Abstract
The study of hemodynamic alterations following the creation of an arteriovenous fistula (AVF) is relevant to vascular adaptive responses and hemodialysis access dysfunction. This study examined such alterations in a murine AVF created by anastomosing the carotid artery to the jugular vein. AVF blood flow was markedly increased due to reduced AVF vascular resistance. Despite such markedly increased basal blood flow, AVF blood flow further increased in response to acetylcholine. This AVF model exhibited increased cardiac output and decreased systemic vascular resistance; the kidney, in contrast, exhibited decreased blood flow and increased vascular resistance. Augmentation in AVF blood flow was attended by increased arterial heme oxygenase-1 (HO-1) mRNA and protein expression, the latter localized to smooth muscle cells of the AVF artery; AVF blood flow was substantially reduced in HO-1−/− mice compared with HO-1+/+ mice. Finally, in a murine model of a representative disease known to exhibit impaired hemodynamic responses (sickle cell disease), the creation of an AVF was attended by decreased AVF flow and impaired AVF function. We conclude that this AVF model exhibits markedly increased AVF blood flow, a vasodilatory reserve capacity, increased cardiac output, decreased renal blood flow, and a dependency on intact hemodynamic responses, in general, and HO-1 expression, in particular, in achieving and maintaining AVF blood flow. We suggest that these findings support the utility of this model in investigating the basis for and the consequences of hemodynamic stress, including shear stress, and the pathobiology of hemodialysis AVF dysfunction.
Keywords: vascular adaptation, shear stress, heme oxygenase-1, vascular access dysfunction, sickle cell disease
the study of hemodynamic alterations incurred by the creation of an arteriovenous fistula (AVF) is significant from at least two perspectives. The first pertains to the nature of biologic responses that occur in the vasculature following hemodynamic and other types of vascular stress. An AVF creates a conduit between the high-pressure, relatively low-capacitance arterial segment of the vasculature and the low-pressure, high-capacitance venous segment, thereby circumventing the resistors of the peripheral circulation. An AVF thus subjects the venous limb to augmented intraluminal pressure, and the arterial limb to substantially increased blood flow, the basis and consequence of which are of substantial biologic interest (4, 18).
The second perspective centers on the use of an AVF as a hemodialysis vascular access, the latter critically dependent on the increased blood flow that occurs in an AVF (2, 9, 19). The AVF is the preferred form of vascular access for maintenance hemodialysis because, as compared with other dialysis vascular accesses, it exhibits greater longevity, and its use is associated with less patient morbidity and lower costs. However, more than 50% of AVFs may never adequately mature such that they could sustain hemodialysis, and after variable periods of usage, subsets of AVFs eventually cease to function (2, 9, 19). In our recent clinical analysis of AVF outcomes, arterial size was the best predictor for AVF patency, a finding that likely reflects, at least in part, the necessity of heightened arterial blood flow in the maturation and continued function of an AVF (32). Conversely, impaired AVF blood flow, along with processes such as neointimal hyperplasia and thrombogenesis, comprises the essential pathobiologic mechanisms underpinning dysfunction and failure of hemodialysis AVFs.
AVF dysfunction and failure are increasingly explored in valid AVF models, the latter including a murine AVF model in which the carotid artery is anastomosed to the jugular vein (7). This model exhibits increased AVF blood flow, juxta-anastomotic venous neointimal hyperplasia, and eventual AVF closure (7). While technically challenging, the appeal of this model resides in its recapitulation of the essential features of functional and failing hemodialysis AVFs, and in the insights it may offer, when imposed on relevant genetically altered mice, on the functional significance of candidate genes (7, 15, 17, 24).
The present study provides the first comprehensive analysis of the regional and systemic hemodynamic alterations that occur in this murine AVF model. We evaluated, by Doppler flow studies, regional blood flow through the AVF, AVF vasodilatory reserve capacity, and blood flow in a distant vascular bed, the kidney; employing murine echocardiography, we also determined cardiac function and systemic hemodynamics following the creation of the AVF. We next sought to determine the contribution of a vasorelaxant system, specifically, heme oxygenase-1 (HO-1), in enabling the augmented blood flow that occurs in the AVF. Finally, to examine whether AVF flow and function would be compromised in a representative disease attended by impaired hemodynamic responses, AVF blood flow and patency were assessed in a murine disease model in which critical aspects of hemodynamic responses (vasorelaxation and blood rheology) are known to be substantially perturbed (11, 25, 26).
MATERIALS AND METHODS
Murine AVF model.
All studies were approved by the Institutional Animal Care and Use Committee of Mayo Clinic and performed in accordance with National Institutes of Health guidelines. For all studies, an AVF was constructed, as described in our previous studies, by an end-to-side anastomosis between the right carotid artery and jugular vein (15, 17, 24). Male C57BL6J mice (Jackson Laboratory, Bar Harbor, ME) ranging from 9 to 12 wk of age were employed to characterize the regional and systemic hemodynamic responses to AVF placement. In these studies, carotid arteries from sham-operated mice were used as controls; sham operation included anesthesia and exposure of the right carotid artery and jugular vein.
For the assessment of the AVF in the setting of HO-1 deficiency, HO-1−/− and HO-1+/+ mice were utilized as we previously described (28, 34, 35). Colonies of these mice, having targeted disruption of the HO-1 gene as described by Poss and Tonegawa (29), were genotyped at the time of weaning using PCR to amplify the wild-type and mutant alleles of genomic DNA from tail samples. HO-1+/+ mice from these colonies were used as controls. AVFs were created in HO-1−/− and HO-1+/+ mice that were age-matched (between the ages of 40 and 45 wk) and sex-matched, and the nonoperated, contralateral carotid artery was utilized as a control. Two weeks after the creation of the AVF, assessment of flow through the arterial limb of the AVF and the contralateral carotid artery was undertaken.
AVFs were also created in the transgenic murine model of sickle cell disease (SCD), employed in our previous studies (16, 22, 23, 26). This SCD murine model is on a C57BL/6 background, is homozygous for the murine β-globin deletion, and carries the two transgenes αHβS and αHβS−Antilles. The AVF was created in age-matched, wild-type and sickle mice with similar numbers of males and females in each group. The nonoperated, contralateral carotid artery was used as a control. Studies were continued for 4 wk after the creation of the AVF, at which time AVF patency was assessed and measurement of flow through the arterial limb of the AVF and the contralateral carotid artery was performed in wild-type and sickle mice.
Flow through the AVF.
Measurements of flow through the arterial limb of the AVF and the control carotid artery were performed on mice anesthetized with pentobarbital sodium (60 mg/kg body wt ip) and placed on a temperature-regulated table to maintain body temperature at 37°C. The artery was exposed using gentle dissection after which a perivascular flow probe (0.5 PSB, Transonic Systems, Ithaca, NY) was carefully placed on the vessel. After a 3-min equilibration period, flow measurements were recorded for 3 min using a perivascular flow meter (TS420, Transonic Systems); we employed this methodology previously to determine blood flow through a femoral AVF in the rat (8). In some studies, mean arterial pressure (MAP), simultaneous with these flow measurements, was recorded via a PE10 catheter inserted in the right femoral artery.
In a subset of animals in which flow through the AVF or carotid artery in the sham-operated mice was measured, blood flow responses to acetylcholine (ACh) were determined in vivo (13, 20). Following preparation as described above for measurement of AVF blood flow, baseline data were collected, the artery was bathed in a solution of ACh (100 μM; Sigma, St. Louis, MO) in normal saline, and AVF flow was determined again 10 min after bathing of the vessel commenced. The bathing solution was refreshed every 2 min for the 10 min of the study. This dose of ACh did not cause changes in systemic MAP.
Echocardiography.
Cardiac structure and function were quantified at 2 wk postsurgery by echocardiography (Vevo2100 with a MS-400 30-MHz transducer, Visual Sonics, Toronto, Canada) under light anesthesia (1–2% of isoflurane) (36–38). Dimensions were measured from three consecutive cardiac cycles using the leading edge convention of the American Society of Echocardiography. Left ventricular (LV) cardiac output was determined by the product of heart rate, the aortic root cross-section area, and the velocity time integral, taken from peak Doppler tracing of flow across the aortic valve. Similarly, right ventricular cardiac output was calculated as the product of heart rate, the pulmonary artery cross-section area, and the velocity time integral, taken from flow across the pulmonary valve. LV weight (mg) was derived as [(LVDD + IVST + PWT)3 − LVDD3] × 1.055, where LVDD is left ventricular end-diastolic dimension, IVST is interventricular septum thickness (mm), and PWT is posterior wall thickness (mm). LV ejection fraction (%) was defined as [(LVVolD − LVVolS)/LVVolD] × 100, where LVVolD = LV end-diastolic volume, and LVVolS = LV end-systolic volume.
Renal hemodynamics.
Studies of renal hemodynamics were performed as described in our prior studies (14, 16, 34, 35). Briefly, mice were anesthetized with pentobarbital sodium (60 mg/kg body wt ip) and placed on a temperature-regulated table to maintain body temperature at 37°C. The trachea was catheterized with PE-160 tubing; PE-10 tubing was used to cannulate the carotid artery, and the jugular vein to monitor MAP and to infuse fluids, respectively. A solution of 0.9% saline containing 2.25% bovine serum albumin was infused into the jugular vein (0.25 μl·min−1·g body wt−1) so as to maintain euvolemic conditions. A perivascular flow probe (0.5VB; Transonic Systems) was placed around the left renal artery to measure renal blood flow (RBF). Thirty minutes after instrumentation, RBF and MAP measurements were obtained. Renal vascular resistance (RVR) was determined as the ratio of MAP/RBF.
HO-1 mRNA expression by quantitative real-time RT-PCR.
HO-1 gene expression in the arterial limb of the AVF and contralateral control carotid artery was assessed by quantitative real-time RT-PCR as previously described (17, 28). Total RNA was extracted and further purified using the TRIzol method (Invitrogen, Carlsbad, CA) and an RNeasy Mini kit (Qiagen, Valencia, CA), respectively, and cDNA was synthesized employing random hexamers (Transcriptor First Strand cDNA Synthesis kit, Roche Applied Science, Indianapolis, IN). The probe and primers were obtained as an assay set (TaqMan Gene Expression Assay Mm00516004_m1, Applied Biosystems, Foster City, CA) and employed using the following parameters: 10 min at 95°C, followed by 40 cycles of amplification for 15 s at 95°C, and 1 min at 60°C. Expression of 18S rRNA was used for standardization.
Immunohistochemical localization of HO-1 expression.
Immunohistochemical analysis of HO-1 expression was performed as described previously on 5-μm sections prepared from formalin-fixed, paraffin-embedded arterial limbs of the AVF and in contralateral control carotid arteries using a rabbit polyclonal antibody (catalog no. ADI-SPA-895, Enzo Life Sciences, Plymouth Meeting, PA) as a primary antibody (17).
Western analysis.
Western blot analysis for HO-1 protein expression in the arterial limb of the AVF and in contralateral control arteries was performed as described previously (24, 28) employing a rabbit polyclonal antibody (catalog no. ADI-SPA-896, Enzo Life Sciences).
Statistical analysis.
Results are expressed as means ± SE and considered statistically significant for P < 0.05. The Student's t-test was used for parametric data and Mann-Whitney U-test was employed for nonparametric data. Patency was assessed using Fisher's exact test.
RESULTS
Regional hemodynamic response in the AVF in wild-type mice.
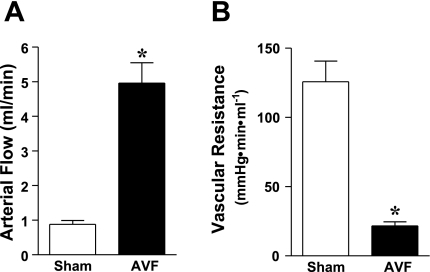
Two weeks following the creation of the AVF, there was a marked increase in AVF blood flow (Fig. 1). Using the individual MAP in each mouse in the sham and AVF groups [104 ± 3 vs. 101 ± 2 mmHg, respectively, n = 5 in each group, P = not significant (NS)], the calculated vascular resistance in the AVF compared with the sham groups was significantly reduced (Fig. 1).
Fig. 1.
Blood flow in the arterial limb of the arteriovenous fistula (AVF) and in the carotid artery of sham-operated mice (sham), and calculated vascular resistance 2 wk after the creation of the AVF. Arterial flow was measured using a perivascular flow probe (A) and vascular resistance (B) was calculated from measured values of blood flow and mean arterial pressure; n = 5 in each group. *P < 0.05 vs. sham.
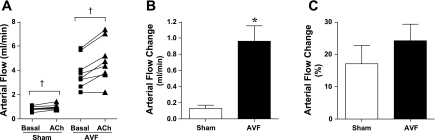
This marked increase in AVF blood flow raised the question whether vasorelaxant responses of the AVF were at their limit or whether a vasodilatory reserve capacity still existed in vivo. To address this question, we examined the vasodilation that occurred in response to locally applied ACh. Such application of ACh allowed an examination of the regional AVF vasodilatory response without a significant change in MAP; MAPs under basal condition and after the application of ACh were not significantly different whether in the sham group (99 ± 3 vs. 99 ± 3 mmHg, n = 5, P = NS) or in the AVF group (95 ± 2 vs. 97 ± 2, mmHg, n = 5, P = NS). As shown in Fig. 2, increases in blood flow occurred in both the sham and AVF groups with a significantly greater increase in absolute blood flow occurring in the AVF group, the percent increase in blood flow not being significantly different between the two groups (Fig. 2).
Fig. 2.
Blood flow in the arterial limb of the AVF and the carotid artery of sham mice in response to acetylcholine (ACh) 2 wk after the creation of the AVF. Measurements of arterial flow were made using a perivascular flow probe before and after treatment with ACh (100 μM; A), and absolute increase in blood flow (B) and percent increase in blood flow (C) were calculated; n = 7 and n = 8 in sham and AVF groups, respectively. †P < 0.05, paired analysis before and after treatment with ACh within a given group; *P < 0.05 vs. sham.
Systemic hemodynamic response in the AVF in wild-type mice.
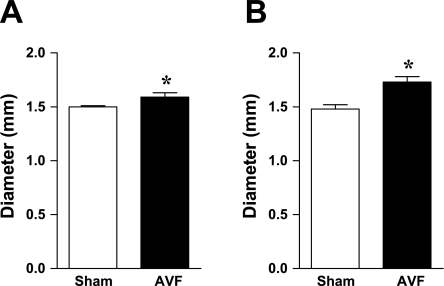
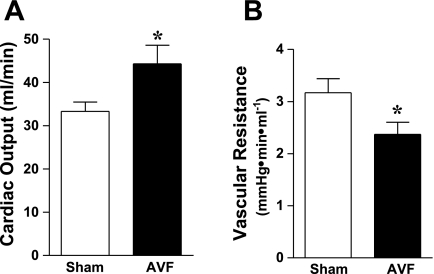
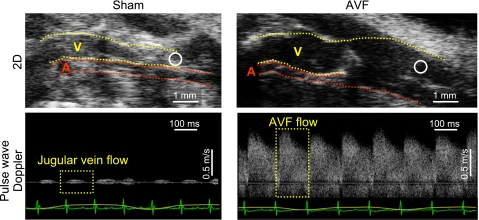
We also evaluated systemic hemodynamics in this AVF model by performing murine echocardiography. As shown in Fig. 3, the root diameters of the aorta and pulmonary artery were significantly increased in mice with the AVF, thereby indicating enlargement of the major vessels of the systemic and pulmonary circulation in vivo. LV cardiac output was increased in mice with an AVF (Fig. 4). Figure 5 shows the enlarged vein of the AVF (Fig. 5, top, 2D echocardiography) and arterialization and augmentation of venous flow (Fig. 5, bottom, pulse wave Doppler); such increased venous flow imposes increased cardiac preload, and, in turn, increased cardiac output. Ejection fraction was comparable in the two groups (Table 1); calculated systemic vascular resistance was decreased in mice with an AVF (Fig. 4).
Fig. 3.
Diameter of the aorta and pulmonary artery in mice with AVF and in sham mice 2 wk after the creation of the AVF. Measurements of the root diameter of the aorta (A) and the pulmonary artery (B) were performed using echocardiography as described in materials and methods; n = 9 and n = 8 in sham and AVF groups, respectively. *P < 0.05 vs. sham.
Fig. 4.
Cardiac output and systemic vascular resistance in mice with AVF and in sham mice 2 wk after the creation of the AVF. Assessment of left ventricular cardiac output was performed using echocardiography (A). Systemic vascular resistance (B) was calculated from these measurements and measurements of mean arterial pressure (not shown); n = 9 and n = 8 in sham and AVF groups, respectively. *P < 0.05 vs. sham.
Fig. 5.
Assessment of the AVF by ultrasound. Top: enlarged jugular vein in the AVF is displayed (Right) and compared with the intact jugular vein in sham mice (left). The jugular vein is labeled by V, and the carotid artery by A. Bottom: pulse wave Doppler assessment of venous flow in the AVF (right) and in the intact jugular vein in sham (left) at areas represented by circles in the respective top panels. An EKG tracing is shown at the bottom of the lower panels. Regarding orientation of the mouse in the top panels, the cephalic position is to the right and the caudal position is to the left, and the anterior position is topmost.
Table 1.
Cardiac indexes in sham (n = 9) and AVF (n = 8) groups obtained by echocardiography
| Sham | AVF | P Value | |
|---|---|---|---|
| Cardiac output, right ventricle, ml/min | 30.8 ± 2.4 | 46.1 ± 3.1 | 0.001 |
| LVDD, mm | 3.0 ± 0.1 | 3.4 ± 0.1 | 0.03 |
| LVVolD, μl | 36.5 ± 2.6 | 42.3 ± 4.4 | NS |
| LV weight, mg | 89.2 ± 4.6 | 115.7 ± 11.0 | 0.08 |
| LV/BW, mg/g | 3.2 ± 0.2 | 4.0 ± 0.4 | 0.06 |
| FS, % | 35.6 ± 2.2 | 36.6 ± 2.2 | NS |
| EF, % | 63.3 ± 2.3 | 66.7 ± 2.2 | NS |
Values are means ± SE. AVF, arteriovenous fistula; NS, not significant; LVDD, left ventricular end-diastolic dimension; LVVolD, left ventricular end-diastolic volume; LV, left ventricle; LV/BW, LV/body weight; FS, fractional shortening; EF, ejection fraction.
Renal hemodynamic response in the AVF in wild-type mice.
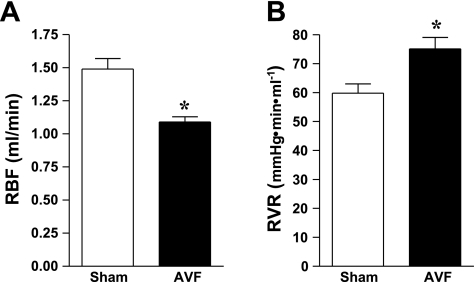
To examine regional blood flow at a vascular bed distant from the AVF, RBF was determined in the sham and AVF groups. As shown in Fig. 6, RBF was significantly decreased in mice with an AVF. During these studies performed under euvolemic conditions, MAP was significantly decreased in mice with the AVF compared with the sham group (86 ± 2 vs. 80 ± 2 mmHg, n = 13 and n = 10, P < 0.05). Calculated RVR was significantly increased in mice with an AVF compared with the sham group (Fig. 6). Thus, while regional AVF and systemic vascular resistances were decreased, other vascular beds, specifically, the kidney, showed increased vascular resistance.
Fig. 6.
Renal hemodynamic parameters in mice with AVF and in sham mice 2 wk after the creation of the AVF. Renal blood flow (RBF) was measured using a perivascular flow probe placed on the renal artery (A). Renal vascular resistances (RVR; B) were calculated from RBF and measurements of mean arterial pressure; n = 13 and n = 10 in sham and AVF groups, respectively. *P < 0.05 vs. sham.
Examination of HO-1 as a mechanism mediating increased AVF blood flow.
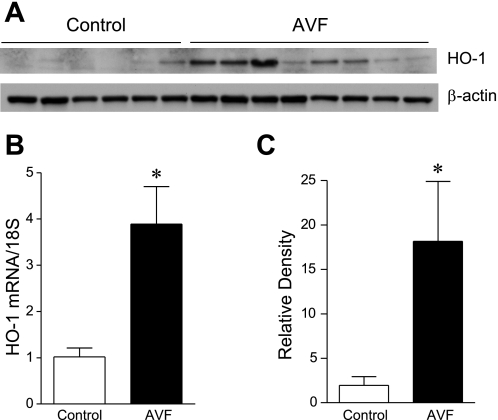
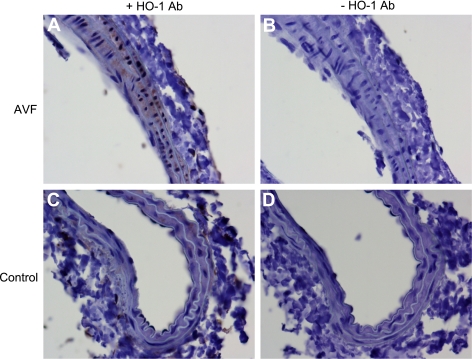
Prior studies indicate that a genetic deficiency of endothelial nitric oxide synthase (eNOS) fails to influence the increase in blood flow in the murine AVF (6). Because HO-1 is known to exert vasodilatory effects (10, 12, 21), we examined whether HO-1 is induced in the arterial limb and the functional significance of such vasodilation. One week after the creation of the AVF, there was almost a fourfold increase in expression of HO-1 mRNA (Fig. 7). Such increased expression of HO-1 mRNA was accompanied by increased expression of HO-1 protein in the arterial limb of the AVF (Fig. 7). The increased expression of HO-1 in the arterial limb of the AVF was localized primarily to smooth muscle cells in the AVF, and occasional adventitial leukocytes (Fig. 8).
Fig. 7.
Expression of heme oxygenase-1 (HO-1) in the arterial limb of the AVF. Western analysis of HO-1 protein expression (A), HO-1 mRNA expression by real-time RT-PCR (B), and standardized densitometric analysis of HO-1 protein expression (C) in the arterial limb of the murine AVF and the contralateral (control) carotid artery 1 wk after AVF creation. For real-time RT-PCR analysis, n = 9 in each group. For densitometric analysis, n = 6 and n = 8 in control and AVF groups, respectively. *P < 0.05 vs. control.
Fig. 8.
Immunohistochemical (IHC) localization of HO-1 expression in the arterial limb of the murine AVF 2 wk after AVF creation. IHC staining for HO-1 (+HO-1 Ab) in the arterial limb of the AVF (A) and in the nonoperated contralateral (control) carotid artery (C) is shown. Incubations with nonimmune rabbit IgG in the AVF and control artery (−HO-1 Ab; B and D, respectively) displayed no such staining. HO-1 expression, localizing to the smooth muscle cells, is more prominent in the arterial limb of the AVF compared with the control artery.
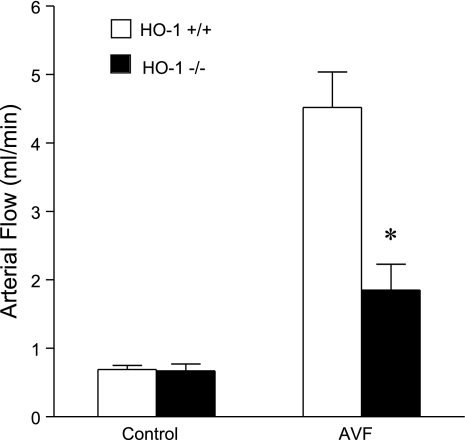
To evaluate the functional significance of HO-1 induction in the artery of the AVF, AVF blood flow in HO-1+/+ and HO-1−/− mice was determined 2 wk after the creation of the AVF. While contralateral carotid blood flow was similar in HO-1+/+ and HO-1−/− mice, AVF blood flow in HO-1−/− mice was markedly reduced compared with HO-1+/+ mice, attaining values that were less than half of that observed in HO-1+/+ mice (Fig. 9).
Fig. 9.
Blood flow in the AVF and control carotid artery in HO-1+/+ and HO-1−/− mice 2 wk after AVF creation. Blood flow was measured in the arterial limb of the AVF and the contralateral carotid artery (control) in HO-1+/+ and HO-1−/− mice using a perivascular flow probe; n = 7 in each group. *P < 0.05 vs. AVF in HO-1+/+ mice.
AVF outcomes in a transgenic murine model of SCD.
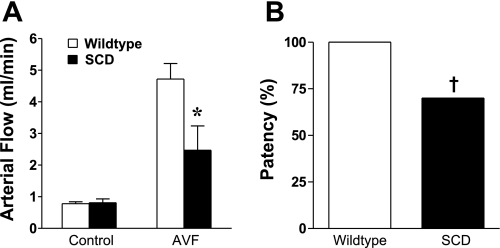
In view of the marked increase in blood flow that occurred in wild-type mice following the creation of an AVF, we posited that a disease that exhibits impaired hemodynamic responses would fail to mount the expected increase in AVF flow and preservation of AVF function. The rheologic abnormalities in SCD have long been recognized (11, 25), and our prior studies demonstrated that the vasculature in a transgenic murine model of SCD exhibits impairment in endothelium-dependent and endothelium-independent vasorelaxant responses (26). We thus employed this murine model as a representative disease to test this hypothesis. Four weeks after the creation of the AVF in wild-type mice and mice with SCD, AVF blood flow was significantly lower in mice with SCD (Fig. 10). Moreover, more than a third of all AVFs in mice with SCD showed loss of patency (Fig. 10). Thus, in a disease state attended by impaired hemodynamic responses, both AVF flow and AVF function are significantly compromised.
Fig. 10.
Blood flow through the arterial limb of the AVF and AVF patency in a murine model of sickle cell disease (SCD) and in wild-type mice 4 wk after creation of the AVF. A: blood flow through the arterial limb of the AVF and the contralateral carotid artery (control) in SCD and wild-type mice (n = 9 in each group). B: patency of the AVF in SCD (n = 17) and wild-type mice (n = 16). *P < 0.05 vs. AVF in wild-type mice; †P < 0.05 vs. AVF patency in wild-type mice.
DISCUSSION
Our present findings demonstrate that AVF blood flow, measured 2 wk after the creation of the AVF, is markedly increased because of reduced vascular resistance at the site of the AVF. Such reduction in AVF vascular resistances greatly outstrips the reduction in systemic vascular resistance, thereby underscoring vascular responses vicinal to the AVF. Despite this marked increase in blood flow, the vasorelaxant limit was not reached in the AVF in that local application of ACh elicited further increments in AVF blood flow. Thus, whatever the mechanisms accounting for the reduced resistances that enable increased AVF blood flow, a vasorelaxant reserve capacity still exists, at least to ACh-dependent pathways.
To determine a potential mechanism for the increase in AVF blood flow, our attention was directed to HO-1 for several reasons. First, prior studies demonstrate that genetic deficiency of eNOS, surprisingly, does not impair the increase in blood flow that occurs in this murine AVF model (6). Second, HO-1 is recognized as a vasorelaxant system because HO activity generates carbon monoxide, a vasorelaxant gaseous product; additionally, HO produces bile pigments that can scavenge superoxide anion, the latter representing an oxidant that avidly consumes nitric oxide (10, 12, 21). Third, in our prior studies, we noted that, at a later time point in this model, AVF patency was lost in more than 30% of HO-1−/− mice but was preserved in all HO-1+/+ mice (17). In these prior studies, we examined HO-1 expression in the venous limb of the AVF in HO-1+/+ mice and observed that it was induced. As neointimal hyperplasia was increased in the venous limb of the AVF in HO-1−/− mice at this later time point, we ascribed premature AVF failure in HO-1−/− mice to these changes in the venous limb. Our prior studies did not examine either the arterial limb or the significance of AVF blood flow in such premature AVF failure. Since a failure in adaptive responses in the arterial limb and impaired AVF blood flow are among the key harbingers of eventual loss of AVF patency, our present studies were thus designed to include an investigation of this issue. Our present data demonstrate that HO-1 mRNA and protein are induced in the arterial segment of the AVF, that such upregulation localizes to smooth muscle cells in the media of the AVF artery, and that the deficiency of HO-1 is attended by an attenuation in the rise in AVF blood flow. From these findings, we conclude that induction of HO-1 in the arterial limb contributes to the adaptive responses in the arterial limb that account for the increase in AVF blood flow, the latter critically required in enabling and sustaining AVF patency.
Hemodynamic alterations involve changes in the vasculature and in blood rheology, both of which are altered in SCD (11, 25, 26). Our prior studies demonstrate that arteries in a murine model of SCD exhibit impaired endothelium-dependent and endothelium-independent vasorelaxation (26), and substantial literature attests to the occurrence of a vasculopathy in SCD (11, 25). We hypothesized that such a vasculopathy in concert with the recognized abnormal blood rheology of SCD would compromise hemodynamic responses needed to ensure augmentation in AVF flow and preservation of AVF function; indeed, we observed that such AVF outcomes were impaired in this model of SCD. We suggest that these findings merit consideration for the following reasons. First, they underscore the dependency of AVF blood flow and AVF function on the integrity and adequacy of the underlying hemodynamic responses. Second, AVF dysfunction (a known contributor to the morbidity and mortality of dialysis patients), if also increased in sickle patients on hemodialysis, may underlie the increased mortality observed in this patient population (1, 27, 30). Third, shear stress-induced vasodilation, which is generally considered a vasoprotective response, is impaired in human SCD (3); this murine AVF model increases shear stress, and thus it provides a useful approach in investigating the basis for this abnormal response in SCD.
In the course of analyzing the hemodynamic changes in this AVF model, we provide the first assessment of cardiac hemodynamics in this model. We demonstrate that this murine AVF model, at this stage, is attended by increased cardiac output, preserved ejection fraction, and decreased systemic vascular resistance, thereby recapitulating hemodynamic changes observed in patients in whom an AVF is created for hemodialysis (31, 33). As a subset of these patients with AVFs develops high-output cardiac failure and pulmonary hypertension, this model may prove useful in examining adaptive mechanisms that preserve cardiac function following the creation of an AVF.
Hemodynamic responses in vascular beds distant from the AVF, specifically, the kidney, were divergent from those observed at the AVF and the systemic circulation: RBF was decreased and RVR was increased. This model may thus be used to explore the basis for renal vasoconstriction, altered sodium reabsorption, and other renal responses that occur when cardiac preload is increased, considerations relevant to the pathogenesis of the cardio-renal syndrome (5). An additional consideration is that as RBF is one of the four determinants of glomerular filtration rate (GFR), it is conceivable that GFR may decrease as RBF is reduced following the creation of the AVF. It is intriguing whether such decrement in renal function occurs in patients with chronic kidney disease in whom an AVF is created in anticipation of the arrival of end-stage kidney disease; if so, such adverse renal hemodynamic effects may be relevant to the loss of kidney function that occurs in these patients, and which is generally ascribed to the ineluctable progression of chronic kidney disease.
In summary, the present studies characterize the regional and systemic hemodynamic alterations following the creation of an AVF, identify the induction of HO-1 in the arterial limb, delineate its role in augmenting AVF blood flow, and illustrate how AVF blood flow and function may be impaired in a representative disease state compromised in its ability to elicit the requisite hemodynamic responses. We suggest that the reliance of the functionality of an AVF on the integrity and adequacy of underlying hemodynamic responses is germane to the relatively high rates of maturational failure (50%) and limited longevity of hemodialysis AVFs: AVFs are placed in settings (uremia) and diseases (diabetic nephropathy/vasculopathy, atherosclerosis, hypertensive arteriosclerosis, vasculitides, cardiac dysfunction, etc.) wherein impaired hemodynamic responses may be expected. Finally, as there are relatively few valid models in vivo for the study of the biology of increased shear stress, this model affords such utility, facilitating, in particular, an understanding of the effect of specific diseases and the functional significance of specific genes.
GRANTS
These studies were supported by National Institutes of Health Grants DK47060, HL55552, and DK70124.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge the secretarial expertise of T. Engel and the technical expertise of D. Jech and A. Ackerman.
REFERENCES
- 1. Abbott KC, Hypolite IO, Agodoa LY. Sickle cell nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol 58: 9–15, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Allon M. Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Belhassen L, Pelle G, Sediame S, Bachir D, Carville C, Bucherer C, Lacombe C, Galacteros F, Adnot S. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress-mediated vasodilation. Blood 97: 1584–1589, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Ben Driss A, Benessiano J, Poitevin P, Levy BI, Michel JB. Arterial expansive remodeling induced by high flow rates. Am J Physiol Heart Circ Physiol 272: H851–H858, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Boerrigter G, Burnett JC., Jr Cardiorenal syndrome in decompensated heart failure: prognostic and therapeutic implications. Curr Heart Fail Rep 1: 113–120, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res 97: 533–540, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Castier Y, Lehoux S, Hu Y, Foteinos G, Tedgui A, Xu Q. Characterization of neointima lesions associated with arteriovenous fistulas in a mouse model. Kidney Int 70: 315–320, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Croatt AJ, Grande JP, Hernandez MC, Ackerman AW, Katusic ZS, Nath KA. Characterization of a model of an arteriovenous fistula in the rat: the effect of l-NAME. Am J Pathol 176: 2530–2541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dixon BS. Why don't fistulas mature? Kidney Int 70: 1413–1422, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Dulak J, Deshane J, Jozkowicz A, Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation 117: 231–241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hebbel RP, Vercellotti G, Nath KA. A systems biology consideration of the vasculopathy of sickle cell anemia: the need for multi-modality chemo-prophylaxsis. Cardiovasc Hematol Disord Drug Targets 9: 271–292, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hill-Kapturczak N, Agarwal A. Haem oxygenase-1–a culprit in vascular and renal damage? Nephrol Dial Transplant 22: 1495–1499, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci 2: 157–161, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Juncos JP, Grande JP, Croatt AJ, Hebbel RP, Vercellotti GM, Katusic ZS, Nath KA. Early and prominent alterations in hemodynamics, signaling, and gene expression following renal ischemia in sickle cell disease. Am J Physiol Renal Physiol 298: F892–F899, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Juncos JP, Grande JP, Kang L, Ackerman AW, Croatt AJ, Katusic ZS, Nath KA. MCP-1 contributes to arteriovenous fistula failure. J Am Soc Nephrol 22: 43–48, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Juncos JP, Grande JP, Murali N, Croatt AJ, Juncos LA, Hebbel RP, Katusic ZS, Nath KA. Anomalous renal effects of tin protoporphyrin in a murine model of sickle cell disease. Am J Pathol 169: 21–31, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Juncos JP, Tracz MJ, Croatt AJ, Grande JP, Ackerman AW, Katusic ZS, Nath KA. Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney Int 74: 47–51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol Heart Circ Physiol 239: H14–H21, 1980 [DOI] [PubMed] [Google Scholar]
- 19. Kanwar YS. Functional duality of progenitor cells influxing into arteriovenous fistula during its neoangiogenesis. Am J Physiol Renal Physiol 293: F468–F469, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Michel F, Duriez M, Levy BI, Boulanger CM. Minimally invasive, in vivo exploration of mouse small artery reactivity. J Cardiovasc Pharmacol 43: 271–275, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int 70: 432–443, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Nath KA, Grande JP, Croatt AJ, Frank E, Caplice NM, Hebbel RP, Katusic ZS. Transgenic sickle mice are markedly sensitive to renal ischemia-reperfusion injury. Am J Pathol 166: 963–972, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol 158: 893–903, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nath KA, Grande JP, Kang L, Juncos JP, Ackerman AW, Croatt AJ, Katusic ZS. β-Catenin is markedly induced in a murine model of an arteriovenous fistula: the effect of metalloproteinase inhibition. Am J Physiol Renal Physiol 299: F1270–F1277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nath KA, Katusic ZS, Gladwin MT. The perfusion paradox and vascular instability in sickle cell disease. Microcirculation 11: 179–193, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Nath KA, Shah V, Haggard JJ, Croatt AJ, Smith LA, Hebbel RP, Katusic ZS. Mechanisms of vascular instability in a transgenic mouse model of sickle cell disease. Am J Physiol Regul Integr Comp Physiol 279: R1949–R1955, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Ojo AO, Govaerts TC, Schmouder RL, Leichtman AB, Leavey SF, Wolfe RA, Held PJ, Port FK, Agodoa LY. Renal transplantation in end-stage sickle cell nephropathy. Transplantation 67: 291–295, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA. MCP-1 is upregulated in unstressed and stressed HO-1 knockout mice: pathophysiologic correlates. Kidney Int 68: 611–622, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA 94: 10919–10924, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM, Johnson C. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 115: 614–620, 1991 [DOI] [PubMed] [Google Scholar]
- 31. Santoro D, Savica V, Bellinghieri G. Vascular access for hemodialysis and cardiovascular complications. Minerva Urol Nefrol 62: 81–85, 2010 [PubMed] [Google Scholar]
- 32. Schinstock C, Albright R, Williams A, Dillon J, Bergstralh E, Jenson B, McCarthy J, Nath KA. Outcomes of arteriovenous fistula creation after the fistula first initiative. Clin J Am Soc Nephrol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stern AB, Klemmer PJ. High-output heart failure secondary to arteriovenous fistula. Hemodial Int In press [DOI] [PubMed] [Google Scholar]
- 34. Tracz MJ, Juncos JP, Croatt AJ, Ackerman AW, Grande JP, Knutson KL, Kane GC, Terzic A, Griffin MD, Nath KA. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int 72: 1073–1080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Rajagopalan G, Knutson KL, Badley AD, Griffin MD, Alam J, Nath KA. Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1−/− mice. Am J Pathol 170: 1820–1830, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol 577: 1053–1065, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamada S, Nelson TJ, Behfar A, Crespo-Diaz RJ, Fraidenraich D, Terzic A. Stem cell transplant into preimplantation embryo yields myocardial infarction-resistant adult phenotype. Stem Cells 27: 1697–1705, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamada S, Nelson TJ, Crespo-Diaz RJ, Perez-Terzic C, Liu XK, Miki T, Seino S, Behfar A, Terzic A. Embryonic stem cell therapy of heart failure in genetic cardiomyopathy. Stem Cells 26: 2644–2653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]