Abstract
We previously reported that reactive oxygen species generated by NADPH oxidase 2 (Nox2) induces sensory plasticity of the carotid body, manifested as a progressive increase in baseline sensory activity or sensory long-term facilitation (sLTF). ANG II, a peptide generated within the carotid body, is a potent activator of Nox2. In the present study, we tested the hypothesis that ANG II evokes sLTF of the carotid body via Nox2 activation. Experiments were performed on carotid bodies ex vivo from adult rats and mice. Sensory activity was recorded from the carotid sinus nerve. Repetitive (5 times for 30 s each at 5-min intervals), but not continuous (for 150 s), application of 60 pM ANG II evoked robust sLTF of the carotid body. ACh, ATP, substance P, and KCl, when applied repetitively, stimulated the carotid body but did not evoke sLTF. Reactive oxygen species levels increased in response to repetitive applications of ANG II, and this effect was blocked by apocynin, an inhibitor of Nox2, as well as losartan, an angiotensin type 1 (AT1) receptor antagonist. Losartan, apocynin, and 4-(2-aminoethyl)benzenesulfonyl fluoride prevented ANG II-induced sLTF, which was absent in mice deficient in gp91phox, the catalytic subunit of the Nox2 complex. These results demonstrate that repetitive application of ANG II induces sLTF of the carotid body via activation of Nox2 by AT1 receptors.
Keywords: angiotensin type 1 receptors, reactive oxygen species, chronic intermittent hypoxia
hypoxemia augments the sensory activity of the carotid body, and the ensuing reflexes mediate autonomic responses, including stimulation of breathing and increased sympathetic nerve activity. Mammalian carotid bodies express a variety of excitatory modulators, including ACh (17), ATP (16, 19, 20), and substance P (SP) (1). Recent studies reported that carotid bodies also synthesize ANG II via a renin-independent pathway (5). Glomus cells of the carotid body express angiotensin-converting enzyme (6, 7) and angiotensinogen, the precursor of ANG II, as well as angiotensin type 1 (AT1) receptor (AT1R) (6, 7). Although the role of ANG II in “normal” carotid body function remains to be established, it has been suggested that ANG II mediates the exaggerated chemoreflex response to hypoxia in experimental models of congestive heart failure (8).
The carotid body expresses NADPH oxidase 2 (Nox2) (10). We previously reported that activation of Nox2 by chronic intermittent hypoxia (CIH) (10) or by exogenous application of 5-HT (12), a biogenic amine expressed in the glomus cells, evokes a progressive increase in baseline activity of the carotid body, a phenomenon termed sensory long-term facilitation (sLTF). Given that ANG II is a potent activator of Nox2 (2–4, 8), we hypothesized that exogenous application of ANG II evokes sLTF of the carotid body via Nox activation. We tested this possibility on carotid bodies from adult rats and mice deficient in the Nox2 isoform (gp91phox−/Y).
METHODS AND MATERIALS
General preparation of animals.
Experiments were approved by the Institutional Animal Care and Use Committee of the University of Chicago and were performed on adult male rats (Sprague-Dawley, 250–300 g) and mice (wild-type and gp91phox−/Y, 25–30 g; Jackson Laboratories). Rats and mice were anesthetized with urethane (1.2 g/kg ip); 10% of the initial dose of anesthetic was given as a supplement, when necessary, and the animals were allowed to breathe spontaneously. Tracheas were intubated to facilitate breathing, and a femoral vein was cannulated for systemic administration of heparin (150 U/kg) to prevent blood clots in the carotid body. Core body temperature was monitored by a rectal thermistor probe and maintained at 37 ± 1°C by a heating pad. After ligation of the common carotid and internal and external carotid arteries, carotid bodies were removed and placed in ice-cold physiological saline. Subsequently, animals were killed by intracardiac injection (0.1 ml) of euthanasia solution (390 mg of pentobarbital sodium and 50 mg of phenytain sodium per milliliter; Beuthanasia-D Special, Schering-Plough, Kenilworth, NJ).
Measurements of carotid body sensory activity.
The protocol for recording sensory activity from ex vivo carotid bodies was essentially the same as that described previously (12, 13). Briefly, after the connective tissue was cleaned, the carotid body, along with the sinus nerve, was placed in a recording chamber (250 μl volume) and superfused at a rate of 2.5 ml/min with warm (36°C) physiological saline (in mM: 125 NaCl, 5 KCl, 1.8 CaCl2, 2 MgSO4, 1.2 NaH2PO4, 25 NaHCO3, 10 d-glucose, and 5 sucrose). The medium was bubbled with 21% O2-5% CO2. Po2 (141 ± 2.8 Torr), Pco2 (35 ± 3 Torr), and pH (7.36 ± 0.01) were determined by a blood-gas analyzer (model ABL 5, Radiometer, Copenhagen, Denmark). To facilitate recording of clearly identifiable action potentials, the sinus nerve was treated with 0.1% collagenase for 5 min. Action potentials (2–4 active units) were recorded from one of the nerve bundles with a suction electrode, amplified (AC preamplifier, 100–3,000 Hz bandwidth; model P511K, Grass Instrument), displayed on an oscilloscope (model 5B12N, Tektronix), and stored in a computer via an analog-to-digital translation board (PowerLab/8P, ADInstruments). “Single” units were selected on the basis of the height and duration of the individual action potentials using a spike discrimination program (Spike Histogram Program, PowerLab, ADInstruments).
Application of drugs.
Known concentrations of ANG II or other excitatory modulators were added to the reservoirs containing the physiological saline that was used for carotid body irrigation. The reservoirs were connected to a timed-application device that allowed repetitive or continuous application of drugs.
Drugs and chemicals.
Acetovanillone (apocynin), ACh, 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), ATP, ANG II, KCl, and SP were obtained from Sigma; losartan was obtained from Cayman. All solutions were prepared fresh in physiological saline at the time of the experiment.
Measurement of malondialdehyde levels.
Malondialdehyde (MDA) levels were determined in carotid bodies as an index of reactive oxygen species (ROS), as described previously (15). Carotid bodies were pooled (6 carotid bodies per experiment) and homogenized in 10 volumes of 20 mM phosphate buffer (pH 7.4) at 4°C, and the resulting homogenate was centrifuged at 500 g for 10 min at 4°C. One hundred microliters of tissue homogenate or a suitable concentration of MDA standard were incubated with 50 μl of SDS (8.1%), 375 μl of acetic acid (20%), and 375 μl of thiobarbituric acid (0.8%) at 90°C for 60 min and then at 4°C for 10 min. The reaction mixture was centrifuged at 800 g for 15 min, and the relative fluorescence intensity of the resulting supernatant was monitored using excitation and emission wavelengths of 530 and 550 nm, respectively. Data are expressed as nanomoles of MDA formed per milligram of protein.
Experimental protocols.
In series 1, the effects of ANG II on carotid body sensory activity were determined. After baseline sensory activity was recorded for 15 min, the carotid body was challenged with repetitive applications of 60 pM ANG II (5 times for 30 s each at 5-min intervals, n = 6 rats and 11 carotid bodies) or continuous application of 60 or 300 pM ANG II (for 150 s, n = 6 rats and 10–11 carotid bodies each). After termination of either mode of ANG II application, sensory activity was continuously monitored for an additional 60 min.
In series 2, the effects of repetitive applications of ACh (10 μM), ATP (100 μM), SP (1 μM), and KCl (40 mM) on carotid body activity (n = 6–7 rats and 11–13 carotid bodies for each substance) were examined. The protocols were the same as those described for series 1. In a given experiment, the effect of only one substance was examined. The choice of concentrations for the agents tested in series 1 and 2 was based on preliminary studies, wherein the indicated concentration of a given agent reproducibly increased sensory discharge ≥20% above baseline activity.
In series 3, the effects of repetitive applications of ANG II were examined in the presence of vehicle (control) or losartan (3 μM), an AT1R antagonist (n = 6 rats and 11 carotid bodies each).
In series 4, the effects of ANG II on MDA levels were determined in the carotid bodies. Carotid bodies were placed in the recording chamber and superfused with warm physiological saline and challenged with repetitive applications of ANG II. Carotid bodies were collected at minute 60 of the poststimulation period and frozen in liquid nitrogen for later measurements of MDA levels. The protocols were repeated in the presence of losartan (3 μM), an AT1R antagonist, or apocynin (500 μM), an inhibitor of Nox (n = 4 individual experiments, 6 carotid bodies per experiment). In another group of experiments, carotid bodies were challenged with continuous application of 60 pM ANG II (for 150 s). Tissues were taken at minute 5 or 60 of the poststimulation period for measurements of MDA levels. The protocols were repeated in the presence of 3 μM losartan or 500 μM apocynin (n = 4 individual experiments, 6 carotid bodies per experiment).
In series 5, the effects of repetitive application of ANG II on carotid body sensory activity were examined in the presence of vehicle or apocynin or AEBSF (500 μM each), two structurally distinct inhibitors of Nox (n = 7 rats, 14 carotid bodies in each).
In series 6, the effects of repetitive applications of ANG II on carotid body sensory activity were examined in wild-type (WT) and gp91phox−/Y mice (n = 6 mice, 11 carotid bodies each).
Data analysis.
Baseline chemoreceptor activity (“single-unit” discharge) was averaged every 5 min for 15 min. After termination of repetitive or continuous application of drugs, sensory activity was averaged every 5 min for 60 min. Changes in sensory activity are expressed as impulses per second, as well as percentage of baseline values. Values are means ± SE. Two-way repeated-measures ANOVA followed by Holm-Sidak's test (Sigmaplot version 11.0, Systat Software, San Jose, CA) was employed to determine the statistical differences between baseline and post-ANG II stimulation values, as well as between groups, i.e., repetitive vs. continuous, vehicle vs. Nox2 inhibitors or losartan, or WT vs. gp91phox−/Y. In the experiments involving the effects of ACh, ATP, SP, and KCl, statistical significance between baseline and poststimulation values was assessed with one-way repeated-measures ANOVA followed by Holm-Sidak's test. Statistical significance of changes in MDA levels was assessed by unpaired t-test. P < 0.05 was considered significant.
RESULTS
ANG II evokes sLTF of the carotid body.
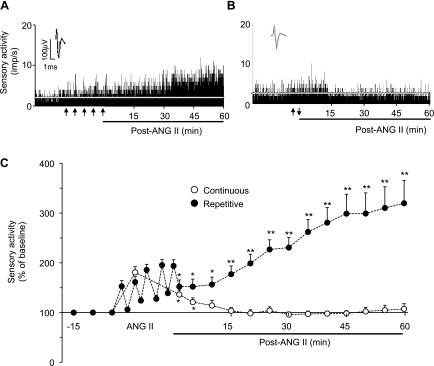
An ex vivo carotid body preparation was employed to test the effects of ANG II to exclude the confounding influence from cardiovascular changes caused by ANG II in intact rats. Repetitive applications of 60 pM ANG II (5 times for 30 s each at 5-min intervals) caused modest, but significant, stimulation of the carotid body with each application (1.8 ± 0.1 and 2.7 ± 0.3 impulses/s for baseline and repetitive application of ANG II, respectively, +53 ± 12%, P < 0.05). After termination of the fifth application of ANG II, baseline activity progressively increased during the ensuing 60 min of the poststimulation period (Fig. 1, A and C). The progressive increase in baseline activity resembled the sLTF of the carotid body reported previously (11, 12).
Fig. 1.
Effect of ANG II on carotid body sensory activity. A and B: sensory responses [impulses (imp)/s] of the carotid body to repetitive (5 times for 30 s each at 5-min intervals, arrows in A) and continuous (for 150 s, arrows in B) applications of 60 pM ANG II. Insets: superimposed action potentials of a “single” fiber from which data were derived. White line represents baseline activity. C: sensory activity. Values are means ± SE from 11 carotid bodies each. Significant difference from baseline: *P < 0.05; **P < 0.01. For clarity, statistical significance is presented only during poststimulation period.
Continuous application of 60 pM ANG II for 150 s also caused a modest stimulation of carotid body activity (Fig. 1B). However, sensory activity returned to baseline within 10 min after termination of the stimulus and remained at this level during the poststimulation period (Fig. 1, B and C). Challenging the carotid bodies with 300 pM ANG II, a concentration five times higher than 60 pM, caused a greater initial sensory excitation (+241 ± 74% increase in baseline), which also returned to baseline within 10 min after termination of the stimulus.
Effects of repetitive application of other excitatory modulators on carotid body activity.
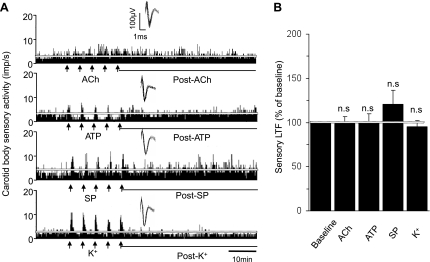
The effects of ACh, ATP, and SP, as well as KCl, a nonselective depolarizing agent, were examined to determine whether repetitive application of excitatory modulators other than ANG II also evokes sLTF of the carotid body. ACh, SP, and KCl stimulated carotid body activity during each application, whereas ATP caused an initial excitation followed by depression (Fig. 2A). After termination of the repetitive applications of each of these substances, sensory activity promptly returned to baseline values and remained at these levels in the poststimulus period (Fig. 2).
Fig. 2.
Effects of repetitive applications of excitatory modulators on carotid body sensory activity. A: carotid body sensory responses to repetitive applications (5 times for 30 s each at 5-min intervals at arrows) of ACh (10 μM), ATP (100 μM), substance P (SP, 1 μM), and KCl (40 mM). Insets: superimposed action potentials of a single fiber from which data were derived. White line represents baseline activity. B: average data of the sensory long-term facilitation (LTF; sensory activity averaged over the entire 60 min of the postapplication period). Values are means ± SE; n = 13 for ACh and ATP, n = 12 for SP, and n = 11 for KCl. NS, P > 0.05 compared with prestimulation baseline activity.
AT1Rs mediate ANG II-induced sLTF.
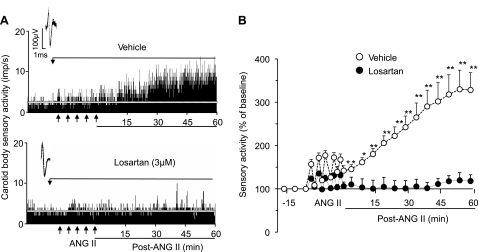
To determine the role of AT1Rs in ANG II-evoked sLTF, the effects of repetitive applications of ANG II were determined in the presence of 3 μM losartan, an established AT1R antagonist, or vehicle (control). In the presence of losartan, ANG II did not induce sLTF (2.0 ± 0.1 and 2.3 ± 0.3 impulses/s at baseline and at the end of 60 min of repetitive application of ANG II, respectively, P > 0.05), whereas sLTF was elicited in the presence of vehicle (1.6 ± 0.1 and 5.2 ± 0.6 impulses/s at baseline and during 60 min of repetitive application of ANG II, respectively, P < 0.01; Fig. 3).
Fig. 3.
Effect of losartan on ANG II-evoked carotid body sensory LTF (sLTF). A: carotid body sensory response to repetitive application of 60 pM ANG II (5 times for 30 s each at 5-min intervals at arrows) in the presence of vehicle (top) and 3 μM losartan (bottom). Insets: superimposed action potentials of a single fiber from which data were derived. White line represents baseline activity. B: sensory activity. Values are means ± SE from 11 carotid bodies each of vehicle and losartan. Statistical significance: *P < 0.05; **P < 0.01 vs. baseline activity. For clarity, statistical significance is presented only during poststimulation period.
ANG II activates Nox2 in the carotid body.
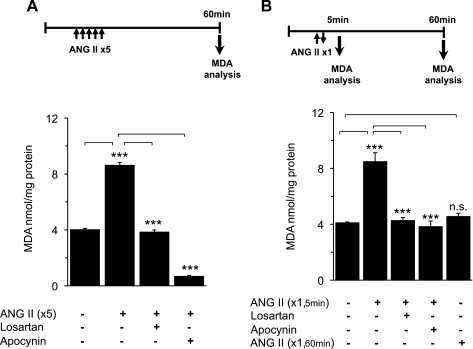
Carotid bodies were challenged with repetitive applications of ANG II, and MDA levels were determined as an index of ROS generation (15). MDA levels were determined at the end of 60 min of the poststimulation period. MDA levels were nearly twofold higher in carotid bodies challenged with repetitive application of ANG II than in controls, and this increase in MDA levels was absent with combined application of ANG II and losartan (3 μM), an AT1R antagonist, or apocynin (500 μM), a Nox2 inhibitor (Fig. 4A).
Fig. 4.
Effect of ANG II on reactive oxygen species (ROS) levels in the carotid body. Malondialdehyde (MDA) levels were analyzed in carotid body extracts as an index of ROS. Top: protocols for MDA analysis with repetitive and continuous applications of ANG II. Analysis of MDA at the end of 60-min superfusion without ANG II served as controls. A: carotid bodies were challenged with repetitive application of 60 pM ANG II (5 times for 30 s each at 5-min intervals), and MDA levels were determined at minute 60 of the poststimulation period. Protocols were repeated in the presence of 3 μM losartan, an angiotensin type 1 (AT1) receptor antagonist, or 500 μM apocynin, an inhibitor of NADPH oxidase 2 (Nox2). B: effect of continuous application of 60 pM ANG II for 150 s on MDA levels in the carotid body. MDA levels were determined at 5 min (ANG II ×1, 5 min) or 60 min (ANG II ×1, 60 min). MDA levels were elevated when measured at 5 min, but not 60 min, after termination of continuous application of ANG II and losartan (3 μM) or apocynin (500 μM) blocked this effect. Values are means ± SE from 4 individual experiments performed in triplicate. ***P < 0.001. NS, P > 0.05 compared with vehicle-treated controls.
The effects of continuous application of 60 pM ANG II on MDA levels were determined. MDA levels were determined at 5 or 60 min poststimulation. When measured at 5 min poststimulation, MDA levels were significantly elevated, and this effect was blocked by 3 μM losartan, as well as by 500 μM apocynin. On the other hand, when measured at 60 min of poststimulation, MDA levels were nearly the same as controls (Fig. 4B).
Nox2 is required for ANG II-induced sLTF of the carotid body.
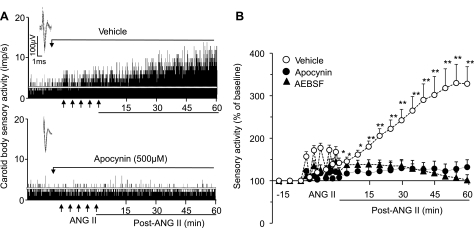
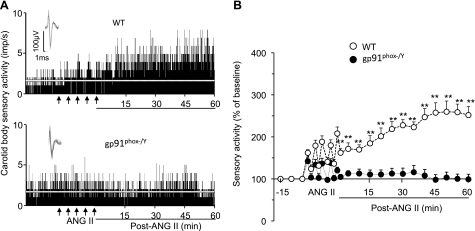
The effects of apocynin and AEBSF, two structurally distinct inhibitors of Nox2, on ANG II-induced sLTF were examined. Repetitive applications of ANG II induced sLTF in the presence of vehicle, and this effect was absent in the presence of apocynin (500 μM) or AEBSF (500 μM; Fig. 5). To further establish the involvement of Nox2, a genetic approach was employed. To this end, the effects of repetitive applications of ANG II were examined on carotid bodies from WT mice and mice deficient in gp91phox, the catalytic subunit of Nox2 (gp91phox−/Y). Effects of ANG II on carotid body activity from both genotypes of mice and the average data are shown in Fig. 6. Repetitive application of ANG II evoked sLTF of the carotid body in WT mice, and this effect was absent in gp91phox−/Y mice.
Fig. 5.
NADPH oxidase inhibitors prevent ANG II-induced sLTF of the carotid body. A: sensory responses of the carotid body to repetitive application of 60 pM ANG II (5 times for 30 s each at 5-min intervals at arrows) in the presence of vehicle (top) and apocynin (bottom). Insets: superimposed action potentials of a single fiber from which data were derived. White line represents baseline activity. B: sensory activity in response to repetitive applications of ANG II in the presence of vehicle, apocynin (Apo, 500 μM), or 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF, 500 μM). Values are means ± SE; n = 14 carotid bodies in each group. Statistical significance: *P < 0.05; **P < 0.01 compared with baseline activity. For clarity, statistical significance is presented only during poststimulation period.
Fig. 6.
Absence of ANG II-induced sLTF in mice deficient in gp91phox. A: carotid body sensory response to repetitive applications of 60 pM ANG II (5 times for 30 s each at 5-min intervals at arrows) in wild-type (WT; top) and gp91phox−/Y (bottom) mice. Insets: superimposed action potentials of a single fiber from which data were derived. White line represents baseline activity. B: sensory activity in response to repetitive applications of ANG II in both genotypes. Values are means ± SE; n = 11 carotid bodies each of WT and gp91phox−/Y. **P < 0.01 compared with WT. For clarity, statistical significance is presented only during the poststimulation period.
DISCUSSION
The results of the present study demonstrate that repetitive application of ANG II induces sLTF of rat and mouse carotid bodies. ANG II-induced sLTF is unlikely due to vascular changes, because it was seen in superfused carotid bodies, wherein vascular influences on sensory activity are effectively absent. Previously, we reported that repetitive applications of 5-HT (12) also induce sLTF when given in nanomolar doses. However, current findings demonstrate that picomolar concentrations of ANG II are adequate to evoke sLTF, suggesting that ANG II is a more potent inducer of sLTF than 5-HT.
How might ANG II induce sLTF of the carotid body? Previous studies have shown that activation of Nox2 and the ensuing generation of ROS are critical for evoking sLTF of the carotid body (10, 12). Given that ANG II is a potent activator of Nox2 in the carotid body (8), as well as in other tissues (2–4), we were prompted to hypothesize that Nox2/ROS signaling mediates ANG II-induced sLTF of the carotid body. To test this possibility, we determined the effects of ANG II on MDA levels in the carotid body as an index of ROS (13, 15) in the presence of the Nox 2 inhibitor (18). This approach allowed us to simultaneously determine the effects of ANG II on ROS, as well as the Nox2 contribution to ROS generation, in a given tissue sample. The following observations demonstrate that Nox2/ROS signaling mediates ANG II-induced sLTF. 1) Repetitive application of ANG II produced a long-lasting increase in carotid body ROS levels, as evidenced by elevated MDA levels measured 60 min after termination of the stimulus. 2) A Nox inhibitor prevented the ANG II-induced increase in ROS, as well as sLTF. 3) ANG II-induced sLTF was absent in mice deficient in gp91phox, the catalytic subunit of Nox2. Our results further suggest that ANG II-induced Nox activation and sLTF require AT1Rs.
Interestingly, continuous application of 60 pM ANG II (i.e., the concentration used for repetitive application) did not induce sLTF. The inability of continuous application to evoke sLTF is unlikely due to the concentration of ANG II, because continuous application of 300 pM, which is five times higher than 60 pM, was equally ineffective in evoking sLTF. However, unlike repetitive applications, continuous application of ANG II caused a transient increase in ROS, as evidenced by elevated MDA levels at minute 5, but not minute 60, of the poststimulation period. This increase in ROS levels is mediated by AT1R-induced Nox activation, as they could be prevented by losartan and apocynin. It follows that the absence of sLTF in response to continuous application of ANG II is due to its inability to produce long-lasting Nox activation and the ensuing ROS generation. While these observations suggest that the mode of ANG II application is critical for evoking sLTF, the mechanisms underlying long-lasting vs. transient Nox activation produced by repetitive and continuous application of ANG II, respectively, require further investigation.
Repetitive application of other excitatory modulators of the carotid body, such as ACh (17, 19), ATP (16, 19, 20), and SP (1), was also ineffective in evoking sLTF. The absence of sLTF by these modulators is unlikely due to inadequate concentrations, because, at the doses tested, all these modulators stimulated carotid bodies, albeit to different degrees. Furthermore, KCl, a nonselective depolarizing agent, which caused robust carotid body stimulation, was equally ineffective in evoking sLTF. The absence of sLTF after exposure to these excitatory stimuli is likely due to lack of Nox2 activation, a possibility that requires further investigation. Nonetheless, these observations suggest that 1) substances that produce carotid body stimulation need not necessarily induce sLTF and 2) the induction of sLTF is unique to certain modulators such as ANG II, as shown in the present study, as well as 5-HT, as reported previously (12), and they do so by generating ROS via Nox activation.
What might be the significance of ANG II-induced sLTF of the carotid body? We previously reported that CIH induces sLTF of the carotid body in rats and mice via 5-HT-mediated Nox2 activation and subsequent generation of ROS (10, 11, 13). It was proposed that sLTF mediates persistent reflex activation of the sympathetic nervous system in CIH-exposed rodents (14). A recent study reported that CIH-exposed rats exhibit augmented excitation of the sympathetic nervous system, which involves heightened carotid body chemoreflex, and blockade of AT1Rs prevents sympathetic excitation (9). The findings of this previous study (9), taken together with the present results, raise the possibility that CIH-induced sLTF of the carotid body requires interaction between ANG II and 5-HT, which in turn contributes to the persistent activation of the sympathetic nervous system.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-76537 and HL-90554.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Cragg PA, Runold M, Kou YR, Prabhakar NR. Tachykinin antagonists in carotid body responses to hypoxia and substance P in the rat. Respir Physiol 95: 295–310, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol 302: 148–158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Germano G, Sanguigni V, Pignatelli P, Caccese D, Lenti L, Ragazzo M, Lauro R, Violi F. Enhanced platelet release of superoxide anion in systemic hypertension: role of AT1 receptors. J Hypertens 22: 1151–1156, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal 4: 899–914, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Lam SY, Leung PS. A locally generated angiotensin system in rat carotid body. Regul Pept 107: 97–103, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Lam SY, Leung PS. Chronic hypoxia activates a local angiotensin-generating system in rat carotid body. Mol Cell Endocrinol 203: 147–153, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Leung PS, Lam SY, Fung ML. Chronic hypoxia upregulates the expression and function of AT1 receptor in rat carotid body. J Endocrinol 167: 517–524, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Li YL, Gao L, Zucker IH, Schultz HD. NADPH oxidase-derived superoxide anion mediates angiotensin II-enhanced carotid body chemoreceptor sensitivity in heart failure rabbits. Cardiovasc Res 75: 546–554, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol 171: 36–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci 29: 4903–4910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol 576: 289–295, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prabhakar NR, Peng YJ, Kumar GK, Pawar A. Altered carotid body function by intermittent hypoxia in neonates and adults: relevance to recurrent apneas. Respir Physiol Neurobiol 157: 148–153, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Raghuraman G, Kalari A, Dhingra R, Prabhakar NR, Kumar GK. Enhanced neuropeptide Y synthesis during intermittent hypoxia in the rat adrenal medulla: role of reactive oxygen species-dependent alterations in precursor peptide processing. Antioxid Redox Signal 14: 1179–1190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci 23: 11315–11321, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shirahata M, Balbir A, Otsubo T, Fitzgerald RS. Role of acetylcholine in neurotransmission of the carotid body. Respir Physiol Neurobiol 157: 93–105, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Suzuki Y, Wang W, Vu TH, Raffin TA. Effect of NADPH oxidase inhibition on endothelial cell ELAM-1 mRNA expression. Biochem Biophys Res Commun 184: 1339–1343, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Varas R, Alcayaga J, Iturriaga R. ACh and ATP mediate excitatory transmission in cat carotid identified chemoreceptor units in vitro. Brain Res 988: 154–163, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signaling at rat carotid body chemoreceptors. J Physiol 525: 143–158, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]