Abstract
The incidence of tendon degeneration and rupture increases with advancing age. The mechanisms underlying this increased risk remain unknown but may arise because of age-related changes in tendon mechanical properties and structure. Our purpose was to determine the effect of aging on tendon mechanical properties and collagen fibril morphology. Regional mechanical properties and collagen fibril characteristics were determined along the length of tibialis anterior (TA) tendons from adult (8- to 12-mo-old) and old (28- to 30-mo-old) mice. Tangent modulus of all regions along the tendons increased in old age, but the increase was substantially greater in the proximal region adjacent to the muscle than in the rest of the tendon. Overall end-to-end modulus increased with old age at maximum tendon strain (799 ± 157 vs. 1,419 ± 91 MPa) and at physiologically relevant strain (377 ± 137 vs. 798 ± 104 MPa). Despite the dramatic changes in tendon mechanical properties from adulthood to old age, collagen fibril morphology and packing fraction remained relatively constant in all tendon regions examined. Since tendon properties are influenced by their external loading environment, we also examined the effect of aging on TA muscle contractile properties. Maximum isometric force did not differ between the age groups. We conclude that TA tendons stiffen in a region-dependent manner throughout the life span, but the changes in mechanical properties are not accompanied by corresponding changes in collagen fibril morphology or force-generating capacity of the TA muscle.
Keywords: tendon mechanics, tangent modulus, contractile properties, collagen fibril diameter
tendons serve as intermediates between muscle and bone, transferring force produced by contractile elements of muscle to the skeleton and allowing for movement. A tendon's ability to perform this functional role is compromised in old age, contributing to the high incidence of frailty and impaired locomotion in the elderly population (16, 21). The age-associated increase in the incidence of tendinopathy has been implicated as one contributor to this decline in function. At least one-third of persons >70 yr old present with some degree of tendon degeneration or tearing (28, 33). In addition to the high incidence of documented tendon degeneration, tendinopathy is often asymptomatic and increases the risk of tendon rupture (22). This increased risk is of great consequence in the elderly population, where tendon rupture can result from a minor slip or fall (29).
The mechanisms underlying the increased risk of tendon degeneration and rupture with old age are unknown but may arise because of age-associated changes in tendon mechanical properties (8). Furthermore, tendon mechanical properties are governed by the characteristics of its underlying structural components, but data correlating mechanical properties with underlying structure in tendons of old animals are scant. Even attempts to develop structure-function relationships in tendons from younger animals have been inconclusive. Derwin and Soslowsky (13) found a positive correlation between collagen fibril diameter and tendon stiffness, but others failed to demonstrate such correlations (18, 25). These inconsistencies may arise because of the mechanical and structural heterogeneity of tendons. The tibialis anterior (TA) tendon of healthy adult rats has a compliant region near the muscle and then gradually becomes stiffer toward the bone insertion (1). Additionally, anterior fascicles from human patellar tendons are stronger and stiffer than posterior fascicles (18, 20). Collagen characteristics of tendons are region-dependent as well, with rabbit patellar tendons showing local variations in collagen fibril diameter and area fraction (36) and chicken gastrocnemius tendons displaying regional differences in collagen content (11).
The local dependency of tendon properties coupled with conflicting results in the literature and a lack of data on the effects of age has made it difficult to develop clear structure-function relationships that can be applied to aging tendon. Further complexities arise since the external loading environment also influences tendon mechanical properties (6, 23). Significant reductions in muscle force output with aging are well documented in many muscles (4, 5, 12), but the adaptive response of tendons to those age-associated changes remains unclear. Previous studies have demonstrated decreases in tendon stiffness following unloading due to prolonged bed rest (30) or spinal cord injury (27). Conversely, our group showed that decreased muscle activity associated with denervation results in tendon stiffening that is most pronounced in the region nearest the muscle (1). Therefore, the goals of this work were to examine how regional TA tendon mechanics are related to collagen fibril morphology and TA muscle contractile properties in adult and old mice. Our working hypothesis is that decreased force generation with aging will lead to stiffening of the tendon, with the region nearest the muscle most affected. We tested the specific hypotheses that maximum isometric force of TA muscles will be lower and the stiffness of TA tendons will be greater in old than adult mice, primarily because of changes in the region of the tendon nearest the muscle, and that regional mechanical properties will correlate with collagen fibril diameter and packing fraction within individual tendons and between age groups.
METHODS
Animals.
Twenty 8- to 12-mo-old and eleven 28- to 30-mo-old C57BL/6 male mice were obtained from Charles River Laboratories (Wilmington, MA). Body mass was 34.6 ± 2.5 and 32.9 ± 3.7 (SD) g for the 8- to 12-mo-old and 28- to 30-mo-old mice, respectively (P > 0.4). Mice were randomly assigned to groups for testing tendon mechanics or muscle contractile properties or for electron microscopy. Tendon mechanical properties were determined for 10 tendons from adult mice and 5 tendons from old mice. TA muscle contractile properties were determined for eight adult muscles and six old muscles. Three tendons per age group were examined with electron microscopy. Animals were housed in a specific-pathogen-free barrier facility in the Unit for Laboratory Animal Medicine at the University of Michigan, with food and water provided ad libitum. All procedures were approved by the University of Michigan Committee for the Use and Care of Animals.
Determination of tendon mechanical properties.
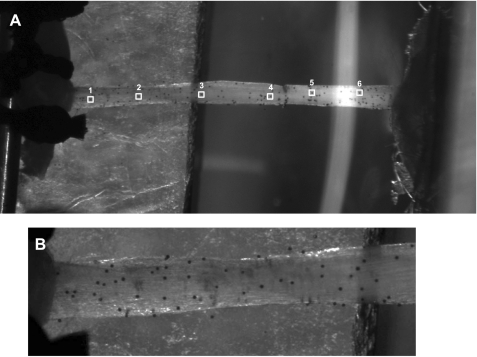
Mice were anesthetized with an intraperitoneal injection of tribromoethanol, which was supplemented as necessary during the procedure. The TA muscle-tendon unit (TA muscle, TA tendon, and 1st metatarsal) was removed from one hindlimb, and overlying connective tissue was carefully removed. Samples were stored in sterile PBS at 4°C until testing, which was within 3 h of tissue removal. The stress-strain response of each tendon was determined using a previously validated testing setup (24) with modified grips to accommodate the small size of the mouse TA muscle-tendon unit. The TA muscle-tendon unit was placed in a room temperature PBS bath, and the TA muscle and first metatarsal were secured to custom grips, so that the tendon was free-standing (Fig. 1A). Tendon cross-sectional area (CSA) was determined by optically measuring diameter at six equally spaced points along the tendon at 0° and 90° rotation using a calibrated microscope eyepiece and fitting the values to an ellipse (Table 1). Polystyrene beads (25 μm; IMT Laboratories, Irvine, CA) were brushed along the length of the tendon to serve as optical markers for strain measurements (Fig. 1B). The tendon was stretched to a preload of ∼0.02 N, which served as the zero point. The sample was then subjected to a load-unload cycle of 10% grip-to-grip strain at a constant strain rate of 0.01/s. Since preconditioning can shift the stress-strain response of collagenous tissue (20, 32), no preconditioning protocol was used. Synchronized force and image recordings were obtained and compiled using LabVIEW (Austin, TX). Bead positions were tracked using MetaMorph software (Molecular Devices, Sunnyvale, FL), and nominal strains in the proximal (near the muscle), central, and distal (near the bone) tendon regions were calculated as the change in separation between two beads in each region divided by their initial separation. Regional nominal stress was determined by dividing raw load data by the local CSA. Overall tendon strain was calculated using beads that were positioned one tendon diameter length proximal to the bone insertion and one diameter length distal to the muscle insertion (Fig. 1A). This bead positioning avoided any strain concentrations created by the grips. Overall tendon stress was calculated using average tendon CSA. Stress-strain data were fit to a third-degree polynomial, and maximum tangent modulus of each tendon region was calculated as the maximum slope of the curve. Generally, the polynomials fit the raw mechanics data very well. Fits were poorest in the distal tendon region (average r2 = 0.756). Fits in the remaining regions were similar to each other (average r2 = 0.935). Tangent modulus served as our measure of tendon stiffness.
Fig. 1.
Mouse tibialis anterior (TA) muscle-tendon unit positioned in mechanical test fixture. A: typical measurement regions along the tendon. Mechanical properties were determined at the distal (points 1 and 2), central (points 3 and 4), and proximal (points 5 and 6) tendon regions. Overall tendon response was also measured (points 1 and 6). Samples were loaded to 10% grip-to-grip strain at a rate of 0.01/s. B: close-up of distal region of the tendon showing distribution of microspheres used for optical determination of surface strains. Contrast has been increased in A and B.
Table 1.
TA tendon morphological properties from adult and old C57BL/6 mice
| Adult (n = 10) | Old (n = 5) | |
|---|---|---|
| Length, mm | 6.90 ± 0.19 | 6.94 ± 0.21 |
| CSA, mm2 | ||
| Distal | 0.048 ± 0.011 | 0.050 ± 0.011* |
| Central | 0.078 ± 0.008 | 0.084 ± 0.014* |
| Proximal | 0.099 ± 0.010 | 0.098 ± 0.010* |
| Average | 0.074 ± 0.009 | 0.077 ± 0.009 |
Values are means ± SD. TA, tibialis anterior; CSA, cross-sectional area.
Significantly different from adult (P < 0.05).
TA muscle contractile properties.
The distal tendon of the TA muscle was exposed by an incision at the ankle of anesthetized mice. The tendon was cut several millimeters distal to the end of the muscle. The tendon was tied with 4.0 nylon suture as close to the muscle attachment as possible, and the tendon was folded back onto itself and tied again. The mouse was placed on a heated platform maintained at 37°C, and the hindlimb was secured by pinning the distal femur and clamping the foot to the platform. The tendon was tied securely to the lever arm of a servomotor (model 305B, Aurora Scientific, Richmond Hill, ON, Canada). The tendon and exposed muscle were kept moist by periodic applications of isotonic saline. The TA muscle was stimulated with 0.2-ms pulses (model S88, Grass) via two needle electrodes that penetrated the skin on either side of the peroneal nerve near the knee. Stimulation voltage and, subsequently, muscle length (Lo) were adjusted for maximum isometric twitch force. While held at Lo, the muscle was stimulated at increasing frequencies, step-wise from 150 Hz by 50 Hz, until a maximum force was reached, typically at 250 Hz. A 1- to 2-min rest period was allowed between each tetanic contraction. On the basis of well-defined anatomic landmarks near the knee and the ankle, muscle length was measured with calipers. Optimum fiber length (Lf) was determined by multiplying Lo by the TA Lf-to-Lo ratio of 0.6 (7).
Transmission electron microscopy.
One TA tendon was removed from three anesthetized mice in each age group. Immediately after excision, tendons were fixed in primary fixative (2.5% glutaraldehyde in 0.1 M Sorensen's buffer), stored at 4°C overnight, and postfixed (1.0% osmium tetroxide in 0.1 M Sorensen's buffer). Samples were then rinsed with Sorensen's buffer, dehydrated in ascending strengths of ethanol, cleared in propylene oxide, and embedded in Epon according to standard procedures. A Reichert-Jung Ultracut E microtome was used to take two transverse sections, one ∼1.5 mm proximal to the bone insertion and one ∼1.5 mm distal to the muscle insertion, from each tendon. Sections were stained with uranyl acetate and lead citrate and viewed using a transmission electron microscope (model CM 100, Philips) at an accelerating voltage of 60 kV. Ten random micrographs were obtained from each section (×64,000 magnification, 2.97 μm2 each). The six tendons thus yielded a total of 120 images. The major and minor axes of all complete fibrils in each micrograph were measured using ImageJ software (National Institutes of Health). Fibril diameter was defined as the major axis for each fibril. Fibril area was calculated by using each fibril's major and minor axes and fitting to an ellipse. Packing fraction was defined as the proportion of area in each micrograph occupied by collagen fibrils. Data from all 10 images from each section were combined and considered representative of the fibril profile for that tendon region. All measurements were performed by a single, blinded investigator.
Statistics.
Values are means ± SD. Statistical analyses were performed using SigmaPlot software (San Jose, CA). Differences in mean values for tendon regional mechanical and collagen fibril data were determined using a two-way ANOVA, with age and tendon region as independent factors. Differences in mean values for muscle contractility data were determined using a Student's t-test. Significance was set at P < 0.05.
RESULTS
Tendon morphology.
Within each age group, tendon CSA gradually increased from the distal bone insertion to the muscle, but no age-associated changes were observed in regional tendon CSA (Table 1). Similarly, tendon length remained constant with age.
Regional stress-strain responses.
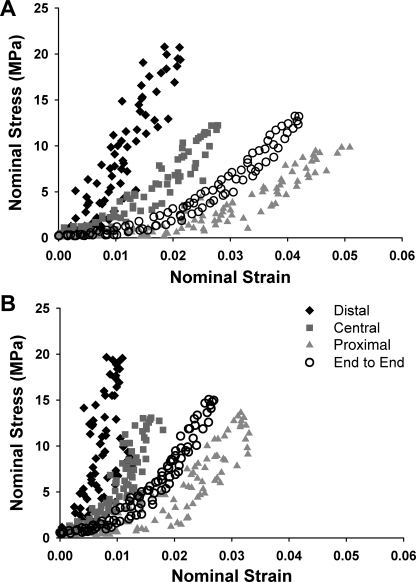
Tendons from adult (Fig. 2A) and old (Fig. 2B) mice show regional variations in their stress-strain responses. For both age groups, strains were smaller and stresses were larger in the distal end than in the other tendon regions. In addition, end-to-end tendon responses were different from any of the individual regional responses. In adult mice, the proximal region of tendons displayed a large toe region that was sharply truncated for tendons of old mice. Maximum strains also decreased with old age in the proximal tendon region (P = 0.020). Finally, for the end-to-end response of tendons of old mice, maximum strains were substantially decreased (P = 0.018), and toe regions were diminished compared with those of adult mice.
Fig. 2.
Representative stress-strain responses of TA tendons from adult (A) and old (B) mice undergoing a 10% grip-to-grip strain load-unload cycle. In both age groups, tendon mechanical response is regionally dependent, with distal region showing greater stresses and lower strains than central and proximal regions. Also, overall tendon response (○) differs significantly from any of the regional responses. Maximum strains in central and proximal tendon regions diminish in old age, leading to a stiffer tendon overall.
Regional differences in tangent modulus.
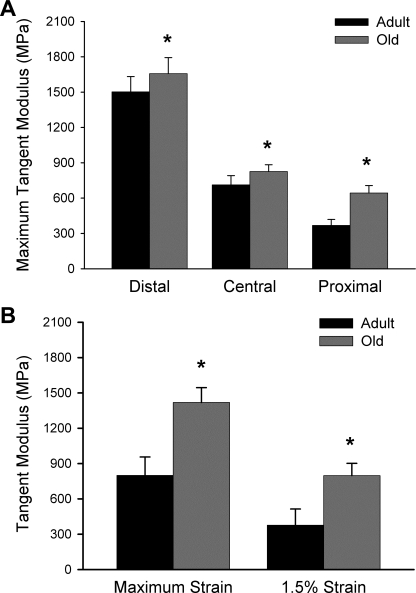
Maximum tangent modulus varied dramatically along the length of TA tendons, displaying an increase of approximately fourfold between the proximal and distal regions in adult mice (Fig. 3A). In all tendon regions, modulus increased significantly with old age, but the increase was not uniform; rather, it was substantially more pronounced in the proximal tendon than in the other regions. The nonuniformity of the changes with aging along the tendons resulted in a loss of heterogeneity in mechanical properties, such that the range in modulus values between the proximal and distal ends was only ∼2.5-fold for old mice. Tangent modulus determined from the overall end-to-end stress-strain responses was also greater for tendons of old than adult mice at maximum tendon strain (799 ± 157 vs. 1,419 ± 91 MPa, P < 0.001) and 1.5% tendon strain (377 ± 137 MPa vs. 798 ± 104 MPa, P < 0.001; Fig. 3B).
Fig. 3.
Effect of aging on regional (A) and overall (B) tangent modulus of mouse TA tendons. In both age groups, maximum tendon tangent modulus was greatest in the distal region and smallest in the proximal region of the sample. Tendons from old mice showed increased modulus values among all tendon regions compared with adult mice. End-to-end tangent modulus also increased with old age at maximum tendon strain and at 1.5% tendon strain. Values are means ± SD. *Statistical difference between age groups at P = 0.05.
Collagen fibril distribution.
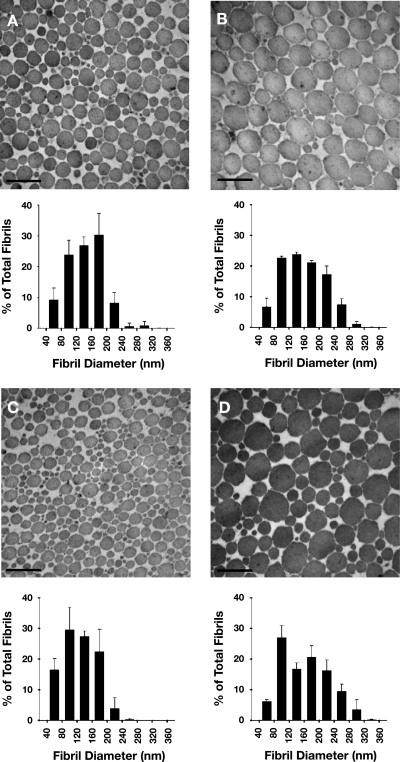
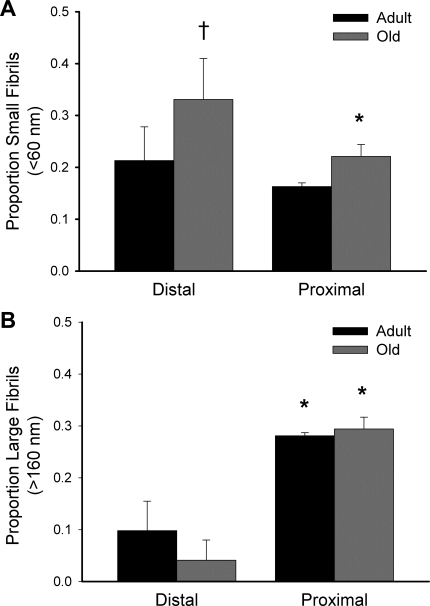
Representative transmission electron microscopic images and tendon collagen fibril diameter distributions are shown in Fig. 4. Regional dependency of fibril density was only observed in tendons from old mice, where fibril density was greater in the distal than proximal region (1,027 ± 205 vs. 490 ± 67 fibrils/μm2, P = 0.030; Table 2). In contrast to the fibril density, fibril packing fraction was greater in the proximal than distal region for tendons of both age groups [0.90 ± 0.03 vs. 0.79 ± 0.03 for adult (P = 0.046) and 0.88 ± 0.03 vs. 0.76 ± 0.09 for old (P = 0.022)], but no effect of age was observed (Table 2). Mean fibril diameter was similar across all age groups and tendon regions. Despite no difference in mean fibril diameter between tendon regions or between tendons of adult and old mice, the proportion of small-diameter (<60 nm) fibrils in tendons of old mice was greater in the distal (0.311 ± 0.079) than proximal (0.211 ± 0.023) region of the same tendons (P = 0.033) and the distal region of tendons from adult mice (0.213 ± 0.065, P = 0.025; Fig. 5A). For both age groups, the proportion of large-diameter (>160 nm) fibrils was greater in the proximal than distal region [0.281 ± 0.006 vs. 0.098 ± 0.057 in adult (P < 0.001) and 0.294 ± 0.023 vs. 0.041 ± 0.039 in old (P < 0.001)], but no changes were seen with aging (Fig. 5B).
Fig. 4.
Representative electron micrographs and diameter distributions of collagen fibrils within distal (A and C) and proximal (B and D) regions of TA tendons of adult (A and B) and old (C and D) mice. In both age groups, diameters of fibrils tended to be larger in the proximal than distal region. Original magnification, ×64,000. Scale bar, 300 nm.
Table 2.
Summary of TA tendon collagen fibril properties
| Fiber Density, no./μm2 |
Fibril Diameter, nm |
Packing Fraction |
||||
|---|---|---|---|---|---|---|
| Distal | Proximal | Distal | Proximal | Distal | Proximal | |
| Adult | 775 ± 200 | 531 ± 48 | 102.2 ± 45.4 | 122.7 ± 56.1 | 0.79 ± 0.03 | 0.90 ± 0.03* |
| Old | 1,027 ± 205 | 490 ± 67* | 86.7 ± 41.8 | 122.5 ± 62.0 | 0.76 ± 0.09 | 0.88 ± 0.03* |
Values are means ± SD.
Significantly different from distal within age group (P < 0.05).
Fig. 5.
Proportion of small (A) and large (B) collagen fibrils in TA tendons. Proportion of large fibrils was significantly greater in the proximal than distal region for both age groups. The only age-related change was an increase in proportion of small fibrils in the distal tendon region. Values are means ± SD. *Significantly different from distal within age group (P < 0.05). †Statistically different from adult within tendon region (P < 0.05).
TA muscle contractile properties.
TA muscle mass decreased by ∼30% between adulthood and old age (P = 0.003; Table 3). Despite the substantial TA muscle atrophy, maximum isometric force was not different between the age groups (Table 3). The maintenance of force-generating capability with age was due in part to architectural changes within the muscle. A decrease in fiber length of ∼15% for muscles of old compared with adult mice (P = 0.014) partially compensated for the decreased mass to maintain force levels.
Table 3.
TA muscle morphological and contractile properties from adult and old C57BL/6 mice
| Adult (n = 8) | Old (n = 6) | |
|---|---|---|
| Mass, mg | 56.7 ± 5.0 | 43.6 ± 7.6* |
| Lf, mm | 9.36 ± 0.49 | 8.27 ± 0.74* |
| Po, mN | 1,464 ± 236 | 1,371 ± 238 |
Values are means ± SD. Lf, optimal fiber length; Po, maximum isometric force.
Significantly different from adult (P = 0.05).
DISCUSSION
The major finding of the present study was that TA tendons of mice display regionally dependent changes with aging in mechanical properties. We showed that the tangent modulus of TA tendons increased sharply for tendons of old compared with adult mice, with the most pronounced increase in the proximal region of the tendon nearest the muscle. Also significant was the finding that the dramatic mechanical changes were not accompanied by major changes in collagen fibril morphology. Moreover, the maximum force-generating capacity of TA muscles remained relatively constant throughout the age range studied. These results indicate that aging has a considerable effect on the mechanical behavior of TA tendons, which is independent of collagen morphology or skeletal muscle contractile force.
Data in the literature regarding the impact of aging on tendon mechanical properties are inconsistent. The modulus of rat tail tendons has been reported to increase (34) and remain constant (17, 35) from young adulthood to old age. Additionally, some studies show that the modulus of human patellar tendons decreases with advancing age (2), but others failed to show such declines (8, 10, 14). The discrepancies likely arise in part from methodological differences in the procedures used for mechanical testing. When tendons are severed from their bone and muscle attachments and gripped directly, collagen fibril slippage and stress-strain concentrations can be introduced at the grip interfaces. These artifacts can result in measured mechanical properties that are underestimated by up to 50% (20, 37). Further disagreements in the literature may be due to difficulties in comparing results from in vivo and in vitro experiments. In vivo testing requires that the tendon be loaded by the subject's own muscle contraction, a situation under which the rate of tendon loading cannot be easily controlled. Tendon is a viscoelastic material, with mechanics that are dependent on the rate of loading (9, 31); as such, any inconsistency in that variable has the opportunity to affect the measured mechanical properties. By keeping the muscle-tendon unit intact and controlling the rate of tendon loading, the testing method presented in this study prevents damage to the tendon being tested and creates uniformity across age groups in parameters that are important for determination of consistent mechanical properties in the tissue.
The present data corroborate the findings of Arruda and colleagues (1) that the TA tendon is functionally graded, with a compliant proximal region and a substantially stiffer region toward the bone insertion. Regional variations in mechanical properties of the patellar tendon have also been reported (18, 20). Thus tendons should be considered heterogeneous in mechanics studies, and the midtendon mechanical response typically will not represent the response of the tendon as a whole (present study; 1). Moreover, global mechanical measurements mask the complicated regional behavior of the tissue (1). This study expands on the work of Arruda and colleagues by establishing that the tangent modulus of TA tendons increases dramatically with advanced age. The observation that the increases were more pronounced in the proximal tendon region with age also demonstrates that the effects of aging cannot be assumed to be uniform across all tendon tissues. Furthermore, prominent increases in tangent modulus were observed at physiologically relevant strain levels. In vivo data suggest that human TA tendons experience strains between 0% and 2.5% during normal physiological movement (26). In the present study, tendons from old mice showed twofold larger modulus than tendons from younger animals within that strain range. Therefore, a complete characterization of changes in tendon mechanical properties with aging cannot be determined unless regional properties and various strain levels are examined.
Our data demonstrate that, despite a significant age-associated increase in tangent modulus for all tendon regions, fibril morphology remains relatively constant from adulthood to old age. Furthermore, fibril packing fraction was significantly greater in the proximal than distal tendon region, indicating that a larger volume of collagen did not lead to increased mechanical properties. The lack of an association between fibril morphology and mechanical properties is contrary to studies that have established a positive correlation between tendon mechanical properties and collagen fibril diameter (13) and packing fraction (17). These correlations, however, were generally established in tendons from young animals. As such, the observed correlations may be coincident with additional changes associated with biological development and maturation. Furthermore, if it is assumed that collagen fibrils can be considered as cylindrical rods, the tangent modulus of the fibril should be independent of the fibril's diameter from a purely mechanical standpoint (15). Tangent modulus is dependent on the properties of the fibril itself and is independent of the amount of material present. In contrast, an increased diameter of the fibril will affect its bending stiffness (15), which may be of consequence at low strain levels, where the mechanical response to the strain is associated with the uncrimping of collagen fibrils (19). A comprehensive study of low-strain tendon mechanical properties coupled with structural analysis would be required to address the hypothesis that fibril diameter influences crimp behavior.
Tendons are responsible for transferring the force produced by the muscle to the skeleton with high fidelity while avoiding musculoskeletal injury. Thus muscle and its associated tendon must function in concert, and the mechanical properties of one tissue will undoubtedly influence the mechanical response of the other. In fact, in vivo investigations demonstrated that tendon stiffness positively correlates with muscle power in young adult humans (3). Despite this important combined functional role, the association between muscle force output and tendon mechanical properties in aged animals and humans is poorly understood. The significant reductions in mass and force output with aging are well documented in many muscles (4, 5, 12). Since tendon tissue clearly adapts to its loading environment (for reviews see Refs. 6 and 23), decreased muscle force output has the potential to dramatically affect the behavior and properties of the tendon. A substantial effect of reduced loading by the muscle on the tendon was demonstrated by Arruda and colleagues (1), who reported a dramatic increase in TA tendon modulus following 5 wk of muscle denervation. Despite the dramatic age-associated increase in modulus in TA tendons in the present study, associated declines in TA muscle maximum force generation were not observed. The relative constancy of TA force generation across the age groups studied indicates that the increased modulus we observed in tendons from old animals was caused by intrinsic age-related changes in the tendon tissue itself, rather than an adaptation to alterations of muscle force-generating capability.
Our study is not without limitations. Ideally, mechanical and structural properties would be determined from the same tendons. We performed our electron microscopic analysis on fresh tendons fixed immediately upon removal from the animal to minimize degradation of the tissue or artifacts in structure resulting from the mechanical testing. Consequently, we are assuming that the mechanical properties of the tendons on which we performed the structural analysis were similar to those measured on a separate group of tendons from mice of the same age and strain. Furthermore, although we examined electron micrographs from random areas within tendon cross sections, regional differences in collagen fibril distributions may exist, and we cannot rule out the possibility that specific regions of some cross sections were inadvertently favored. In addition, our reported overall tendon strain values fall below the imposed strain level of 10%. This is primarily a result of the bead placement, which leaves a portion of the tendon unsampled. Measuring strain over the entire muscle-tendon unit length would alter our results most noticeably by increasing strain values in the compliant proximal region, accentuating, rather than eliminating, the local variations in mechanical properties. Finally, it is important to acknowledge that the results of this investigation may not generalize to all tendons or all species. Specific tendons are uniquely suited for specific functions. The TA tendon is the primary dorsiflexor of the mouse hindlimb and, therefore, is highly important for locomotion, but differences in loading environment of other tendons may affect how individual tendons respond to aging.
In conclusion, we demonstrated that the tangent modulus of TA tendons increases in a region-specific fashion throughout the life span, with the most pronounced increases in the proximal tendon region. This increase was not accompanied by dramatic alterations in collagen fibril morphology, suggesting that fibril size is not a significant contributor to the changes in tendon mechanical properties with aging. We also showed that the force-generating capacity of TA muscles did not decrease significantly over the age range of the present study, providing evidence that age-related changes in TA tendon mechanics are not an adaptation to wasting and weakness of the TA muscle but are, in fact, a result of intrinsic alterations in the tendon tissue itself. These results have important implications for understanding the natural aging process in tendons, which may help elucidate the underlying causes of the increasing incidence of tendon dysfunction in the elderly.
GRANTS
Financial support for this work was provided by National Institute on Aging Grant AR-055624.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to Adriana Lingl and Perry Atangcho for help with data collection. We thank the staff at the Microscopy and Image Analysis Laboratory at the University of Michigan for assistance with preparation and imaging of the tendon samples.
REFERENCES
- 1. Arruda EM, Calve S, Dennis RG, Mundy K, Barr K. Regional variation of tibialis anterior tendon mechanics is lost following denervation. J Appl Physiol 101: 1113–1117, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Blevins FT, Hecker AT, Bigler GT, Boland AL, Hayes WC. The effects of donor age and strain rate on the biomechanical properties of bone-patellar tendon-bone allografts. Am J Sports Med 22: 328–333, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Bojsen-Moller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structure. J Appl Physiol 99: 986–994, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown M, Taylor J, Gabriel R. Differential effectiveness of low-intensity exercise in young and old rats. J Gerontol A Biol Sci Med Sci 58: B889–B894, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Buchanan CI, Marsh RL. Effect of exercise on the biomechanical, biochemical and structural properties of tendons. Comp Biochem Physiol A 133: 1101–1107, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 221: 117–190, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol 105: 1907–1915, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clemmer J, Liao J, Davis D, Horstemeyer MF, Williams LN. A mechanistic study for strain rate sensitivity of rabbit patellar tendon. J Biomech 43: 2785–2791, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Couppe C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol 107: 880–886, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Curwin SL, Roy RR, Vailas AC. Regional and age variations in growing tendon. J Morphol 221: 309–320, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Degens H, Alway SE. Skeletal muscle function and hypertrophy are diminished in old age. Muscle Nerve 27: 339–347, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Derwin KA, Soslowsky LJ. A quantitative investigation of structure-function relationships in a tendon fascicle model. J Biomech Eng 121: 598–604, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F, Boivin GP. A potential mechanism for age-related declines in patellar tendon biomechanics. J Orthop Res 20: 1315–1322, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Gere JM. Mechanics of Materials. Toronto, ON, Canada: Thompson, 2006 [Google Scholar]
- 16. Gill TM, Hardy SE, Williams CS. Underestimation of disability in community-living older persons. J Am Geriatr Soc 50: 1492–1497, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Goh KL, Holmes DF, Lu HY, Richardson S, Kadler KE, Purslow PP, Wess TJ. Ageing changes in the tensile properties of tendon: influence of collagen fibril volume fraction. J Biomed Eng 130: 021011, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Hansen P, Haraldsson BT, Aagaard P, Kovanen V, Avery NC, Qvortrup K, Larsen JO, Krogsgaard M, Kjaer M, Magnusson SP. Lower strength of the human posterior patellar tendon seems unrelated to mature collagen cross-linking and fibril morphology. J Appl Physiol 108: 47–52, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Hansen KA, Weiss JA, Barton JK. Recruitment of tendon crimp with applied tensile strain. J Biomech Eng 124: 72–77, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Haraldsson BT, Aagaard P, Krogsgaard M, Alkjaer T, Kjaer M, Magnusson SP. Region-specific mechanical properties of the human patella tendon. J Appl Physiol 98: 1006–1012, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Jette AM, Branch LG. The Framingham Disability Study. II. Physical disability among the aging. Am J Public Health 71: 1211–1216, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kannus P, Josza L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am 73: 1507–1525, 1991 [PubMed] [Google Scholar]
- 23. Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84: 649–698, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Larkin LM, Calve S, Kostrominova TY, Arruda EM. Structural and functional evaluation of tendon-skeletal muscle constructs engineered in vivo. Tissue Eng 12: 3149–3158, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lavagnino M, Arnoczky SP, Frank K, Tian T. Collagen fibril diameter distribution does not reflect changes in the mechanical properties of in vitro stress-deprived tendons. J Biomech 38: 69–75, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Maganaris CN. Tensile properties of in vivo human tendinous tissue. J Biomech 35: 1019–1027, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K, Haan AD. Adaptive response of human tendon to paralysis. Muscle Nerve 33: 85–92, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. J Bone Joint Surg Br 77B: 296–298, 1995 [PubMed] [Google Scholar]
- 29. Ouzounian TJ, Anderson R. Anterior tibial tendon rupture. Foot Ankle Int 16: 406–410, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Reeves ND, Maganaris CN, Ferretti G, Narici MV. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J Appl Physiol 98: 2278–2286, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Robinson PS, Lin TW, Reynolds PR, Derwin KA, Iozzo RV, Soslowsky LJ. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J Biomech Eng 126: 252–257, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Sverdlik A, Lanir Y. Time-dependent mechanical behaviors of sheep digital tendons, including the effects of preconditioning. J Biomech Eng 124: 78–84, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg 8: 296–299, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Viidik A, Nielsen HM, Skalicky M. Influence of physical exercise on aging rats. II. Life-long exercise delays aging of tail tendon collagen. Mech Ageing Dev 88: 139–148, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Vogel HG. Influence of maturation and age on mechanical and biochemical parameters of connective tissue of various organs in the rat. Connect Tissue Res 6: 161–166, 1978 [DOI] [PubMed] [Google Scholar]
- 36. Williams LN, Elder SH, Horstemeyer MF, Harbarger D. Variation of diameter distribution, number density and area fraction of fibrils within five areas of the rabbit patellar tendon. Ann Anat 190: 442–451, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Wu JZ, Brumfield A, Miller GR, Metheny R, Cutlip RG. Comparison of mechanical properties of rat tibialis anterior tendon evaluated using two different approaches. Biomed Mater Eng 14: 13–22, 2004 [PubMed] [Google Scholar]