Abstract
Cytochrome P-450 metabolites of arachidonic acid, the epoxyeicosatrienoic acids (EETs) and hydrogen peroxide (H2O2), are important signaling molecules in the kidney. In renal arteries, EETs cause vasodilation whereas H2O2 causes vasoconstriction. To determine the physiological contribution of H2O2, catalase is used to inactivate H2O2. However, the consequence of catalase action on EET vascular activity has not been determined. In rat renal afferent arterioles, 14,15-EET caused concentration-related dilations that were inhibited by Sigma bovine liver (SBL) catalase (1,000 U/ml) but not Calbiochem bovine liver (CBL) catalase (1,000 U/ml). SBL catalase inhibition was reversed by the soluble epoxide hydrolase (sEH) inhibitor tAUCB (1 μM). In 14,15-EET incubations, SBL catalase caused a concentration-related increase in a polar metabolite. Using mass spectrometry, the metabolite was identified as 14,15-dihydroxyeicosatrienoic acid (14,15-DHET), the inactive sEH metabolite. 14,15-EET hydrolysis was not altered by the catalase inhibitor 3-amino-1,2,4-triazole (3-ATZ; 10–50 mM), but was abolished by the sEH inhibitor BIRD-0826 (1–10 μM). SBL catalase EET hydrolysis showed a regioisomer preference with greatest hydrolysis of 14,15-EET followed by 11,12-, 8,9- and 5,6-EET (Vmax = 0.54 ± 0.07, 0.23 ± 0.06, 0.18 ± 0.01 and 0.08 ± 0.02 ng DHET·U catalase−1·min−1, respectively). Of five different catalase preparations assayed, EET hydrolysis was observed with two Sigma liver catalases. These preparations had low specific catalase activity and positive sEH expression. Mass spectrometric analysis of the SBL catalase identified peptide fragments matching bovine sEH. Collectively, these data indicate that catalase does not affect EET-mediated dilation of renal arterioles. However, some commercial catalase preparations are contaminated with sEH, and these contaminated preparations diminish the biological activity of H2O2 and EETs.
Keywords: arteries, EDHF, epoxyeicosatrienoic acids, hydrogen peroxide
the vascular endothelium produces vasoactive substances including nitric oxide (NO), prostacyclin (PGI2), and a group of compounds called endothelium-derived hyperpolarizing factors (EDHFs) (6, 11, 15, 23). EDHFs hyperpolarize the underlying smooth muscle to cause vascular relaxation (4). In the coronary circulation, two candidates of EDHF activity are the reactive oxygen species (ROS), hydrogen peroxide (H2O2) (17, 21), and the epoxyeicosatrienoic acids (EETs), cytochrome P-450 (CYP) epoxygenase metabolites of arachidonic acid (AA) (3, 17, 27). In many vasculatures, EETs mediate vasodilation through activation of smooth muscle large-conductance calcium-activated potassium (BKCa) channels (1, 18, 19, 31). Alternatively, an endothelial cell autocrine effect of EETs has been noted with EETs increasing intracellular calcium through transient receptor potential (TRP) channel translocation and activation of endothelial small-conductance (SKCa) and intermediate-conductance (IKCa) channels (9, 10).
EETs are hydrolyzed by soluble epoxide hydrolase (sEH) to produce dihydroxyeicosatrienoic acids (DHETs) (29). In human coronary arteries and rat renal afferent arterioles, DHET relaxations are less potent than the corresponding EET regioisomer (16, 18). Thus sEH metabolism of EETs with subsequent DHET formation could regulate EET bioavailability and consequently EDHF activity.
H2O2 is formed from the dismutation of superoxide (O2·−). In vascular endothelial cells, a likely source of H2O2 is the superoxide dismutase (SOD) family of enzymes (24, 25). Besides the coronary circulation, H2O2 mediates EDHF activity in human mesenteric and mouse mesenteric arteries (22, 24). In contrast, in isolated rat renal arteries, H2O2 functions as an endothelium-derived contracting factor (12). To examine the role of H2O2, catalase is often employed as a pharmacological tool to decompose H2O2 and limit its availability.
The initial purpose of this study was to determine whether EET activity in renal arteries is altered by H2O2. In rat renal afferent arterioles, we observed that catalase reduced EET dilations, but this effect varied depending upon the specific commercial catalase preparation. In vitro analysis was performed to examine the chemical interaction of EETs with catalase. We provide evidence that EET stability and EET dilations of renal afferent arterioles are not altered by catalase per se. Alternatively, contamination of select catalase preparations by sEH catalyzes EET hydrolysis. Thus sEH contamination is responsible for decreasing EET bioavailability and consequently for limiting EET vascular activity in the presence of certain catalase products.
MATERIALS AND METHODS
Vascular Activity
Experiments were carried out according to the guidelines of the Medical College of Wisconsin Institutional Animal Care and Use Committee. Male Sprague-Dawley rats weighing 296 ± 6 g (Charles River Laboratories) were used. Experiments were conducted using the in vitro perfused juxtamedullary nephron preparation described previously (16). Isolated kidneys were perfused with renal artery perfusion pressure set to 100 mmHg. A single afferent arteriole was chosen from each kidney for each experiment. Following a 20-min equilibration, a control diameter was determined. Phenylephrine (0.5 μM) was added to decrease the diameter by 50%. Thereafter, diameter responses to 14,15-EET (0.001–1 μM) were determined. Catalases, Sigma bovine liver (SBL; 1,000 U/ml) or the Calbiochem bovine liver (CBL; 1,000 U/ml) or trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (tAUCB; 1 μM), were added 20 min before determination of the responses to 14,15-EET. Vascular internal diameters were measured at a single site using a calibrated image-shearing monitor (model 901, Instrumentation for Physiology and Medicine, San Diego, CA). Diameters are expressed as percent control diameter.
Catalase Incubations
Incubations were performed in 1 ml HEPES buffer (in mM: 10 HEPES, 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, and 6 glucose, pH 7.4). SBL catalase (5, 50, 500 U/ml) was incubated in HEPES for 5 min at 37°C. 3H-14,15-EET (10 μl) was added, and the samples were incubated for an additional 30 min. For metabolite mass spectrometry analysis, EET regioisomers (10 μM) were incubated in HEPES buffer as above with 500 U/ml SBL catalase. For EET regioisomer hydrolysis assays, EETs were incubated in HEPES for 5 min. SBL catalase (100 U/ml) was added, and the incubation was continued for an additional 30 min. All other hydrolysis assays were performed with 14,15-EET (10 μM). In some instances, the catalase inhibitor 3-amino-1,2,4-triazole (3-ATZ; 10, 25, 50 mM) or the sEH inhibitor BIRD-0826 (1, 5, 10 μM) was added with the catalase. Similar incubations were performed using different commercial catalase preparations [500 U/ml, SBL, Sigma mouse liver (SML), Sigma human erythrocytes (SHE), Sigma Aspergillus niger (SAN), Calbiochem bovine liver (CBL)] (Table 1). Ethanol (95%, 333 μl) was added to all samples to quench the reactions. Samples were extracted by solid-phase extraction (Varian C18 extraction columns) as previously described (28), dried under N2 gas, and frozen.
Table 1.
Catalase preparations
| Catalase Preparation | Product No. | Lot No. | Specific Activity U/mg protein |
|---|---|---|---|
| Sigma bovine liver (SBL) | C9322 | 020K7260 | 3,940 |
| Sigma bovine liver (C40) | C40 | 120H7060 | 25,000 |
| Sigma mouse liver (SML) | C8531 | 027H7050 | 1,200 |
| Sigma human erythrocytes (SHE) | C3556 | 082K1210 | 93,200 |
| Sigma Aspergillus niger (SAN) | C3515 | 042K3792 | 5,270 |
| Calbiochem bovine liver (CBL) | 219001 | D00009037 | 39,012 |
Reverse-Phase HPLC
[14C]AA and 3H-14,15-EET metabolites were resolved by reverse-phase HPLC as previously described (28). Briefly, sample extracts were dissolved in 200 μl acetonitrile-H2O-acetic acid (50:50:0.01). Samples were separated on a Nucleosil-C18 column (5 μm, 4.6 × 250 mm) using a 40-min linear solvent gradient from 50% solvent B (acetonitrile+0.1% glacial acetic acid) in solvent A (H2O) to 100% solvent B. The flow rate was 1 ml/min. The effluent was collected in 200-μl fractions (5 fractions/min), and radioactivity was counted using liquid scintillation spectrometry.
For EET hydrolysis assays, standards and samples were resolved and measured by a Phenomenex Kromasil C18 column (2.0 × 250 mm) and a Hewlett-Packard 1090 Series II liquid chromatograph (Hewlett-Packard, Palo Alto, CA). The flow rate was 0.2 ml/min. Solvent A was deionized water with 0.1% glacial acetic acid, and solvent B was acetonitrile with 0.1% glacial acetic acid. The program consisted of a linear gradient from 55 to 60% B over 10 min, 10 min at 60% B, a linear gradient from 60% to 75% B over 15 min, and a linear gradient of 75–100% over 5 min. Absorbance (235 nm) was recorded with a UV diode array detector and analyzed with Chemstation software. 15-HETE (1 μg) was added to each sample and served as an internal standard. A DHET standard curve was used to quantify DHET metabolites. Each regioisomer was tested in triplicate, and data are presented as means ± SE.
EET Metabolite Mass Spectrometry
Liquid chromatography-mass spectrometry (LC-MS) was performed (Agilent 1100 LC/MSD, S1 model) as previously described (26). Corresponding deuterated EETs ([2H8]-EETs) were added to the samples as an internal standard. Samples were separated on a reverse-phase C18 column (5 μm, 2 × 150 mm, Kromasil). The mobile phase was H2O-acetonitrile with 0.005% acetic acid and a flow rate of 0.2 ml/min. Nitrogen was used as the drying gas (12 l/min, at 350°C). Detection was done in the negative scanning mode.
Electrophoresis and Coomassie Staining
Protein from each catalase preparation (5 μg) was loaded onto a 12% Tris·HCl gel. The gel was developed for 1 h at 150 V and subsequently washed in H2O for three 5-min intervals. The washed gel was incubated in Coomassie stain for 1 h and washed for 72 h in H2O.
Western Immunoblotting
Proteins from each catalase preparation (100 μg each) were loaded onto a 12% Tris·HCl gel and resolved at 100 V for 1 h. Proteins were transferred to a nitrocellulose membrane. Nonspecific binding was blocked with 5% milk in Tris-buffered saline (TBS; buffer containing 20 mM Tris base, 150 mM NaCl, 0.1% sodium azide, and 3% bovine serum albumin) containing 0.1% Tween (TBST) for 2 h at room temperature. Membranes were washed in TBST and incubated with rabbit anti-sEH antibody (1:2,000 in 5% TBST, kind gift from Dr. Bruce Hammock, UC Davis, Davis, CA) overnight at 4°C. The membranes were rinsed with TBST buffer, incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody (1:2,000 in 5% milk in TBS) for 1 h at room temperature, and washed with TBS buffer. Immunoreactive bands were identified using the Renaissance chemiluminescence detection kit and Kodak BioMax ML film.
Protein Mass Spectroscopy
Sample preparation.
From the SDS-PAGE gel, the SBL catalase protein bands at ∼57 kDa (1-mm segments) were cut, minced, and placed into a low binding Eppendorf tube. Gel segments were washed with 10 volumes of distilled water (∼200 μl) for 10 min with sonication. The segments were equilibrated with 200 μl of 25 mM ammonium bicarbonate (NH4HCO3, pH 8.0) with sonication for 10 min. The supernatant was discarded, and the remaining gel particles were dried (∼10 min in a vacuum centrifuge). Gel slices were digested in 25 μl trypsin (20 ng/μl, Promega) in 25 mM NH4HCO3 for 16 h at 37°C. Digest solution was extracted with one volume (35 μl) 80% acetonitrile in 1% formic acid with sonication for 20 min. Peptides were dried and resuspended in 16 μl distilled water with 0.1% formic acid, passed through a C18 ZipTip, and eluted in 50% acetonitrile in water with 0.1% formic acid.
LC-Fourier transformed ion cyclotron resonance MS.
LC analysis was performed on an Agilent 1100 LC system connected to an IonSpec 7.0 Tesla Fourier transformed ion cyclotron resonance MS (FTMS), which was controlled using IonSpec Omega software. A Proteo 90A column (Jupiter, 150 × 0.50 mm, 4 μm) was used to separate the peptides. The mobile phase consisted of solvent A (0.1% formic acid in H2O) and solvent B (0.1% formic acid in acetonitrile) with a gradient of 10% B at 0 min to 100% B at 60 min. The flow rate was set to 10 μl/min, and run time was 85 min. Probe and ESI chamber heaters were 80°C, and the source heater was 130°C. The FTMS was operated in the positive mode. The ion guide was optimized for 700 m/z. The peptide calibration mixture contained angiotensin II, bombesin, substance P, and melitin. The data-dependent function of the Omega software was used to acquire the MS/MS data. Precursor ions were dissociated by sustained off-resonance irradiation (SORI). Nitrogen gas was used for fragmentation. Transients of both the MS and MS/MS data were collected.
Backward database searching.
The PubMed Protein website was used to obtain the amino acid sequences of bovine catalase and sEH. Theoretical trypsin digestions (2 missed cleavage sites allowed) were determined using the Peptide Cutter Program (http://us.expasy.org/tools/peptidecutter) to acquire possible tryptic fragments. These were compared against the list of deconvoluted masses in the MS data. Theoretical fragments of peptides dissociated for MS/MS were generated using the fragment ion calculator (http://db.systemsbiology.net:8080/proteomicsToolkit/FragIonServlet.html). Resulting possible fragment ions were compared against ions generated by MS/MS.
Data collection.
MS spectra 48–81 were summed, and the resulting spectrum was deconvoluted. Masses were submitted to the MASCOT search engine using the MSDB database. Two missed cleavages and a 1.5-Dalton peptide mass tolerance was allowed. Similarly, MS/MS data selected and dissociated by the Omega software between spectra 48 and 81 were submitted to MASCOT.
Epoxygenase Activity Assay
CYP2C9 microsomes (250 pM, BD Gentest Supersomes, Franklin Lakes, NJ) were incubated in 400 μl assay buffer of the following composition: 0.05 M Tris·HCl, 0.15 M KCl, 0.01 M MgCl2·6H2O, pH = 7.5 with no catalase or with either CBL and SBL catalase (500 U/ml) at 37°C for 10 min in a shaking water bath. Isocitrate (1 mM), isocitrate dehydrogenase (0.025 U/ml), AA (10−7 M), 3 μl 14C-AA, and NADPH (0.4 mM) were added, and the samples were incubated at 37°C for an additional 30 min. Ethanol (25% final concentration) was added to quench the reactions. All samples were extracted and separated with reverse-phase HPLC as previously described (28). EET/DHET ratios were estimated by summing the total radioactive counts under the respective peaks.
Materials
The various commercial catalase preparations are described in Table 1. EETs and 3H-EETs were synthesized using published methods (3, 7, 28). All chemicals were purchased from Sigma unless noted otherwise. The sEH inhibitor tAUCB was a gift from Dr. Bruce Hammock (Univ. of California-Davis).
Statistical Analysis
Data were evaluated using a one-way ANOVA with repeated measures when applicable. Differences between group means were determined using a Newman-Kuels multiple range test. P values <0.05 were considered statistically different. All values are reported as means ± SE.
RESULTS
In buffer-perfused and pressurized rat renal afferent arterioles, 14,15-EET caused concentration-dependent dilations (Fig. 1, maximum dilation = 45 ± 9%). The dilations were not altered by pretreatment with the CBL catalase (1,000 U/ml) but were significantly inhibited by the SBL catalase (1,000 U/ml, maximum dilation = 25 ± 6%). Dilations to 14,15-EET in the presence of the SBL catalase were restored to control values by the sEH inhibitor tAUCB (1 μM, maximum dilation = 49 ± 12%). This suggests that the SBL catalase contains an enzyme that metabolizes the EET to a less vasoactive metabolite.
Fig. 1.
Effect of Sigma bovine liver (SBL) or Calbiochem bovine liver (CBL) catalase on 14,15-epoxyeicosatrienoic acid (EET)-induced dilation of rat renal afferent arterioles. 14,15-EET dilations were examined in arteries under control conditions without catalase, with SBL or CBL catalase (1,000 U/ml) or SBL catalase with the soluble epoxide hydrolase inhibitor trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (tAUCB; 1 μM). n = 3–8 each. *Significantly different from control, P < 0.05.
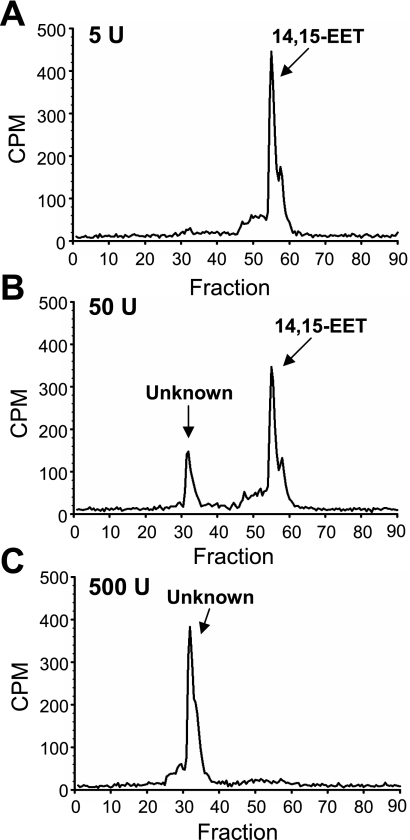
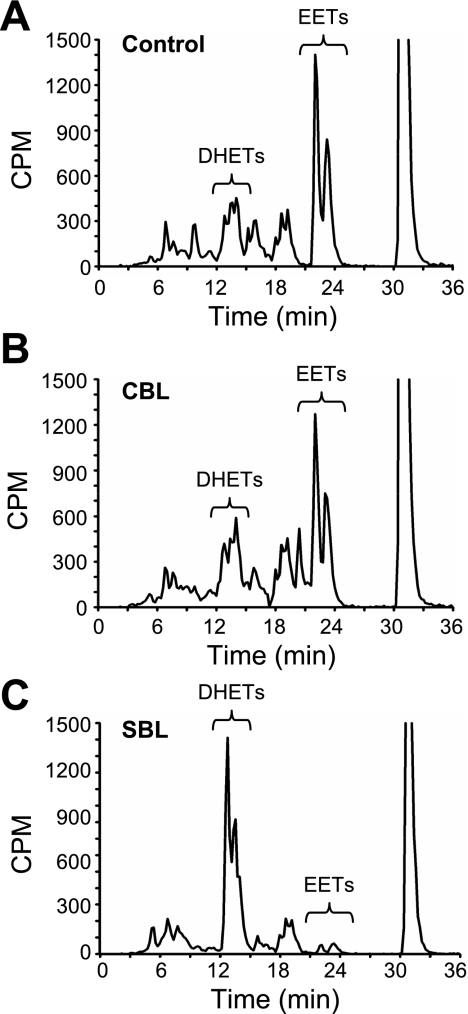
3H-14,15-EET was incubated with SBL catalase (5, 50 and 500 U/ml). Metabolites were extracted, separated by reverse-phase HPLC, and radioactivity was counted. With 5 U/ml SBL catalase (Fig. 2A), only one radioactive peak was observed that comigrated with the 14,15-EET standard. With higher catalase concentrations (Fig. 2, B and C), a more polar peak was evident. Only the polar peak was observed when the EET was incubated with 500 U/ml catalase (Fig. 2C).
Fig. 2.
Effect of SBL catalase on 14,15-EET hydrolysis. 3H-14,15-EET (10 μM) was incubated with 5 (A), 50 (B), or 500 (C) U/ml SBL catalase for 30 min. Metabolites were extracted and analyzed by reverse-phase HPLC. Migration times of EET standards are noted on each chromatogram. CPM, counts per minute.
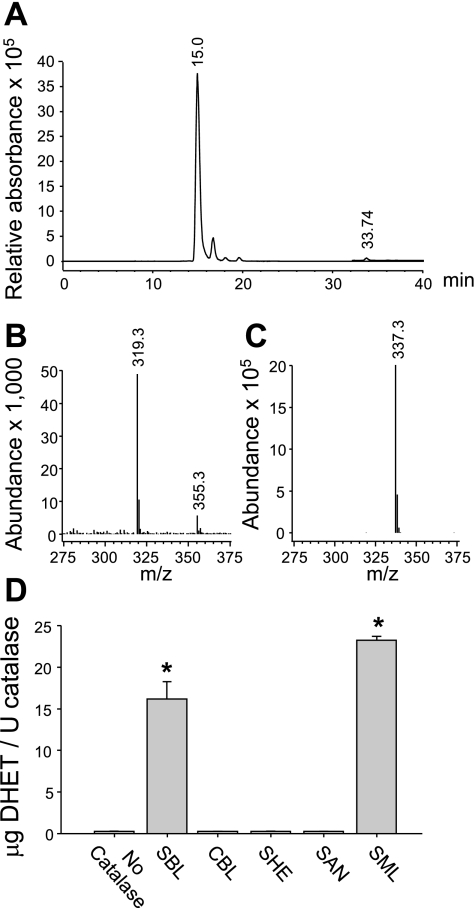
LC-MS analysis was separately performed to identify the SBL catalase metabolites of 14,15-EET as well as metabolites of 11,12-, 8,9-, and 5,6-EET. EETs and the corresponding metabolites eluted as two distinct peaks: 15.0 and 33.7 min, 16.7 and 36.1 min, 18.1 and 36.9 min, and 19.8 and 37.8 min, for 14,15-, 11,12-, 8,9-, and 5,6-EET, respectively. The 33- to 38-min peaks were consistent with the elution time of EET standards and the 15- to 20-min peaks comigrated with the DHET standards. 14,15-EET eluded the column at 33.7 min (Fig. 3A) and demonstrated an [M-1] ion with a mass/charge (m/z) of 319 (Fig. 3B). The hydrolysis metabolite of 14,15-EET eluded the column at 15.0 min and demonstrated an [M-1] ion with an m/z of 337 (Fig. 3C). This is consistent with the molecular mass of 14,15-DHET. The other EET regioisomers produced similar results (data not shown).
Fig. 3.
Liquid chromatography (LC)-mass spectrometric (MS) analysis of 14,15-EET metabolism by SBL catalase (A–C) and 14,15-EET hydrolysis by various commercial catalase preparations (D). 14,15-EET (10 μM) was incubated with 500 U/ml of SBL catalase for 30 min. Metabolites were extracted and analyzed by LC-MS. A: metabolite LC elution profile. B: MS [M-1] ion of the 33.74-min peak. C: MS [M-1] ion of the 15.0-min peak. D: 14,15-EET (10 μM) was incubated with the same amount of catalase activity (100 U/ml) of different catalase preparations. Metabolites were extracted and separated by reverse phase HPLC. *Significantly different from no catalase control, P < 0.05; n = 3 each.
We evaluated the effect of other commercially available catalase preparations on 14,15-EET hydrolysis. Properties of these preparations are listed in Table 1. There were no differences between control incubations without catalase or incubations with the CBL, SHE, and SAN preparations (≤ 0.3 μg DHET/U catalase, Fig. 3D). Significant DHET production was measured in incubations with the SBL and SML preparations (≥16.0 μg DHET/U catalase). Of note, EET hydrolysis was limited to liver-derived catalase preparations obtained from Sigma with low catalase specific activity.
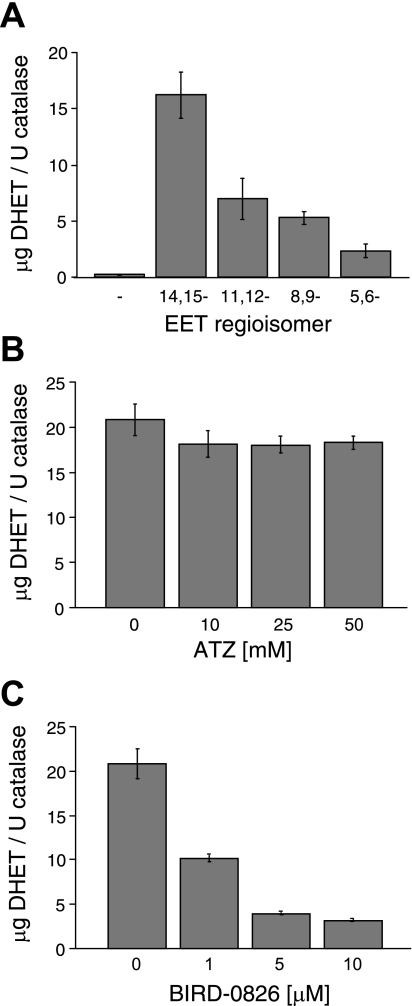
The rate of EET hydrolysis by the SBL catalase demonstrated regioisomer specificity. For the 30-min incubation, DHET production was greatest for 14,15- followed by 11,12-, 8,9-, and 5,6-EET (Fig. 4A). This reflects hydrolysis rates of 0.54 ± 0.07, 0.23 ± 0.06, 0.18 ± 0.01, and 0.08 ± 0.02 μg DHET/U catalase/min for 14,15-, 11,12-, 8,9-, and 5,6-EET, respectively. This profile is similar to EET hydrolysis by purified sEH (27). Together, these results suggest that sEH contamination of the SBL catalase is responsible for the observed EET metabolism.
Fig. 4.
EET hydrolysis by SBL catalase. EETs (10 μM) were incubated with SBL catalase (100 U/ml), and metabolites were extracted and analyzed by reverse-phase HPLC. A: 14,15-, 11,12-, 8,9-, and 5,6-EET hydrolysis. B: effect of the catalase inhibitor 3-amino-1,2,4-triazole (3-ATZ; 10, 25, or 50 mM), on 14,15-EET hydrolysis. C: effect of the sEH inhibitor BIRD-0826 (1, 5, or 10 μM) on 14,15-EET hydrolysis. *Significantly different from control, P < 0.05; n = 3 each.
To further clarify the role of sEH and catalase in the observed hydrolysis, 14,15-EET hydrolysis assays with the SBL catalase were repeated in the presence and absence of either the catalase inhibitor 3-ATZ (10–50 mM) or the sEH inhibitor BIRD-0826 (1–10 μM). 14,15-DHET production did not differ between the control and samples treated with 3-ATZ (Fig. 4B). Conversely, BIRD-0826 caused concentration-dependent inhibition of 14,15-DHET production (Fig. 4C). These results further indicate that EET hydrolysis to DHET is due to sEH activity and not catalase activity.
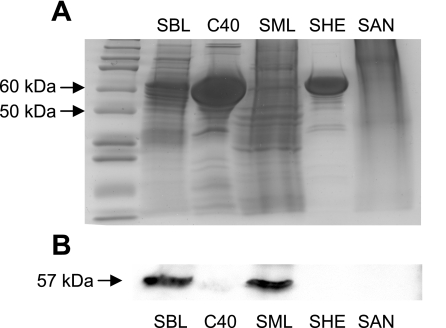
The various catalase preparations were examined by electrophoresis and Coomassie staining for protein content and Western immunoblotting for sEH expression. Coomassie staining (Fig. 5A) revealed prominent bands between 50 and 75 kDa in the SBL, C40, and SHE preparations, corresponding to the ∼60-kDa mass of catalase and mammalian sEH. Multiple bands or streaking was seen in all preparations except the SHE preparation. Strong sEH expression (57-kDa band) (Fig. 5B) was observed in the SBL and SME catalase preparations. Notably, these preparations also demonstrated significant EET hydrolysis.
Fig. 5.
Electrophoresis and Coomassie staining (A) and sEH Western immunoblotting (B) of commercial catalase preparations.
The 57-kDa band from lanes loaded with the SBL catalase were cut from the gel and prepared for LC-FTMS for protein identification. MS analysis detected 28 peptides that matched bovine sEH (Table 2). Further MS/MS analysis identified three peptide fragments that matched segments of bovine sEH (Table 3). Two fragments had scores that indicated extensive homology for bovine sEH. As a positive control, MS results were compared against tryptic fragments of bovine catalase. Seventeen peptide fragments were detected that matched bovine catalase (data not shown).
Table 2.
MS peptide analysis of SBL catalase: peptides matching bovine sEH
| Residues | Expected Mr | Calculated Mr | Sequence |
|---|---|---|---|
| 40–52 | 1342.03 | 1341.72 | K.AFLRGGPDGPSIR.V |
| 44–52 | 853.39 | 854.42 | R.GGPDGPSIR.V |
| 44–55 | 1212.64 | 1212.63 | R.GGPDGPSIRVMK.G |
| 90–94 | 664.16 | 663.36 | K.QIFEK.I |
| 90–99 | 1219.10 | 1219.69 | K.QIFEKILSSR.K |
| 90–99 | 1219.60 | 1219.69 | K.QIFEKILSSR.K |
| 90–100 | 1346.71 | 1347.79 | K.QIFEKILSSRK.I |
| 168–174 | 834.40 | 834.49 | K.FVLDTLK.I |
| 276–287 | 1332.64 | 1333.71 | R.YQIPALAQAGFR.V |
| 288–294 | 773.07 | 774.43 | R.VLAVDMK.G |
| 288–294 | 773.40 | 774.43 | R.VLAVDMK.G |
| 288–294 | 773.73 | 774.43 | R.VLAVDMK.G |
| 295–315 | 2282.16 | 2283.08 | K.GYGESSAPPEIEEYSLEVLS.K |
| 295–315 | 2283.14 | 2284.08 | K.GYGESSAPPEIEEYSLEVLS.K |
| 400–410 | 1401.79 | 1401.75 | K.NLSRTFKSFFR.S |
| 400–410 | 1402.73 | 1401.75 | K.NLSRTFKSFFR.S |
| 456–467 | 1478.75 | 1479.77 | K.KSGFRGPLNWYR.N |
| 457–467 | 1350.64 | 1351.68 | K.SGFRGPLNWYR.N |
| 457–471 | 1839.99 | 1839.88 | K.KSGFRGPLNWYRNMDK.N |
| 457–471 | 1840.99 | 1839.88 | K.KSGFRGPLNWYRNMDK.N |
| 472–478 | 966.53 | 965.44 | K.NWEWGFK.G |
| 472–482 | 1321.63 | 1322.62 | K.NWEWGFKGSGR.K |
| 472–483 | 1451.79 | 1450.71 | K.NWEWGFKGSGRK.I |
| 483–495 | 1424.61 | 1425.86 | R.KILIPALMVTAEK.D |
| 496–505 | 1130.59 | 1131.58 | K.DLVLTPEMSK.H |
| 496–505 | 1131.10 | 1131.58 | K.DLVLTPEMSK.H |
| 496–505 | 1131.58 | 1131.58 | K.DLVLTPEMSK.H |
| 537–555 | 2210.10 | 2209.24 | R.ILIEWLETDARDLPVVSKL |
MS, mass spectrometry; sEH, soluble epoxide hydrolase.
Table 3.
MS/MS peptide analysis of SBL catalase: peptide fragments matching bovine sEH
| Fragment Ion | Expected Mr | Calculated Mr | Peptide | Ion Score |
|---|---|---|---|---|
| 610.3147 (2+) | 1218.6148 | 1219.6924 | QIFEKILSSR | 32 |
| 786.4867 (2+) | 1570.9588 | 1572.7970 | AVASLNTPFMPSNPK | 44* |
| 795.4453 (2+) | 1588.8760 | 1588.9010 | KINYPMLQAAVTLK | 49* |
Ion score indicative of extensive homology or identity (>36).
To eliminate the possibility that catalase is directly altering cytochrome P-450 activity to inhibit EET production, epoxygenase assays were performed using microsomes overexpressing CYP2C9. Microsomes were incubated with [14C]AA without catalase or with SBL or CBL catalase (500 U/ml). Metabolites were extracted and analyzed by reverse-phase HPLC. In control incubations, 14C metabolites comigrating with EET and DHET standards were observed, and the EET/DHET ratio was 2.0/1 with AA conversion to EET+DHET of 25% (Fig. 6A). Incubation with the CBL catalase did not alter the [14C]AA metabolic profile (EET/DHET ratio = 1.5/1, and AA conversion of EET+DHET of 23%) (Fig. 6B). However, the SBL catalase dramatically reduced the magnitude of the metabolites that comigrated with the EETs and increased the peaks that comigrated with the DHETS (EET/DHET ratio = 0.1/1 with AA conversion of EET+DHET of 31%) (Fig. 6C). This indicates that catalase itself does not alter CYP2C9 epoxygenase activity, and the CYP2C9 microsome incubation with the SBL catalase showed a greatly reduced EET/DHET ratio, suggesting enhanced EET hydrolysis.
Fig. 6.
Effect of CBL and SBL catalase on [14C]arachidonic acid (AA) metabolism by microsomes overexpressing CYP2C9. Microsomes were incubated with [14C]AA without catalase (A), with CBL (B), or with SBL (C) catalase (500 U/ml). Metabolites were extracted and analyzed by reverse-phase HPLC. Migration times of EET and DHET standards are noted on each chromatogram.
DISCUSSION
The major finding from this study is that some, but not all, commercial catalase preparations are contaminated with sEH. This finding is particularly relevant since EETs and H2O2 have opposing activity in renal vascular studies (12, 16) and both EETs and H2O2 have been identified as EDHFs in other vascular beds (3, 17, 21, 22, 27). Catalase is a fundamental pharmacological tool widely used to characterize the role of H2O2 in vascular function. However, if the catalase preparation also contains sEH, inhibition of vascular activity could be secondary to limited EET availability and not decreased H2O2.
The effect of contaminated catalase preparations would only be apparent in arteries that demonstrate a clear difference between the dilator ability of the EET and the DHET with more potent dilations occurring with the EETs. In rat renal afferent arteries, 11,12-EET causes a potent dilation whereas 11,12-DHET causes constriction (16). In these arterioles, the sEH-contaminated SBL catalase reduced 14,15-EET dilations whereas the noncontaminated CBL catalase was without effect. The catalase-associated reduction in EET dilation was rescued with sEH inhibition. Thus sEH contamination of catalase can significantly diminish EET availability and consequently reduce EET dilations. Since H2O2 functions as a contracting factor in renal arteries (12), inhibition of EET vascular dilation by the contaminated catalase could mask the reduction of H2O2 availability and the associated contraction.
Identification and characterization of sEH as the contaminating enzyme in the SBL catalase preparation was demonstrated through multiple mechanisms. The identification of DHETs as the primary EET metabolite suggested hydrolysis activity. sEH is highly expressed in numerous mammalian tissues including the liver and is the primary endogenous enzyme responsible for EET hydrolysis (8, 30). Thus sEH was a primary candidate for the hydrolysis activity. In addition to our study, Guenthner and colleagues (13) described sEH preparations contaminated with catalase and vice versa. sEH contamination of catalase preparations likely stems from structural similarities between the two enzymes (14). Both are cytosolic enzymes, but mammalian catalase is a homotetramer whereas mammalian sEH is a homodimer. However, the subunits are of similar size, ∼60 kDa.
Catalase-dependent 14,15-EET hydrolysis was blocked by sEH inhibition but not catalase inhibition. In addition, catalase-dependent EET hydrolysis demonstrated a similar pattern of EET regioisomer specificity as purified sEH (30). This pharmacological and hydrolytic profile further suggested sEH as the enzyme responsible for the hydrolysis. In addition, Western immunoblotting detected sEH protein in some commercial catalase preparations. The expression of sEH by Western immunoblotting correlated with EET hydrolysis activity. Additionally, the presence of bovine sEH in the SBL catalase was confirmed by MS analysis. Together, our evidence clearly illustrates the role of sEH in the EET hydrolysis by the SBL catalase.
The catalase sEH contamination should only alter EET availability and not EET production. This was clarified by measuring epoxygenase activity of CYP2C9 microsomes. The EET/DHET production was not reduced by the sEH-contaminated catalase; however, the EET/DHET ratio was dramatically reduced.
The two sEH-contaminated catalase preparations that were identified by Western immunoblotting and EET hydrolysis assays were derived from mammalian liver, purchased from Sigma and had low levels of catalase enzyme activity (SBL = 3,940 U/mg protein, SML = 1,200 U/mg protein) (Table 1). The lack of sEH contamination in the CBL liver-catalase demonstrates that contamination is independent of tissue source. The contaminated Sigma catalases were prepared from both murine and bovine sources, showing that contamination is independent of source species. Sigma as a vendor is also not a determining factor since Sigma provided the apparently sEH-free SAN and SHE catalases. In addition, low catalase activity in itself does not correlate with sEH contamination because the SAN preparation had low activity (catalase activity = 5,270 U/mg protein) and did not show evidence of EET hydrolysis or sEH protein expression. Thus it is important to determine the purity of a catalase preparation before use.
To illustrate the importance of our findings to the American Journal of Physiology-Renal Physiology readership, searching the journal website for the terms hydrogen peroxide plus catalase resulted in 2,090 citations. From the first 50 relevant original articles listed, 18 articles used catalase in their experimental design. Of these 18, Sigma was the source in 12 of the articles, 5 articles did not note a catalase source, and 1 used Boehringer Ingelheim Biochemical as their source. Thus Sigma catalase accounts for >65% of catalase use by American Journal of Physiology-Renal Physiology contributors. It should be noted that we verified sEH contamination of the Sigma bovine and mouse liver-derived catalases of the specific product and lot numbers tested (see Table 1). This article should alert researchers to the problem so investigators need to check for sEH contamination of other product and lot numbers. Notation of product and lot numbers are not typically included in manuscript method descriptions.
Catalase is an indispensible tool for studying the endogenous effects of H2O2, and suitable alternative pharmacological tools to inactivate H2O2 are not available. An alternative approach is to pharmacologically inhibit H2O2 production. However, vascular H2O2 production occurs at many sources, including dismutation of O2·− either spontaneously or by SOD catalysis or enzymatic production by xanthine oxidase or glucose oxidase (2, 24, 25). Thus pharmacological regulation of H2O2 at the site of production remains complex.
Purification of commercial catalase by various chromatography applications (5, 20) is feasible but time consuming. Purchase of high-specific activity, purified catalase preparations remains a more practical approach to eliminate sEH contamination. In this regard, all high-activity catalase preparations tested for this publication were without apparent sEH contamination.
In summary, our findings demonstrated that certain commercial catalase preparations are contaminated with active sEH. Because of the dual nature of the contaminated catalase preparations to degrade H2O2 and hydrolyze EETs, their use could lead to erroneous interpretations regarding EET- and H2O2 -dependent vascular responses. These studies indicate that published studies using SBL or SML catalase to establish a role for H2O2 as a mediator of endothelium-dependent responses need to be reevaluated and their conclusions questioned.
GRANTS
These studies were supported by grants from the National Heart, Lung and Blood Institute (HL-51055 to W. B. Campbell), HL-59699 (to J. D. Imig), and HL-080704 (to D. D. Gutterman) and the National Institute of Digestive and Kidney Diseases (DK38226 to J. R. Falck).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Gretchen Barg for secretarial assistance, Dr. Bruce Hammock (Univ. of California-Davis) for the sEH inhibitor tAUCB and sEH antibody, and Dr. Richard Ingraham (Boehringer Ingelheim Pharmaceutical) for the sEH inhibitor BIRD0826.
REFERENCES
- 1. Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BKCa channels. Circulation 107: 769–776, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res 68: 26–36, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Campbell WB, Harder DR. Prologue: EDHF-what is it? Am J Physiol Heart Circ Physiol 280: H2413–H2416, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Chatterjee U, Sanwal GG. Purification and characterization of catalase from goat (Capra capra) lung. Mol Cell Biochem 126: 125–133, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization: beyond nitric oxide and cyclic GMP. Circulation 92: 3337–3349, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Corey EJ, Niwa H, Falck JR. Selective epoxidation of eicosa-cis-5,8,11,14-tetraenoic (arachidonic) acid and eicosa-cis-8,11,14-trienoic acid. J Am Chem Soc 101: 1586–1587, 1979 [Google Scholar]
- 8. Enayetallah AE, French RA, Thibodeau MS, Grant DF. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J Histochem Cytochem 52: 447–454, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Fleming I, Busse R. Endothelium-derived epoxyeicosatrienoic acids and vascular function. Hypertension 47: 629–633, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate Trp-channel-dependent Ca signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol 27: 2612–2618, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Furchgott RF, Zawadzki JW. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 12. Gao YJ, Lee RM. Hydrogen peroxide is an endothelium-dependent contracting factor in rat renal artery. Br J Pharmacol 146: 1061–1068, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guenthner TM, Hjelle JT, Whalen R. Selective inhibition of cytosolic epoxide hydrolase activity in vitro by compounds that inhibit catalase. J Biochem Toxicol 4: 241–249, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Guenthner TM, Qato M, Whalen R, Glomb S. Similarities between catalase and cytosolic epoxide hydrolase. Drug Metab Rev 20: 733–748, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res 61: 866–879, 1987 [DOI] [PubMed] [Google Scholar]
- 16. Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR. Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol 7: 2364–2370, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome P450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res 102: 59–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BKCa channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol 290: H491–H499, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li PL, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ Res 80: 877–884, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Maimoni GV, Cezar de Cerqueira LL, Raw I, Cabrera-Crespo J. Purification of catalase from human placenta. Biotechnol Appl Biochem 29: 73–77, 1999 [PubMed] [Google Scholar]
- 21. Matoba T, Shimokawa H, Morikawa K, Kubota H, Kunihiro I, Urakami-Harasawa L, Mukai Y, Hirakawa Y, Akaike T, Takeshita A. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler Thromb Vasc Biol 23: 1224–1230, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 2106: 1521–1530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev 30: 293–331, 1979 [PubMed] [Google Scholar]
- 24. Morikawa K, Fujiki T, Matoba T, Kubota H, Hatanaka M, Takahashi S, Shimokawa H. Important role of superoxide dismutase in EDHF-mediated responses of human mesenteric arteries. J Cardiovasc Pharmacol 44: 552–556, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Morikawa K, Shimokawa H, Matoba T, Kubota H, Akaike T, Talukder MA, Hatanaka M, Fujiki T, Maeda H, Takahashi S, Takeshita A. Pivotal role of Cu,Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J Clin Invest 112: 1871–1879, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nithipatikom K, Grall AJ, Holmes BB, Harder DR, Falck JR, Campbell WB. Liquid chromatographic-electrospray ionization-mass spectrometric analysis of cytochrome P450 metabolites of arachidonic acid. Anal Biochem 298: 327–336, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Pinto A, Abraham NG, Mullane KM. Arachidonic acid-induced endothelial-dependent relaxation of canine coronary arteries: contribution of a cytochrome P450-dependent pathway. J Pharmacol Exp Ther 240: 856–863, 1987 [PubMed] [Google Scholar]
- 28. Roslowsky M, Campbell WB. Role of PGI2 and epoxyeicosatrienoic acids in relaxation of bovine coronary arteries to arachidonic acid. Am J Physiol Heart Circ Physiol 264: H327–H335, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res 87: 992–998, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, Capdevila JH. Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J Biol Chem 268: 6402–6407, 1993 [PubMed] [Google Scholar]
- 31. Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K+-channel activity. Am J Physiol Renal Fluid Electrolyte Physiol 270: F822–F832, 1996 [DOI] [PubMed] [Google Scholar]