, Hohimer AR. Hemodynamics and organ blood flow in fetal sheep subjected to chronic anemia. Am J Physiol Regul Integr Comp Physiol 261: R1542–R1548, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Faber JJ, Anderson DF. The placenta in the integrated physiology of fetal volume control. Int J Dev Biol 54: 391–396, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faber JJ, Anderson DF, Binder ND. Delayed vasoconstriction of the umbilico-placental circulation by angiotensin in fetal sheep. Placenta 19: 675–676, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Giammattei CE, Strandhoy JW, Rose JC. Regulation of in vitro renin secretion by ANG II feedback manipulation in vivo in the ovine fetus. Am J Physiol Regul Integr Comp Physiol 277: R1230–R1238, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Giraud GD, Faber JJ, Jonker SS, Davis L, Anderson DF. Effects of intravascular infusions of plasma on placental and systemic blood flow in fetal sheep. Am J Physiol Heart Circ Physiol 291: H2884–H2888, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Hanssens M, Keirse MJ, Vankelecom F, Van Assche FA. Fetal and neonatal effects of treatment with angiotensin converting enzyme inhibitors in pregnancy. Obstet Gynecol 78: 128–135, 1991 [PubMed] [Google Scholar]
- 11. Hardman JG, Limbird LE, Gilman AG. Goodman & Gilman's The Pharmacological Basis of Therapeutics (10th ed.). New York: McGraw-Hill, 2001 [Google Scholar]
- 12. Jonker SS, Faber JJ, Anderson DF, Thornburg KL, Louey S, Giraud GD. Sequential growth of fetal sheep cardiac myocytes in response to simultaneous arterial and venous hypertension. Am J Physiol Regul Integr Comp Physiol 292: R913–R919, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Mott JC. Control of the foetal circulation. J Exp Biol 100: 129–146, 1989 [DOI] [PubMed] [Google Scholar]
- 14. O'Tierney PF, Anderson DF, Faber JJ, Louey S, Thornburg KL, Giraud GD. Reduced systolic pressure load decreases cell cycle activity in the fetal sheep heart. Am J Physiol Regul Integr Comp Physiol 299: R573–R578, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rudolph A. Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ Res 57: 811–820, 1985 [DOI] [PubMed] [Google Scholar]
- 16. Stevenson KM, Gibson KJ, Lumbers ER. Effects of losartan on the cardiovascular system, renal haemodynamics and function and lung liquid flow in fetal sheep. Clin Exp Pharm Physiol 23: 125–133, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Thornburg KL, Bissonette JM, Faber JJ. Absence of fetal placental waterfall phenomenon in chronically prepared fetal lambs. Am J Physiol 230: 886–892, 1976 [DOI] [PubMed] [Google Scholar]
- 18. Westward Pharmaceuticals Enalaprilat Injection (medication insert) Eatontown, NJ, 02/2009 [Google Scholar]