Abstract
Renal fibrosis is a final stage of many forms of kidney disease and leads to impairment of kidney function. The molecular pathogenesis of renal fibrosis is currently not well-understood. microRNAs (miRNAs) are important players in initiation and progression of many pathologic processes including diabetes, cancer, and cardiovascular disease. However, the role of miRNAs in kidney injury and repair is not well-characterized. In the present study, we found a unique miRNA signature associated with unilateral ureteral obstruction (UUO)-induced renal fibrosis. We found altered expression in UUO kidneys of miRNAs that have been shown to be responsive to stimulation by transforming growth factor (TGF)-β1 or TNF-α. Among these miRNAs, miR-21 demonstrated the greatest increase in UUO kidneys. The enhanced expression of miR-21 was located mainly in distal tubular epithelial cells. miR-21 expression was upregulated in response to treatment with TGF-β1 or TNF-α in human renal tubular epithelial cells in vitro. Furthermore, we found that blocking miR-21 in vivo attenuated UUO-induced renal fibrosis, presumably through diminishing the expression of profibrotic proteins and reducing infiltration of inflammatory macrophages in UUO kidneys. Our data suggest that targeting specific miRNAs could be a novel therapeutic approach to treat renal fibrosis.
Keywords: chronic kidney disease, kidney failure
progressive renal fibrosis is the final stage of various chronic kidney diseases that results in renal function impairment and ultimate kidney failure (11, 47). Renal fibrosis is characterized by leukocytic cell infiltration, tubular cell apoptosis and necrosis, tubulointerstitial fibroblast proliferation, and elevated matrix production (11, 47). Despite fundamental advances in understanding the pathophysiology of renal fibrosis, definitive therapies remain limited (4, 41). Therapeutic approaches aimed at novel targets are urgently needed to effectively prevent and/or treat this disorder.
Unilateral ureteral obstruction (UUO) is a well-characterized experimental model of injury leading to renal inflammation and tubulointerstitial fibrosis (6). Many cellular and molecular characteristics have been identified in UUO kidneys that undergo fibrotic transition, including enhanced expression of proapoptotic genes and profibrotic growth factors, increased infiltration of macrophages, and initiation of epithelial mesenchymal transition (EMT) and endothelial mesenchymal transition (11, 20). Transforming growth factor-β1 (TGF-β1) is one of the most important mediators of fibrogenesis and also regulates recruitment of macrophages in kidneys (18, 22, 25). Furthermore, TGF-β is a crucial inducer of EMT, a key process in the generation of interstitial fibroblasts (46).
microRNAs (miRNAs) are noncoding small RNAs, 22 nt. in length, which bind to the 3′-UTR of target genes and, thereby, repress translation and/or induce degradation of target gene mRNAs (37). miRNAs have been shown to regulate numerous molecular and cellular processes (37). Aberrant expression of miRNAs is associated with initiation and progression of pathologic processes including diabetes, cancer, and cardiovascular disease (7, 21, 33, 39). However, the role of miRNAs in kidney injury and repair is not well-characterized. Improved understanding of the roles that specific miRNAs play in the pathogenesis of kidney injury and repair is likely to suggest important new directions for prevention and treatment of this condition.
In the present study, we found a unique miRNA signature associated with renal fibrosis. We found altered expression in UUO kidneys of miRNAs that have been shown to be responsive to stimulation by TGF-β1 or TNF-α. Among these miRNAs, miR-21 demonstrated the greatest increase in fibrotic kidneys. The enhanced expression of miR-21 was primarily located in the tubular epithelial cells. miR-21 expression was upregulated in response to treatment with TGF-β1 or TNF-α in human renal tubular epithelial cells in vitro. We found that blocking miR-21 in vivo attenuated UUO-induced renal fibrosis, presumably through diminishing the expression of profibrotic proteins and reducing infiltration of inflammatory macrophages in UUO kidneys.
MATERIALS AND METHODS
Experimental renal fibrosis model.
The study protocol was approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. UUO surgery was performed as previously described (16). Mice were anesthetized with inhalation of isoflurane (2.5%). In the UUO group, the left ureter was exposed through a midabdominal incision and ligated twice, ∼1 cm below the renal hilum, using a 4–0 silk suture. Sham operation was done in a similar manner, without ureteral ligation. At the indicated time point, all mice were killed and kidneys were harvested.
miRNA array.
Total RNAs were isolated from mouse kidneys harvested at 0, 3, and 7 days after UUO with miRNAeasy Mini Kit (Qiagen). The miRNA array was performed by Exiqon using miRCURY LNA microRNA Array (Exiqon).
Reagents.
LNA-modified control knockdown and miR-21 knockdown probes for in vivo applications were synthesized by Exiqon.
Northern blotting.
The assay was performed as previously described (27). Briefly, total RNA (10 μg) was resolved on a 12% denatured polyacrylamide gel containing 8 M urea. The RNA was then transferred to a Hybond nylone membrane (GE Life Sciences). After UV crosslinking, the membrane was incubated in prehybridization buffer [50% formamide (USB), 0.5% SDS, 5 × SSC (USB), 5 × Denhardt's solution (USB)] and 20 μg/ml sheared, denatured, salmon sperm DNA (Invitrogen) at 55°C for 30 min and then hybridized with specific γ-P32-labeled human LNA miR-21 probes (Exiqon) at 55°C for 24 h. The membrane was washed for 10 min three times with buffer (0.5% SDS, 2 × SSC) and exposed to film. After hybridization with miR-21 probes, the membrane was stripped and reblotted with specific γ-P32-labeled mouse U6 probes (Exiqon) as loading controls.
In situ hybridization.
The assay was performed as previously described (26). Briefly, mice were killed and the kidneys were embedded with OCT (Fisher Scientific). Ten-micrometer-thick frozen sections were prepared. The sections were dried at room temperature for 30 min, followed by fixation in 4% paraformaldehyde (Fisher) for 30 min. The sections were treated in acetylation solution for 10 min and then in PBS containing 10 μg/ml proteinase K (Sigma) for 5 min. Sections were then blocked with hybridization solution for 4 h at room temperature and incubated with digoxigenin (Dig)-conjugated miR-21 probes (Exiqon) or Dig-conjugated control probes with scrambled sequence (Exiqon) overnight. The sections were washed with 0.2 × SSC followed by incubation with horseradish peroxidase-conjugated anti-Dig antibody (Roche) overnight at 4°C. After being washed three times with buffer B1, the sections were developed with NBT/BCIP (Roche) for 24 h, with light blue cytoplasmic staining being positive. The sections were double stained with fast red to manifest nuclei.
Cell culture.
The human renal tubular epithelial cell line, HK-2, was purchased from American Type Culture Collection (ATCC) and cultured according to the ATCC instructions.
Real-time PCR.
The assay was performed as previously described (27). TaqMan probes for hsa-miR-21, hsa-miR-142–3p, hsa-miR-142–5p, hsa-miR-214, hsa-miR-223, hsa-miR-101a, hsa-miR-193, hsa-miR-218, and mouse Sno135 were purchased from Applied Biosystems. The expression of α-smooth muscle actin (SMA), fibronectin, Col1A1, Col1A2, PAI-1, TGF-β1 was determined using SYBR Green Master Mix kit (Roche). GAPDH or HPRT was used as an internal control. The sequences of the primers: mouse HPRT: sense, 5′-GGGACATAAAAGTTATTGGTGGAGATG-3′; antisense, 5′-CAACAACAAACTTGTCTGGAATTTCAA-3′; human fibronectin: sense, 5′-GTGTTGGGAATGGTCGTGGGGAATG-3′; antisense, 5′-CCAATGCCACGGCCATAGCAGTAGC-3′; mouse fibronectin: sense, 5′-TCTGGGAAATGGAAAAGGGGAATGG-3′; antisense, 5′-CACTGAAGCAGGTTTCCTCGGTTGT-3′; human α-SMA: sense, 5′-CATCACCAACTGGGACGACATGGAA-3′; antisense, 5′-GCATAGCCCTCATAGATGGGGACATTG-3′; mouse α-SMA: sense, 5′-GACGCTGAAGTATCCGATAGAACACG-3′; antisense 5′-CACCATCTCCAGAGTCCAGCACAAT-3′; mouse TGF-β1: sense, 5′-AGCGGACTACTATGCTAAAGAGGTCACCC-3′; antisense, 5′-CCAAGGTAACGCCAGGAATTGTTGCTATA-3′; mouse Col1A1: sense, 5′-GGAGGGCGAGTGCTGTGCTTT-3′; antisense, 5′-GGGACCAGGAGGACCAGGAAGT 3′; mouse Col1A2: sense, 5′-TGGTCTTACTGGGAACTTTGCTGC-3′; antisense, 5′-ACCCTGTGGTCCAACGACTCCTCTC-3′; mouse GAPDH: sense, 5′-CGACTTCAACAGCAACTCCCACTCTTCC-3′; antisense, 5′-TGGGTGGTCCAGGGTTTCTTACTCCTT-3′; human GAPDH: sense, 5′-GCTGGCGCTGAGTACGTCGTGGAGT-3′; antisense, 5′-CACAGTCTTCTGGGTGGCAGTGATGG-3′.
Picrosirius red staining to determine collagen deposition.
The kidney sections were deparaffinized with xylene and then rehydrated in water through graded ethanol. The sections were incubated with Weigert's hematoxylin for 8 min to stain nuclei and then washed with water for 10 min. The sections were incubated in picrosirius red for 1 h followed by two washes with acidified water. The sections were then dehydrated, cleared, and mounted in a resinous medium. The area of collagen deposition, stained red by picrosirius red staining, was measured by color image analysis software (Image-Pro Plus, Media Cybernetics).
Immunofluorescence.
Mouse kidneys were fixed with 10% formalin solution and embedded in paraffin. Ten-micrometer-thick sections were prepared, deparaffinized with xylene, and then rehydrated in water through graded ethanol. Antigen retrieval was performed in a pressure cooker in Tris-EDTA solution, pH 9.0, for 5 min. After incubation in TBST buffer for 10 min, the sections were blocked with affinity-purified goat anti-mouse IgG (H+L). The sections were then incubated with mouse anti-CD11b (Abcam, Cambridge, MA) antibodies overnight at 4°C and FITC-conjugated secondary antibody for 60 min. Finally, the sections were counterstained with DAPI. A total of 13 images (5 cortex, 5 medulla, and 3 papilla) was taken from each section. CD11b-positive cells were counted and the total number of cells per section was used to calculate the average.
Statistical analysis.
One-way ANOVA followed by the Holm-Sidak or Tukey-Kramer test was performed for multiple group comparisons. The Student's t-test was used for comparison between two groups. P < 0.05 was considered significant.
RESULTS
miRNA expression is altered in kidneys with UUO-induced fibrosis.
To determine whether miRNAs play a role in the initiation and progression of kidney fibrosis, we performed a miRNA expression profiling in kidneys with UUO-induced fibrosis and in the contralateral kidneys. We found multiple miRNAs that demonstrated altered expression at days 3 and 7 following UUO compared with the contralateral kidneys (Fig. 1). Most notable among the upregulated miRNAs were miR-21, 142–3p, 142–5p, 214, and 223, while miR-101a, 193, and 218 were significantly downregulated. Given that miRNAs are important in many pathophysiological processes, these data suggest that specific miRNAs may participate in the perpetuation of renal fibrosis in response to kidney injury caused by UUO.
Fig. 1.
microRNA (miRNA) expression is altered in kidneys with unilateral ureteral obstruction (UUO)-induced fibrosis. At 0, 3, and 7 days after UUO surgery, mice were killed and the obstructive kidneys (left kidneys) were harvested. Kidney RNA was isolated and miRNA array analysis was performed. Unsupervised hierarchical clustering is presented (n = 3/group).
To confirm the findings with the miRNA array analysis, we performed real-time PCR assays on selected miRNAs that demonstrated the greatest alterations in the UUO kidneys. As shown in Fig. 2 and consistent with the miRNA array analysis, the expression of miR-21, miR-142–3p, miR-142–5p, miR-214, and miR-223 was significantly increased, whereas the expression of miR-101a, miR-193, and miR-218 was substantially decreased, in the UUO kidneys. Of note, many of the miRNAs with altered expression in the UUO kidneys have been previously shown to be responsive to profibrotic growth factors, such as TGF-β1, or to participate in inflammatory responses (2, 8, 9, 15, 36, 38). Given that inflammation, as well as profibrotic growth factors, including TGF-β1, is centrally involved in abnormal repair of various kidney injuries and in kidney fibrosis (10, 18, 22, 25, 31, 32, 35), the present data suggest that specific miRNAs may play a role in kidney injury repair and the associated fibrosis through regulating the inflammatory and fibrotic responses in the kidney.
Fig. 2.
Expression of specific miRNAs is altered in kidneys with UUO-induced fibrosis. A and B: mice experiments were performed as in Fig. 1. The levels of specific miRNAs were determined by real-time PCR assays. *P < 0.05, **P < 0.01, ***P < 0.001 vs. day 0.
miR-21 expression is upregulated in fibrotic kidneys.
As shown in the miRNA array and the real-time PCR analyses, miR-21 expression demonstrated the greatest increase in the UUO kidneys compared with that in the contralateral and sham kidneys. To further characterize the kinetics of miR-21 expression in the kidneys with UUO, we performed Northern blotting and found that miR-21 expression remained unchanged within the first 6 h after UUO, but it was markedly enhanced at 24 h after UUO (Fig. 3A). The expression of miR-21 continued to rise 1 day after the obstruction and remained elevated even at 7 days after UUO. These data suggest that miR-21 may participate in the pathogenesis of renal fibrosis caused by UUO.
Fig. 3.
miR-21 expression is upregulated in fibrotic kidneys. A: mice underwent sham or UUO surgery. At 0 and 6 h and 1, 2, 3, and 7 days after UUO, the mice were killed and both kidneys [left: obstructive kidneys (UUO); right: contralateral kidneys (CL)] were harvested. Kidney RNA was isolated and Northern blotting was performed. The PAGE gels were stained with ethidium bromide before transfer to manifest 5S rRNA serving as loading controls. B: at 7 days after UUO, the mice were killed and both kidneys were harvested. Frozen sections were prepared and in situ hybridization (ISH) assays were performed as described in materials and methods using specific miR-21 probes and probes with scrambled sequence serving as negative controls. The original digital microphotographs were ×4. C: experiments were performed as in B. The original digital microphotographs were ×40. The images in the squares were enlarged for detailed manifestation (UUO ×120).
To determine the localization of the enhanced expression of miR-21, we performed in situ hybridization (ISH) assays. As shown in Fig. 3B, miR-21 was expressed primarily in the cortex, with minimal expression in the medulla and papilla of the contralateral kidneys. The expression of miR-21 was strikingly enhanced in kidneys with UUO for 7 days (Fig. 3B). Furthermore, we found that the enhanced expression of miR-21 was localized mainly in the cytoplasm of distal tubular epithelial cells within the cortex, medulla, and papilla of the UUO kidneys (Fig. 3C). Of note, the ISH assays using probes with a scrambled sequence demonstrated minimal background staining (Fig. 3, B and C, scramble), suggesting that the staining with miR-21 probes was specific for miR-21.
miR-21 expression is upregulated in TGF-β1-, TNF-α-, or aristolochic acid-treated human renal epithelial cells.
Numerous studies demonstrated that TGF-β1 is one of the most important mediators of tissue fibrosis (22, 25). It has been also shown that macrophages play a central role in renal fibrosis by producing a number of proinflammatory cytokines, such as TNF-α, growth factors, and reactive oxygen species (31, 32, 35). Since miR-21 expression is significantly upregulated in the tubular epithelial cells of UUO kidneys, we next investigated whether TGF-β1 or TNF-α enhances miR-21 expression in human renal tubular epithelial cells in vitro. As shown in Fig. 4A, TGF-β1 or TNF-α treatment resulted in a modest but significant increase in miR-21 expression in renal epithelial cells. Of note, the expression of TGF-β1 was also significantly increased in UUO kidneys (Fig. 4B). These data suggest that the enhanced expression of miR-21 in UUO kidneys is mediated by TGF-β1 and/or TNF-α. In addition, using aristolochic acid (AA), which is a well-known profibrotic molecule, we observed similar pattern of increase in the level of miR-21 expression (Fig. 4C). Of note, AA treatment led to increased expression of fibronectin and collagen 1A1, consistent with previous studies (45).
Fig. 4.
miR-21 expression is upregulated in TNF-α-, transforming growth factor (TGF)-β1-, or aristolochic acid (AA)-treated human renal epithelial cells. A: human renal tubular epithelial cells were treated without or with 10 ng/ml TNF-α or 10 ng/ml TGF-β1 for 48 h. Cells were harvested and RNA was isolated. The levels of miR-21 were determined by Northern blotting. B: at 0 and 7 days after UUO, mice were killed and both kidneys were harvested. Kidney RNA was isolated and TGF-β1 expression was determined by real-time PCR (n = 7). C: renal tubular epithelial cells were treated without or with 5 μg/ml AA for 48 h. Cells were harvested and RNA was isolated. The levels of miR-21, Col1A1, and smooth muscle actin (SMA)-α were determined by real-time PCR assays; n = 3/group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. time 0.
Blocking miR-21 in vivo diminishes UUO-induced kidney fibrosis.
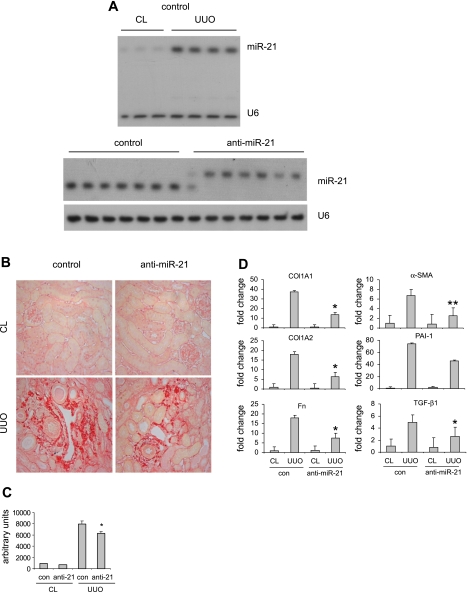
We showed that miR-21 expression is remarkably increased in fibrotic kidneys. We next determined whether blocking miR-21 in kidneys affects UUO-induced renal fibrosis. To do this, mice were injected intraperitoneally with control probes or anti-miR-21 probes before UUO. As shown in Fig. 5A, top, miR-21 expression was increased in the kidneys with UUO, as expected. We found that miR-21 was sequestered in the kidneys of mice that were given anti-miR-21 probes, as demonstrated by the retarded migration of the miR-21:anti-miR-21 dimers in PAGE gels (Fig. 5A, bottom). Next, we determined the extent of kidney fibrosis in these mice. As shown in Fig. 5, B and C, collagen deposition in UUO kidneys was increased compared with that in the contralateral kidneys. However, blocking miR-21 significantly attenuated collagen deposition in UUO kidneys (Fig. 5, B and C). We also found that blocking miR-21 diminished the enhanced expression of collagen 1A1, collagen 1A2, fibronectin, SMA-α, and PAI-1 in UUO kidneys, consistent with reduced collagen deposition after antagonizing miR-21 (Fig. 5D). Furthermore, we found that blocking miR-21 decreased the enhanced expression of TGF-β1 (Fig. 5D).
Fig. 5.
Blocking miR-21 in vivo suppresses UUO-induced kidney fibrosis. A: mice were injected intraperitoneally with control probes or anti-miR-21 probes (10 mg/kg in 200 μl saline) 3 times separated by 1 day between injections. On the second day after the last injection, UUO surgery was performed. At 12 days after UUO, mice were killed and both kidneys were harvested. Kidney RNA was isolated and the levels of miR-21 and U6 were determined by Northern blotting. B: experiments were performed as in A. Kidney sections were prepared and Picrosirius red staining was performed to manifest collagen deposition. C: ten random fields were selected from each section for digital quantification (n = 7/group). *P < 0.05 vs. UUO kidneys in the control group. D: experiments were performed as in A. Kidney RNA was isolated and the expression of Col1A1, Col1A2, Fn, SMA-α, PAI-1, and TGF-β1 was determined by real-time PCR (n = 7/group). *P < 0.05, **P < 0.01 vs. UUO kidneys in the control group.
Blocking miR-21 reduces macrophage infiltration in UUO kidneys.
It has been previously shown that macrophages are key players in UUO-induced renal fibrosis (31, 32, 35). As shown in Fig. 6, blocking miR-21 significantly decreased macrophage infiltration in UUO kidneys. These data suggest that the inhibitory effects of miR-21 blockage on UUO-induced renal fibrosis may be due to the reduced macrophage infiltration and the associated decrease in production of profibrotic factors, such as TGF-β1.
Fig. 6.
Blocking miR-21 reduces macrophage infiltration in UUO kidneys. A: mice were injected intraperitoneally with control probes or anti-miR-21 probes (10 mg/kg in 200 μl saline) 3 times separated by 1 day between injections. On the second day after the last injection, UUO surgery was performed. At 4 days after UUO, mice were killed and both kidneys were harvested. Kidney sections were prepared and immunofluorescence assays were performed using anti-CD11b antibodies to determine macrophage infiltration in the kidneys. DAPI was used to stain nuclei. B: quantification of macrophage infiltration was performed as described in materials and methods (n = 7/group). *P < 0.05 vs. UUO kidneys in the control group.
DISCUSSION
Renal fibrosis is a final stage of many forms of kidney disease and leads to impairment of kidney function (11, 47). There is currently no definitive treatment for renal fibrosis, which reflects the incomplete understanding of the molecular pathogenesis of this disease (4). miRNAs recently emerged as critical players in many pathophysiological processes and have been shown to participate in the progression of some forms of kidney disorders such as acute kidney injury, renal cell cancer, polycystic kidney disease, chronic transplant rejection, diabetic kidney disease, and others (3, 23, 29). However, how miRNAs regulate renal fibrosis remains unclear. In the present study, we performed miRNA expression profiling in a well-established renal fibrosis model and found a unique miRNA signature associated with UUO.
We found that many miRNAs that demonstrate altered expression in UUO kidneys have been previously shown to be responsive to TGF-β1 stimulation and/or be involved in inflammatory responses. Among those, miR-223 regulates myeloid progenitor proliferation and granulocyte differentiation as well as activation (15). Therefore, miR-223 may participate in renal fibrosis by regulating macrophage activation and differentiation in UUO kidneys. miR-214 inhibits monocyte apoptosis by targeting PTEN (14, 44), which suggests that the enhanced miR-214 expression may contribute to renal fibrosis through its negative regulation of macrophage apoptosis in UUO kidneys. miR-218 regulates cell migration via the SLIT-ROBO pathway (40). The diminished expression of miR-218 could promote the motility of renal tubular epithelial cells that undergo EMT. As shown in numerous previous studies, infiltration of macrophages that produce proinflammatory cytokines and profibrotic growth factors is a crucial event in the pathogenesis of renal fibrosis (10, 31, 32, 35). Therefore, it is likely that the proinflammatory cytokines and profibrotic growth factors produced by macrophages regulate the expression of these specific miRNAs in response to kidney injury. Reciprocally, these miRNAs could regulate the infiltration of macrophages and the production of proinflammatory cytokines and profibrotic growth factors by the macrophages, thereby participating in the fibrogenesis in UUO kidneys.
We found that miR-21 demonstrates the greatest increase of expression in UUO kidneys. miR-21 is a versatile miRNA that is involved in a variety of physiological and pathological events (19). It should be emphasized that the enhanced expression of miR-21 is localized primarily in the tubular epithelial cells of UUO kidneys, suggesting that miR-21 exerts its activity of regulating renal fibrosis via tubular epithelial cells. The role of tubular epithelial cells in renal fibrosis has been well-established in that they are the first responders to kidney injury through producing inflammatory cytokines and chemokines that subsequently promote macrophage infiltration (1). Furthermore, tubular epithelial cells potentially contribute to the origin of matrix-producing interstitial fibroblasts via EMT (11). Therefore, miR-21 may participate in renal fibrosis by regulating the activation and/or EMT of renal tubular epithelial cells. It must be noted that although the evidence to support EMT is overwhelming, there are still controversial questions that remain to be unequivocally answered, particularly with recent work highlighting the role of pericytes and nontubular epithelial cells as precursor cells of myofibroblasts in models of renal fibrosis (12, 13, 20, 24, 28). Whereas proximal tubules are the primary site of various forms of kidney diseases, our data show that the enhanced expression of miR-21 appears to localize mainly to the distal tubular epithelial cells in UUO kidneys. Further colocalization studies using nephron segment-specific markers would be required to confirm these findings. Given that miR-21 blockade demonstrates beneficial effects in attenuating UUO-induced renal fibrosis, our finding showing enhanced miR-21 expression in distal tubular epithelial cells may shed new light on the role of distal tubules in obstructive nephropathy.
Previous studies, including those from our laboratory, showed that miR-21 regulates both lung and heart fibrosis by enhancing the fibrogenic activity or promoting the proliferation of interstitial fibroblasts (26, 39). The mode of action for miR-21 in renal fibrosis could be distinct from that in the heart or lungs because the cell populations that harbor the enhanced miR-21 expression are different and the pathogenesis of kidney injury is unique based on the diverse nature of cells in the kidney (26, 39, 47). It is also likely that miR-21 participates in fibrogenic events in kidneys, lungs, heart, or other organs by regulating a unique array of targets. Of note, Smad7 and Spry1, the two genes that miR-21 targets in the heart and lungs (26, 39), appear not to be modulated in kidneys by miR-21 (data not shown). Therefore, we acknowledge that deciphering the target molecules of miR-21 in renal fibrosis is essential and will be elucidated in the future.
Although miR-21 expression is remarkably enhanced in UUO kidneys, treatment with TGF-β1 or TNF-α alone of renal tubular epithelial cells in vitro only leads to a modest upregulation compared with the in vivo results. These data suggest that the enhanced expression of miR-21 in UUO kidneys could be a result of combined actions of multiple cytokines and growth factors, such as TNF-α, FGF-2, and TGF-β1. In addition, it has been previously shown that specific miRNAs are differentially expressed in hearts that undergo pressure overload and in endothelial cells in response to pulsatile shear flow (34, 39, 42). These data suggest that the mechanical stimuli that are caused by ureteral obstruction may also generate direct or indirect effects on miRNA expression in UUO kidneys. Nevertheless, the present data suggest that the elevated expression of miR-21 in UUO kidneys is mediated by proinflammatory cytokines and profibrotic growth factors, which are crucial in the pathogenesis of renal fibrosis (22, 25).
We found that miR-21 blockage attenuated UUO-induced renal fibrosis, which suggests that miR-21 could be a potential target for development of novel therapeutic approaches to treat renal fibrosis. However, it should be noted that the efficiency of miR-21 blockade in treating renal fibrosis is not as robust compared with those demonstrated in the heart and lungs (26, 39). These findings suggest that the effectiveness of miR-21 blockade could be tissue-type dependent because the enhanced miR-21 expression is located in different cell populations of the heart, kidneys, and lungs. miR-21 expression is enhanced primarily in renal tubular epithelial cells. Given that previous studies demonstrated that miR-21 is anti-apoptotic and that apoptosis leads to loss of tubular epithelial cells, decreased reepithelialization, and sustained inflammation, thereby promoting kidney interstitial fibrosis (5, 6, 10, 17, 30, 43), the anti-fibrotic activity of miR-21 blockade could be partially offset by a potential increase in tubular epithelial cell apoptosis, which reflects a complicated consequence after miR-21 blockade. Nevertheless, we identified a few miRNAs with prominent alterations in UUO kidneys that are involved in regulation of apoptosis. Therefore, it is warranted to target these specific miRNAs, simultaneously or sequentially, with miR-21 blockade to improve the efficiency in treating renal fibrosis.
GRANTS
This study was funded by National Institutes of Health Grants HL105473 (to Gang Liu), HL097218 (to Gang Liu), a pilot grant from the UAB-UCSD O'Brien Center [P30DK079337 (to Gang Liu)], and an American Heart Association award 10SDG4210009 (to Gang Liu).
Author contributions: Gang Liu and Anupam Agarwal conceived and designed the study; Abolfazl Zarjou, Shanzhong Yang, Edward Abraham, Anupam Agarwal, and Gang Liu analyzed the data; Abolfazl Zarjou, Shanzhong Yang, and Gang Liu performed the experiments; Abolfazl Zarjou, Anupam Agarwal, and Gang Liu wrote the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Lingling Guo and Yuanyuan Guo of UAB for technical assistance in mouse studies.
REFERENCES
- 1. Bani-Hani AH, Campbell MT, Meldrum DR, Meldrum KK. Cytokines in epithelial-mesenchymal transition: a new insight into obstructive nephropathy. J Urol 180: 461–468, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bellon M, Lepelletier Y, Hermine O, Nicot C. Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood 113: 4914–4917, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhatt K, Mi QS, Dong Z. microRNAs in kidneys: biogenesis, regulation, and pathophysiological roles. Am J Physiol Renal Physiol 300: F602–F610, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 6: 643–656, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65: 6029–6033, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 10: 704–714, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454: 56–61, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell 39: 373–384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia-Sanchez O, Lopez-Hernandez FJ, Lopez-Novoa JM. An integrative view on the role of TGF-beta in the progressive tubular deletion associated with chronic kidney disease. Kidney Int 77: 950–955, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Grande MT, Lopez-Novoa JM. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol 5: 319–328, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Jindra PT, Bagley J, Godwin JG, Iacomini J. Costimulation-dependent expression of microRNA-214 increases the ability of T cells to proliferate by targeting Pten. J Immunol 185: 990–997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 451: 1125–1129, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Kie JH, Kapturczak MH, Traylor A, Agarwal A, Hill-Kapturczak N. Heme oxygenase-1 deficiency promotes epithelial-mesenchymal transition and renal fibrosis. J Am Soc Nephrol 19: 1681–1691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim J, Kim DS, Park MJ, Cho HJ, Zervos AS, Bonventre JV, Park KM. Omi/HtrA2 protease is associated with tubular cell apoptosis and fibrosis induced by unilateral ureteral obstruction. Am J Physiol Renal Physiol 298: F1332–F1340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kopp JB. TGF-beta signaling and the renal tubular epithelial cell: too much, too little, and just right. J Am Soc Nephrol 21: 1241–1243, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med 13: 39–53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest 121: 468–474, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol 6: 419–429, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Li JH, Zhu HJ, Huang XR, Lai KN, Johnson RJ, Lan HY. Smad7 inhibits fibrotic effect of TGF-β on renal tubular epithelial cells by blocking Smad2 activation. J Am Soc Nephrol 13: 1464–1472, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Liang M, Liu Y, Mladinov D, Cowley AW, Jr, Trivedi H, Fang Y, Xu X, Ding X, Tian Z. MicroRNA: a new frontier in kidney and blood pressure research. Am J Physiol Renal Physiol 297: F553–F558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ling H, Li X, Jha S, Wang W, Karetskaya L, Pratt B, Ledbetter S. Therapeutic role of TGF-beta-neutralizing antibody in mouse cyclosporin A nephropathy: morphologic improvement associated with functional preservation. J Am Soc Nephrol 14: 377–388, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 207: 1589–1597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci USA 106: 15819–15824, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21: 212–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lorenzen JM, Haller H, Thum T. MicroRNAs as mediators and therapeutic targets in chronic kidney disease. Nat Rev Nephrol 7: 286–294, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature 467: 86–90, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, Roncal CA, Glushakova OY, Chiodo VA, Atkinson MA, Hauswirth WW, Flotte TR, Rodriguez-Iturbe B, Johnson RJ. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol 16: 3651–3660, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Nishida M, Hamaoka K. Macrophage phenotype and renal fibrosis in obstructive nephropathy. Nephron Exp Nephrol 110: e31–36, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Pandey AK, Agarwal P, Kaur K, Datta M. MicroRNAs in diabetes: tiny players in big disease. Cell Physiol Biochem 23: 221–232, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Patrick DM, Montgomery RL, Qi X, Obad S, Kauppinen S, Hill JA, van Rooij E, Olson EN. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest 120: 3912–3916, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary J, Ruan Q, Johnson DP, Chen Y, O'Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol 11: 141–147, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol 9: 219–230, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Sun Y, Varambally S, Maher CA, Cao Q, Chockley P, Toubai T, Malter C, Nieves E, Tawara I, Wang Y, Ward PA, Chinnaiyan A, Reddy P. Targeting of microRNA-142–3p in dendritic cells regulates endotoxin induced mortality. Blood 117: 6172–6183, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456: 980–984, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S, Guo X, Wang B, Gang Y, Zhang Y, Li Q, Qiao T, Zhao Q, Nie Y, Fan D. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet 6: e1000879, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vilayur E, Harris DC. Emerging therapies for chronic kidney disease: what is their role? Nat Rev Nephrol 5: 375–383, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Wang KC, Garmire LX, Young A, Nguyen P, Trinh A, Subramaniam S, Wang N, Shyy JY, Li YS, Chien S. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc Natl Acad Sci USA 107: 3234–3239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang CH, Yue J, Fan M, Pfeffer LM. IFN induces miR-21 through a signal transducer and activator of transcription 3-dependent pathway as a suppressive negative feedback on IFN-induced apoptosis. Cancer Res 70: 8108–8116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang H, Kong W, He L, Zhao JJ, O'Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res 68: 425–433, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yeh YC, Wei WC, Wang YK, Lin SC, Sung JM, Tang MJ. Transforming growth factor-β1 induces Smad3-dependent β1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am J Pathol 177: 1743–1754, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21: 1819–1834, 2010 [DOI] [PubMed] [Google Scholar]