Abstract
Hypokalemia is a common electrolyte disorder that increases renal ammonia metabolism and can cause the development of an acid-base disorder, metabolic alkalosis. The ammonia transporter family members, Rh B glycoprotein (Rhbg) and Rh C glycoprotein (Rhcg), are expressed in the distal nephron and collecting duct and mediate critical roles in acid-base homeostasis by facilitating ammonia secretion. In the current studies, the effect of hypokalemia on renal Rhbg and Rhcg expression was examined. Normal Sprague-Dawley rats received either K+-free or control diets for 2 wk. Rats receiving the K+-deficient diet developed hypokalemia and metabolic alkalosis associated with significant increases in both urinary ammonia excretion and urine pH. Rhcg expression increased in the outer medullary collecting duct (OMCD). In OMCD intercalated cells, hypokalemia resulted in more discrete apical Rhcg expression and a marked increase in apical plasma membrane immunolabel. In principal cells, in the OMCD, hypokalemia increased both apical and basolateral Rhcg immunolabel intensity. Cortical Rhcg expression was not detectably altered by immunohistochemistry, although there was a slight decrease in total expression by immunoblot analysis. Rhbg protein expression was decreased slightly in the cortex and not detectably altered in the outer medulla. We conclude that in rat OMCD, hypokalemia increases Rhcg expression, causes more polarized apical expression in intercalated cells, and increases both apical and basolateral expression in the principal cell. Increased plasma membrane Rhcg expression in response to hypokalemia in the rat, particularly in the OMCD, likely contributes to the increased ammonia excretion and thereby to the development of metabolic alkalosis.
Keywords: acid-base, ammonia, collecting duct, potassium
renal ammonia1 metabolism is the predominant component of renal new bicarbonate generation and thereby has a central role in acid-base homeostasis (20). In contrast to the majority of urinary solutes, whose excretion involves glomerular filtration, renal ammonia metabolism does not involve significant glomerular filtration, but instead is an integrated process of ammoniagenesis and renal epithelial cell transport involving specific membrane transporters (54, 55). This process of selective transport of ammonia is critical to acid-base homeostasis. Ammonia excreted into the urine results in net acid excretion and new bicarbonate formation. Ammonia not excreted is returned via the renal veins to the systemic circulation, where the liver metabolizes it in a bicarbonate-consuming process, and does not result in net acid excretion. Accordingly, understanding the mechanisms and regulation of renal epithelial cell ammonia transport is critical to understanding acid-base homeostasis.
Recent studies have identified a new family of proteins that mediate transmembrane ammonia transport and are widely expressed in both prokaryotic and eukaryotic organisms (27, 37, 51). Mammalian members of this ammonia transporter family are the Rh glycoproteins, Rh A Glycoprotein, Rh B Glycoprotein (Rhbg), and Rh C Glycoprotein (Rhcg). These are secondarily active, ammonia-specific transporters with no apparent affinity for sodium, potassium, or other known renal solutes (37, 54). In the kidney, the nonerythroid Rh glycoproteins, Rhbg and Rhcg, are expressed in the distal convoluted tubule through the inner medullary collecting duct (13, 21, 38, 49, 52), sites responsible for secretion of ∼60–80% of total urinary ammonia (20, 55). Collecting duct ammonia transport involves carrier-mediated mechanisms with characteristics similar to those identified for Rhbg and Rhcg (24, 25), and genetic deletion of either Rhbg or Rhcg impairs renal ammonia excretion (5, 6, 33, 34).
In many conditions, changes in Rhbg and/or Rhcg expression parallel changes in ammonia excretion and contribute to the maintenance of acid-base homeostasis. In models of metabolic acidosis, where the primary mechanism of increased net acid excretion involves increased ammonia excretion, there is increased renal Rh glycoprotein expression in both rat (Rhcg) (40) and mouse (Rhcg and Rhbg) (5, 33, 34). In conditions of decreased nephron number, where increased single-nephron ammonia excretion contributes to acid-base homeostasis, both Rhbg and Rhcg expression are increased (31). Thus changes in Rhbg- and Rhcg-mediated ammonia transport can assist in the maintenance of acid-base homeostasis.
However, there are also conditions in which altered ammonia metabolism contributes to development of acid-base disturbances. In hypokalemia, there is increased ammonia excretion despite the development of metabolic alkalosis. The roles of Rhbg and Rhcg in this response are unexamined. If Rhbg and Rhcg expression is primarily related to systemic acid-base homeostasis, then, in view of the coexistent metabolic alkalosis, hypokalemia should decrease their expression. If their expression can be regulated by mechanisms other than those directed at systemic acid-base homeostasis, then increased Rhbg and/or Rhcg expression might contribute to the increased urinary ammonia excretion and development of metabolic alkalosis.
We designed the current studies to determine the effect of hypokalemia on renal expression of the nonerythroid ammonia transporter family members, Rhbg and Rhcg. We used normal Sprague-Dawley rats adapted to a nominally K+-free or control diet for 2 wk and then examined renal Rhbg and Rhcg expression. Our results demonstrate that hypokalemia is associated with increased Rhcg expression, particularly in the outer medullary collecting duct, and that this involves increased apical Rhcg expression in intercalated cells and increased apical and basolateral Rhcg expression in principal cells.
METHODS
Animals.
Normal, adult Sprague-Dawley rats were used in these studies. Hypokalemia was induced by substituting a nominally K+-free diet (catalog no. 960189, MP Biomedicals, Solon, OH) for the usual chow for 2 wk. Control rats received a control diet (catalog no. 905453, MP Biomedicals). In some studies, the K+-free diet was Teklad Diet TD95006 and the control diet was TD88238 (Harlan Laboratories, Madison, WI). Similar results were obtained, and the results are combined for purpose of analysis. All animals had free access to water and food throughout the experiment. On day 14, a subset of animals was placed in metabolic cages and urine was collected for 24 h under mineral oil. The urine volume was recorded, and an aliquot was frozen for later analysis. On the day of experiment, animals were euthanized using pentobarbital sodium. In a subset of rats, blood samples were obtained from the abdominal aorta under general anesthesia; then, the kidneys were perfused through the aorta with PBS, removed, and the cortex and outer medulla were dissected and frozen in liquid nitrogen. In other animals, following PBS perfusion the kidneys were perfused with 2% paraformaldehyde-lysine-periodate (PLP) and embedded in polyester wax, as described previously (31, 40, 49). For tissues used for immunogold electron microscopy, the fixatives used included 4% paraformaldehyde, 2% PLP, and 1% glutaraldehyde; samples were embedded in Lowicryl K4M acrylic resin (Polysciences, Warrington, PA) and polymerized under UV light for 24 h at −20°C, followed by 60 h under UV light at room temperature. Similar results were obtained with the different fixatives. All animal interventions were reviewed and approved by the Institutional Animal Care and Use Committees of Ewha Womans University, University of Colorado, University of Florida, and the North Florida/South Georgia Veterans Health System.
Antibodies.
We used affinity-purified antibodies to Rhbg and to Rhcg generated in our laboratory and characterized previously. This characterization involves showing specificity using peptide-blocking approaches, using gene knockout animals, and using heterologous expression in oocytes (5, 30, 33, 34, 36, 49). We obtained antibodies to the B1/2 subunit of H+-ATPase from Santa Cruz Biotechnology (Santa Cruz, CA) and antibodies to the anion exchanger AE1 from Alpha Diagnostics (San Antonio, TX).
Electrolyte analysis.
A portion of blood obtained from anesthetized rats was placed in a heparinized tube for pH and Pco2 measurement. The remaining blood was placed in a nonheparinized tube for serum collection. After centrifugation, the serum was placed in microfuge tubes, frozen, and stored for later analysis. Arterial blood-gas analysis was performed using an ABL555 pH/blood-gas analyzer (Radiometer Nederland, Copenhagen, Denmark). Serum electrolytes were measured using the VetACE clinical chemistry system (Alfa Wassermann, West Caldwell, NJ). Urine ammonia was determined using a commercially available kit (B7550-75, Pointe Scientific) according to the manufacturer's protocol.
Immunoblotting procedure.
Proteins were isolated from tissues using T-PER Tissue Protein Extraction Reagent (ThermoScientific, Rockford, IL). An aliquot was obtained for protein determination using a BCA assay, and the remainder was stored frozen at −70°C until used. Five to twenty micrograms of renal protein were electrophoresed on 10% PAGE ReadyGel (Bio-Rad, Hercules, CA). Gels were then transferred electrophoretically to nitrocellulose membranes, blocked with 5 g/dl nonfat dry milk, and incubated for 2 h with primary antibody diluted in Blotto buffer (50 mM Tris, 150 mM NaCl, 5 mM Na2EDTA, and 0.05% Tween 20, pH 7.6) with 5 g/dl nonfat dry milk. Rhbg antibodies were used at a dilution of 1:100 and Rhcg antibodies at a dilution of 1:500. Loading and transfer equivalence were assessed with Ponceau S staining. After washing, membranes were exposed to secondary antibody conjugated to horseradish peroxidase (goat anti-rabbit IgG; Promega, Madison, WI) at a dilution of 1:5,000. Sites of antibody-antigen reaction were visualized by using enhanced chemiluminescence (SuperSignal West Pico Substrate, Pierce, Rockford, IL) and a Kodak Image Station 440CF digital imaging system. Band density was quantified using Kodak 1D, version 3.5.4 software (Kodak Scientific Imaging, New Haven, CT).
Immunohistochemistry.
Immunolocalization was accomplished using standard immunoperoxidase procedures detailed previously (30, 33). The sections were dewaxed in ethanol, rehydrated, and then rinsed in PBS. Endogenous peroxidase activity was blocked by incubating the sections in Peroxidase Blocking Reagent (DakoCytomation, Carpinteria, CA) for 45 min. The sections were blocked for 15 min with Serum-Free Protein Block (DakoCytomation) and then incubated overnight at 4°C with primary antibody. Antibodies to Rhbg and to Rhcg were used at a dilution of 1:5,000. The sections were washed in PBS and incubated for 30 min with polymer-linked peroxidase-conjugated goat anti-rabbit IgG (MACH2, Biocare Medical, Concord, CA), again washed with PBS, then exposed to diaminobenzidine for 5 min. The sections were washed in distilled water, then dehydrated in a graded series of ethanols and xylene, mounted, and observed by light microscopy. Comparisons of labeling were made only between sections of the same thickness from the same immunohistochemistry experiment. Sections were examined on a Nikon E600 microscope equipped with DIC optics and photographed using a DXM1200F digital camera and ACT-1 software (Nikon).
Double-immunolabeling procedure.
Double immunolabeling was accomplished using sequential immunoperoxidase procedures described in detail previously (21–23). Briefly, tissue sections were labeled with the first primary antibody following the procedure described above, using Vector SG (Vector Laboratories) as the chromogen to produce a blue label. After the Vector SG reaction, sections were washed in PBS and then blocked using the Peroxidase Blocking Reagent and Serum-Free Protein Block as described in the single-label procedure. The above procedure was repeated with the substitution of a second primary antibody and the substitution of DAB for Vector SG. Antibodies to H+-ATPase and AE1 were both used at dilutions of 1:200. The sections were then washed with glass-distilled water, dehydrated with xylene, mounted with Permount, and observed by light microscopy.
Immunogold labeling.
Briefly, the immunogold labeling procedure was performed by exposure of the ultrathin tissue sections, mounted on Formvar/carbon-coated nickel grids, to the Rhcg antibody at a dilution of 1:500 and then to a goat anti-rabbit IgG secondary antibody conjugated to 0.8-nm colloidal gold particles (Aurion Ultra Small Immuno Gold Reagent, Electron Microscopy Sciences, Ft. Washington, PA). Unless noted otherwise, all steps were done by floating the grids on droplets of solution at room temperature. The sections were exposed to 0.1 M NH4Cl for 1 h, rinsed with PBS, treated with the blocking solution (5% BSA, 0.1% cold-water fish-skin gelatin, and 5% normal goat serum, in PBS) for 30 min, washed with incubation solution [0.2% acetylated BSA (Aurion BSA-c, Electron Microscopy Sciences) and 10 mM NaN3, in PBS, pH 7.4], and then incubated in a humidified chamber overnight at 4°C with the affinity-purified primary antibody diluted in incubation solution with 0.1% each of Tween 20 and Triton X-100 added. The sections were washed with incubation solution with 0.1% Tween 20 and exposed for 1.5 h to the secondary antibody diluted in incubation solution with 0.1% Tween 20. The sections were washed with incubation solution with 0.1% Tween 20, washed with PBS, postfixed with 1.25% glutaraldehyde in PBS, washed with PBS, and then with distilled water. Silver enhancement of the gold particles was done using Aurion EM SE (Electron Microscopy Sciences) for 45 min, and then the grids were washed again with distilled water, dried overnight, and counterstained with saturated uranyl acetate. Each group of sections subjected to the immunogold procedure included a control section that was exposed to incubation buffer in place of the primary antibody. Ultrathin sections were examined by an observer blinded to the experimental conditions using a Zeiss EM10A transmission electron microscope equipped with a Peltier-cooled CCD camera (SIA-7C) controlled by Maxim DL software (Scientific Instrument and Applications, Atlanta, GA). The outer stripe of the outer medullary collecting duct (OMCDo) was identified by its characteristic heterogeneous epithelial cell population, which included principal cells and intercalated cells.
Statistics.
Results are presented as means ± SE. Statistical analyses were performed using Student's unpaired t-test, and P < 0.05 taken as statistically significant.
RESULTS
Physiological data.
Results of arterial blood-gas and plasma electrolyte analyses are summarized in Table 1. Rats treated with a nominally K+-free diet for 2 wk developed significant hypokalemia [K+ concentration, 4.6 ± 0.2 (control) vs. 2.8 ± 0.2 (K+-free), P < 0.05]. The K+-free diet also caused metabolic alkalosis, with a significantly increased arterial pH [7.37 ± 0.01 (control) vs. 7.42 ± 0.02 (K+-free), P < 0.05], and increased serum bicarbonate [24.9 ± 1.9 (control) vs. 28.6 ± 1.4 mmol/l (K+ free), P < 0.05].
Table 1.
Plasma and urine electrolytes with K+ depletion
| Control | Hypokalemia | |
|---|---|---|
| Plasma Na+ | 138 ± 1.7 (5) | 141 ± 3.8 (5) |
| Plasma K+ | 4.6 ± 0.2 (5) | 2.8 ± 0.2 (5)* |
| pH | 7.37 ± 0.01 (5) | 7.42 ± 0.02* |
| HCO3− | 24.9 ± 1.9 (5) | 28.6 ± 1.4 (5)* |
| Pco2 | 40.6 ± 1.9 (5) | 40.5 ± 1.6 |
| Urine volume, ml/day | 14.4 ± 1.6 (7) | 22.6 ± 4.9 (8) |
| Urine osmolality, mosmol/kgH2O | 1,756 ± 387 (7) | 767 ± 305 (8)* |
| Urine ammonia concentration, mmol/l | 24.9 ± 6.6 (5) | 171.5 ± 51.8 (8)* |
| Urine ammonia content, mmol/day | 0.39 ± 0.16 (10) | 2.77 ± 0.41 (12)* |
| Urine pH | 6.83 ± 0.13 (5) | 7.60 ± 0.28 (5)* |
Values are means ± SE. Numbers in parentheses indicate number of animals studied.
P < 0.05.
Table 1 also summarizes the analyses of 24-h urine collections obtained at the end of the 2 wk of the K+-free diet. Urinary ammonia excretion increased significantly in response to hypokalemia [0.39 ± 0.16 (control) vs. 2.77 ± 0.41 mmol/day (K+-free), P < 0.05]. Urine total ammonia concentration also increased significantly [24.9 ± 6.6 (control) vs. 171.5 ± 51.8 mmol/l (K+-free), P < 0.05]. Urine pH was significantly increased [6.83 ± 0.13 (control) vs. 7.60 ± 0.28 (K+-free), P < 0.002]. Increased urinary ammonia simultaneous with urine alkalinization suggests that increased rates of distal nephron NH3 secretion are an important component of the increase in urinary total ammonia excretion.
Mild polyuria was present [volume = 14.4 ± 1.9 (control) vs. 22.6 ± 4.9 ml/day (K+-free), n = 5/group, P < 0.05], consistent with hypokalemia's known effect to inhibit urine concentrating ability. Thus the increased total ammonia excretion involves increases in both urine volume and urinary ammonia concentration.
Thus 2 wk of a K+-free diet caused hypokalemia in association with metabolic alkalosis and increased urine ammonia excretion despite increased urine pH. The increase in urinary ammonia excretion is similar to that which occurs in response to metabolic acidosis (40); however, in contrast to metabolic acidosis, where the increased ammonia excretion leads to correction of an acid-base disorder, in hypokalemia the increased urinary ammonia excretion likely contributes to development of an acid-base disorder, namely metabolic alkalosis.
Rhcg protein expression by immunoblot analysis.
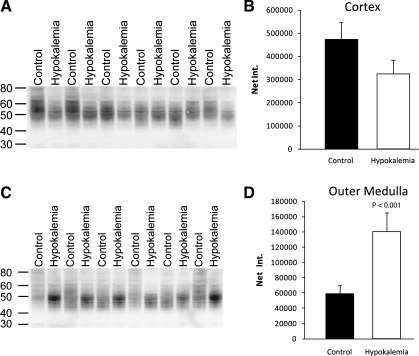
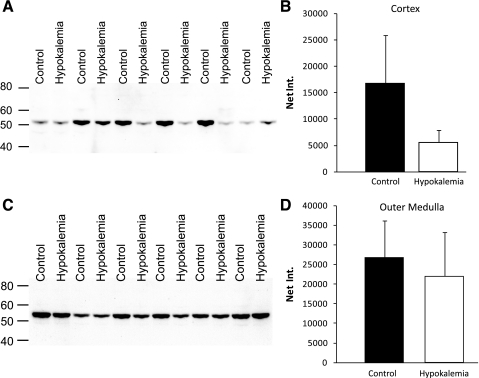
The most dramatic changes in ammonia transporter family member expression in the rat kidney in response to other conditions associated with increased urinary ammonia excretion, such as metabolic acidosis and reduced renal mass, involve increased Rhcg expression (31, 40, 41). Thus we next examined changes in Rhcg expression. Immunoblot analysis showed that hypokalemia slightly decreased Rhcg protein expression in the cortex, and significantly increased expression in the outer medulla (Fig. 1).
Fig. 1.
Rh C glycoprotein (Rhcg) protein expression in response to hypokalemia. A: immunoblot for Rhcg in cortex of control and hypokalemic kidney. B: quantification of Rhcg protein expression. C: immunoblot analysis of Rhcg protein expression in the outer medulla of control and hypokalemic kidney. D: quantification of outer medullary Rhcg protein expression. Hypokalemia increased outer medullary Rhcg expression significantly. Results are from 6 control and 6 hypokalemic rats.
Rhcg immunolocalization.
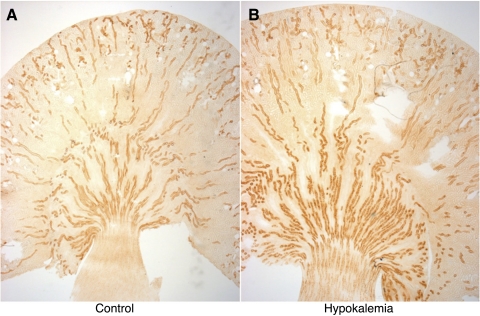
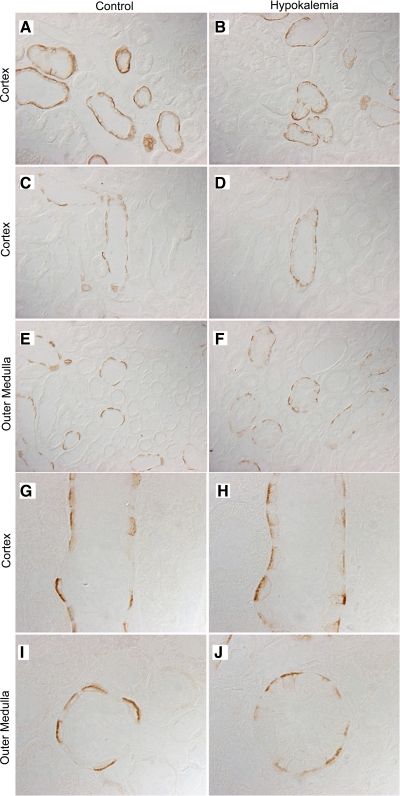
To further examine changes in Rhcg expression in response to hypokalemia, we examined Rhcg's localization using immunohistochemistry (Fig. 2). In the cortex, hypokalemia did not induce detectable changes in either the intensity or the subcellular distribution of Rhcg in cortical epithelial cells in the distal convoluted tubule, connecting tubule, and cortical collecting duct segments (Fig. 3).
Fig. 2.
Rhcg expression in the rat kidney in response to hypokalemia. A: low-power micrograph of Rhcg expression in control kidney. B: Rhcg localization in hypokalemic kidney. In the cortex, hypokalemia did not induce evident changes in Rhcg expression. In the outer medulla, hypokalemia increased the intensity of Rhcg expression. Results are representative of findings from 5 control and 5 hypokalemic rats.
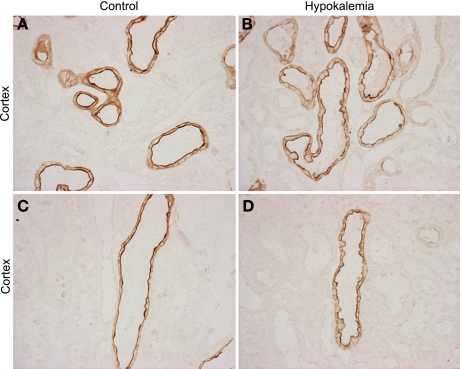
Fig. 3.
High-power micrographs of Rhcg immunolabel in cortex of control and hypokalemic rat kidneys. A: Rhcg immunolabel in distal convoluted tubule (DCT) and connecting tubule (CNT) of control kidney. C: expression in the cortical collecting duct (CCD). B: Rhcg immunolabel in the DCT and CNT of hypokalemic kidney. D: expression in CCD of hypokalemic kidney. There is no evidence of differences in the expression or subcellular distribution of Rhcg in the DCT, CNT, or CCD in response to hypokalemia. Results are representative of findings from 5 control and 5 hypokalemic rats.
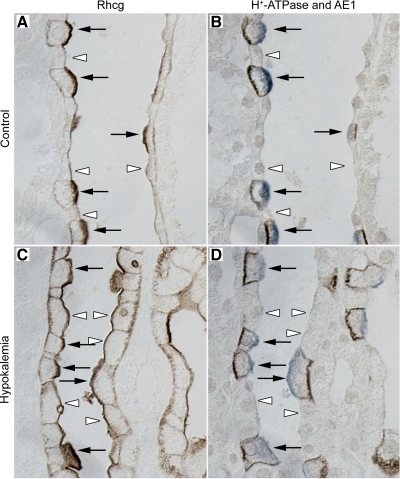
By contrast, in the outer medulla hypokalemia induced substantial changes in Rhcg expression. In the control kidney, all OMCD cells exhibited apical and basolateral Rhcg immunolabel. Apical immunolabel was more intense than basolateral immunolabel, and a subset of cells with the appearance of intercalated cells exhibited more intense apical and basolateral Rhcg expression than did the majority of cells (principal cells) (Fig. 4). Double-immunolabel using H+-ATPase and AE1 in serial sections confirmed this identification. In the OMCD of hypokalemic rats, a subpopulation of OMCD cells protruded into the tubule lumen. These cells had greater Rhcg apical immunolabel intensity than the majority of OMCD cells, but apical Rhcg intensity was increased substantially in the majority of OMCD cells, thus including principal cells. Consequently, the difference in apical Rhcg immunolabel intensity between different cell types was substantially less than in control kidneys. Hypokalemia also substantially increased basolateral Rhcg immunolabel intensity in the OMCD. This was most evident in the majority population, i.e., principal cells, in the OMCD.
Fig. 4.
Colocalization of Rhcg with H+-ATPase and anion exchanger AE1 in control and hypokalemic kidney. A: Rhcg in OMCD of control kidney. B: expression of H+-ATPase (blue) and AE1 (brown) in serial sections. Intercalated cells, identified by apical H+-ATPase and basolateral AE1 immunolabel (arrows), exhibit more intense apical and basolateral Rhcg expression than do principal cells (arrowheads) in the control kidney. C: Rhcg immunolabel in hypokalemic rat kidney. D: H+-ATPase and AE1 immunolabel in serial sections. Both intercalated cells (arrows) and principal cells (arrowheads) exhibit intense apical and basolateral Rhcg immunolabel. Principal cells in the hypokalemic kidney exhibit more intense apical and basolateral Rhcg than observed in control kidney. In intercalated cells, hypokalemia appears to be result in more intense basolateral Rhcg immunolabel intensity than observed in principal cells. Results are representative of findings in at 5 control and 5 hypokalemic rat kidneys.
To confirm the identification of intercalated and principal cells in the hypokalemic kidney, we used double-immunolabel with antibodies to H+-ATPase and AE1 in serial sections. Principal cells were identified by the absence of apical H+-ATPase and basolateral AE1 immunoreactivity in serial sections. Principal cells were clearly hypertrophied in hypokalemic kidneys compared with control kidneys. Principal cells also exhibited dramatically increased Rhcg immunoreactivity, with increases in both apical and basolateral Rhcg immunoreactivity, compared with the control kidney. OMCD intercalated cells, identified by apical H+-ATPase and basolateral AE1 immunoreactivity, correlated with the minority cell population that protruded into the tubule lumen in both the control and hypokalemic kidney. Apical Rhcg immunolabel in intercalated cells in the OMCD of hypokalemic kidneys was more discretely localized than in the control kidney, where it exhibited a more diffuse apical expression pattern.
Immunogold localization of Rhcg in OMCD intercalated cells.
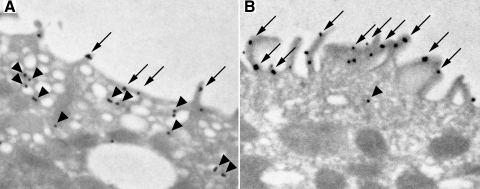
Changes in the subcellular distribution of Rhcg are an important component of the response to both metabolic acidosis and ⅚ ablation-infarction, two conditions associated with increased single-nephron ammonia excretion (31, 41). To determine whether similar mechanisms were present in the response to hypokalemia, we used immunogold electron microscopy to localize subcellular Rhcg expression. In the control kidney, immunogold electron microscopy showed that in the intercalated cells in the OMCDo the majority of apical Rhcg was present in subapical vesicles, with only a minority of Rhcg being present in the apical plasma membrane (Fig. 5A). These findings in the control rat kidney are similar to those we reported previously (41). In the hypokalemic kidney, there were dramatic expansion of the apical plasma membrane and increased microvilli, as reported previously (14, 26). Rhcg immunolabel was localized almost exclusively in the apical plasma membrane; cytoplasmic Rhcg immunolabel was only rarely identified and was clearly less than observed in control kidney (Fig. 5B). Thus hypokalemia induces substantial changes in Rhcg's subcellular distribution in intercalated cells in the OMCD.
Fig. 5.
Subcellular distribution of Rhcg in intercalated cells in the outer stripe of the OMCD (OMCDo) in control and hypokalemic kidney. A: immunogold electron microscopy localization of Rhcg in the apical region of intercalated cells in the OMCDo of control rat kidney. The majority of immunolabel is present in subapical vesicles (arrowheads), and only a minority is present in the apical plasma membrane (arrows). B: immunogold electron microscopy localization of Rhcg in the apical region of intercalated cells in the OMCDo of hypokalemic rat kidney. The predominant component of apical Rhcg is present in the apical plasma membrane (arrow), and only a small amount is present in subapical vesicles (arrowheads). Results are representative of results from 4 control and 5 hypokalemic rat kidneys.
Rhbg protein expression.
Rhbg is another member of the ammonia transporter family that mediates an important role in metabolic acidosis-stimulated renal ammonia excretion (5). Immunoblot analysis of tissues from control and hypokalemic rat kidneys showed a slight decrease in Rhbg expression in the cortex and no significant change in the outer medulla between control and hypokalemic kidneys (Fig. 6).
Fig. 6.
Quantification of Rh B glycoprotein (Rhbg) protein expression in control and hypokalemic kidney. A: immunoblot analysis of Rhbg protein expression in cortex of control and hypokalemic kidney. B: quantification of Rhbg protein expression. Hypokalemia did not significantly alter cortical Rhbg expression. C: immunoblot analysis of Rhbg protein expression in the outer medulla of control and hypokalemic kidney. D: quantification of Rhbg protein expression. Hypokalemia did not significantly alter outer medullary cortical Rhbg expression. Results are from 6 control and 6 hypokalemic rats.
Rhbg immunolocalization in control and hypokalemic kidneys.
To more fully determine the effect of hypokalemia on Rhbg expression, we examined the localization of Rhbg protein using immunohistochemistry (Fig. 7). In the cortex, hypokalemia was associated with a slight decrease in Rhbg immunolabel intensity in both the CNT and the CCD, but otherwise there was no detectable change in its expression pattern. In the OMCD, Rhbg immunolabel did not detectably change in response to hypokalemia. Thus changes in Rhbg expression appear unlikely to contribute to the increased ammonia excretion that occurs in the rat in response to hypokalemia.
Fig. 7.
Rhbg immunolabel in control and hypokalemic kidney. A–D: low-power micrographs of Rhbg immunolabel in the cortex of control and hypokalemic kidney. No difference in the pattern of Rhbg localization is present. E and F: low-power micrographs of Rhbg immunolabel in the OMCD of control (E) and hypokalemic (F) kidneys. A slight decrease in Rhbg immunolabel intensity is present, but no change in the distribution or localization of Rhbg protein. G and H: high-power micrographs of Rhbg immunolabel in the CCD of control (G) and hypokalemia (H) kidneys. A slight decrease in Rhbg immunolabel intensity is present, but otherwise no change in the distribution or localization of Rhbg protein. I and J: high-power micrographs of Rhbg immunolabel in the OMCD of control (I) and hypokalemia (J) kidneys. No detectable changes in Rhbg immunolabel were evident in the rat OMCD. Results are representative of findings in 5 control and 5 hypokalemic rat kidneys.
DISCUSSION
The current study demonstrates important new findings regarding the renal response to dietary potassium restriction. A K+-free diet for 2 wk caused hypokalemia, metabolic alkalosis, and a significant increase in urinary ammonia excretion despite the metabolic alkalosis. These changes were accompanied by increased Rhcg protein expression, particularly in the OMCD, and more discrete apical expression in intercalated cells and increased apical and basolateral expression in principal cells in this region. These observations indicate that enhanced Rhcg-mediated collecting duct ammonia transport is likely to contribute to increased urinary ammonia excretion and the development of metabolic alkalosis, and that Rhcg expression, particularly in the OMCD, can be regulated through mechanisms independent of those required for systemic acid-base homeostasis.
Dietary potassium restriction is a common clinical occurrence and is associated with development of systemic acid-base disorders. In humans and rats, dietary potassium restriction is associated with development of metabolic alkalosis (44–46), whereas in dogs it causes metabolic acidosis (7). The reason for these species-dependent differences in acid-base homeostasis is likely to be due to differences in renal ammonia metabolism. Chronic hypokalemia increases urinary ammonia excretion in humans and rats (43–46, 58), whereas in dogs it decreases ammonia excretion (7). The observation that hypokalemia causes metabolic alkalosis only in species in which ammonia excretion increases strongly suggests that the increased ammonia excretion causes the acid-base alteration.
The majority of urinary ammonia is secreted in the distal nephron and collecting duct, and at least in the collecting duct involves parallel H+ and NH3 secretion (54, 55). H+ secretion occurs through both H+-ATPase and H+-K+-ATPase, both of which are activated by hypokalemia. Although it is theoretically possible that increased H+ secretion, by increasing luminal H+ concentration and thus titrating luminal NH3 to NH4+, lowers luminal NH3 concentration and thereby enhances transepithelial NH3 secretion, this is unlikely to be the primary mechanism of increased ammonia secretion. In particular, hypokalemia was associated with an increase in urine pH of ∼0.8 pH units, indicating a >80% decrease in urinary H+ concentration in the current study, and multiple other studies also show that hypokalemia induces urine alkalinization (43–46, 58). Instead, increased urine ammonia excretion associated with urine alkalinization indicates that increased NH3 secretion directly contributes to increased renal ammonia excretion.
Our results suggest that Rhcg contributes to the increased urinary ammonia excretion observed with hypokalemia. Rhcg transports NH3 and is expressed in distal renal tubular epithelium in the kidney (13, 21, 49). Its expression increases in both metabolic acidosis and reduced renal mass, two other common conditions associated with increased single-nephron ammonia excretion (31, 40, 41). Gene deletion studies, involving global, collecting duct-, and intercalated cell-specific Rhcg deletion, have shown that Rhcg mediates a central role in both basal and metabolic acidosis-stimulated ammonia excretion (6, 33, 34). In hypokalemia, Rhcg expression increases in the rat kidney, particularly in the OMCD, a site that is critical for both K+ reabsorption and ammonia secretion. The current study thereby adds to previous studies by demonstrating a third common clinical condition of altered renal ammonia metabolism in which rat Rhcg expression increases in parallel with renal ammonia excretion.
Rhcg expression increases in hypokalemia in both intercalated cells and principal cells in the OMCD, suggesting that both cell types contribute to increased transepithelial ammonia transport in this model. The role of intercalated cells in acid-base transporters is well established, with extensive previous work showing adaptations in multiple proteins involved in intercalated cell-mediated acid-base transport (19, 35, 39). Moreover, studies examining intercalated cell-specific Rhcg deletion have shown the importance of transcellular ammonia transport by intercalated cells, both under basal conditions and in response to metabolic acidosis (34).
The role of principal cells in acid-base homeostasis is less well recognized, but increasing numbers of studies suggest that the principal cell contributes to acid-base homeostasis through Rh glycoprotein-mediated ammonia transport. Principal cells, particularly in the OMCD, express apical H+-ATPase and H+-K+-ATPase activity (53), basolateral Cl−/HCO3 exchange activity (56), cytoplasmic carbonic anhydrase (11), and multiple H+-K+-ATPase subunits, including HKα1, HKα2, and HKβ (1, 9, 50). Principal cells also express the ammonia transporter family members Rhbg and Rhcg (40, 41, 49). Physiologically, principal cell apical and basolateral Rhcg expression changes in parallel with renal ammonia excretion in multiple models of altered ammonia metabolism, including metabolic acidosis (40, 41), reduced renal mass (31), and hypokalemia (current study). Last, comparison of mice with intercalated cell-specific Rhcg deletion with those with Rhcg deletion from both intercalated and principal cells demonstrates that principal cell Rhcg expression contributes to both basal and metabolic acidosis-stimulated ammonia excretion (33, 34). The current study, by showing that hypokalemia increases principal cell Rhcg expression almost to the level observed in intercalated cells adds to the growing evidence that principal cells contribute to ammonia excretion and thereby to renal acid-base homeostasis.
At least two mechanisms, increased total protein expression and changes in its subcellular distribution, can regulate plasma membrane Rhcg expression (31, 40, 41). The current study shows that both mechanisms regulate Rhcg expression in response to hypokalemia. Immunoblot analysis studies demonstrated increased Rhcg protein expression in the outer medulla, observations similar to those we observed previously in the response to metabolic acidosis (40). Hypokalemia also induced a substantial increase in apical Rhcg polarization, demonstrated using both immunohistochemistry and immunogold electron microscopy, with increased apical plasma membrane expression and a decrease in subapical vesicular Rhcg. Similar changes in Rhcg's subcellular distribution occur in response to both metabolic acidosis and reduced renal mass (31, 41). Thus the two regulatory mechanisms operative in hypokalemia, increased Rhcg protein expression and changes in its subcellular distribution, are similar to those utilized in other conditions.
Basolateral Rhcg expression appears to mediate an important role in renal ammonia excretion. Increased basolateral Rhcg expression, particularly in principal cells, occurs in metabolic acidosis (41), reduced renal mass (31) and hypokalemia (current study). In the mouse kidney, there are substantial differences in basolateral Rhcg expression among different mouse strains under both basal and acidosis-stimulated conditions (30, 57), and these differences correlate with the ability to increase urinary ammonia excretion in response to an acid load (57). Thus the increased basolateral Rhcg expression that occurs in hypokalemia, and is particularly evident in OMCD principal cells, likely contributes to increased ammonia excretion.
An important aspect of the current studies is that they suggest that Rhcg expression in the kidney is regulated, at least in part, by mechanisms other than extracellular pH. In other models, such as metabolic acidosis (6, 33, 34, 40, 41). and ⅚ renal ablation-infarction (31), changes in Rhcg expression facilitate maintenance of acid-base homeostasis. In contrast, in hypokalemia Rhcg expression increases despite the development of metabolic alkalosis (current study), and likely contributes to the development of the metabolic alkalosis. Thus one or more factors other than those involved in extracellular acid-base homeostasis may regulate Rhcg expression. In previous studies, we suggested that aldosterone may regulate Rhcg expression (31), but aldosterone is unlikely to mediate the increased expression in hypokalemia as hypokalemia decreases aldosterone production (10, 28).
Hypokalemia induces multiple other adaptations that contribute to increased renal ammonia metabolism. These include increased renal glutamine uptake (29), increased ammoniagenesis (47), and increased expression of the ammoniagenic enzymes, phosphoenolpyruvate carboxykinase and phosphate-dependent glutaminase and the glutamine transporter SNAT3 (8, 44). In the collecting duct, there is increased expression of H+-K+-ATPase (2, 3, 12, 18, 32), increased apical polarization of the vacuolar proton pump, H+-ATPase (4, 42) and increased carbonic anhydrase II expression (48). Finally, ammonia itself directly stimulates H+-K+-ATPase-mediated collecting duct H+ secretion (15–17). Thus the increased ammonia excretion observed in response to hypokalemia likely involves the integrated involvement of multiple processes that promote ammoniagenesis, acid secretion, and ammonia secretion.
Rhbg, another member of the ammonia transporter family, did not undergo detectable changes in expression in the current study. Rhbg expression decreased slightly in the cortex and was not altered in the OMCD. One explanation of these observations is that Rhbg does not contribute significantly to the increase in renal ammonia excretion, at least in the rat kidney. However, we cannot exclude the possibility that mechanisms, such as posttranslational modification, can regulate Rhbg-mediated ammonia transport, but are undetectable by immunoblot analysis and immunohistochemistry. Precedent for this possibility derives from studies of the role of Rhbg in the renal response to metabolic acidosis, where in the rat Rhbg expression does not change (40), whereas in the mouse Rhbg expression increases and Rhbg expression is necessary for the normal increase in urinary ammonia excretion in response to metabolic acidosis (5). Finally, it is also possible that because of the coexisting hypertrophy, particularly in the outer medulla, and because immunoblot analysis determines changes in Rhbg expression relative to total protein expression, that absolute Rhbg protein expression actually increases, albeit in parallel with total protein expression, particularly in the OMCD.
In summary, hypokalemia produced by dietary potassium restriction in the rat is associated with increased Rhcg expression in both intercalated and principal cells in the OMCD. This increase involves both increased protein expression and subcellular redistribution from subapical sites to the apical plasma membrane. Basolateral Rhcg expression increases, particularly in principal cells. Therefore, increased Rhcg expression during hypokalemia likely contributes in the rat to the increased collecting duct ammonia secretion and the development of metabolic alkalosis.
GRANTS
These studies were supported by funds from the National Institutes of Health (R01-DK045788, R21-047624), a Department of Veterans Affairs Merit Review Grant, the Gatorade Research Foundation, the Research Service of the North Florida/South Georgia Veterans Health System, and the National Research Foundation of Korea (2009-0073733 and 2011-0016068).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Gina Cowsert for secretarial assistance.
Footnotes
The term ammonia refers to the combination of both NH3 and NH4+. When referring specifically to either of these molecular species, we use the specific term “NH3” or “NH4+.”
REFERENCES
- 1. Ahn KY, Kone BC. Expression and cellular localization of mRNA encoding the “gastric” isoform of the H+-K+-ATPase α-subunit in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 268: F99–F109, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Ahn KY, Park KY, Kim KK, Kone BC. Chronic hypokalemia enhances expression of the H+-K+-ATPase α2-subunit gene in renal medulla. Am J Physiol Renal Fluid Electrolyte Physiol 271: F314–F321, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Ahn KY, Turner PB, Madsen KM, Kone BC. Effects of chronic hypokalemia on renal expression of the “gastric” H+-K+-ATPase α-subunit gene. Am J Physiol Renal Fluid Electrolyte Physiol 270: F557–F566, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Bailey MA, Fletcher RM, Woodrow DF, Unwin RJ, Walter SJ. Upregulation of H+-ATPase in the distal nephron during potassium depletion: structural and functional evidence. Am J Physiol Renal Physiol 275: F878–F884, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Bishop JM, Verlander JW, Lee HW, Nelson RD, Weiner AJ, Handlogten ME, Weiner ID. Role of the Rhesus glycoprotein, Rh B Glycoprotein, in renal ammonia excretion. Am J Physiol Renal Physiol 299: F1065–F1077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Burnell JM, Teubner EJ, Simpson DP. Metabolic acidosis accompanying potassium deprivation. Am J Physiol 227: 329–333, 1974 [DOI] [PubMed] [Google Scholar]
- 8. Busque SM, Wagner CA. Potassium restriction, high protein intake, and metabolic acidosis increase expression of the glutamine transporter SNAT3 (Slc38a3) in mouse kidney. Am J Physiol Renal Physiol 297: F440–F450, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Campbell-Thompson ML, Verlander JW, Curran KA, Campbell WG, Cain BD, Wingo CS, McGuigan JE. In situ hybridization of H-K-ATPase β-subunit mRNA in rat and rabbit kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F345–F354, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Cooke CR, Horvath JS, Moore MA, Bledsoe T, Walker WG. Modulation of plasma aldosterone concentration by plasma potassium in anephric man in the absence of a change in potassium balance. J Clin Invest 52: 3028–3032, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dobyan DC, Magill LS, Friedman PA, Hebert SC, Bulger RE. Carbonic anhydrase histochemistry in rabbit and mouse kidneys. Anat Rec 204: 185–197, 1982 [DOI] [PubMed] [Google Scholar]
- 12. DuBose TD, Codina J, Burges A, Pressley TA. Regulation of H+-K+-ATPase expression in kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F500–F507, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Elger M, Bankir L, Kriz W. Morphometric analysis of kidney hypertrophy in rats after chronic potassium depletion. Am J Physiol Renal Fluid Electrolyte Physiol 262: F656–F667, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Frank AE, Weiner ID. Effects of ammonia on acid-base transport by the B-type intercalated cell. J Am Soc Nephrol 12: 1607–1614, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Frank AE, Wingo CS, Andrews PM, Ageloff S, Knepper MA, Weiner ID. Mechanisms through which ammonia regulates cortical collecting duct net proton secretion. Am J Physiol Renal Physiol 282: F1120–F1128, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Frank AE, Wingo CS, Weiner ID. Effects of ammonia on bicarbonate transport in the cortical collecting duct. Am J Physiol Renal Physiol 278: F219–F226, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Garg LC. Respective roles of H-ATPase and H-K-ATPase in ion transport in the kidney. J Am Soc Nephrol 2: 949–960, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Gluck SL, Iyori M, Holliday LS, Kostrominova T, Lee BS. Distal urinary acidification from Homer Smith to the present. Kidney Int 49: 1660–1664, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol Renal Fluid Electrolyte Physiol 253: F595–F605, 1987 [DOI] [PubMed] [Google Scholar]
- 21. Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, Rh C Glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol 17: 2670–2679, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han KH, Kim HY, Croker BP, Reungjui S, Lee SY, Kim J, Handlogten ME, Adin CA, Weiner ID. Effects of ischemia-reperfusion injury on renal ammonia metabolism and the collecting duct. Am J Physiol Renal Physiol 293: F1342–F1354, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Han KH, Lee SY, Kim WY, Shin JA, Kim J, Weiner ID. Expression of the ammonia transporter family members, Rh B Glycoprotein and Rh C Glycoprotein, in the developing rat kidney. Am J Physiol Renal Physiol 299: F187–F198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 287: F628–F638, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 289: F347–F358, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Hansen GP, Tisher CC, Robinson RR. Response of the collecting duct to disturbances of acid-base and potassium balance. Kidney Int 17: 326–337, 1980 [DOI] [PubMed] [Google Scholar]
- 27. Heitman J, Agre P. A new face of the Rhesus antigen. Nat Genet 26: 258–259, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Henrich WL, Katz FH, Molinoff PB, Schrier RW. Competitive effects of hypokalemia and volume depletion on plasma renin activity, aldosterone and catecholamine concentrations in hemodialysis patients. Kidney Int 12: 279–284, 1977 [DOI] [PubMed] [Google Scholar]
- 29. Kamm DE, Strope GL. Glutamine and glutamate metabolism in renal cortex from potassium-depleted rats. Am J Physiol 224: 1241–1248, 1973 [DOI] [PubMed] [Google Scholar]
- 30. Kim HY, Verlander JW, Bishop JM, Cain BD, Han KH, Igarashi P, Lee HW, Handlogten ME, Weiner ID. Basolateral expression of the ammonia transporter family member, Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 296: F545–F555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293: F1238–F1247, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Kraut JA, Hiura J, Besancon M, Smolka A, Sachs G, Scott D. Effect of hypokalemia on the abundance of HKα1 and HKα2 protein in the rat kidney. Am J Physiol Renal Physiol 272: F744–F750, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C Glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296: F1364–F1375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee HW, Verlander JW, Bishop JM, Nelson RD, Handlogten ME, Weiner ID. Effect of intercalated cell-specific Rh C Glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 299: F369–F379, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madsen KM, Verlander JW, Tisher CC. Relationship between structure and function in distal tubule and collecting duct. J Electron Microsc Tech 9: 187–208, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. Am J Physiol Renal Physiol 290: F297–F305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakhoul NL, Hamm LL. Non-erythroid Rh glycoproteins: a putative new family of mammalian ammonium transporters. Pflügers Arch 447: 807–812, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14: 545–554, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Schuster VL. Function and regulation of collecting duct intercalated cells. Annu Rev Physiol 55: 267–288, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F1443–F1452, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Silver RB, Breton S, Brown D. Potassium depletion increases proton pump (H+-ATPase) activity in intercalated cells of cortical collecting duct. Am J Physiol Renal Physiol 279: F195–F202, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Tannen RL. The effect of uncomplicated potassium depletion on urine acidification. J Clin Invest 49: 813–827, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tannen RL. Relationship of renal ammonia production and potassium homeostasis. Kidney Int 11: 453–465, 1977 [DOI] [PubMed] [Google Scholar]
- 45. Tannen RL. Ammonia and acid-base homeostasis. Med Clin North Am 67: 781–798, 1983 [DOI] [PubMed] [Google Scholar]
- 46. Tannen RL. Effect of potassium on renal acidification and acid-base homeostasis. Semin Nephrol 7: 263–273, 1987 [PubMed] [Google Scholar]
- 47. Tannen RL, McGill J. Influence of potassium on renal ammonia production. Am J Physiol 231: 1178–1184, 1976 [DOI] [PubMed] [Google Scholar]
- 48. Thongboonkerd V, Chutipongtanate S, Kanlaya R, Songtawee N, Sinchaikul S, Parichatikanond P, Chen ST, Malasit P. Proteomic identification of alterations in metabolic enzymes and signaling proteins in hypokalemic nephropathy. Proteomics 6: 2273–2285, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Verlander JW, Moudy RM, Campbell WG, Cain BD, Wingo CS. Immunohistochemical localization of H-K-ATPase α2C subunit in rabbit kidney. Am J Physiol Renal Physiol 281: F357–F365, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Weiner ID. The Rh gene family and renal ammonium transport. Curr Opin Nephrol Hyper 13: 533–540, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Weiner ID. Expression of the non-erythroid Rh glycoproteins in mammalian tissues. Transfus Clin Biol 13: 159–163, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weiner ID, Frank AE, Wingo CS. Apical proton secretion by the inner stripe of the outer medullary collecting duct. Am J Physiol Renal Physiol 276: F606–F613, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weiner ID, Verlander JW. Role of NH3 and NH4+ transporters in renal acid-base transport. Am J Physiol Renal Physiol 300: F11–F23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weiner ID, Wingo CS, Hamm LL. Regulation of intracellular pH in two cell populations of the inner stripe of the rabbit outer medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F406–F415, 1993 [DOI] [PubMed] [Google Scholar]
- 57. Weiner ID, Verlander JW. Molecular physiology of the Rh ammonia transport proteins. Curr Opin Nephrol Hypertens 19: 471–477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Welt LG, Hollander W, Blythe WB. The consequences of potassium depletion. J Chronic Dis 11: 213–254, 1960 [DOI] [PubMed] [Google Scholar]