Abstract
A two-arm, prospective, randomized, controlled trial study was conducted to investigate the effects of movement velocity during progressive resistance training (PRT) on the size and contractile properties of individual fibers from human vastus lateralis muscles. The effects of age and sex were examined by a design that included 63 subjects organized into four groups: young (20–30 yr) men and women, and older (65–80 yr) men and women. In each group, one-half of the subjects underwent a traditional PRT protocol that involved shortening contractions at low velocities against high loads, while the other half performed a modified PRT protocol that involved contractions at 3.5 times higher velocity against reduced loads. Muscles were sampled by needle biopsy before and after the 14-wk PRT program, and functional tests were performed on permeabilized individual fiber segments isolated from the biopsies. We tested the hypothesis that, compared with low-velocity PRT, high-velocity PRT results in a greater increase in the cross-sectional area, force, and power of type 2 fibers. Both types of PRT increased the cross-sectional area, force, and power of type 2 fibers by 8–12%, independent of the sex or age of the subject. Contrary to our hypothesis, the velocity at which the PRT was performed did not affect the fiber-level outcomes substantially. We conclude that, compared with low-velocity PRT, resistance training performed at velocities up to 3.5 times higher against reduced loads is equally effective for eliciting an adaptive response in type 2 fibers from human skeletal muscle.
Keywords: permeabilized single fibers, aging
aging is associated with progressive declines in mass, force, and power of limb muscles of mammals in species as divergent in body mass and lifespan as mice (5, 6), rats (7), and humans (23, 34). Humans lose ∼40% of their muscle mass by age 80 yr due to both the loss of fibers and fiber atrophy, with a preferential reduction in the cross-sectional area (CSA) of the fast, powerful fibers that contain type 2 myosin (“type 2 fibers”) (23). Type 2 fibers generate four- to sixfold more power per unit mass than slow fibers that contain type 1 myosin (“type 1 fibers”) (35). Consequently, the preferential loss of type 2 fiber mass with aging results in a deficit in power generation that greatly exceeds the loss in muscle mass. Impaired mobility and a high incidence of falls are characteristic of the frail older person (42). Since powerful countermovements are frequently required for the prevention of falls (30), the deficit in power is an important factor that contributes to the increase in falls in the elderly. Maintenance and improvement of the function of the remaining fibers, particularly the type 2 fibers, thus becomes increasingly important with advancing age. Resistance training programs have the potential to reverse some of these age-related changes and improve the quality of life for the elderly frail population.
Resistance training most commonly entails repetitive slow shortening of skeletal muscles against loads that are large relative to the collective force-generating capacity of the participating muscles. A program of such training results in increases in the maximum forces that the muscles can generate. During a course of progressive resistance training (PRT), loads are typically increased as the training progresses, such that the force generated remains a high percentage of maximum, and the velocities at which the muscles shorten remain low. In both young (43) and older (16, 36, 37) human subjects, 12 wk of PRT increases the CSA, maximum isometric force (Fo) and peak power (Pmax) of type 2 fibers within the trained muscles.
Recognition of the importance of maintaining or improving power-generating capabilities has given rise to studies in which more powerful, high-velocity movements are employed during PRT in older subjects (2, 12, 24). Results from these studies indicate that “high-velocity” PRT is more effective than traditional, “low-velocity” PRT with respect to increasing the power output of whole muscle groups, but the mechanisms responsible for this advantage have not been identified. The goal of the present study was to determine, at the level of individual muscle fibers, whether high-velocity PRT results in greater enhancements in force and power compared with low-velocity PRT. The study included young and older male and female subjects who were randomized to either low-velocity or high-velocity PRT groups. Muscles were sampled by needle biopsy before and after a 14-wk training period, and functional tests were performed on individual fiber segments isolated from the biopsies. We tested the hypothesis that, compared with low-velocity PRT, a modified PRT regimen that consists of faster movements results in additional increases in the CSA of type 2 fibers, with corresponding improvements in the ability of the fibers to generate force and power. Secondary hypotheses were that neither sex nor age affects the responses to PRT. The results indicated that both types of PRT increased the CSA, force, and power of type 2 fibers, independent of the subjects' sex and age. Contrary to our primary hypothesis, the velocities at which the PRT was performed did not substantially affect the outcomes.
MATERIALS AND METHODS
Human subjects.
Healthy young men (24.2 ± 2.3 yr, mean ± SD), older men (76.0 ± 3.8 yr), young women (24.9 ± 2.7 yr), and older women (75.2 ± 4.3 yr) underwent medical history and physical examination screening by a nurse practitioner under the supervision of a physician geriatrician. Participants had no evidence of musculoskeletal, neurological, or cognitive impairment and had not participated in PRT in the past 6 mo. Written, informed consent was obtained from each participant after all procedures were approved by the University of Michigan Institutional Review Boards of the UM Medical School.
Experimental design.
Subjects were block-randomized into one of two arms of a 14-wk PRT exercise intervention aimed at strengthening the lower extremity extensor muscle strength and power. One group performed “high-velocity” PRT of the leg muscles, whereas the other performed the traditional “low-velocity” PRT of the leg muscles (details below). Subcutaneous needle biopsies of the vastus lateralis (VLT) were performed both before and at the conclusion of the 14-wk training period. Details of the training and biopsy protocols are provided below. Fifteen young men (low-velocity group: 6; high-velocity group: 9) and 14 older men (low-velocity group: 7; high-velocity group: 7) completed the training. A total of 18 young women (low-velocity group: 9; high-velocity group: 9) and 16 older women (low-velocity group: 8; high-velocity group: 8) completed the training. Approximately 40 fibers were tested for each subject, including ∼10 type 1 and 10 type 2 fibers from both the pre- and posttraining biopsies.
PRT.
Upon being accepted for entry into the study, subjects attended three strength training sessions each week for 14 wk. The first 2 wk of training were performed using relatively light weights and served as a “ramp-up” period during which subjects became accustomed to using standard seated leg press (model 1203 Leg Press, Magnum Fitness Systems, South Milwaukee, WI) and standing hip flexion (model 1010 Multi-hip, Magnum Fitness Systems) machines with adjustable weight stacks. The target muscles were the hip and knee extensors for the leg press and hip flexors for the hip flexion machine, which was included because one of the knee extensors also flexes the hip.
During each training session, subjects received real-time visual and audio feedback on the angular velocity of their knee flexion angle (Training Buddy, Bio Logic Engineering, Dexter, MI). Subjects were required to keep the peak velocity within specified limits, depending on their group assignment. If the movement velocity exceeded the upper bound, a high warning tone was emitted, and if the velocity dropped below the lower bound limit, a low warning tone was emitted. Both the subject and the trainer monitored the maximum velocity so that it remained within the set bounds. The velocities employed by the “high-velocity” group were selected, during a series of preliminary experiments, to be significantly greater than those of the “low-velocity” group and closer to the velocities at which maximum power is developed in vivo, but not so fast as to increase the risk of injury to the participants. For the hip training, the upper and lower limits for the high- and low-velocity groups were 350 and 250°/s, and 90 and 30°/s, respectively. The corresponding limits for the knee training were 160 and 100°/s, and 40 and 20°/s, respectively. New weights were assigned by the trainer every 2 wk, such that the subject could just perform two sets of 10 repetitions. This was followed by a third set during which failure to maintain the desired movement velocity through the full range of motion occurred after 5–15 repetitions. The number of repetitions completed in the third set was recorded.
Whole limb strength testing.
Every 2 wk, subjects were tested to determine their “1-repetition maximum” (1 RM, a measure of their maximum strength) on the leg press and hip flexor machines at a self-selected velocity. After warm-up exercises, the 1 RM was established as the maximum weight that a subject could move once through the range of motion, with both feet in contact with the foot plate (leg-press machine) and while standing on one leg with the padded arm in contact with the flexed distal thigh (hip flexion machine).
Muscle biopsy.
A core needle biopsy of the VLT muscle was performed on each subject before any training or functional testing. All biopsies were taken from the right leg, midthigh, laterally overlying the VLT muscle belly. Following shaving and preparation of the skin with Betadine, 3 ml of 2% lidocaine with 1:200,000 epinephrine were infiltrated subcutaneously for local anesthesia. Care was taken to avoid injection of the VLT with the local anesthetic. A 5-mm incision was made with a scalpel in the skin overlying the biopsy site. Approximately 50 mg of VLT were obtained atraumatically with a University College Hospital (London, UK) skeletal muscle core biopsy needle inserted through the incision and into the muscle. At the end of the 14-wk training period, 3–4 days after the final assessment of strength and power, a second muscle biopsy was obtained using the same procedure. The second biopsy was obtained from the same leg, 3–4 cm distal to the site of the first biopsy.
Permeabilized single-fiber procedures and apparatus.
The muscle sample was removed quickly from the biopsy needle and placed immediately into cold skinning solution. Fiber bundles ∼4–6 mm in length and 0.5 mm in diameter were dissected from the samples. Following dissection, bundles were immersed for 30 min in skinning solution to which the nonionic detergent Brij 58 had been added (0.5% wt/vol). Fiber bundles were then placed in storage solution and maintained for 24 h at 4°C, followed by storage at −20°C. On the day of an experiment, a bundle of permeabilized fibers was removed from storage solution and placed in relaxing solution on ice. Individual permeabilized fibers were pulled manually from the bundle with fine forceps and transferred to an experimental chamber containing relaxing solution maintained at 15°C. One end of the fiber was secured to a force transducer (Aurora Scientific, model 403A) using two ties of 10–0 monofilament nylon suture. The other end of the fiber was attached in a similar manner to the lever arm of a servomotor (Aurora Scientific, model 322C). The solution-changing system (Aurora Scientific, model 802A) consisted of six separate glass-bottom chambers machined into a moveable, temperature-controlled, stainless-steel plate. Movement of the plate with respect to the fiber was achieved by remote control of two stepper motors: one to lower and raise the chamber array and the other to translate the plate to a new chamber position. For all experiments reported here, the length of the fiber was adjusted to obtain an average sarcomere length of 2.5–2.6 μm, determined by projecting a laser diffraction pattern produced by the fiber onto a calibrated target screen. The laser beam had a diameter of ∼180 μm and passed through the fiber at a location approximately midway between the attachment points. Published thin-filament lengths for human skeletal muscle (40) that came to our attention late in the study indicate that a more appropriate initial sarcomere length for these experiments would have been 2.7 μm. That is, our measurements were likely made at sarcomere lengths on the ascending limb of the length-tension relationship, near the plateau. After setting sarcomere length, fiber length (Lf) was measured by first aligning the innermost tie at one end of the fiber with the crosshairs of a microscope eyepiece graticule, then translating the entire apparatus with respect to the microscope using a micrometer drive with digital readout until the innermost tie at the other end of the fiber was aligned with the crosshairs. Fiber CSA was estimated with the fiber length held at Lf using fiber width and depth measurements from high-magnification digital images of both top and side views of the fiber. Side views were obtained using a prism embedded in the side of the bath. Five width-depth measurement pairs were obtained at ∼100-μm intervals along the midsection of the fiber. Fiber CSAs were calculated for each width-depth pair, assuming an elliptical cross section, and overall CSA was estimated by averaging the five individual areas. Great care was taken to determine the CSA of each fiber because the value was used to: 1) indicate fiber size, 2) normalize absolute Fo determined at pCa 4.5 (Fo; mN) to obtain specific force (sFo; kPa), and 3) normalize peak power (Pmax, nW) to obtain normalized peak power (nPmax; W/l). Relaxed single fibers were activated by first immersing them in a chamber containing a low- Ca2+ concentration ([Ca2+]) preactivating solution for 3 min and then immersing them in a chamber containing high-[Ca2+] activating solution (pCa ∼4.5) to elicit Fo. The preactivating solution was weakly buffered for Ca2+, resulting in very rapid activation and force development upon introduction of the activating solution (26). Fibers were stored for up to 12 mo before testing, and sFo, velocity, and power exhibited small declines in value that correlated with storage time. These trends were removed by first plotting the measurements as functions of storage time and then fitting lines by least-squares regression to determine the rates of decline. Measured values were then scaled as a function of storage time, such that the slope of the resulting regression line was zero, but the intercept was unchanged. Before detrending, the rates of decline of force, velocity, and power, expressed in percent per day, were 0.07, 0.05, and 0.13, respectively, for type 1 fibers and 0.05, 0.08, and 0.13 for type 2 fibers. Force responses and motor position were acquired at 5k samples/s through a 16-bit analog-digital board (NI-6052-PCI, National Instruments) and displayed and stored on a personal computer using a custom-designed LabVIEW program (National Instruments). The position of the motor was updated at a rate of 10k/s by the LabVIEW program via a digital-analog channel on the acquisition board.
Solutions.
The skinning solution was composed of the following (in mM): 125 potassium propionate; 20 imidazole; 5 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA); 2 MgCl2; 2 ATP; pH 7.0. The composition of the storage solution was identical to skinning solution, with the exception that glycerol was substituted for 50% of the water volume. The relaxing, preactivating, and activating solutions were modified from Moisescu and Thieleczek (26). The relaxing solution (pCa ∼9.0) was composed of the following (in mM): 90 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES); 10.3 Mg (total); 1.0 Mg2+; 50 EGTA; 8.0 ATP; 10.0 CrP; 1.0 NaN3; 36 Na (total); 125 K (total); pH 7.1. Preactivating solution (pCa ∼8.0) was as follows (in mM): 90 HEPES; 8.50 Mg (total); 1.0 Mg2+; 0.10 EGTA; 50 1,6-diaminohexane-N,N,N′,N′-tetraacetic acid (HDTA); 8.0 ATP; 10.0 CrP; 1.0 NaN3; 36 Na (total); 125 K (total); pH 7.1. The activating solution (pCa ∼4.5) was as follows (in mM): 90 HEPES; 8.12 Mg (total); 1.0 Mg2+; 50 EGTA; 50 Ca2+ (total); 8.0 ATP; 10.0 CrP; 1.0 NaN3; 36 Na (total); 125 K (total), pH 7.1, and was confirmed, through a separate series of force-pCa experiments, to have a [Ca2+] that exceeded that required to elicit Fo. Potassium propionate was obtained from TCI America, and all other compounds were obtained from Sigma Chemical.
Force-velocity and power measurements.
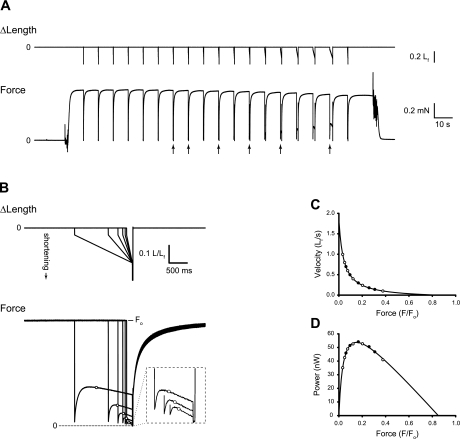
Force-velocity characteristics were evaluated by applying a series of step-ramp shortening movements to the fully activated fiber from an initial length of Lf + 10% of Lf. The step ranged in amplitude from 3–5% of Lf, and its purpose was to discharge the strained series elasticity before the ramp, thereby reducing the time required for the tension maintained during the ramp to reach a steady state. The total amplitude of the step-ramp was 20% of Lf. Immediately following each ramp, an additional shortening step equivalent to 10% of Lf was applied to the fiber, followed 20 ms later by a step return to the original length. The final shortening step slackened the fiber briefly, and its purposes were to 1) indicate the location of the force baseline in the experimental recording; 2) ensure that the subsequent rapid reextension of the fully activated fiber to original length did not damage the fiber; and 3) maintain the homogeneity of the striation spacing by being functionally equivalent, when coupled with the reextension, to the rapid shortening-reextension cycle described by Brenner (4). After force regeneration was complete following the return to original length (8–20 s, depending on fiber type), the next step-ramp was applied. Ramps were administered in sequence from fast to slow. The force associated with a given shortening velocity was measured at the time that the fiber had shortened by 0.1 Lf, and, consequently, its length was passing through Lf. Force (F) was measured relative to the baseline revealed by the postramp step and was normalized by the force immediately preceding the step-ramp (Fo) to obtain F/Fo. Force-velocity pairs for which F/Fo was < 0.025 were excluded from the analysis. This procedure resulted in 8–12 data points to which a rectangular hyperbola of the form V = (Fo/Fo − F/Fo)/(a/Fo + F/Fo) was fitted, where V is velocity of shortening, F′o is the intercept with the force axis, and a and b are the force and velocity asymptotes, respectively. This is the force-velocity relationship described by Hill (19), with the added parameter F′o, which eliminates the requirement that the fitted hyperbola intersect the force axis at F/Fo = 1 (3, 9, 11, 21). Values for a, b, and F′o were determined using a curve-fitting algorithm that computed the least squares best fit of the hyperbola to the V and F/Fo measurements. The addition of F′o as a fitting parameter improves the fit of the hyperbola throughout the range of measured F/Fo values, including the F/Fo at which Pmax is generated. Experimental recordings and the procedure for performing the force-velocity analysis are illustrated in Fig. 1 for a representative fiber. The maximum power-generating capacity (Pmax, the peak of the force-power curve, Fig. 1D) was calculated from the parameters of the fitted curve, according to Eq. 1, and then divided by fiber volume (Lf × CSA) to obtain nPmax (W/l).
| (1) |
Fig. 1.
Determination of fiber force-velocity and force-power characteristics. A: step-ramp shortening movements (top) applied by means of the servomotor, and corresponding force (F) responses (bottom) for a representative type 2 (fast) fiber. Arrows beneath the force record indicate responses that are shown in greater detail in B. B: superimposed fiber length (top) and force response (bottom) records shown on expanded time scales. For clarity, only a subset of the events shown in A (see arrows) are reproduced in B. Force records have been scaled such that all have matching prerelease values. The force value associated with a given ramp velocity was recorded as fiber length passed through optimal length (Lf) during the ramp shortening, and those values are indicated with open circles on the force records. The inset replicates, on expanded scales (×4 for both time and force), the force responses to the three highest-velocity shortening ramps shown in the main figure. C: plot of the force-velocity pairs and the fitted rectangular hyperbola. D: force-power relationship obtained by multiplying force × velocity for all measured pairs and for the fitted curve shown in C. For both C and D, all measured force-velocity pairs are shown, and the open symbols indicate the subset of measurements illustrated in B. Fiber 150-2-01-12. Fo, maximum isometric force.
Fiber types.
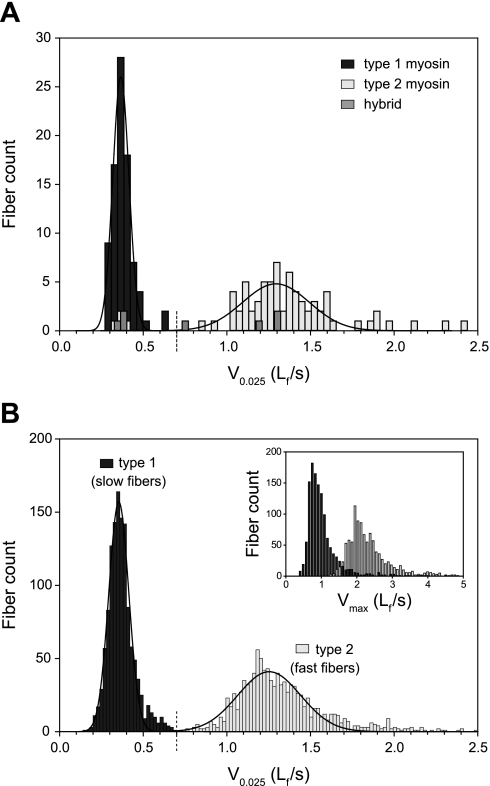
Myosin heavy chain (MHC) isoform analysis was performed on 168 of the 2,471 fibers tested to determine whether the MHC isoform present in a fiber could be predicted from its force-velocity characteristics. Complete force-velocity relationships were determined for the 168 fibers, and a rectangular hyperbola was fitted to each set of force-velocity measurements as described above. The velocity at which the hyperbola passed through a force of F/Fo = 0.025 (V0.025, Lf/s) served as the index of intrinsic fiber shortening velocity. This velocity was chosen because it required little or no extrapolation of the fitted hyperbola beyond measured data points. Avoidance of extrapolation is particularly important when analyzing the shortening velocity characteristics of human fibers because of the high degree of curvature (low a/Fo values) that the human fiber force-velocity relationship exhibits (3, 35, 43). As a consequence of the extreme curvature, the fitted curve approaches the velocity axis at a very acute angle, causing small variations in fit to have a large influence on the value at which the curve intersects the velocity axis, Vmax. Fibers were classified as fast if the V0.025 was >0.7 Lf/s and slow if V0.025 was <0.7 Lf/s. Fibers were stored at −80°C until analyzed for MHC isoform by polyacrylamide gel electrophoresis (PAGE). Each fiber was assigned to one of three MHC groups based on isoform content. Type 1 fibers were those containing MHC1 exclusively. Type 2 fibers contained MHC2a, MHC2x, or a combination of 2a and 2x isoforms. Fibers that contained MHC1, combined with either or both of the MHC2 isoforms, were assigned to the “hybrid” MHC group. The histogram of V0.025 values shown in Fig. 2A superimposes the two classification systems by using the shading of the bars to indicate MHC type. For the 168 fibers analyzed, 93 were slow (V0.025 < 0.7 Lf/s) and 75 were fast (V0.025 > 0.7 Lf/s). Of the 93 slow fibers, 86 (92.5%) were MHC type 1, 4 (4.3%) were MHC type 2, and 3 (3.2%) were MHC hybrids. For the 75 fast fibers, 71 (94.7%) were MHC type 2, 4 (5.3%) were MHC hybrids, and none was MHC type 1. We concluded that, for fibers from VLT muscles of humans (tested at 15°C), V0.025 = 0.7 Lf/s is a good discriminator of MHC type and, consequently, we did not perform PAGE analyses on all fibers. For the fibers in which MHC content was not determined by PAGE in this study, those having a V0.025 < 0.7 Lf/s were classified as “type 1”, and those having a V0.025 > 0.7 Lf/s were classified as “type 2”. Figure 2B shows the V0.025 distribution for all 2,471 fibers tested in this study and exhibits two well-discriminated subdistributions that share a border at V0.025 = 0.07 Lf/s. The inset in Fig. 2B shows that the Vmax distribution, although clearly bimodal and qualitatively similar to the V0.025 distribution, is not sufficiently discriminating to provide unambiguous classification of fiber type.
Fig. 2.
Fiber-type classification. A: histogram showing the distributions of fiber shortening velocities [velocity of shortening at F/Fo = 0.025 (V0.025)] for fibers containing type 1 myosin (dark-shaded bars), type 2 myosins (light-shaded bars), and combinations of type 1 myosin with type 2 myosins (“hybrid”, medium-shaded bars). Myosin isoform content was determined by polyacrylamide gel electrophoresis (PAGE). The solid lines are Gaussian functions fitted, separately, to the type 1 and type 2 fiber data. The dashed vertical line that crosses the velocity axis at V0.025 = 0.7 Lf/s indicates the velocity boundary used to assign fiber type in the absence of PAGE results (see B). B: histogram showing the distribution of fiber shortening velocities (V0.025) for all fibers in the study. Fibers were assigned to a fiber-type group based exclusively on their shortening velocity; fibers with V0.025 < 0.7 Lf/s were designated “type 1” (dark-shaded bars), and those with V0.025 > 0.7 Lf/s were designated “type 2” (light-shaded bars). The solid lines are Gaussian functions fitted, separately, to the type 1 and type 2 fiber data. The inset shows the maximal velocity (Vmax) distribution for all fibers, but maintains the fiber-type designation based on V0.025 as in the main graph.
Statistical analysis.
The dependent variables (DV) for the fibers included CSA, Fo, sFo, V0.025, Pmax, and nPmax. The effects of various predictors on each DV were studied separately for type 1 and type 2 fibers using linear mixed-effects models of analysis of variance (38, 41). Linear mixed-effects models were chosen to accommodate the expected correlation of pretraining and posttraining measurements for multiple fibers taken from the same subject. The main advantage of using this class of models rather than classical repeated-measures analysis of variance is that they allow for observations missing at random and for unbalanced data, both encountered in this study. We considered models of the following form:
where DV includes measures of a selected dependent variable for all fibers of a given type (1 or 2). RI (random intercept for subject) is included to account for the possibility that DV measures taken from a given subject are correlated. Age group and sex are factors associated with the effects of age and sex on a given DV. Two other predictors, time and training group, are associated with the effects of time (pre- or posttraining) and training group (high velocity or low velocity), respectively. The time × training group interaction term was of primary interest in the analysis, because it allowed testing of the primary hypothesis that, for a given DV and fiber type, the pre-post training difference was greater for the high-velocity group compared with the low-velocity group. The effects of covariates, including the time × training group interaction term, on DV were tested using F-tests. For cases in which the time × training group interaction term was not significant, it was omitted from the model, and results from the reduced model are reported. To satisfy the normality assumptions of linear mixed-effects models, the DV were transformed before analysis using a logarithmic function. Consequently, an exponential back-transformation was applied before expressing the effects of predictors on DV (in percent difference) in Figs. 5 and 6. Models were fitted, and computations were performed using PROC MIXED (SAS 9.1). Sample size calculations were based on the work of Galecki et al. (15). The level of significance was set a priori at P < 0.05.
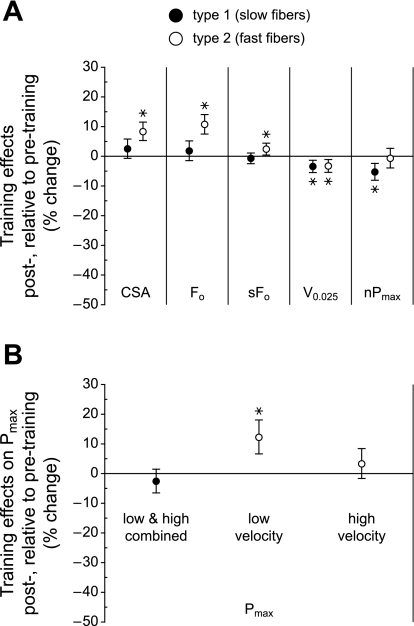
Fig. 5.
Effects of training. A: the 95% confidence intervals for the effects of training (posttraining minus pretraining), adjusted for sex, age, and training group, are shown for the following independent variables: CSA, Fo, specific force (sFo), V0.025, and nPmax. A positive change indicates that the measurement was higher in fibers obtained from subjects after PRT relative to before PRT; a negative change indicates that the measurement was lower following PRT. B: effects of training on Pmax. Confidence intervals (95%) are plotted as in A, except that the results from low-velocity and high-velocity PRT have been separated for type 2 fibers because the posttraining increase could only be attributed to the low-velocity training. For the type 2 fibers, n = 230 and 268 for low-velocity pre- and posttraining and n = 261 and 290 for high-velocity pre- and posttraining, respectively. *P < 0.05.
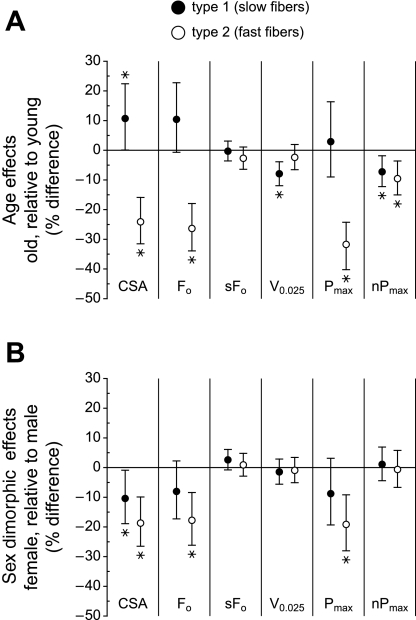
Fig. 6.
Effects of age and sex on type 1 and type 2 fibers. A: the 95% confidence intervals for the effects of age (older minus young), adjusted for sex, trained state (pre or post), and training group, are shown. A positive change indicates that the measurement was higher in fibers obtained from older subjects relative to young subjects; a negative change indicates that the measurement was lower in older subjects. B: the 95% confidence intervals for the effects of sex (female minus male), adjusted for age, trained state (pre or post), and training group, are shown. A positive change indicates that the measurement was higher in fibers obtained from female subjects relative to male subjects; a negative change indicates that the measurement was lower in female subjects. *P < 0.05.
RESULTS
Training velocities.
Across all groups and time points, the “high-velocity” group trained on the leg-press machine at a mean (SD) knee extension velocity of 111°/s (SD 31), or 3.5× faster than the “low-velocity” group, which trained at 31°/s (SD 3). The corresponding velocities for the hip flexion training were 276°/s (SD 37) for the “high-velocity” group and 72°/s (SD 8) for the “low-velocity” group.
Increases in training weight at the whole limb level.
Over the 14-wk PRT period, all groups exhibited increases in the weight that they were capable of lifting in the leg press. For young men, the average weight lifted in the last 2 wk of leg-press training compared with that lifted during weeks 3 and 4 of training increased by a mean (range) of 49% (38–90%) and 56% (3–93%) for the low-velocity and high-velocity PRT groups, respectively; for the older men, it increased by 54% (22–101%) and 39% (8–100%), respectively. The corresponding increases in leg-press weight for the young women were 38% (23–113%) and 36% (7–165%), whereas for the older women increases were 41% (15–79%) and 26% (0–48%), respectively. Similar, but more pronounced, results were observed for the weight lifted during hip flexor muscle training: in the young men the increases were 70% (47–151%) and 87% (28–154%) for the low-velocity and high-velocity PRT groups, whereas they were 76% (25–171%) and 59% (18–197%), respectively, for the older men; for the young women increases were 60% (16–132%) and 82% (13–252%), and for the older women increases were 62% (2–88%) and 96% (44–160%), respectively. Over the last 12 wk of the leg-press PRT, the mean (range) training weight increased from 72% (65–80%) to 84% (64–96%) of the 1 RM across all groups.
Fiber types.
In the subset of fibers for which myosin isoforms were identified by PAGE (168 of 2,471), two distinct velocity populations were evident, and they corresponded to two distinct myosin isoform populations (Fig. 2A). Of the 2,471 fibers tested, 1,299 were classified as type 1, and 1,172 were classified as type 2 using velocity of shortening as the classification criterion (see materials and methods). The two distinct populations of velocities apparent in Fig. 2A remained in evidence in the velocity distribution that included all fibers (Fig. 2B). This observation provided strong support for the validity of the velocity-based classification criterion.
Effects of training on fiber CSA, force, velocity, and power.
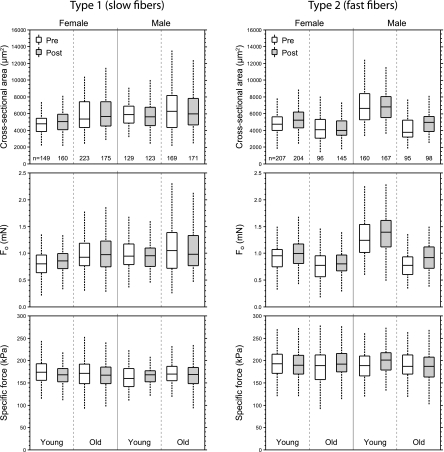
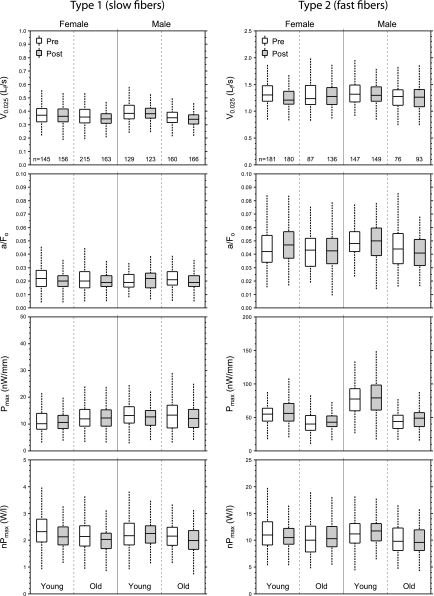
Size and force data for all fibers, separated by fiber type, subject sex, age, and training status, are displayed as box plots in Fig. 3. Corresponding box plots for velocity and power measurements are shown in Fig. 4. Type 1 fibers exhibited no training-related increases in any of the fiber characteristics that were measured (Fig. 5). In contrast, the PRT resulted in increases of 8.3% in CSA, 10.7% in absolute force (Fo), and 12.2% in absolute power (Pmax) of type 2 fibers (Fig. 5). The posttraining increase observed in the Pmax of type 2 fibers was not due to training-induced changes in the shape of the force-velocity relationship (13, 43); posttraining a/Fo values were not different from the pretraining values (P = 0.235). For both type 1 and type 2 fibers, the training-related changes shown in Fig. 5 were independent of subject sex and age.
Fig. 3.
Cross-sectional area (CSA) and force empirical distributions. The box plots indicate the median and the 25th and 75th quartiles. The dashed vertical lines that originate from the top and bottom surfaces of the boxes extend to the outermost data points that fall within 1.5× the difference between the 75th and 25th quartiles. Unshaded boxes correspond to values from fibers obtained before the subjects undertook the progressive resistance training (PRT); shaded boxes correspond to values from fibers obtained after training. The number of fibers in each group is indicated at the bottom of the topmost panels. Data from the low-velocity and high-velocity PRT groups are combined in these plots. Results of the statistical analyses of main effects appear in Figs. 5 and 6.
Fig. 4.
Velocity and power empirical distributions. The box plots indicate the median and the 25th and 75th quartiles. The dashed vertical lines that originate from the top and bottom surfaces of the boxes extend to the outermost data points that fall within 1.5× the difference between the 75th and 25th quartiles. Unshaded boxes correspond to values from fibers obtained before the subjects undertook the PRT; shaded boxes correspond to values from fibers obtained after training. The number of fibers in each group is indicated at the bottom of the topmost panels. Note that, with the exception of a/Fo, the vertical scales on the plots for type 2 fibers differ from the vertical scales on the plots for type 1 fibers. Also note that the fibers that contributed to the data shown in Fig. 4 are, for most groupings, a subset of the fibers that contributed to the data shown in Fig. 3, because not all fibers survived the rigorous force-velocity experimental protocol. Data from the low-velocity and high-velocity PRT groups are combined in these plots. Results of the statistical analyses of main effects appear in Figs. 5 and Fig. 6. Pmax, peak power; nPmax, normalized peak power.
Contrary to our hypothesis that high-velocity training results in preferential increases in the size, force, and power of type 2 fibers, the responses to training were almost completely independent of the velocity at which the PRT was performed. The only exception was that the increase in the Pmax of type 2 fibers could only be attributed to the low-velocity training (Fig. 5B).
Age and sex effects on single-fiber characteristics.
Across all fibers, the effects of age on fiber size and function were most pronounced for type 2 fibers (Fig. 6A), with large reductions observed in CSA (24.1%), absolute force (26.3%), and power (32.7%) in fibers from older subjects. The only positive change observed with aging was an 11% increase in the CSA of type 1 fibers.
The CSA of both type 1 and type 2 fibers obtained from female subjects were 10.4% and 18.7% smaller, respectively than the corresponding fibers from male subjects. Type 2 fibers from female subjects generated 17.8% less force and 19.2% less power than those from male subjects (Fig. 6B). The intrinsic force- (sFo) and power-generating (nPmax) capabilities were independent of sex.
DISCUSSION
Compared with traditional “low-velocity” PRT, training that is performed using higher velocity movements has been reported to increase the power output of the participating muscle groups as measured in vivo (2, 12, 24). The aim of the present study was to determine whether high-velocity PRT results in greater enhancements in force and power at the level of individual muscle fibers. To the best of our knowledge, this is the first report to compare directly the functional properties of individual fibers obtained before and after both low-velocity and high-velocity PRT. The main finding was that, whereas both types of PRT produced moderate increases in the size, force, and power of type 2 fibers, there was no evidence at the single-fiber level that the high-velocity PRT resulted in greater increases than the low-velocity PRT. Indeed, the increased power-generating capability observed in type 2 fibers following training could only be attributed to the low-velocity PRT (Fig. 5B). Thus our findings do not support the hypothesis that the positive results observed in vivo by others arise from enhanced fiber function. An alternative explanation for the enhanced in vivo response to high-velocity PRT is that it results from neural adaptations (18, 27) rather than muscle fiber adaptations.
Effects of training on fiber size and force.
Increases in the CSA of type 2 fibers are among the most consistently reported consequences of PRT, with young men (22, 25, 43), young women (22, 25, 31), older men (22, 25, 33, 37, 39), and older women (8, 22, 25, 33) responding, although the absence of a response in older women has also been reported (16, 31, 36). Our results are in general agreement with past findings; type 2 fiber CSA increased in response to PRT, independent of subject sex and age. In addition, we report the novel finding that the increases in type 2 fiber size are independent of the velocity at which the training was performed.
There is less agreement regarding the effects of PRT on the CSA of type 1 fibers. Several groups have reported PRT-induced increases in the CSA of type 1 fibers in young subjects (22, 25, 43) and older subjects (16, 36, 37), but others report no change in young subjects (1, 17, 31) or older subjects (8, 14, 22, 25, 33, 39). We found no change in the CSA of type 1 fibers in response to either type of PRT, independent of subject sex and age. All of these training studies employed movements in which the trained muscles were shortening against resistance. During muscle shortening at any velocity, participating type 2 (fast) fibers operate at a higher fraction of their maximum force capacity (F/Fo) than coparticipating type 1 (slow) fibers due to the differences in their respective force-velocity characteristics. This could be the basis for the differential response of the two fiber types reported here and elsewhere. Studies in which isometric training was included in addition to low-velocity PRT have reported preferential increases in whole-muscle Fo in the isometric groups (10, 20). One interpretation of these results is that the adaptive stimulus is more equally extended to both fiber types during isometric training than during training in which shortening movements are employed.
The increase in maximum force production (Fo, mN) in type 2 fibers was similar in magnitude to the increase in CSA, indicating that neither low-velocity nor high-velocity PRT affected substantially the intrinsic force-generating capability (sFo, kPa) of the constituent myofibrils. This finding is in agreement with those from previous studies in which low-velocity PRT was employed (14, 16, 36, 37, 43), although a much longer PRT duration (52 wk) has been reported to increase sFo in both young (28) and older (29) women.
Effects of training on fiber shortening velocity and power.
Relative to pretraining values, very small declines in our measure of intrinsic shortening velocity (V0.025) were observed after PRT, independent of the velocity at which the training was carried out (Fig. 5A). Coupled with the absence of a training effect on the shape of the force-velocity relationship, as indicated by the absence of an effect on a/Fo, this suggests that the training-induced increase in absolute power observed in type 2 fibers is attributable primarily to the increase in their absolute force-generating capacity, a direct consequence of their larger CSA. Moreover, the statistical analysis indicated that the increase in power of the type 2 fibers could only be attributed to the low-velocity PRT protocol, not the high-velocity PRT (Fig. 5B). The PRT-induced changes in measurements affected by intrinsic myofibrillar shortening velocity (V0.025, nPmax) were either minor or absent (Fig. 5A).
Effects of age and sex on fiber size and function.
Although age and sex effects were not the main focus of this study, the inclusion of two age groups and both sexes, coupled with the large number of fibers tested, allow comment on age-related (Fig. 6A) and sex-related (Fig. 6B) differences in fiber properties. The most striking effect of increased age was a large, sex-independent decrease in the CSA of type 2 fibers that resulted in substantial reductions in their absolute force and power-generating capabilities (Fig. 6A). In contrast, the CSA of type 1 fibers increased moderately with age. No substantive changes were observed in any of the intrinsic properties (sFo, V0.025, nPmax) of either type 1 or type 2 fibers, indicating that the function of the myofibrils within fibers from older subjects is not fundamentally different from that of fibers from younger subjects. Our results showing the changes in fiber properties with age are in general agreement with those from a similar study (35), with the notable exception that, compared with type 2 fibers from younger men, no changes in size, absolute force, or absolute power were observed in the type 2 fibers from older men in the previous study.
When comparisons were made on the basis of sex (Fig. 6B), type 2 fiber CSA was substantially smaller in women than in men, and type 1 fiber CSA was moderately smaller, independent of subject age. The smaller size of the type 2 fibers resulted in similarly reduced absolute force and power in the fibers from women. As with age effects, the intrinsic properties of the fibers obtained from women were not different from those of fibers obtained from men; the functional differences observed were due to differences in fiber size.
Relationship between fiber adaptations and weight lifted during PRT.
The observation that the magnitude of the gains in weight lifted in response to PRT (26–96%) exceeded the gains in type 2 fiber CSA, strength, and power (8–12%) is in agreement with earlier findings that have implicated neural recruitment mechanisms as contributors to large gains in muscle strength in vivo during PRT (e.g., Ref. 27). The greater increases recorded for the hip flexor weight lifted than the leg-press weight lifted likely reflect the additional motor learning required during the hip PRT for the subject to balance on the contralateral limb while lifting and lowering increasingly heavier weights at the assigned velocity.
Study limitations.
A significant limitation inherent to all in vivo training studies is uncertainty regarding motor unit recruitment. Despite careful control of in vivo training protocols, it is impossible to know precisely the training stimulus that was applied to a given fiber. This could result in a worst-case circumstance in which a fiber from a posttraining biopsy had received no training. The recruitment uncertainty could thus contribute to the variability in the measurements that is apparent in Figs. 3 and 4. Combining results from type 2a and type 2x fibers is another potential source of variability; type 2x fibers have higher intrinsic shortening velocities than type 2a fibers (3, 44) and have also been reported to have higher sFo (44). Furthermore, differential adaptations of type 2a and type 2x fibers cannot be ruled out.
Summary.
The dominant effect of PRT on individual muscle fibers was an increase in the size (CSA) of type 2 fibers. The increased size, in turn, produced increases in absolute force (Fo) and absolute power (Pmax). The PRT-induced improvements were independent of subject sex and age and, with the exception of power, independent of the velocity at which the PRT was performed. Measures of myofibrillar function (sFo, V0.025, nPmax) appear to be fixed, with very little variation noted within a fiber type, regardless of sex, age, or training state. A practical implication of these results is that, for type 2 fibers, older adults can use lighter weights and move them more rapidly to get the same training effect at the fiber level as low-velocity PRT. If, as has been suggested (32), the use of lighter weights moved more rapidly proves to be more attractive than low-velocity PRT, then high-velocity PRT might encourage wider acceptance of resistance training among the elderly population. The significance for whole muscle would be enhancement of the powerful type 2 fiber population that is most at risk for reduced functionality in the elderly.
GRANTS
This work was supported by grants AG 015434 and AG 024824 from the National Institute on Aging and by a grant from the State of Michigan Life Science Corridor program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Charles Cole for developing the software used to monitor training velocities.
REFERENCES
- 1. Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Halkjaer-Kristensen J, Simonsen EB. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol 534: 613–623, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bottaro M, Machado SN, Nogueira W, Scales R, Veloso J. Effect of high versus low-velocity resistance training on muscular fitness and functional performance in older men. Eur J Appl Physiol 99: 257–264, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol 495: 573–586, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenner B. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys J 41: 99–102, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brooks SV, Faulkner JA. Forces and powers of slow and fast skeletal muscles in mice during repeated contractions. J Physiol 436: 701–710, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol Cell Physiol 256: C1262–C1266, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Charette SL, McEvoy L, Pyka G, Snow-Harter C, Guido D, Wiswell RA, Marcus R. Muscle hypertrophy response to resistance training in older women. J Appl Physiol 70: 1912–1916, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Claflin DR, Faulkner JA. The force-velocity relationship at high shortening velocities in the soleus muscle of the rat. J Physiol 411: 627–637, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duchateau J, Hainaut K. Isometric or dynamic training: differential effects on mechanical properties of a human muscle. J Appl Physiol 56: 296–301, 1984 [DOI] [PubMed] [Google Scholar]
- 11. Edman KAP. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol 291: 143–159, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, Fiatarone Singh MA. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc 50: 655–662, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Fitts RH. Effects of regular exercise training on skeletal muscle contractile function. Am J Phys Med Rehabil 82: 320–331, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Frontera WR, Hughes VA, Krivickas LS, Kim SK, Foldvari M, Roubenoff R. Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve 28: 601–608, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Galecki AT, Chen S, Faulkner JA, Ashton-Miller JA, Burzykowski T. Statistical power calculations for clustered continuous data. Int J Knowl Eng Soft Data Paradig 1: 40–48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Godard MP, Gallagher PM, Raue U, Trappe S. Alterations in single muscle fiber calcium sensitivity with resistance training in older women. Pflügers Arch 444: 419–425, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Häkkinen K, Alen M, Komi PV. Changes in isometric force- and relaxation-time, electromyographic and muscle fibre characteristics of human skeletal muscle during strength training and detraining. Acta Physiol Scand 125: 573–585, 1985 [DOI] [PubMed] [Google Scholar]
- 18. Häkkinen K, Pakarinen A, Kraemer WJ, Häkkinen A, Valkeinen H, Alen M. Selective muscle hypertrophy, changes in EMG and force, and serum hormones during strength training in older women. J Appl Physiol 91: 569–580, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci 126: 136–195, 1938 [DOI] [PubMed] [Google Scholar]
- 20. Jones DA, Rutherford OM. Human muscle strength training: the effects of three different regimens and the nature of the resultant changes. J Physiol 391: 1–11, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Julian FJ, Rome LC, Stephenson DG, Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol 370: 181–199, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 101: 531–544, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50A: 11–16, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Marsh AP, Miller ME, Rejeski WJ, Hutton SL, Kritchevsky SB. Lower extremity muscle function after strength or power training in older adults. J Aging Phys Act 17: 416–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martel GF, Roth SM, Ivey FM, Lemmer JT, Tracy BL, Hurlbut DE, Metter EJ, Hurley BF, Rogers MA. Age and sex affect human muscle fibre adaptations to heavy-resistance strength training. Exp Physiol 91: 457–464, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Moisescu DG, Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol 275: 241–262, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moritani T, deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58: 115–130, 1979 [PubMed] [Google Scholar]
- 28. Pansarasa O, Rinaldi C, Parente V, Miotti D, Capodaglio P, Bottinelli R. Resistance training of long duration modulates force and unloaded shortening velocity of single muscle fibres of young women. J Electromyogr Kinesiol 19: e290–e300, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Parente V, D'Antona G, Adami R, Miotti D, Capodaglio P, De VG, Bottinelli R. Long-term resistance training improves force and unloaded shortening velocity of single muscle fibres of elderly women. Eur J Appl Physiol 104: 885–893, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Pijnappels M, Bobbert MF, van Dieër JH. How early reactions in the support limb contribute to balance recovery after tripping. J Biomech 38: 627–634, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol 106: 1611–1617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sayers SP. High velocity power training in older adults. Curr Aging Sci 1: 62–67, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Singh MA, Ding W, Manfredi TJ, Solares GS, O'Neill EF, Clements KM, Ryan ND, Kehayias JJ, Fielding RA, Evans WJ. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol Endocrinol Metab 277: E135–E143, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Svanborg A. A medical-social intervention in a 70-year-old Swedish population: is it possible to postpone functional decline in aging? J Gerontol 48 Spec No: 84–88, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fiber contractile properties in young and old men and women. J Physiol 552: 47–58, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol 281: C398–C406, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol 89: 143–152, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer-Verlag, 2000 [Google Scholar]
- 39. Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci 64: 332–339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walker SM, Schrodt GR. I segment lengths and thin filament periods in skeletal muscle fibers of the Rhesus monkey and the human. Anat Rec 178: 63–81, 1974 [DOI] [PubMed] [Google Scholar]
- 41. West BT, Welch KB, Galecki AT. Linear Mixed Models: A Practical Guide Using Statistical Software. Boca Raton, FL: Chapman & Hall/CRC, 2007 [Google Scholar]
- 42. Whipple RH, Wolfson LI, Amerman PM. The relationship of knee and ankle weakness to falls in nursing home residents: an isokinetic study. J Am Geriatr Soc 35: 13–20, 1987 [DOI] [PubMed] [Google Scholar]
- 43. Widrick JJ, Stelzer JE, Shoepe TC, Garner DP. Functional properties of human muscle fibers after short-term resistance exercise training. Am J Physiol Regul Integr Comp Physiol 283: R408–R416, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Widrick JJ, Trappe SW, Blaser CA, Costill DL, Fitts RH. Isometric force and maximal shortening velocity of single muscle fibers from elite master runners. Am J Physiol Cell Physiol 271: C666–C675, 1996 [DOI] [PubMed] [Google Scholar]