Abstract
Intermittent hypoxia (IH) resulting from sleep apnea can lead to pulmonary hypertension. IH causes oxidative stress that may limit bioavailability of the endothelium-derived vasodilator nitric oxide (NO) and thus contribute to this hypertensive response. We therefore hypothesized that increased vascular superoxide anion (O2−) generation reduces NO-dependent pulmonary vasodilation following IH. To test this hypothesis, we examined effects of the O2− scavenger tiron on vasodilatory responses to the endothelium-dependent vasodilator ionomycin and the NO donor S-nitroso-N-acetylpenicillamine in isolated lungs from hypocapnic-IH (H-IH; 3 min cycles of 5% O2/air flush, 7 h/day, 4 wk), eucapnic-IH (E-IH; cycles of 5% O2, 5% CO2/air flush), and sham-treated (air/air cycled) rats. Next, we assessed effects of endogenous O2− on NO- and cGMP-dependent vasoreactivity and measured O2− levels using the fluorescent indicator dihydroethidium (DHE) in isolated, endothelium-disrupted small pulmonary arteries from each group. Both E-IH and H-IH augmented NO-dependent vasodilation; however, enhanced vascular smooth muscle (VSM) reactivity to NO following H-IH was masked by an effect of endogenous O2−. Furthermore, H-IH and E-IH similarly increased VSM sensitivity to cGMP, but this response was independent of either O2− generation or altered arterial protein kinase G expression. Finally, both H-IH and E-IH increased arterial O2− levels, although this response was more pronounced following H-IH, and H-IH exposure resulted in greater protein tyrosine nitration indicative of increased NO scavenging by O2−. We conclude that IH increases pulmonary VSM sensitivity to NO and cGMP. Furthermore, endogenous O2− limits NO-dependent vasodilation following H-IH through an apparent reduction in bioavailable NO.
Keywords: sleep apnea, pulmonary hypertension, superoxide anion, endothelial nitric oxide synthase, protein kinase G
sleep apnea (SA) affects ∼20% of the adult population in the United States (47). In addition to causing systemic hypertension (32), intermittent hypoxia (IH) associated with SA exacerbates pulmonary hypertension (PH) in patients with chronic obstructive pulmonary disease and may be an independent risk factor for PH (14). Although the cardiovascular sequelae leading to sleep apnea-induced PH remain poorly characterized, recent studies using rodent models of the disorder have begun to elucidate these mechanisms of PH, which include vasoconstriction, arterial remodeling, and polycythemia (2, 6, 8, 40). Such models have employed chronic exposure to a variety of hypoxia-reoxygenation cycles (0.5–3 min, 7–12 h/day for 2–8 wk) to mimic the repetitive apneic episodes observed in patients with SA (6, 11, 27, 40). In addition, because IH alone leads to cyclical reductions in arterial Pco2 and consequent alkalemia, other studies have introduced supplemental CO2 into the cycling protocol to prevent hypocapnia and thus more closely approximate arterial blood gases in human SA (1, 10, 11, 40, 41).
Whereas the endogenous vasodilator and antimitogenic factor, nitric oxide (NO), may be protective in limiting the severity of IH-induced PH, little is known regarding effects of IH on NO-dependent vasoreactivity or NO signaling in pulmonary vascular smooth muscle (VSM). NO is a highly labile reactive nitrogen species that is produced by endothelial nitric oxide synthase (eNOS) in vascular endothelial cells. NO elicits pulmonary vasodilation through activation of the soluble guanylyl cyclase (sGC)-protein kinase G (PKG) signaling axis, leading to a reduced VSM intracellular calcium concentration ([Ca2+]i) and myofilament Ca2+ desensitization (19). While a compensatory increase in eNOS expression occurs in some models of PH (22, 39, 42), NO-dependent pulmonary vasoreactivity is often compromised by reduced NO synthesis (26), inactivation of NO by reactive oxygen species (ROS) (13, 21, 27), or impaired NO signal transduction in VSM (17).
IH is associated with increased oxidative stress (24, 27, 41) that results from enhanced levels of ROS, including superoxide anion (O2−) and hydrogen peroxide. O2− may limit the bioavailability of NO by the formation of peroxynitrite (ONOO−) (21) or as a result of eNOS uncoupling (46). Additionally, ROS may directly interfere with NO signaling in VSM (45). We therefore hypothesized that increased vascular O2− generation following IH reduces NO-dependent pulmonary vasodilation. We tested our hypothesis by assessing the role of ROS in NO-dependent vasoreactivity in isolated lungs and small pulmonary arteries from hypocapnic IH, eucapnic IH, and sham-treated control rats. Our findings reveal a novel effect of IH to increase pulmonary VSM sensitivity to NO and cGMP. Furthermore, NO-dependent pulmonary vasodilation is reduced by a nonendothelial source of O2− following hypocapnic-IH, but not eucapnic-IH.
METHODS
All protocols and surgical procedures employed in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico School of Medicine (Albuquerque, NM). Male Wistar rats (body wt 250–350 g, age 3–4 mo; Harlan Industries) were used for all experiments. Animals were maintained on a 12:12-h light-dark cycle.
Experimental Groups
Rats were housed in Plexiglas chambers with free access to food and water and divided into three experimental groups. The first group was exposed to IH alone (3 min cycles of 5% O2/air flush), which leads to cyclical reductions in arterial Po2 and Pco2, and consequent alkalemia (40), and is therefore termed hypocapnic IH (H-IH). A second group was provided with supplemental CO2 during hypoxic cycling (3 min cycles of 5% O2, 5% CO2/air flush) to more closely approximate the arterial blood gas and pH changes that occur during apneic episodes in patients with sleep-disordered breathing (12). Previous studies from our laboratory indicate that this method of CO2 supplementation maintains normal arterial Pco2 and pH during hypoxic episodes (40), and is therefore termed eucapnic IH (E-IH). Both H-IH and E-IH animals were exposed to these conditions for 7 h/day for 4 wk. A third control group was exposed to sham conditions (3 min air/air cycles) for equal duration. For sham exposure, the inflow gas was always room air but the solenoid switches and inlets approximated the airflow and noise disturbances of the IH protocols. In addition to evidence of PH following H-IH and E-IH exposure (40), this model of E-IH causes systemic hypertension in rats (1, 20), consistent with known effects of SA in humans (47).
Assessment of Right Ventricular Weight and Hematocrit
Blood samples were obtained by direct cardiac puncture at the time of lung isolation for measurement of hematocrit. Right ventricular hypertrophy was assessed as an index of IH-induced PH as described previously (17, 36).
Isolated Lung Protocols
Methods for lung isolation and perfusion have been previously reported (36). Lungs were perfused with a physiological salt solution and ventilated with normoxic gas (6% CO2 in room air) to eliminate possible complicating influences of differential hypoxic pulmonary vasoconstriction between groups on NO-dependent vasodilatory responses.
Effects of IH on endothelium-dependent vasodilation.
To compare effects of H-IH and E-IH exposure on endothelium-derived NO (EDNO)-dependent pulmonary vasoreactivity, vasodilatory responses to the Ca2+ ionophore ionomycin (Calbiochem) were assessed in lungs from each group of rats. Experiments were conducted in the presence of vehicle (saline), the O2− scavenger 4,5-dihydroxy-1,3-benzene-disulfonic acid (10 mM, tiron, Sigma), or in the combined presence of tiron and the NO synthase (NOS) inhibitor Nω-nitro-l-arginine (l-NNA; 300 μM, Sigma). Following equilibration, U-46619 (Cayman Chemical) was added in cumulative doses to the perfusate reservoir until a stable arterial pressor response of ∼10 mmHg was achieved. The vasculature was then dilated with ionomycin (100 nM). Ionomycin was chosen as a non-receptor-mediated EDNO-dependent vasodilator in this study since interpretation of responses to receptor-mediated agonists could be complicated by possible changes in receptor number or affinity in response to sham, H-IH, or E-IH exposure. Furthermore, we have previously shown that ionomycin elicits vasodilatory responses in this preparation that are sensitive to NOS inhibition (35, 36). Ionomycin was dissolved in anhydrous dimethyl sulfoxide (Sigma) and stored at 4°C.
Effects of IH on vasoreactivity to exogenous NO.
Vasodilatory responses to the NO donor S-nitroso-N-acetylpenicillamine (SNAP; 1 μM, Sigma) were assessed in separate sets of U-46619-constricted lungs from rats from each group. Experiments were conducted in the presence of vehicle or tiron (10 mM) to further assess the influence of ROS on these responses. All protocols were performed in the presence l-NNA (300 μM) to minimize potential complicating influences of endogenous NO on vascular reactivity.
Isolated Vessel Protocols
Effects of H-IH and E-IH on VSM reactivity to NO/cGMP were evaluated in isolated, cannulated small pulmonary arteries from each group of rats. Inner diameter (ID) and fura-2 340/380 emission ratios (F340/F380) were measured simultaneously to assess vasoreactivity and changes in VSM [Ca2+]i as previously described (3, 19). All arteries were studied at a transmural pressure of 12 Torr under normoxic conditions (equilibrated with a 10% O2, 6% CO2, and balance N2 gas mixture) to eliminate possible complicating influences of differential hypoxic pulmonary vasoconstriction between groups on the subsequent vasodilatory responses to NO. Arteries were endothelium-disrupted to directly evaluate effects of H-IH and E-IH on VSM sensitivity to NO and cGMP independent of complicating influences of NO or ROS produced by the endothelium. The effectiveness of endothelial disruption was verified by the lack of a vasodilatory response to ACh (1 μmol/l) in UTP-constricted vessels.
Effects of IH on NO- and cGMP-dependent vasodilation and changes in VSM [Ca2+]i.
Concentration-response curves to the NO donor SNAP (10−9–10−5 M, Sigma) were conducted in isolated pulmonary arteries from each group. Following cannulation and equilibration, as described above, vessels were preconstricted with UTP (5 μM) to ∼40% of baseline ID. Vasodilatory responses and changes in VSM [Ca2+]i were assessed in the presence of vehicle, tiron, or polyethylene glycol (PEG)-catalase (250 U/ml, Sigma) (7) to determine the effects of O2− and H2O2 on NO-dependent reactivity in these arteries.
Effects of IH cGMP-mediated pulmonary vasodilation were assessed by performing cumulative concentration-response curves to the cell-permeable, phosphodiesterase-5-resistant cGMP analog 8-para-chlorophenylthio (8-pCPT)-cGMP (10−6–10−4 M, Sigma) (19), in UTP-constricted endothelium-disrupted arteries from each group. Vasodilatory and VSM [Ca2+]i responses were measured in the presence of vehicle or tiron to determine potential influences of endogenous O2− on reactivity to 8-pCPT-cGMP.
Effects of PKG inhibition on NO-dependent reactivity.
The role of PKG in mediating NO-dependent vasodilation was determined by performing cumulative concentration-response curves to SNAP in the presence of the PKG inhibitor KT-5823 (10 μM, Sigma) as previously described (18, 19).
Effects of IH on arterial O2− generation.
Fluorescence detection of dihydroethydium (DHE, Molecular Probes) oxidation was used to compare effects of H-IH and E-IH treatment on basal levels of O2− in pressurized, endothelium-disrupted pulmonary arteries as previously reported (3).
Western Blotting: Effects of IH on Arterial eNOS, PKG-1, and Nitrotyrosine Levels
Pulmonary arterial eNOS and PKG-1 expression were compared between sham-treated, H-IH, and E-IH rats using previously published methods (35). Nitrotyrosine levels were additionally determined by Western blotting as a measure of ONOO− formation (30) in arteries from each group. Arteries used for nitrotyrosine blots were incubated for 30 min at 37° C, then treated with SNAP (10−6 M; 5 min), and snap-frozen in liquid nitrogen. A polyclonal anti-nitrotyrosine antibody (1:1,000, Millipore) was used to detect nitrotyrosine residues. Bands were compared between blots following normalization to an identical control sample. In addition, all bands of interest were normalized to those of beta-actin on the same blot.
Calculations and Statistics
For isolated lung experiments, total pulmonary vascular resistance was calculated as the difference between Pa and Pv divided by flow (30 ml·min−1·kg body wt−1). Vasodilatory responses were calculated as a percent reversal of U-46619-induced vasoconstriction. Vasodilatory responses in isolated arteries are expressed as a percent reversal of UTP-induced constriction. All data are presented as means ± SE, and n refers to the number of animals in each group. A one-way, two-way, or repeated-measures ANOVA was used to make comparisons when appropriate. If differences were detected by ANOVA, individual groups were compared using the Student-Newman-Keuls test. A probability of P ≤ 0.05 was considered significant for all comparisons.
RESULTS
IH-Induces Right Ventricular Hypertrophy and Polycythemia
Both H-IH and E-IH exposure produced right ventricular (RV) hypertrophy indicative of PH, as evidenced by greater RV-to-total ventricular weight (RV/T) and RV/body weight (BW) ratios compared with sham-treated controls (Table 1). However, these indexes of PH were significantly lower in E-IH compared with H-IH rats. No significant differences in left ventricle plus septum weight (LV + S)/BW were observed between groups, demonstrating a lack of LV hypertrophy in response to IH. Exposure to H-IH and E-IH resulted in lower BW compared with sham-treated rats, suggesting that IH attenuates weight gain independent of hypocapnic or eucapnic treatment. H-IH rats exhibited greater hematocrit vs. sham-treated rats, whereas no such response was observed in the E-IH group. We have previously reported similar effects of supplemental CO2 to attenuate RV hypertrophy and polycythemia following 2 wk IH exposure (40).
Table 1.
Body weight, ventricular weight ratios, and hematocrit
| BW, g | RV/T, mg/mg | RV/BW, mg/g | (LV+S)/BW, mg/g | Hct, % | n | |
|---|---|---|---|---|---|---|
| Treatment | ||||||
| Sham | 324 ± 4 | 0.172 ± 0.005 | 0.497 ± 0.015 | 2.402 ± 0.041 | 46.9 ± 0.7 | 19 |
| H-IH | 302 ± 3* | 0.239 ± 0.009* | 0.701 ± 0.021* | 2.252 ± 0.065 | 61.6 ± 1.2* | 19 |
| E-IH | 299 ± 3* | 0.212 ± 0.005*† | 0.590 ± 0.026*† | 2.183 ± 0.061 | 49.9 ± 0.8† | 19 |
Values are means ± SE; n, no. of rats. BW, body weight; RV, right ventricle weight; T, total ventricular weight; LV + S, left ventricle plus septum weight; Hct, hematocrit; IH, intermittent hypoxia; H-IH, hypocapnic IH; E-IH, eucapnic IH
P < 0.05 vs. sham treated.
P < 0.05 vs. H-IH.
IH Enhances EDNO-Dependent Vasodilation in Isolated Lungs: Role of O2−
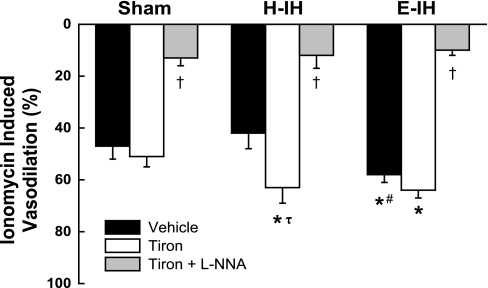
No differences in Po2, Pco2, or pH were observed in isolated lung perfusate effluent samples between groups. There were also no differences in baseline vascular resistance or U-46619-induced increases in resistance between groups in isolated lung protocols. Vasodilatory responses to the EDNO-dependent vasodilator ionomycin were greater in lungs from E-IH rats compared with those from sham-treated animals, whereas responses to ionomycin were unaltered following H-IH exposure (Fig. 1). However, the O2− scavenger tiron augmented EDNO-dependent reactivity in the H-IH group only, revealing greater vasodilation compared with similarly treated sham lungs. NOS inhibition with l-NNA greatly reduced vasodilation in all three groups, thus confirming the NO dependency of these responses.
Fig. 1.
Vasodilatory responses to ionomycin (100 nM) in the presence of vehicle, tiron (10 mM), or tiron + Nω-nitro-l-arginine (l-NNA) (300 μM) in lungs isolated from sham-treated, hypocapnic-intermittent hypoxia (H-IH), and eucapnic-IH (E-IH) rats. Values are means ± SE of n = 6 rats/group. *P < 0.05 vs. sham treated. #P < 0.05 vs. H-IH. τP < 0.05 vs. vehicle. †P < 0.05 vs. tiron.
Pulmonary Arterial eNOS Expression is Not Altered Following IH
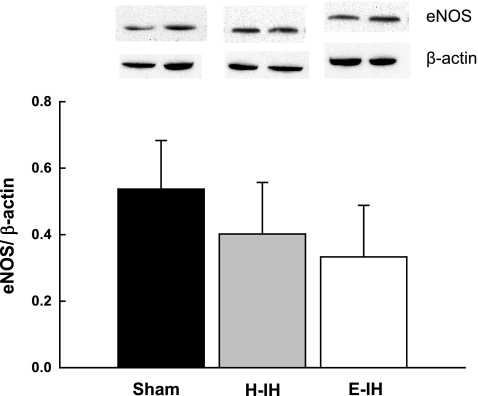
To address whether greater EDNO-dependent pulmonary vasodilation following H-IH and E-IH (Fig. 1) is associated with increased eNOS expression, we measured eNOS protein levels in intrapulmonary arteries from each group by Western blotting. However, levels of pulmonary arterial eNOS were unaltered after either H-IH or E-IH exposure (Fig. 2).
Fig. 2.
Representative Western blots and mean densitometric data for endothelial nitric oxide synthase (eNOS) normalized to β-actin in intrapulmonary arteries from sham-treated, H-IH, and E-IH rats. eNOS was identified as a single band at ∼140 kDa. Values are means ± SE of n = 4–5 rats/group. There are no significant differences.
IH Augments Vasodilation to Exogenous NO in Isolated Lungs: Regulation by O2−
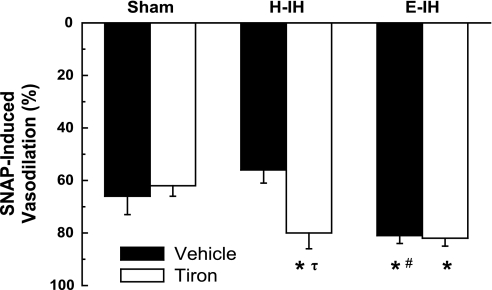
Augmented EDNO-mediated vasodilation following IH could be a function of either increased NO synthesis (independent of a change in eNOS expression) or enhanced VSM sensitivity to NO. NO-dependent vasoreactivity was therefore examined by measuring vasodilatory responses to exogenous NO in lungs isolated from each group of rats. Similar to EDNO-mediated vasodilation (Fig. 1), vasodilatory responses to the NO donor SNAP were augmented in lungs from rats exposed to E-IH, but not H-IH (Fig. 3). Furthermore, tiron elevated NO-dependent vasoreactivity in the H-IH group only (Fig. 3). These findings suggest that both H-IH and E-IH increase pulmonary VSM sensitivity to NO, a response that is masked by endogenous O2− following H-IH.
Fig. 3.
Vasodilatory responses to the NO donor SNAP (1 μM) in the presence of vehicle or tiron in lungs isolated from sham-treated, H-IH, and E-IH rats. All experiments were conducted in the presence l-NNA (300 μM). Values are means ± SE of n = 6 rats/group. *P < 0.05 vs. sham treated. #P < 0.05 vs. H-IH. τP < 0.05 vs. vehicle.
IH Increases VSM Reactivity to NO in Isolated Small Pulmonary Arteries: Role of O2−
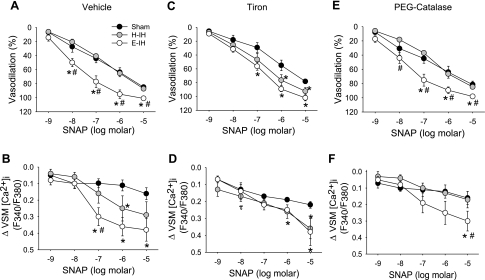
Basal ID, VSM [Ca2+]i, UTP-induced constriction, and UTP-induced increases in [Ca2+]i were similar between isolated small pulmonary arteries from sham-treated, H-IH, and E-IH rats (Table 2). To directly assess effects of IH on VSM reactivity to NO, we measured vasodilatory responses and changes in VSM [Ca2+]i to SNAP in endothelium-disrupted small pulmonary arteries from each group of rats. Consistent with observations from isolated lung studies above, E-IH augmented vasodilatory responses to SNAP, whereas H-IH treatment was without effect on NO-dependent reactivity (Fig. 4A). This enhanced vasodilatory responsiveness to SNAP following E-IH corresponded to a greater fall in VSM [Ca2+]i compared with sham-treated arteries (Fig. 4B). Decreases in VSM [Ca2+]i to SNAP also tended to be greater in H-IH vs vessels from sham-treated rats, although significance was achieved only at 10−6 M SNAP. Also in agreement with our results from isolated lungs (Fig. 3), O2− scavenging with tiron revealed greater vasodilation and [Ca2+]i responses to SNAP in arteries from H-IH vs. sham-treated rats (Fig. 4, C and D), while having no significant effect on reactivity to NO in the E-IH group. In contrast, vasodilatory and VSM [Ca2+]i responses to SNAP in the presence of the H2O2 scavenger, PEG-catalase (Fig. 4, E and F), generally reflected those in the presence of vehicle (Fig. 4, A and B), with arteries from E-IH rats displaying significantly greater vasodilation and changes in VSM [Ca2+]i vs. sham-treated arteries, whereas no differences were observed between sham-treated and H-IH arteries.
Table 2.
Basal inner diameter (ID) and VSM [Ca2+]i, and UTP-induced constrictor and VSM [Ca2+]i responses in isolated pulmonary arteries
| Basal ID, μm | Basal [Ca2+]i | %Constriction | Δ[Ca2+]i | n | |
|---|---|---|---|---|---|
| Treatment | |||||
| Sham | 189.5 ± 7.3 | 1.13 ± 0.20 | 34.3 ± 3.3 | 0.19 ± 0.04 | 9 |
| H-IH | 184.1 ± 6.9 | 1.14 ± 0.15 | 38.4 ± 2.5 | 0.19 ± 0.03 | 10 |
| E-IH | 185.3 ± 7.1 | 1.10 ± 0.11 | 37.8 ± 2.9 | 0.22 ± 0.05 | 10 |
Values are means ± SE; n, number of rats. Constriction was calculated as a percent change from basal inner diameter (ID). Basal vascular smooth muscle (VSM) intracellular Ca2+ concentration ([Ca2+]i) is expressed as 340/380-nm emission ratios. Changes in VSM [Ca2+]i (Δ[Ca2+]i) are calculated as the difference in 340/380-nm emission ratios from basal. There are no significant differences.
Fig. 4.
Vasodilatory responses (A, C, E) and changes in vascular smooth muscle (VSM) intracellular Ca2+ concentration ([Ca2+]i) (B, D, F) to the NO donor SNAP in the presence of vehicle (A, B), tiron (C, D; 10 mM), or PEG-catalase (E, F; 250 U/ml) in isolated pulmonary arteries from sham-treated, H-IH, and E-IH rats. Values are means ± SE of n = 4–5 rats/group. *P < 0.05 vs. sham. #P < 0.05 vs. H-IH. τP < 0.05 vs. vehicle.
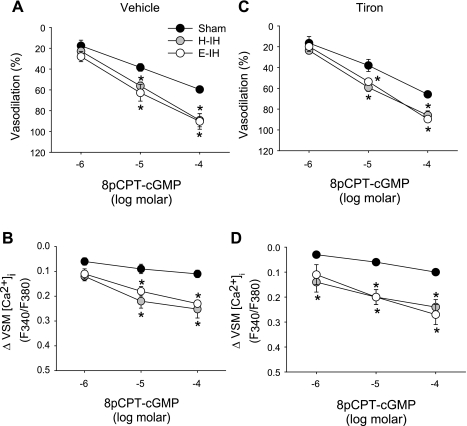
IH Augments cGMP-Dependent Vasodilation Independently of O2− in Isolated Small Pulmonary Arteries
Increased vasodilation to NO following IH may be mediated by greater VSM sensitivity to the second messenger cGMP. To examine this possibility, we evaluated effects of both H-IH and E-IH exposure on responses to the cGMP analog 8-pCPT-cGMP in endothelium-disrupted pulmonary arteries. Dilation to 8-pCPT-cGMP was enhanced in arteries from both H-IH and E-IH rats compared with those of sham-treated controls (Fig. 5A). This increased vasoreactivity following H-IH and E-IH was associated with a greater fall in VSM [Ca2+]i (Fig. 5B). The addition of tiron resulted in no significant changes in either vasodilatory or VSM [Ca2+]i responses to 8-pCPT-cGMP in any of the groups (Fig. 5, C and D).
Fig. 5.
Vasodilatory responses (A, C) and changes in VSM [Ca2+]i (B, D) to the cell-permeable cGMP analog 8-pCPT-cGMP in the presence of vehicle (A, B) or tiron (C, D) in isolated pulmonary arteries from sham-treated, H-IH, and E-IH rats. Values are means ± SE of n = 4–5 rats/group. *P < 0.05 vs. sham.
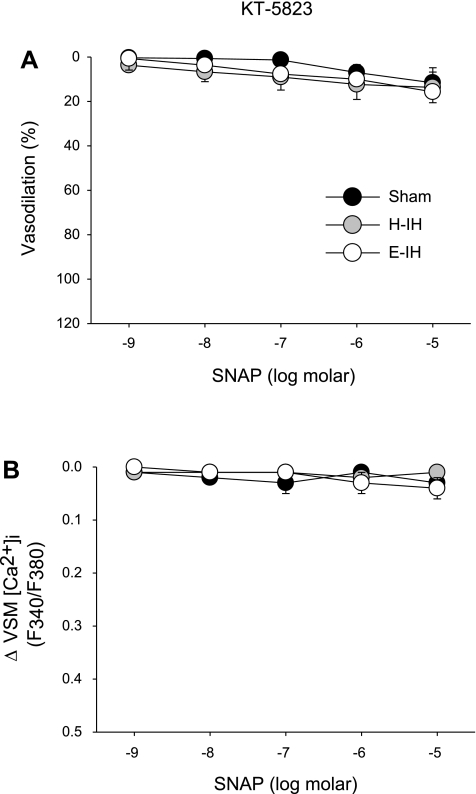
PKG Inhibition Prevents NO-Dependent Responses in Isolated Small Pulmonary Arteries
To determine whether NO-dependent vasodilation following IH and sham treatments is mediated by PKG, we evaluated responses to varying concentrations of SNAP in the presence of KT-5823, a PKG inhibitor. No significant vasodilation or change in VSM [Ca2+]i to SNAP was observed in the presence of KT-5823 (Fig. 6, A and B) in arteries from any group.
Fig. 6.
Vasodilatory responses (A) and changes in VSM [Ca2+]i (B) to the NO donor SNAP in the presence of the protein kinase G (PKG) inhibitor KT-5823 (10 μM) in isolated arteries from each group of rats. Values are means ± SE of n = 3 rats/group. There are no significant differences between groups, nor are there significant vasodilatory or VSM [Ca2+]i responses within groups.
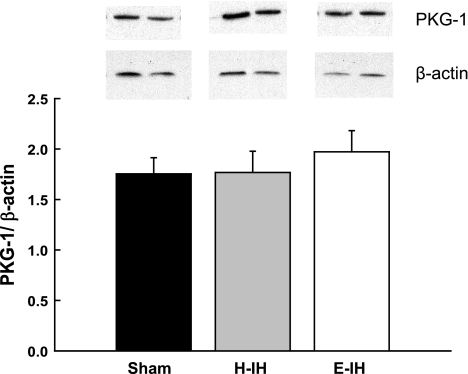
Pulmonary Arterial PKG Expression is not Altered by IH
Western blots revealed no differences in pulmonary arterial PKG-1 levels between groups (Fig. 7), suggesting that increased VSM reactivity to NO and cGMP following IH is not a function of increased PKG-1 expression.
Fig. 7.
Representative Western blots and mean densitometric data for PKG-1 in intrapulmonary arteries from sham-treated, H-IH, and E-IH rats. PKG-1 was identified as a single band at ∼75 kDa. Values are means ± SE of n = 4–5 rats/group. There are no significant differences.
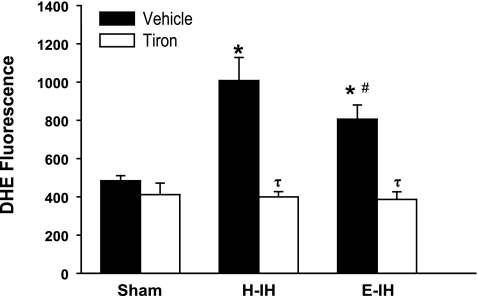
IH Increases Pulmonary Arterial O2− Production
The effect of O2− to selectively attenuate NO-mediated pulmonary vasodilation following H-IH may be due to greater vascular O2− generation in that group. We therefore measured O2− generation using the fluorescent O2− indicator DHE in isolated pressurized arteries from each group. DHE fluorescence was greater in arteries from rats exposed to H-IH compared with sham-treated arteries (Fig. 8). While arteries from E-IH animals also demonstrated enhanced fluorescence vs. the sham-treated vessels, it was significantly less than in the H-IH group. The addition of tiron decreased O2− levels in both H-IH and E-IH arteries to similar levels as expected, without altering fluorescence intensity in sham-treated arteries.
Fig. 8.
DHE fluorescence in the presence of vehicle or tiron in isolated pulmonary arteries from sham-treated, H-IH, and E-IH rats. Values are means ± SE of n = 4–5 rats/group. *P < 0.05 vs. sham treated. #P < 0.05 vs. H-IH. τP < 0.05 vs. vehicle.
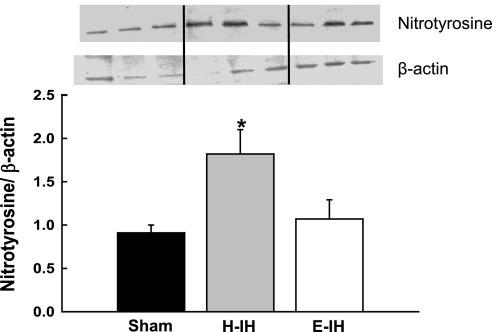
Pulmonary Arterial Nitrotyrosine Levels are Increased Following H-IH
Greater vascular O2− generation following H-IH may limit NO-dependent vasodilation by reacting with NO to form ONOO−, thus reducing NO bioavailability. Therefore, we measured levels of nitrotyrosine-positive proteins by Western blot as an index of ONOO− production (30) in intrapulmonary arteries from sham, H-IH, and E-IH rats. Consistent with increased ONOO− generation following H-IH, we observed greater protein tyrosine nitration in arteries from H-IH vs. sham-treated rats, whereas E-IH exposure had no significant effect on nitrotyrosine levels (Fig. 9).
Fig. 9.
Representative Western blots and mean densitometric data for nitrotyrosine in intrapulmonary arteries from sham-treated, H-IH, and E-IH rats. Nitrotyrosine containing protein was identified as a single band at ∼16 kDa. Values are means ± SE of n = 5–6 rats/group. *P < 0.05 vs. sham treated.
DISCUSSION
The objective of this study was to evaluate mechanisms of altered NO-dependent pulmonary vasodilation in IH-induced PH. Our major findings are that 1) E-IH and H-IH augmented vasodilation to both EDNO and exogenous NO, but these effects were masked in H-IH by increased O2−; 2) greater reactivity to EDNO and exogenous NO following H-IH and E-IH was not associated with increased pulmonary arterial eNOS or PKG-1 expression; 3) H-IH and E-IH similarly increased VSM reactivity to cGMP; however, this response was independent of vascular O2− generation; and 4) H-IH resulted in greater arterial protein tyrosine nitration indicative of increased NO scavenging by O2−. Together, these results demonstrate an effect of IH to increase pulmonary VSM reactivity to both NO and cGMP, and support a role for endogenous O2− to limit NO-dependent pulmonary vasodilation following H-IH by reducing bioavailable NO.
Mechanisms of IH-induced PH are generally considered to be similar to those resulting from chronic sustained hypoxia (CH), including pulmonary vasoconstriction, arterial remodeling, and polycythemia (12). Whereas NO mediates an important protective influence to limit vasoconstriction and arterial remodeling in PH (9, 34), NO bioavailability is often reduced in both experimental and human forms of PH as a result of diminished NO synthesis (26) or increased scavenging of NO by O2−(13). Indeed, lung ROS levels are increased in IH mice (27), and ROS are implicated as contributing factors to both vasoconstrictor (3, 43) and vascular remodeling components of PH (28). However, IH effects on pulmonary vasoreactivity to NO and regulation of this response by ROS have not previously been addressed, and were the focus of the present study.
IH Augments EDNO-Dependent Pulmonary Vasodilation by Increasing VSM Sensitivity to NO and cGMP
CH increases pulmonary arterial eNOS expression and enhances EDNO-dependent pulmonary vasodilation, responses that may mitigate the PH response (15, 31, 35, 36, 38). Although similar alterations in pulmonary vasoreactivity are not apparent following 2 wk IH exposure in rats (40), our present results demonstrate that 4 wk E-IH treatment augments vasodilation to the Ca2+ ionophore, ionomycin. Interestingly, endogenous O2− masked a similar increase in endothelium-dependent vasodilation in lungs from H-IH rats. Therefore, supplemental CO2 during IH exposure appears to protect against the deleterious effects of ROS to limit endothelium-dependent vasoreactivity in the hypertensive pulmonary vasculature. Furthermore, enhanced EDNO-dependent responsiveness following IH was not associated with increased arterial eNOS levels, suggesting that pulmonary eNOS expression is differentially regulated by IH and CH.
IH-mediated increases in EDNO-dependent vasodilation could result from greater VSM sensitivity to NO. In agreement with this possibility, vasoreactivity to the NO-donor SNAP was augmented following E-IH in both isolated lungs and endothelium-disrupted small pulmonary arteries. Furthermore, scavenging O2− revealed a similar increase in reactivity to NO in lungs and arteries isolated from H-IH rats, consistent with effects of O2− to limit EDNO-dependent dilation following H-IH. Because responses to SNAP were PKG-dependent, and since IH also augmented VSM reactivity to the PKG agonist, 8pCPT-cGMP, greater NO/cGMP-mediated vasodilation following IH appears to be mediated in large part by enhanced PKG signaling.
Whereas CH similarly augments pulmonary VSM sensitivity to NO and cGMP, distinct differences exist between CH and IH with respect to the mechanism of this response. For example, enhanced NO induced pulmonary vasodilation following CH is mediated by an increase in PKG-dependent myofilament Ca2+ desensitization through inhibition of the RhoA/Rho kinase signaling pathway (19). Conversely, our present findings demonstrate that augmented VSM sensitivity to NO and 8pCPT-cGMP following IH is associated with a greater fall in VSM [Ca2+]i, suggesting a Ca2+ dependency of this response, although these data do not preclude a potential contribution of changes in Ca2+ sensitivity to the observed responses. Furthermore, in contrast to effects of CH to upregulate PKG expression (18), arterial PKG levels were unaltered by IH exposure. Therefore, enhanced NO-dependent vasodilation following IH is likely a function of greater cGMP-mediated PKG activation or amplification of distal components of this signaling pathway. Our present observation that IH augments vasodilation to the PDE-resistant cGMP analog 8pCPT-cGMP (5) argues against a role of altered PDE activity in this response. Future studies, however, are needed to dissect the mechanism by which IH potentiates VSM PKG signal transduction to mediate this response, including possible increases in sarcolemmal Ca2+ extrusion, greater sarcoplasmic reticulum Ca2+ sequestration, or reduced Ca2+ entry.
Enhanced Pulmonary VSM Reactivity to NO following Hypocapnic-IH is Masked by O2−
Candidate ROS species that limit pulmonary vasodilation to both endogenous and exogenous NO following H-IH include O2− and H2O2, both of which have been implicated in the regulation of pulmonary vascular tone (4). However, a primary role for O2− in this response is indicated by the effect of the O2− spin trap tiron to selectively restore NO-dependent vasoreactivity in both isolated lungs and arteries from H-IH rats, whereas responses to NO were unaltered by H2O2 scavenging with PEG-catalase. This effect of O2− to attenuate NO-mediated vasodilation may result from increased vascular O2− generation following H-IH exposure as suggested by greater DHE fluorescence in arteries from this group compared with both E-IH and sham-treated vessels. Although it is not clear why tiron was without effect on NO-mediated vasoreactivity following E-IH despite significantly elevated O2− levels, it is possible that E-IH leads to O2− synthesis within subcellular domains that limit access of O2− to NO or distal targets of NO signaling. Alternatively, it may be that a threshold level of O2− is required to blunt NO-mediated vasodilation.
Based on our findings that endogenous O2− does not alter cGMP-mediated dilation in arteries from H-IH rats, we postulate that O2− limits NO-dependent responses proximal to PKG activation, possibly through oxidation of sGC (17) or direct scavenging of NO to generate ONOO− (13). In agreement with the latter possibility, we found that H-IH increases arterial nitrotyrosine levels, suggesting that production of ONOO− is elevated in response to H-IH. Consistent with this observation, Nisbet and colleagues (27) reported that increases in lung O2− generation following long-term IH exposure in mice are associated with a reduction in bioavailable NO.
Although the vascular cell type responsible for IH-induced ROS generation has yet to be identified, our present finding that O2− inhibits NO-mediated vasodilation in endothelium-disrupted arteries from H-IH rats supports a role for a nonendothelial source of O2− in this response. Therefore, the likely sources of O2− following H-IH are either the VSM (44) or adventitial fibroblasts (23, 37), both of which are reported to be sites of O2− generation in the hypertensive pulmonary circulation (23, 27). The enzyme responsible for increased ROS generation following IH remains unknown. However, NADPH oxidase isoforms (23, 25, 27) and xanthine oxidase (16) are the major sources of ROS implicated in the development of PH, and are candidates for mediating elevated ROS production in response to IH. Indeed, NADPH oxidase-generated ROS play an important role in the development of IH-induced PH and vascular remodeling in mice (27). Alternatively, elevated O2− levels in arteries from H-IH rats could result from decreased superoxide dismutase expression or activity, as previously demonstrated in PH resulting from CH (29). Elucidating the cellular and enzymatic sources of ROS that limit NO-dependent pulmonary vasodilation following H-IH are important areas for future investigation.
Administration of inhaled CO2 has previously been demonstrated to interfere with the development of RV hypertrophy, arterial remodeling, polycythemia, and PH resulting from CH (21, 33) and IH in rats (40). In agreement with these findings are our present observations that CO2 supplementation attenuates both RV hypertrophic and polycythemic responses to 4 wk IH treatment. Although the mechanism responsible for this protective effect of CO2 is currently unknown, it is possible that CO2 reduces lung oxidant stress to attenuate hypoxic PH, as previously demonstrated in response to 10% CO2 exposure in CH neonatal rats (21). Consistent with this possibility, we found that pulmonary arterial O2− levels were lower in arteries from E-IH compared with H-IH rats, and further, that NO-dependent vasodilatory responsiveness was unaltered by O2− scavenging in isolated lungs and arteries from E-IH rats. However, whether supplemental CO2 attenuates the development of IH-induced PH by limiting oxidative stress to increase NO bioavailability remains to be established.
In conclusion, the present study demonstrates a novel effect of IH to augment NO-dependent pulmonary vasodilation by increasing VSM sensitivity to NO and cGMP. However, enhanced pulmonary vasoreactivity to NO following H-IH was masked by increased O2− generation. This effect of O2− is likely mediated by scavenging of NO rather than through attenuation of VSM PKG signaling. Greater NO-dependent vasodilation following IH may have significance in providing a protective influence to limit the severity of PH in this setting, although this response may be compromised by elevated O2− generation associated with H-IH.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-92598 (N. L. Jernigan), HL-82799 (N. L. Kanagy); HL-58124, HL-95640, and HL-07736 (B. R. Walker); HL-88192 and HL-77876 (T. C. Resta).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Minerva Murphy and Dr. Jessica Snow for technical assistance.
REFERENCES
- 1. Allahdadi KJ, Walker BR, Kanagy NL. Augmented endothelin vasoconstriction in intermittent hypoxia-induced hypertension. Hypertension 45: 705–709, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bradford A. Effects of chronic intermittent asphyxia on haematocrit, pulmonary arterial pressure and skeletal muscle structure in rats. Exp Physiol 89: 44–52, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Broughton BR, Jernigan NL, Norton CE, Walker BR, Resta TC. Chronic hypoxia augments depolarization-induced Ca2+ sensitization in pulmonary vascular smooth muscle through superoxide-dependent stimulation of RhoA. Am J Physiol Lung Cell Mol Physiol 298: L232–L242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol Heart Circ Physiol 252: H721–H732, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Butt E, Nolte C, Schulz S, Beltman J, Beavo JA, Jastorff B, Walter U. Analysis of the functional role of cGMP-dependent protein kinase in intact human platelets using a specific activator 8-para-chlorophenythio-cGMP. Biochem Pharmacol 43: 2591–2600, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol 99: 2028–2035, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Dennis KE, Aschner JL, Milatovic D, Shmidt JW, Aschner M, Kaplowitz MR, Zhang Y, Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L596–L607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fagan KA. Selected contribution: pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol 90: 2502–2507, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Fagan KA, Fouty BW, Tyler RC, Morris KG, Hepler L, Sato K, Le Cras TD, Abman SH, Weinberger H, Huang P, McMurtry IF, Rodman DM. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest 103: 291–299, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fletcher EC, Bao G. Effect of episodic eucapnic and hypocapnic hypoxia on systemic blood pressure in hypertension-prone rats. J Appl Physiol 81: 2088–2094, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Fletcher EC, Bao G, Miller CC. Effect of recurrent episodic hypocapnic, eucapnic, and hypercapnic hypoxia on systemic blood pressure. J Appl Physiol 78: 1516–1521, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez Bosc LV, Resta TC, Walker BR, Kanagy NL. Mechanisms of intermittent hypoxia induced hypertension. J Cell Mol Med 14: 3–17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986 [DOI] [PubMed] [Google Scholar]
- 14. Guidry UC, Mendes LA, Evans JC, Levy D, O'Connor GT, Larson MG, Gottlieb DJ, Benjamin EJ. Echocardiographic features of the right heart in sleep-disordered breathing: the Framingham Heart Study. Am J Respir Crit Care Med 164: 933–938, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Isaacson T, Hampl V, Weir E, Nelson D, Archer S. Increased endothelium-derived NO in hypertensive pulmonary circulation of chronically hypoxic rats. J Appl Physiol 76: 933–940, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Jankov RP, Kantores C, Pan J, Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol 294: L233–L245, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Jernigan NL, Resta TC. Chronic hypoxia attenuates cGMP-dependent pulmonary vasodilation. Am J Physiol Lung Cell Mol Physiol 282: L1366–L1375, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Jernigan NL, Walker BR, Resta TC. Pulmonary PKG-1 is upregulated following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 285: L634–L642, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Jernigan NL, Walker BR, Resta TC. Chronic hypoxia augments protein kinase G-mediated Ca2+ desensitization in pulmonary vascular smooth muscle through inhibition of RhoA/Rho kinase signaling. Am J Physiol Lung Cell Mol Physiol 287: L1220–L1229, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension 37: 511–515, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Kantores C, McNamara PJ, Teixeira L, Engelberts D, Murthy P, Kavanagh BP, Jankov RP. Therapeutic hypercapnia prevents chronic hypoxia-induced pulmonary hypertension in the newborn rat. Am J Physiol Lung Cell Mol Physiol 291: L912–L922, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Le Cras TD, Xue C, Rengasamy A, Johns RA. Chronic hypoxia upregulates endothelial and inducible NO synthase gene and protein expression in rat lung. Am J Physiol Lung Cell Mol Physiol 270: L164–L170, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, Hanze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal 10: 1687–1698, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Liu JN, Zhang JX, Lu G, Qui Y, Yang D, Yin GY, Zhang XL. The effect of oxidative stress in myocardial cell injury in mice exposed to chronic intermittent hypoxia. Chinese Med J 123: 74–78, 2010 [PubMed] [Google Scholar]
- 25. Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Nelin LD, Thomas CJ, Dawson CA. Effect of hypoxia on nitric oxide production in neonatal pig lung. Am J Physiol Heart Circ Physiol 271: H8–H14, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol 40: 601–609, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nozik-Grayck E, Stenmark KR. Role of reactive oxygen species in chronic hypoxia-induced pulmonary hypertension and vascular remodeling. Adv Exp Med Biol 618: 101–112, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Nozik-Grayck E, Suliman HB, Majka S, Albietz J, Van Rheen Z, Roush K, Stenmark KR. Lung EC-SOD overexpression attenuates hypoxic induction of Erg-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 295: L422–L430, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohshima H, Friesen M, Brouet I, Bartsch H. Nitrotyrosine as a new marker for endogenous nitrosation and nitration of proteins. Food Chem Toxicol 28: 647–652, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Oka M, Hasunuma K, Webb SA, Stelzner TJ, Rodman DM, McMurtry IF. EDRF suppresses an unidentified vasoconstrictor mechanism in hypertensive rat lungs. Am J Physiol Lung Cell Mol Physiol 264: L587–L597, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Okabe S, Hida W, Kikuchi Y, Taguchi O, Ogawa H, Mizusawa A, Miki H, Shirato K. Role of hypoxia on increased blood pressure in patients with obstructive sleep apnoea. Thorax 50: 28–34, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ooi H, Cadogan E, Sweeney M, Howell K, O'Regan RG, McLoughlin P. Chronic hypercapnia inhibits hypoxic pulmonary vascular remodeling. Am J Physiol Heart Circ Physiol 278: H331–H338, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Ozaki M, Kawashima S, Yanashita T, Ohashi Y, Rikatake Y, Inoue N, Hirata KI, Hayashi Y, Itoh H, Yokoyama M. Reduced hypoxic pulmonary vascular remodeling by nitric oxide from the endothelium. Hypertension 37: 322–327, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Resta TC, Kanagy NL, Walker BR. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am J Physiol Lung Cell Mol Physiol 280: L88–L97, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Resta TC, Walker BR. Chronic hypoxia selectively augments endothelium-dependent pulmonary arterial vasodilation. Am J Physiol Heart Circ Physiol 270: H888–H896, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Rey FE, Pagano PJ. The reactive adventitia: fibroblast oxidase in vascular function. Arterioscler Thromb Vasc Biol 22: 1962–1971, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Russell PC, Emery CJ, Cai YN, Barer GR, Howard P. Enhanced reactivity to bradykinin, angiotensin I and the effect of captopril in the pulmonary vasculature of chronically hypoxic rats. Eur Respir J 3: 779–785, 1990 [PubMed] [Google Scholar]
- 39. Shaul PW, North AJ, Brannon TS, Ujiie K, Wells LB, Nisen PA, Lowenstein CJ, Snyder SH, Star RA. Prolonged in vivo hypoxia enhances nitric oxide synthase type I and type III gene expression in adult rat lung. Am J Respir Cell Mol Biol 13: 167–174, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Snow JB, Kitzis V, Norton CE, Torres SN, Johnson KD, Kanagy NL, Walker BR, Resta TC. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol 104: 110–118, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Troncoso Brindeiro C, da Silva A, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol 293: H2971–H2976, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tyler RC, Muramatsu M, Abman SH, Stelzner TJ, Rodman DM, Bloch KD, McMurtry IF. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 276: L297–L303, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Waypa GB, Schumacker PT. Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. J Appl Physiol 98: 404–414, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Wedgwood S, Dettman RW, Black SA. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 281: L1058–L1067, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Wolin MS, Gupte SA, Neo BH, Gao Q, Ahmad M. Oxidant-redox regulation of pulmonary vascular responses to hypoxia and nitric oxide-cGMP signaling. Cardiol Rev 18: 89–93, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu J, Xie Z, Reece R, Pimental D, Zou MH. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol 26: 2688–2695, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 1217–1239, 2002 [DOI] [PubMed] [Google Scholar]