Abstract
After block of Kv1- and Kv2-mediated K+ currents in acutely dissociated neocortical pyramidal neurons from layers II/III of rat somatosensory and motor cortex, the remaining current is slowly activating and persistent. We used whole cell voltage clamp to show that the Kv7 blockers linopirdine and XE-991 blocked a current with similar kinetics to the current remaining after combined block of Kv1 and Kv2 channels. This current was sensitive to low doses of linopirdine and activated more slowly and at more negative potentials than Kv1- or Kv2-mediated current. The Kv7-mediated current decreased in amplitude with time in whole cell recordings, but in most cells the current was stable for several minutes. Current in response to a traditional M-current protocol was blocked by muscarine, linopirdine, and XE-991. Whole cell slice recordings revealed that the Q10 for channel deactivation was ∼2.5. Sharp electrode current-clamp recordings from adult pyramidal cells demonstrated that block of Kv7-mediated current with XE-991 reduced rheobase, shortened the latency to firing to near rheobase current, induced more regular firing at low current intensity, and increased the rate of firing to a given current injection. XE-991 did not affect single action potentials or spike frequency adaptation. Application of XE-991 also eliminated subthreshold voltage oscillations and increased gain for low-frequency inputs (<10 Hz) without affecting gain for higher frequency inputs. These data suggest important roles for Kv7 channels in subthreshold regulation of excitability, generation of theta-frequency subthreshold oscillations, regulation of interspike intervals, and biasing selectivity toward higher frequency inputs.
Keywords: linopirdine, M current, motor cortex, somatosensory cortex, XE-991
pyramidal cells from layers II/III of somatosensory and motor cortex of rats express substantial persistent potassium currents. We previously found that approximately one-third of this current was unaccounted for after blockade of Kv1 and Kv2 channels (Guan et al. 2006, 2007a). In the present study we used whole cell voltage clamp of acutely dissociated pyramidal cells to test whether Kv7 (KCNQ; Cooper and Jan 2003; Gutman et al. 2003) α-subunits contribute to the remaining current.
Many cell types express a slowly activating potassium current that is referred to as “M current” because of its modulation by muscarinic agonists, as well as other transmitters and signaling pathways (Brown and Adams 1980). M-type currents are generally too slowly activating to repolarize an action potential (AP) but are persistent and active at subthreshold voltages and thus contribute to spike threshold, resting potential, and the regulation of interspike intervals (ISIs) in various neuron types (Aiken et al. 1995; Cooper and Jan 2003; Lamas et al. 1997; Lampe and Brown, 1991; Lawrence et al. 2006; Shen et al. 2005; Wladyka and Kunze 2006). Native M currents are thought to be due to heteromultimeric channels of Kv7.2 and Kv7.3 subunits (Peters et al. 2005; Roche et al. 2002; Selyanko et al. 2002; Wang et al. 1998). In addition, Kv7.5 subunits can form M-type currents when associated with Kv7.3 subunits (Lerche et al. 2000; Wickenden et al. 2001).
M current is of considerable interest because it can be modulated by several transmitters as well as by drugs of abuse (e.g., cannabinoids, opiates, ethanol; Koyama et al. 2007, Moore et al. 1990, 1994; Schweitzer, 2000). Furthermore, reduced Kv7-mediated currents are associated with pathophysiological changes. Mutations in four of the five KCNQ genes underlie human diseases, including deafness (Kv7.4), cardiac arrhythmias (Kv7.1), and epilepsy (Kv7.2, Kv7.3, and Kv7.5; Cooper and Jan 2003; Jentsch 2000). Kv7 channel dysfunction may also contribute to dyskinesias (Richter et al. 2006). Kv7 blockers (e.g., linopirdine and XE-991) enhance the release of transmitters in the central nervous system and improve performance in animal models of learning and memory (Aiken et al. 1996; Nickolson et al. 1990; Zaczek et al. 1998).
In CA1 pyramids, subthreshold activation allows Kv7 channels to prevent firing to constant input, restricts neurons from responding to dynamic input below a certain amplitude, and allows the cell to maintain spike timing precision (Prescott et al. 2006). M current interacts with Ih and persistent sodium conductance to generate subthreshold resonance in CA1 pyramidal cells, which may facilitate network oscillations at theta frequency (Hu et al. 2002). An XE-991-sensitive conductance may have similar effects in neocortical pyramidal cells (Castro-Alamancos et al. 2007; Gutfreund et al. 1995; Higgs et al. 2007) and contribute to oscillations.
In motoneurons, sympathetic ganglia neurons, and CA1 pyramidal neurons (Aiken et al. 1995; Alaburda et al. 2002; Hu et al. 2007; Marrion 1997; Prescott et al. 2006; Storm 1989, 1990; Yue and Yaari 2004), application of linopirdine or XE-991 resulted in reduction of the medium afterhyperpolarization (mAHP), shortened ISIs, and reduced spike frequency adaptation (SFA) (see Higgs et al. 2007 for neocortex and Shen et al. 2005 for medium spiny neurons). Somatodendritic Kv7 channels also control ISIs in hippocampal interneurons (Lawrence et al. 2006). A mutation (Szt1) that decreases functional Kv7.2 expression in mice reduces M-current density and SFA in CA1 pyramidal neurons (Otto et al. 2004). Mice overexpressing a mutant Kv7.2 (G279S) have reduced M current and increased neuronal excitability (Peters et al. 2005). Conversely, in a Kv7.2 dominant negative mouse, CA1 pyramidal cells had increased excitability, reduced SFA, reduced mAHP, and deficits in spatial memory (Peters et al. 2005). Linopirdine or XE-991 enhanced Ca2+ spikes in CA1 pyramidal neurons and increased excitatory postsynaptic potential summation (Hu et al. 2007). Linopirdine or XE-991 also markedly enhanced the fast afterdepolarization in CA1 pyramidal cells, converting single spikes to high-frequency bursts of several spikes (Golomb et al. 2006; Yue and Yaari 2004, 2006; see also Yoshida and Alonso 2007).
Despite extensive study in hippocampal and other neurons, the M current and its functional roles have not been well characterized in neocortical pyramidal cells. A slowly deactivating K+ current reduced by muscarinic agonists has been described in these cells (McCormick and Prince 1986; McCormick and Williamson 1989; Wang and McCormick 1993). Halliwell (1986) identified M current in human neocortical pyramidal neurons based on slow kinetics and voltage sensitivity, persistence in extracellular Cd2+, and muscarinic suppression. Linopirdine-sensitive currents were also reported in cultured neocortical pyramidal neurons (Noda et al. 1998).
Using low doses of the selective KCNQ/Kv7 current blockers linopirdine and XE-991 (Aiken et al. 1995, 1996; Lerche et al. 2000; Shah et al. 2002; Wang et al. 1998; Wickenden et al. 2001; Zaczek et al. 1998), we found that Kv7-containing channels contribute to the persistent K+ current in neocortical pyramidal cells. The Kv7 current contributes to rheobase, regulation of ISIs and repetitive firing (especially at low to intermediate rates), and subthreshold oscillations in the theta range and regulates the gain of firing responses to low-frequency input.
METHODS
These studies were performed on either 1) juvenile rats (Sprague-Dawley; postnatal days P6–P36) for studies of acutely dissociated cells or whole cell recordings in slices or 2) adult rats (8–16 wk) for sharp electrode studies. All procedures were approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center or the Animal Care and Use Committee of the Veterans Affairs Puget Sound Health Care System.
Dissociated cell studies.
Briefly, the animals were anesthetized with isoflurane until they were areflexive. The animals were decapitated, and the brain was removed and held in ice-cold cutting solution for 30–60 s. The cutting solution contained (in mM) 250 sucrose, 2.5 KCl, 1 NaH2PO4, 11 glucose, 4 MgSO4, 0.1 CaCl2, and 15 HEPES (pH 7.3–7.4; 300 mosmol/l). Coronal slices (400 μm) of the frontoparietal regions were cut using a vibrating tissue slicer (World Precision Instruments, Sarasota, FL). The slices were then transferred to a mesh surface in a chamber containing artificial cerebrospinal fluid (aCSF), which was continuously bubbled with a 95% O2-5% CO2 (carbogen) mixture at room temperature (RT). The aCSF contained (in mM) 125 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 20 glucose (pH 7.4; 310 mosmol/l).
Acute isolation of neurons.
The supragranular layers (I–III) from brain slices of the combined primary motor and primary somatosensory cortex were dissected under a stereomicroscope into 3-mm-wide pieces. Four to six tissue pieces were then transferred to oxygenated aCSF (35°C) with added enzyme (protease type XIV, 1.2 mg/ml; Sigma-Aldrich, St. Louis, MO). After 12–30 min of incubation in enzyme, the tissue pieces were washed with sodium isethionate solution, which consisted of (in mM) 140 Na-isethionate, 2 KCl, 4 MgCl2, 23 glucose, 0.1 CaCl2, and 15 HEPES (pH 7.3; 300 mosmol/l). Enzyme-treated tissue pieces were triturated with this solution, using three successively smaller fire-polished pipettes to release individual neuronal somata. The supernatant from each trituration step was collected, transferred to a fresh container, and plated onto a plastic petri dish (Nunc, Rochester, NY) on the stage of an inverted microscope. After 5–8 min of settling time, a uniform background flow of ∼1 ml/min of HEPES-buffered saline solution (HBSS) was established. HBSS consisted of (in mM) 138 NaCl, 3 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 dextrose (pH 7.3; 300–305 mosmol/l).
Electrophysiology.
With the exception of a few whole cell recordings in the slice preparation to determine temperature dependence (see below), all voltage-clamp experiments were performed on acutely dissociated neurons. Whole cell patch-clamp recordings were performed on 141 acutely dissociated pyramidal neurons. Most of the data were from animals between P16 and P36 (56 from P27 to P36, 43 from P21 to P26, and 11 from P16 to P20). Additional experiments were conducted on younger animals (P6–P8, n = 13 cells; P11–P14, n = 18) to test for changes in the Kv7-mediated current with age. Cells were identified as pyramidal by soma shape and the presence of a single apical dendrite (typically 20–30 μm in length). In combination with dissociation of supragranular layers only (see above), this ensured that we recorded from pyramidal cells from layers II/III. No axonal processes were observed. A few cells used to indicate the presence of a slowly activating current remaining after application of Kv1 and Kv2 blockers were from Guan et al. 2007b. All tests with Kv7 blockers and all of the slice recordings were new experiments for the present study.
A multibarrel array of glass capillaries (500-μm outer diameter) was used to apply external recording solutions. Solutions were changed by moving the active barrel (from which the solution flowed) so that it surrounded the recorded cell. To isolate K+ current for recording, the external solution contained (in mM) 140 Na-isethionate, 3 KCl, 1 MgCl2, 12 glucose, 10 HEPES, and 1.6–2 CaCl2 (adjusted so that CaCl2 + CdCl2 = 2 mM), plus 1 μM tetrodotoxin (TTX) and 100–400 μM CdCl2 for blocking Na+ and Ca2+ channels, respectively. Unless otherwise noted, all chemicals were obtained from Sigma-Aldrich. The following peptide toxins were used individually or jointly in the external solution for blocking specific Kv channels: α-dendrotoxin (DTX; 100–200 nM to block Kv1.1, Kv1.2, and Kv1.6), rMargatoxin (MTX; 10–20 nM to block Kv1.3), and rStromatoxin-1 (ScTx; 1 μM to block Kv2.1, Kv2.2, and Kv4.2). All peptide toxins were obtained from Alomone Labs (Jerusalem, Israel). BSA (0.1%) was added to all solutions to prevent peptides from binding to glass and plastic vessels.
Whole cell recordings were made with a DAGAN 8900 (Minneapolis, MN) amplifier at room temperature (21–23°C). Corning 8250 capillary glass (Garner Glass, Claremont, CA) was used to create electrodes on a Sutter Instruments (Novato, CA) model P-87 Flaming/Brown micropipette puller. Electrodes were fire-polished and filled with internal solution. The internal solution consisted of (in mM) 86 KMeSO4, 54 KOH, 2 MgCl2, 40 HEPES, 2 ATP, 0.2 GTP, 9 creatine phosphate, 0.1 leupeptin, and 10 BAPTA (pH 7.2; 270 mosmol/l). Electrode resistances were 1.4–2.2 MΩ. Series resistance (2–4 MΩ before compensation) was compensated by 70–90%. Cells with calculated series resistance errors of >5 mV were discarded [series resistance error (mV) = series resistance after compensation (GΩ) multiplied by peak current (pA)]. Membrane potentials were corrected for the measured liquid junction potential (+8 mV). Data acquisition (20-kHz sampling, filtered at 5 kHz) and analysis were done using pCLAMP 8 software (Axon Instruments, Union City, CA) and Prism 4 (GraphPad Software, San Diego, CA). Linear leak currents and capacitative artifacts were subtracted using an online P/4 or P/6 protocol.
Slice whole cell voltage clamp.
A small number of voltage-clamp recordings were carried out from 300-μm-thick slices made from immature (P21–P30) animals, as described previously (Guan et al. 2007b). Space clamp is compromised in these dendritic neurons, but the M current is slow, and we were interested in the relative changes in kinetics as a function of temperature. Pyramidal neurons in layers II/III were visualized with infrared-differential interference contrast videomicroscopy (Dodt and Zieglgänsberger 1990; Stuart et al. 1993) using a ×40 (0.8 NA) Olympus water-immersion objective and a cooled charge-coupled device camera (Sensicam; PCO Computer Optics, Kehlheim, Germany). Whole cell patch-clamp records were acquired using pCLAMP 10 and a Multiclamp 700B amplifier. Cells were recorded with borosilicate electrodes (4–8 MΩ in the bath) produced with a horizontal electrode puller (Sutter Instruments) and filled with a solution containing (in mM) 130.5 KMeSO4, 10 KCl, 7.5 NaCl, 2 MgCl2, 10 HEPES, 2 ATP, 0.2 GTP, and 5 mM EGTA. Data were collected only from cells forming a 1-GΩ or tighter seal. Series resistances were 8–15 MΩ and were not compensated for. Data were corrected for the measured liquid junction potential (10 mV). The aCSF contained (in mM) 125 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 20 glucose (pH 7.4; 310 mosmol/l) and was bubbled with carbogen. Currents were elicited using an “M-current protocol” where the holding potential was −30 mV and the voltage was stepped to −60 mV for 1 s. TTX (1 μM) was used to eliminate voltage-gated Na+ currents. Deactivation time constants were determined by fitting the current relaxation with a single-exponential function (Clampfit 10). To determine the Q10 values for deactivation kinetics, data were compared for cells recorded at RT (23 ± 1°C) and at 33 ± 1°C. The Q10 was determined as the ratio of the deactivation time constant τ at RT/τ at 33°C.
Sharp electrode intracellular recording.
Current-clamp recordings were obtained from regular-spiking neurons in layers II/III of the motor cortex. For these studies, 300-μm coronal brain slices were prepared from adult (8–16 wk old) rats as described previously (Higgs and Spain 2009). To avoid washout of the Kv7 current during prolonged stimulus protocols, recordings were obtained using sharp electrodes (40–50 MΩ) pulled from borosilicate glass (Sutter BF100-50-10) and filled with 3 M KCl. Current injection and voltage recording were performed at 34 ± 1°C using an Axoclamp-2A amplifier. Data on individual APs were acquired in bridge mode with 10-kHz filtering and 20-kHz data sampling. Most other data were obtained in discontinuous current-clamp mode (5-kHz cycle rate) to minimize voltage errors caused by electrode series resistance.
Statistics.
Data are means ± SE. Prism software (GraphPad Software) was used for statistical tests of significance. Paired or unpaired t-tests were used to compare sample population data. P values <0.05 were considered to be significant. Multiple comparisons used one-way ANOVA combined with Tukey's multiple comparison test for post hoc comparison of means. Sample population data are presented as scatter plots or box plots (Tukey 1977). Box plots indicate the upper and lower quartiles as edges of the box, with the median represented as a line crossing the box. The stems indicate the largest and smallest nonoutlying values, and outliers are indicated by open circles. Outlying values are greater than 1.5 times the quartile boundaries.
RESULTS
Kv7-mediated currents.
(Unless otherwise noted, all voltage-clamp experiments were performed on acutely dissociated neurons.) Our previous studies demonstrated that the major part of the slowly inactivating K+ current in neocortical pyramidal cells is carried by Kv2.1 channels (∼58%; Guan et al. 2007a), with an additional ∼10% of the current due to Kv1 channels (Guan et al. 2006). In the presence of 1 μM TTX (to block Na+ current), 100–400 μM Cd2+ (to block Ca2+-dependent currents), and blockers of Kv1 (100 nM DTX plus 20 nM MTX) and Kv2 channels (1 μM ScTx), 20–30% of the current remained (Guan et al. 2007a; Fig. 1A). This current was typically slow to activate and was persistent (Fig. 1, A and D; Table 1). In many cell types, a similar slow component of the outward current (M current) is due to expression of channels containing Kv7 subunits (Roche et al. 2002; Selyanko et al. 2002; Wang et al. 1998). The present experiments were designed to test whether Kv7 channels underlie the remaining current and to characterize the biophysical properties, development, and function of this current in neocortical pyramidal neurons. If Kv7 channels underlie the remaining current, the current should be slowly activating and persistent (like Kv7-mediated currents), and known Kv7 blockers should block the slowly activating current.
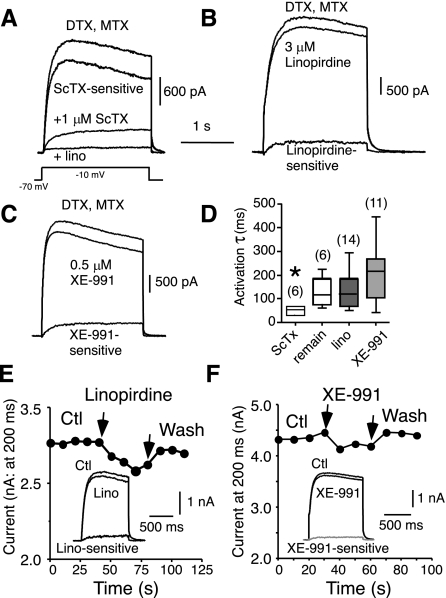
Fig. 1.
Similarity between current remaining after block of Kv1 and Kv2 channels and current sensitive to the Kv7 blockers linopirdine and XE-991 (acutely dissociated layer II/III pyramidal cells). All currents were elicited by 2-s steps to −10 mV from a holding potential of −70 mV. Voltage steps were repeated every 10 s. All recordings were in the presence of 400 μM Cd2+, 100 nM α-dendrotoxin (DTX), and 20 nM margatoxin (MTX). A: example of current remaining after block of Kv1 (DTX and MTX) and Kv2 channels (1 μM stromatoxin; ScTx). Note slow activation and persistence of remaining current (ScTx trace). The current inactivated, and activation was much faster for the ScTx-sensitive current compared with the remaining current. Addition of 100 μM linopirdine blocked all of the slowly activating current. B: current sensitive to block by the Kv7 blocker linopirdine (3 μM) was slowly activating and persistent. C: current sensitive to block by the Kv7 blocker XE-991 (0.5 μM) was also slowly activating and persistent. D: summary data for activation time constant τ for current sensitive to 1 μM ScTx, remaining current after block of Kv1 and Kv2 channels (remain), 1–3 μM linopirdine-sensitive current (lino), and 0.5–1 μM XE-991-sensitive current. Numbers of cells are shown in parentheses. *P < 0.05 indicates significant difference from other 3 groups. E: linopirdine's block was slow and partly reversible for short applications. F: XE-991 block was also reversible for short applications. Ctl, control; Wash, washout.
Table 1.
Activation time constants at room temperature
| Current | τ, ms | n |
|---|---|---|
| Remaining (after Kv1 and Kv2 block) | 137 ± 23 | 6 |
| Linopirdine sensitive (3 μM) | 143 ± 19* | 16 |
| XE-991 sensitive (0.5–1 μM) | 206 ± 34* | 11 |
| Muscarine/Oxo sensitive | 134 ± 11 | 8 |
| ScTx sensitive | 69 ± 21 | 4 |
| Kv2.1 antibody sensitive | 75 ± 25 | 4 |
| DTX sensitive | 11 ± 1 | 33 |
Values are means ± SE (n = no. of cells) for activation time constant τ at −10 mV. Data for current sensitive to stromatoxin (ScTx; putative Kv2 channels) and for current sensitive to intracellular application of a Kv2.1 antibody (Kv2.1 channels) are from Guan et al. 2007a. Data for current sensitive to α-dendrotoxin (DTX; putative Kv1.1, Kv1.2, and Kv1.6 channels) are from Guan et al. 2006. Muscarine/Oxo sensitive, current sensitive to muscarine (0.3–1 μM) or oxotremorine (10 μM).
P < 0.05, significant difference from whole current or blocker-resistant current.
The current remaining in the presence of ScTx, DTX, and MTX activated slowly and did not inactivate during a 2-s test step (to −10 mV from a holding potential of −70 mV), consistent with a role for Kv7 subunits. Activation was well fit with a single-exponential function (r2 > 0.9; Fig. 1D; Table 1). Subsequent addition of the known Kv7 channel blockers linopirdine (0.5–100 μM, n = 6; Aiken et al. 1996; Costa and Brown 1997; Schnee and Brown 1998) or XE-991 (0.1–10 μM, n = 2, Schroeder et al. 2000; Wang et al. 1998) to the external solution blocked additional current in all cells tested. Current remained in the presence of 10–15 μM linopirdine or XE-991. This remaining current was almost completely blocked by 100 μM linopirdine (Fig. 1A, n = 3 cells).
We next tested for a current component blocked by linopirdine or XE-991 in the absence of a Kv2 blocker. Low doses of either linopirdine (≤3 μM) or XE-991 (≤1 μM) blocked a current that activated slowly and did not inactivate over a 2-s step to −10 mV (Fig. 1, B and C; Table 1). We found that in 29/34 cells tested (85%; 8.7 ± 4.0% block, range = 1–17%), linopirdine blocked current. Low doses of XE-991 blocked current in 37/44 cells (84%; 7.0 ± 5.7% block, range = 1–16%). The activation τ at −10 mV was similarly slow for low-dose linopirdine- and XE-991-sensitive currents and for the remaining current after blockade of Kv1 and Kv2 current (Fig. 1; Table 1). Currents sensitive to 3 μM linopirdine or 1 μM XE-991 were significantly slower than control or drug-resistant currents (Table 1; P < 0.001: ANOVA, Tukey's post hoc tests).
The current block by linopirdine was partially reversible with short applications (e.g., Fig. 1E). We found no clear relationship between reversibility and dose or between reversibility and kinetics of the sensitive current (c.f., Wladyka and Kunze 2006). The block by linopirdine was dose dependent. At −10 mV, there was 6.6 ± 7.2% block with 1 μM linopirdine, 13 ± 6% block with 3 μM linopirdine, and 23 ± 9% with 10 μM linopirdine. The block by XE-991 was also reversible (Fig. 1F) and dose dependent, with 5 ± 2% block with 0.3–0.5 μM XE-991, 11 ± 4% with 1 μM XE-991, and 41 ± 10% with 10 μM XE-991 (n = 6 cells).
Both linopirdine and XE-991 have been shown to block channels other than Kv7 at high micromolar doses (Aiken et al. 1995, 1996; Elmedyb et al. 2007; Lamas and Brown 1995; Schnee and Brown 1998; Wang et al. 1998; Wladyka and Kunze 2006). In subsequent voltage-clamp experiments, we chose doses of linopirdine (≤3 μM) or XE-991 (≤1 μM) that are selective for Kv7 channels (Schnee and Brown 1998). These are less than saturating doses, so the current sensitive to these agents is an underestimate of the Kv7 contribution to the whole cell current, but the blocked current is likely to be exclusively due to Kv7 subunits.
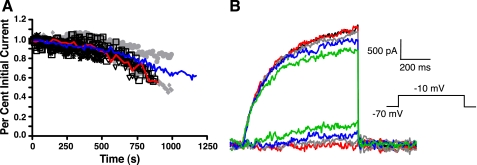
Run-down of Kv7-mediated current.
Kv7-mediated currents are prone to run-down in whole cell recordings (c.f., Schnee and Brown 1998), so we examined run-down in a subset of recordings in which a test command to −10 mV for 500 ms (from a holding potential of −70 mV) was repeated at 10-s intervals over a period of several minutes. Only cells with small and stable leak current and series resistance were accepted for analysis. In five cells the current ran down immediately and quickly, so these cells were discarded. Twenty-one cells were analyzed further. Typically, these cells had stable currents (<3% change) for 4–12 min (mean = 8 ± 3 min; Fig. 2A). After the initial stable period, the whole cell current ran down at 67 ± 0.4 pA/min. Subtraction of current remaining after 3, 6, 9, or 12 min from the initial current revealed that the run-down current was very slowly activating and persistent (Fig. 2B), consistent with the properties of linopirdine- or XE-991-sensitive current (Fig. 1). A similar rate of run-down (87 pA/min) was found for the whole cell current in our previous study of Kv1 channels (Guan et al. 2006). On the basis of these data, we took advantage of our rapid drug application system and the access to the cells afforded by the dissociated cell preparation to make all measurements of putative Kv7-mediated currents within the first 3–5 min of the recordings.
Fig. 2.
Run-down of whole cell outward current with time (acutely dissociated layer II/III pyramidal cells). All data were obtained with a holding potential of −70 mV and steps to −10 mV for 500 ms (repeated every 10 s). A: for this group of 21 neurons, outward K+ currents were unchanged for at least 4 min after break-in to whole cell mode. After a stable period of 4–12 min, there was steady run-down. The blue trace is the mean for all 21 cells. The red trace is an individual example, the traces for which are shown in B. B: traces from a representative neuron (same cell as the red trace in A). Note the slow activation kinetics of the persistent run-down current (obtained by subtraction from the initial current). Red trace = 3 min into recording, gray trace = 6 min, blue trace = 9 min, and green trace = 12 min.
Ontogeny of Kv7-mediated current.
To test whether the density (peak current divided by whole cell capacitance) of current sensitive to XE-991 (1 μM) or linopirdine (3 μM) increased with age, we applied Kv7 blockers to cells from animals from 1 wk of age to 4 wk of age. We assume that sensitivity to these blockers does not change with age. Current density (at −10 mV from a holding potential of −70 mV) increased from 6.9 ± 1.6 pS at P6–P8 (n = 13), to 8.7 ± 2.3 pS at P11–P14, 17.9 ± 3.1 pS at P20–P24, and 17.6 ± 4.9 pS at P26–P30. There was a significant difference in putative Kv7-mediated density with age (P < 0.003: ANOVA), but only the 1-wk data (P6–P8) differed from the other groups, so in subsequent experiments, the data were combined for cells from animals from P16 to P36.
Properties of linopirdine-sensitive current.
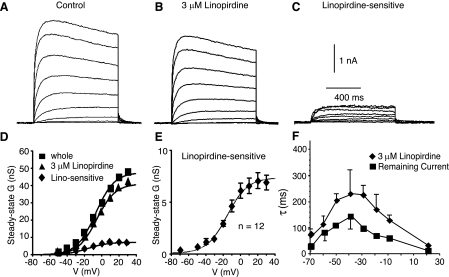
We next took advantage of the reduced processes (and attendant spatial control of membrane voltage) of dissociated cells to characterize the biophysical properties of this current. In a sample of 11 cells, the holding current at −70 mV was shifted inward 2–22 pA by 3 μM linopirdine (7.5 ± 2.2 pA). When cells were held at −80 mV, there was no significant difference in holding current (n = 7 cells).
To examine steady-state voltage dependence, we used a family of 1-svoltage steps from a holding potential of −70 mV. We obtained records in control solution and in the presence of 3 μM linopirdine and analyzed the difference currents (control minus linopirdine) as the linopirdine-sensitive current (Fig. 3). To obtain steady-state activation curves, conductance corresponding to peak currents was calculated [G = I/(E − EK)] and fitted with the Boltzmann equation:
where Gmax is the maximum conductance, V1/2 is the half-activation voltage, and Vc is the slope. All three of these parameters were determined by the fit. For 12 cells, the steady-state activation curve for the linopirdine-sensitive current was well fit by a single Boltzmann function, with V1/2 = −16.7 ± 1.6 mV and slope = 11.8 ± 1.3 mV (Fig. 3). The half-activation for current resistant to linopirdine was also fit by a single Boltzmann function, with V1/2 = −2.1 ± 1.2 mV and slope = 13.2 ± 1.8 mV (n = 12 cells). The V1/2 for the linopirdine-sensitive current was significantly hyperpolarized from that for the linopirdine-resistant current (P < 0.001: paired t-test).
Fig. 3.
Steady-state activation for the current sensitive to the Kv7 blocker linopirdine (acutely dissociated layer II/III pyramidal cells). All currents were elicited by 1-s steps from a holding potential of −70 mV (interstep interval was 10 s). All recordings were in the presence of 400 μM Cd2+, 100 nM DTX, and 20 nM MTX. A: current traces for a representative cell in control solution. B: currents from the same cell in the presence of 3 μM linopirdine. C: subtracted records to illustrate the linopirdine-sensitive current. D: plot of steady-state conductance (G) vs. step voltage (V) for the same cell as in A–C. E: average steady-state activation curve for 12 cells (means ± SE). F: time constants for activation and deactivation as a function of voltage for linopirdine-sensitive and linopirdine-resistant (remaining) current.
We also examined the time constant for activation/deactivation for the linopirdine-sensitive and -insensitive currents. The holding potential was −70 mV. We examined the onset of currents after steps to potentials between −40 and +20 mV and deactivation tail currents on return to −70 mV. Typically, three to five traces at a given potential were averaged before the exponential fit was determined. The time constant for activation was voltage dependent and slower than the linopirdine-insensitive (remaining) current at all voltages (Fig. 3F; Table 2).
Table 2.
Voltage dependence of activation time constant
| τ, ms |
||||||
|---|---|---|---|---|---|---|
| Current | −70 mV | −40 mV | −30 mV | −20 mV | −10 mV | +20 mV |
| Linopirdine sensitive (3 μM) | 80 ± 16 | 292 ± 127 | 236 ± 38 | 180 ± 59 | 142 ± 22 | 41 ± 4 |
| Linopirdine resistant | 28 ± 4 | 169 ± 64 | 102 ± 4 | 63 ± 1 | 60 ± 6 | 19 ± 4 |
Onset of the current was fit with an exponential function. Data are means ± SE (n = 5 cells) of activation time constants for the current resistant to 3 μM linopirdine (linopirdine resistant) and the subtracted current after application of 3 μM linopirdine (linopirdine sensitive). Holding potential was −70 mV. Data were obtained with 1-s steps to potentials between −40 and +20 mV. Data for −70 mV are taken from deactivation tail on return to holding potential.
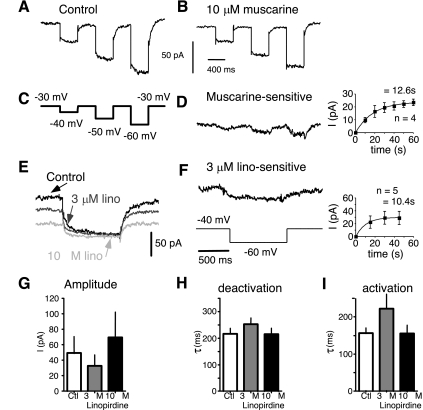
M current.
The traditional way to isolate M current is to hold the cell depolarized (to activate M current and inactivate other currents) and then step back to a more hyperpolarized potential. M current is then revealed as a slow current relaxation due to channel deactivation (Adams et al. 1982; Brown and Adams 1980). M currents (Brown and Adams 1980; Halliwell 1986; Marrion 1997; McCormick and Prince 1986; Wang and McCormick 1993) and Kv7.2/7.3 channels in expression systems (Selyanko et al. 2000; Shapiro et al. 2000; Wang et al. 1998) are modulated by muscarinic receptor activation. We tested whether muscarine and linopirdine were effective in blocking current elicited by a traditional M-current protocol. We held the cells at a potential where M current was activated (typically −40 or −30 mV) and stepped back to −60 or −70 mV to elicit a current relaxation (in the presence of 400 μM Cd2+ to block Ca2+-dependent K+ currents). A slowly deactivating current was revealed by this protocol in 13 cells (for a step from −40 to −60 mV, the decay τ = 214 ± 50 ms, n = 8; Fig. 4, A and C). This current was blocked by 10 μM muscarine (n = 4 cells; Fig. 4, A–D), consistent with the slowly deactivating current being M current. The kinetics of activation and deactivation of the muscarine-sensitive current were slow (Fig. 4, H and I). About one-half of the M current was blocked by 3 μM linopirdine (52 ± 31%, n = 8), and much of the rest was blocked by 10 μM linopirdine (80 ± 24%, n = 6; Fig. 4G).
Fig. 4.
Linopirdine and muscarine blocked current elicited by the traditional M-current protocol (acutely dissociated layer II/III pyramidal cells). All recordings were in the presence of 400 μM Cd2+, 100 nM DTX, and 20 nM MTX. A: current in control solution. Cell was held at −30 mV, and steps were made to −40, −50, and −60 mV (protocol shown in C). B: current in the same cell after application of 10 μM muscarine. Muscarine blocked much of the slow current relaxation. C: voltage protocol for A, B, and D. D: muscarine-sensitive current for the same cell as in A and B. Current is slowly deactivating and slowly activating (on return to holding potential). Inset: time course of block by 10 μM muscarine (means ± SE, n = 4 cells). E: currents from another cell held at −40 mV in response to a step to −60 mV. The slow current relaxation was largely blocked by 3 μM linopirdine and completely blocked by 10 μM linopirdine. F: current sensitive to 3 μM linopirdine (same cell as in E). Protocol for E and F is shown at bottom in F. Inset: time course of block by 3 μM linopirdine (means ± SE, n = 5 cells). G: amplitude histograms of current amplitude in control solution, current blocked by 3 μM linopirdine, and current blocked by 10 μM linopirdine (for G–I: n = 8 cells for control and 3 μM linopirdine, n = 6 for 10 μM linopirdine; holding potential = −40 mV, step to −60 mV). H: histograms for time constant τ for deactivation (at −60 mV). I: histograms for time constant τ for activation (at −40 mV).
When we examined test steps from a holding potential of −70 to −10 mV, the activation kinetics of muscarine- or oxotremorine-sensitive current were similar to the activation kinetics for low-dose linopirdine- or XE-991-sensitive current (Table 1). For muscarine (0.3–1 μM), τ was 119 ± 30 ms (n = 3). For 10 μM oxotremorine, τ was 128 ± 35 ms (n = 5). The current at −10 mV was inhibited by 0.3–1 μM muscarine (11 ± 6%, n = 3 cells; data not shown) or 0.3–10 μM oxotremorine (10 ± 2%, n = 5 cells; data not shown).
Whole cell voltage clamp in slices.
We recorded from 13 layer III pyramidal cells in a slice preparation (data not shown) with whole cell voltage clamp and an M-current protocol (hold at −30 mV, step for 1 s to −60 mV; methods). Data from 6 cells were obtained at RT (23 ± 1°C), and 7 cells were recorded at 33 ± 1°C. The deactivation τ was 114 ± 18 ms at 22°C and 45 ± 5 ms at 32°C, resulting in a Q10 of ∼2.5. The slice kinetics at RT were similar to those for linopirdine- or muscarine-sensitive currents at RT in dissociated cells. To relate kinetics data from our dissociated cell experiments at RT to current-clamp data at 35°C, τ values at RT (Fig. 3F; Tables 1 and 2) should thus be divided by ∼2.5.
Function of Kv7-mediated currents.
We addressed the functional implications of the properties of the Kv7-mediated current for cortical pyramidal neurons by sharp electrode intracellular current-clamp recordings (at 34 ± 1°C) from layer II and III pyramidal cells in brain slices from adult rats. We obtained data in control solution and during bath application of XE-991 (5 μM).
On average, XE-991 had no significant effect on resting membrane potential (−83.4 ± 2 mV in control vs. −82.4 ± 1.8 mV in XE-991, n = 6 cells). XE-991 also had little effect on the AP waveform (Fig. 5A). In cells tested with just-threshold current steps, the peak (overshoot) of the AP potential was 35.0 ± 1.1 mV in control solution and 33.4 ± 1.3 mV in XE-991 (n = 5, P = 0.12), and the spike width at half-height [determined as ½(AP peak − Vthreshold)] was 0.54 ± 0.03 ms in control and 0.55 ± 0.04 ms in drug. Although XE-991 slightly lowered the spike threshold in the cell illustrated, overall this effect was not significant (−52.2 ± 2.6 mV in control vs. −53.5 ± 2.3 mV in XE-991).
Fig. 5.
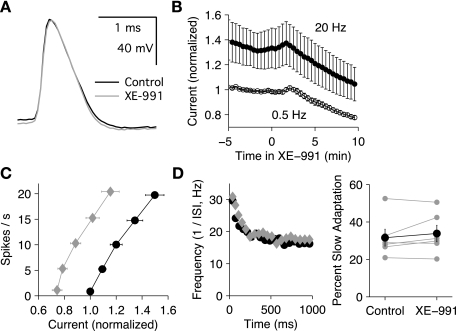
Effects of XE-991 on pyramidal neuron responses to current stimuli (sharp electrode recordings in slice preparation). Control data are shown in black, and XE-991 (5 μM) data are shown in gray. A: example of action potentials in control solution and in XE-991. The drug had little effect on the spike waveform. B: the average time course (n = 6 cells) for the effects of 5 μM XE-991 on the current (normalized by rheobase) required to fire at 2 firing frequencies, 0.5 and 20 Hz (means ± SE, n = 6 cells). C: average firing frequency-current (f-I) relationships (n = 6) showing a leftward shift in XE-991 (diamonds) vs. control (circles). The current was normalized to the control rheobase for each cell. D: example of instantaneous firing frequency-time (f-t) relationship showing that XE-991 (diamonds) had little effect on slow spike frequency adaptation (control; circles). E: summary data showing the lack of effect of XE-991 (5 μM) on slow adaptation.
Although XE-991 had little effect on individual action potentials, it clearly increased the firing rate during prolonged current injection. This effect was quantified by measuring the firing frequency-current (f-I) relationship for 1-s current steps delivered at a 5-s interval. To compare f-I curves over the same ranges of firing rates, and to aid in keeping stable recordings, the step amplitudes were adjusted by computer to maintain target spike counts of 0.5, 5, 10, 15, and 20. The 0.5 spike target defined the current threshold (rheobase, Irh), giving one or more spikes on ∼50% of the trials. The firing frequency for each step was taken as the spike count for the entire 1 s, and the gain was defined as (f20 − frh)/(I20 − Irh), where f20 and frh are the mean spike counts for target counts of 20 and 0.5 and Irh and I20 are the corresponding mean currents. On average, XE-991 lowered Irh from 482 ± 61 to 366 ± 54 pA (P = 0.001) and increased the gain from 98 ± 6 to 116 ± 10 Hz/nA (P = 0.01). Figure 5B indicates the time course of the XE-991 effect, showing the average current (normalized by rheobase) required for cells to fire at either 0.5 or 20 Hz (n = 6 cells). Typical effects of XE-991 on the f-I relationship are illustrated in Fig. 5C, which shows the mean firing rate (n = 6 cells) in control solution as a function of injected current, normalized by Irh. These effects suggest that consistent with our voltage-clamp results, the Kv7 current began to activate below spike threshold, thereby increasing Irh, and Kv7 current increased further with additional current injection, reducing gain.
In pyramidal neurons, slow activation of Kv7 current may contribute to slow SFA (Aiken et al. 1995; Gu et al. 2005; Otto et al. 2006; Prescott and Sejnowski 2008). To investigate this possibility, we determined the instantaneous firing frequency as the inverse of each ISI and plotted the frequency as a function of time from step onset (taken as the average of the 2 spike times). Data for a target spike count of 20 were analyzed (Fig. 5, D and E). To measure slow adaptation, the data points from 100 to 1,000 ms were fitted with a single-exponential function, and the percent slow adaptation was defined by the decrease in the fitted curve from 100 to 1,000 ms. By this measure, XE-991 did not affect the magnitude of slow adaptation (control, 32 ± 4%; XE-991, 34 ± 4%; P = 0.27). This finding suggests that the Kv7 current reached steady state within the first 100 ms of each current step and did not contribute to adaptation on a longer time scale.
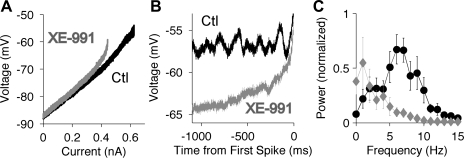
To characterize the effects of Kv7 channels on the subthreshold input resistance, and to investigate their contributions to membrane potential oscillations and the pattern of firing, we applied slow ramp stimuli (20 pA/s) at 1- to 2-min intervals. As predicted from our voltage-clamp data, XE-991 consistently altered the current-voltage (I-V) relationship in the subthreshold range (Fig. 6A). Near resting potential the slope resistance (the slope of the curve) showed little change, but near spike threshold the I-V relationships were relatively linear in control solution but curved upward in XE-991 (depolarized to approximately −70 mV), resulting in a lower rheobase. Membrane potential oscillations were not observed at the resting potential but began to occur slightly below spike threshold (Fig. 6B). The power spectra of the oscillations were computed based on the membrane potential from 1,100 to 100 ms before the first spike, after subtracting a linear fit to the data. In control solution, the power showed a peak located at 7.0 ± 0.6 Hz (n = 6; Fig. 6C). XE-991 greatly reduced the oscillations and eliminated the peak in the power spectra. In the presence of XE-991, the membrane potential was not stable at the level of the control oscillations, but instead swept directly upward into the first spike.
Fig. 6.
Effects of XE-991 on subthreshold response of pyramidal neurons (sharp electrode recordings in slice preparation). Control data are shown in black, and XE-991 (5 μM) data are shown in gray. A: current-voltage (I-V) relationship during a slow ramp stimulus (20 pA/s). B: example of membrane potential oscillations during the ramp, as the membrane potential approached the spike threshold. XE-991 eliminated the oscillations. C: average power spectra (n = 6) of the membrane potential during the ramp, from 1,000 to 100 ms before the first spike. The power was normalized by the peak value in control solution in each cell. The peak located at 6–7 Hz in control solution was eliminated by XE-991.
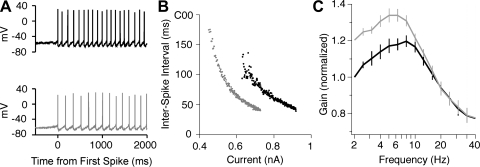
XE-991 also altered the pattern of firing at currents slightly above rheobase (Fig. 7A). In most cells, XE-991 lowered the initial firing rate or caused the firing to become regular at a lower rate. This effect may be appreciated by plotting each ISI as the current was slowly increased (Fig. 7B). In the presence of XE-991, the first ISIs were longer and less variable than those obtained in control solution. To quantify the onset frequency for regular firing (freg), we identified the first pair of consecutive ISIs with <10% difference (Δtreg1 and Δtreg2). We then calculated freg = 2/(Δtreg1 + Δtreg2). On average, XE-991 lowered freg from 8.9 ± 1.0 to 5.6 ± 0.9 Hz (n = 6; P = 0.03). These data suggest that the Kv7 current prevents regular firing at frequencies at or below the frequency of the subthreshold oscillation (fosc). In each cell tested, the value of freg in control solution was greater than fosc. The inability to fire regularly at fosc suggests that the spike afterhyperpolarization advances the phase of the voltage oscillation by accelerating the hyperpolarizing trajectory, which increases the natural frequency.
Fig. 7.
Effects of XE-991 on suprathreshold responses of pyramidal neurons (sharp electrode recordings in slice preparation). Control data are shown in black, and XE-991 (5 μM) data are shown in gray. A: example of the onset of firing during ramps showing a lower onset frequency in XE-991 (bottom). B: interspike intervals (same data as A) plotted as a function of the current. C: gain-frequency relationship (frequency on log axis) determined by noise stimulation and correlation analysis. The data indicate the average depth of firing rate modulation per unit sine wave current and are normalized by the control value at 2 Hz. XE-991 increased gain below 10 Hz but had little effect at higher frequencies.
In addition to altering the firing pattern, Kv7 channels may alter the selectivity of pyramidal neurons for different components of a time-varying input signal. The data obtained with current steps and ramps suggest that the Kv7 current reduces the sensitivity of pyramidal neurons to slowly changing current input. However, because of the slow activation kinetics of Kv7 channels, we hypothesized that the Kv7 current might not reduce the sensitivity to higher frequency input fluctuations. Thus the Kv7 current may increase the selectivity for higher frequency input. To test this hypothesis, we stimulated cells with broadband Gaussian noise current (SD = 250–400 pA, exponential-filtered, τ = 5 ms) and determined the frequency-dependent gain by correlation analysis of the noise current and the digitized spike response, as described previously (Higgs and Spain 2009). Similar firing rates were maintained in control solution (8.4 ± 1.0 Hz) and in the presence of XE-991 (8.9 ± 0.8 Hz) by adjusting the direct current offset. For pooling of data from multiple cells (n = 5), the gain at each frequency was normalized by the gain at 2 Hz in control solution. The average gain data are illustrated in Fig. 7C. Consistent with our hypothesis, XE-991 increased gain at frequencies below 10 Hz but had little effect at higher frequencies.
DISCUSSION
Previously, we showed that Kv1 (Guan et al. 2006) and Kv2 (Guan et al. 2007a) α-subunits contribute to slowly inactivating and persistent K+ current in neocortical pyramidal neurons. We presently report that Kv7 (KCNQ) subunits also contribute to the persistent potassium current in these cells. This conclusion is supported by several observations. 1) The current that remains after blocking Kv1 and Kv2 channels is extremely slow to activate and is persistent. 2) Most cells expressed current sensitive to low doses of known Kv7 blockers (linopirdine or XE-991). 3) Current blocked by low doses of linopirdine or XE-991 had similar slow activation properties and absence of inactivation to the Kv1 and Kv2 blocker-resistant current (and to Kv7-mediated currents in expression systems and M currents in other cell types). 4) Current elicited by the traditional M-current protocol was blocked by linopirdine or muscarinic agonists.
Neocortical expression of Kv7.2, Kv7.3, and Kv7.5 subunit mRNA and protein has been demonstrated by in situ hybridization, Northern blots, immunohistochemistry, and tissue RT-PCR (Devaux et al. 2004; Geiger et al. 2006; Jensen et al. 2005; Lerche et al. 2000; Saganich et al. 2001; Schroeder et al. 2000; Weber et al. 2006; Yus-Najera et al. 2003). Immunocytochemical studies indicate that most Kv7 expression in cortical regions is axonal, although somas and dendrites are also stained (Chung et al. 2006; Cooper et al. 2000; Devaux et al. 2004; Geiger et al. 2006; Hu et al. 2007; Pan et al. 2006; Weber et al. 2006).
We found that the putative Kv7-mediated current was active at potentials depolarized to approximately −70 mV and made up a large fraction of the subthreshold outward current in layer II/III neocortical pyramidal cells. The slow kinetics render this current relatively insensitive to voltage excursions during APs but would allow major contribution to rheobase, subthreshold oscillations, and regulation of ISIs.
Kv7 blockers.
Sensitivity to the cognitive enhancers linopirdine and XE-991 has been considered evidence for Kv7 (and M) channel expression in many cell types (Aiken et al. 1995; Alaburda et al. 2002; Costa and Brown 1997; Gu et al. 2005; Hu et al. 2007; Lawrence et al. 2006; Noda et al. 1998; Oiu et al. 2007; Pena and Alavez-Perez 2006; Rennie et al. 2001; Shen et al. 2005; Wladyka and Kunze 2006; Wang et al. 1998; Yeung and Greenwood 2005; Yue and Yaari 2004, 2006). In expression systems, currents mediated by Kv7.2, Kv7.3, or heteromeric Kv7.2/7.3 channels are blocked by linopirdine with IC50 values of ∼2–10 μM (Schnee and Brown 1998; Wang et al. 1998) and Kv7.5 channels with lower affinity (IC50 ∼16–50 μM, Lerche et al. 2000; Schroeder et al. 2000; Wickenden et al. 2001). XE-991 blocks Kv7.2 channels or Kv7.2/7.3 heteromeric channels in expression systems with IC50 values of 0.6–0.7 μM (Schroeder et al. 2000; Wang et al. 1998). Kv7.5 channels were much less sensitive to XE-991 (65 μM, Schroeder et al. 2000).
M current was blocked by linopirdine with IC50 values of 3–36 μM in several neuron types (Aiken et al. 1995, 1996; Lamas and Brown 1995; Lamas et al. 1997; Passmore et al. 2003), including cultured neocortical pyramidal neurons (Noda et al. 1996). XE-991 blocks native M current with high affinity in several cell types. M current in sympathetic ganglion neurons was blocked with an IC50 = 980 nM (Wang et al. 1998). In ventral tegmental area dopamine neurons, XE-991 blocked M current with an IC50 = 710 nM (Koyama and Appel 2006). In rat dorsal root ganglia cells, XE-991 blocked M current with an IC50 = 260 nM (Passmore et al. 2003). In mammalian nodes of Ranvier, the slow potassium current is blocked by XE-991 (IC50 = 2.2 μM, Schwarz et al. 2006).
We studied putative Kv7 current properties with low concentrations of XE-991 or linopirdine that are selective for slowly activating current. We observed a current sensitive to these agents (or muscarinic agonists) in rat layer II/III pyramidal neurons, indicating that Kv7 subunits contribute to the persistent K+ current in these cells. Since we used near-IC50 concentrations, our percent block data underestimate the contribution of Kv7-mediated current to the overall current.
Properties of putative Kv7-mediated current in neocortical pyramidal cells.
Current sensitive to linopirdine (≤3 μM ) or XE-991 (≤1 μM) was slowly activating and deactivating and essentially noninactivating, similar to Kv7 channels in expression systems and M-type currents in other neuron types (Cooper and Jan 2003; Halliwell and Adams 1982; Hu et al. 2007; Marrion 1997). These properties are also similar to the current remaining in neocortical pyramidal cells after block of Kv1 and Kv2 channels (Guan et al. 2007b).
Activation and deactivation were well fitted with a single exponential in most pyramidal cells, and the kinetics at room temperature were voltage dependent, with τ values of ∼120 ms at −10 mV and ∼20–40 ms at voltages greater than or equal to +10 mV (c.f., Brown and Adams 1980; Wang et al. 1998). Our slice data suggest a Q10 of ∼2.5 for deactivation of M current in layer II/III pyramidal cells. Activation of M currents and Kv7 currents can be fitted with single or double exponentials in different preparations. Our data are similar to those for single τ values reported for neurons at RT by others (Koyama and Appel 2006; Wladyka and Kunze 2006) and similar to the faster τ values in studies reporting biexponential activation/deactivation (e.g., Lawrence et al. 2006; Lerche et al. 2000; Shen et al. 2005; Wang et al. 1998; Wickenden et al. 2001).
In neocortical pyramidal neurons, Kv7 currents were active at approximately −70 mV and more depolarized levels. The steady-state voltage dependence of activation was relatively hyperpolarized (half-activation: −17 mV) compared with that of Kv1 (−3 mV, Guan et al. 2006) or Kv2 channels (+4 mV, Guan et al. 2007a) in these cells. The putative Kv7-mediated current in neocortical pyramidal cells was similar to Kv7.2/Kv7.3 channels in Chinese hamster ovary (CHO) cells or Xenopus oocytes (half-activation = −17.7 mV, Selyanko et al. 2000; −17 mV, Selyanko et al. 2001; −40 mV, Wang et al. 1998) and M current in neurons (−41 mV in medium spiny neurons, Shen et al. 2005; −45 mV in sympathetic ganglion, Wang et al. 1998; −24 mV in nodose ganglion neurons, Wladyka and Kunze 2006). Kv7.3/Kv7.5 channels had a half-activation voltage of −35 mV (CHO cells, Wickenden et al. 2001).
Functional significance.
We investigated the function of Kv7 current with current-clamp recordings in layer II/III pyramidal neurons in cortical slices from adult rats, using sharp intracellular electrodes to avoid washout of intracellular factors. The hyperpolarized activation range and lack of inactivation suggest that Kv7 channels would play critical roles in regulation of subthreshold integration of inputs in neocortical pyramidal neurons. Most layer II/III pyramidal neurons had resting membrane potentials of approximately −80 mV in our current-clamp recordings in slices (see also Guan et al. 2007b; Higgs and Spain 2009) compared with activation of Kv7 currents at approximately −70 mV in our voltage-clamp experiments. Although some cells depolarized in XE-991, overall there was no significant effect of XE-991 on resting membrane potential. Membrane depolarization due to Kv7 channel block has been reported in other cell types with more positive resting membrane potentials (e.g., Aiken et al. 1995; Marrion 1997; Shen et al. 2005; Wladyka and Kunze 2006; Yue and Yaari, 2004).
We found that the putative Kv7 current contributes a large fraction of the outward current at subthreshold voltages. Thus Kv7 channels should be important for regulating threshold and influencing subthreshold integration in pyramidal neurons, as observed in other cell types (Hetka et al. 1999; Hu et al. 2007; Lawrence et al. 2006; Otto et al. 2002; Shen et al. 2005; Wladyka and Kunze, 2006; Yoshida and Alonso 2007; Yue and Yaari 2004). As predicted, our data showed that the XE-991-sensitive (Kv7) current did not shape the somatic AP (c.f., Cooper and Jan 2003) but had significant effects on rheobase and firing rate.
The slow kinetics of putative Kv7-mediated current should cause the conductance to build during a sustained underlying depolarization, facilitating regulation of later ISIs and thus SFA. The Kv7 current appeared to act primarily on an intermediate time scale, longer than a single spike but shorter than the slow phase of SFA, which was also unaffected by XE-991. Our data suggest that Kv7 activation reached steady state within ∼100 ms of depolarization and spiking, consistent with our voltage-clamp data and a Q10 of ∼2.5 for activation/deactivation of the Kv7 current. Experiments using slow current ramps showed that the Kv7 current controlled the voltage response below spike threshold and was necessary for the generation of a theta-frequency subthreshold oscillation (∼7 Hz) that developed slightly below spike threshold in control solution.
The Kv7 current also regulated the onset firing pattern during the ramp stimuli, preventing regular firing below ∼9 Hz. The changes in firing pattern caused by Kv7 may have implications for rate coding by a population of pyramidal neurons. According to some models, irregular firing with minimal synchronization is most ideal for rate coding, because this firing pattern allows the population rate to follow rapid changes in input (Mazurek and Shadlen 2002; Shadlen and Newsome 1998). In this case, Kv7 channels may improve rate coding when the average firing rate is low, by causing a more irregular firing pattern. However, this benefit may be lost at higher rates at which Kv7 allows regular firing.
Our data showed that the Kv7 current regulates firing of pyramidal neurons in response to inputs of relatively long duration, as seen with current steps and ramps. However, the slow activation kinetics of Kv7 channels suggest that responses to higher frequency input components might not be affected. Thus the Kv7 current may make cortical pyramidal neurons more selective for rapid input fluctuations, consistent with previous, largely modeling-based studies of M current in hippocampal pyramidal cells (Prescott et al. 2006; Prescott and Sejnowski 2008). To test this hypothesis, we measured the gain-frequency relationship of layer II/III pyramidal neurons by noise stimulation and correlation analysis (Higgs and Spain 2009). These data quantify the linear modulation of the time-varying spike probability by each frequency component of the noise, providing a measure of the sensitivity to sine wave current of different frequencies in the presence of the applied noise background. We found that, for a given mean firing rate, XE-991 increased gain at frequencies below 10 Hz but had little effect at higher frequencies. Thus the Kv7 current appears to increase the relative sensitivity, or selectivity, for higher frequency components of the synaptic input.
In conclusion, we found that Kv7 currents contribute to outward current in layer II/III pyramidal neurons, have slow activation kinetics, and are activated at more hyperpolarized potentials than other major components of voltage-gated K+ current and subthreshold to AP threshold. As a result, Kv7 channels regulate rheobase, theta-frequency oscillations near threshold voltages, and ISIs during repetitive firing. These effects are most pronounced for low-frequency inputs; thus Kv7 channels serve to bias cell responses toward higher frequency inputs. These findings may partially mediate actions of Kv7 blockers as cognitive enhancers (Aiken et al. 1996; Cook et al. 1990; Schnee and Brown 1998; Wang et al. 1998).
GRANTS
This work was funded by National Institute of Neurological Disorders and Stroke Grant NS044163 (to R. C. Foehring) and a Veterans Affairs Merit Award and Veterans Affairs Epilepsy Center of Excellence (to W. J. Spain).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Tara Toleman for comments on an earlier version of the manuscript.
REFERENCES
- Adams PR, Brown DA, Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurons. J Physiol 330: 537–572, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken SP, Lampe EJ, Murphy PA, Brown BS. Reduction of spike frequency adaptation and blockade of M-current in rat CA1 pyramidal neurons by linopirdine (DuP 996), and neurotransmitter release enhancer. Br J Pharmacol 115: 1163–1168, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken SP, Zaczek R, Brown BS. Pharmacology of the neurotransmitter release enhancer linopirdine (DuP 996), and insights into its mechanism of action. Adv Pharmacol 35: 349–384, 1996 [DOI] [PubMed] [Google Scholar]
- Alaburda A, Perrier JF, Hounsgaard J. An M-like outward current regulates the excitability of spinal motoneurones in the adult turtle. J Physiol 540: 875–881, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neuron. Nature 283: 673–676, 1980 [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Rigas P, Tawara-Hirata Y. Resonance (approximately 10 Hz) of excitatory networks in motor cortex: effects of voltage-dependent ion channel blockers. J Physiol 578: 173–191, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Jan YN, Jan LY. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci USA 103: 8870–8875, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook L, Nickolson VJ, Steinfels GF, Rohrbach KW, DeNoble VJ. Cognition enhancement by the acetylcholine releaser DuP 996. Drug Dev Res 19: 301–314, 1990 [Google Scholar]
- Cooper EC, Aldape KD, Abosch A, Barbaro NM, Berger MS, Peacock WS, Jan LY. Colocalization and coassembly of two brain M-type potassium channel subunits that are mutated in epilepsy. Proc Natl Acad Sci USA 97: 4914–4919, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC, Jan LY. M-channels. Neurological diseases, neuromodulation, and drug development. Arch Neurol 60: 496–500, 2003 [DOI] [PubMed] [Google Scholar]
- Costa AM, Brown BS. Inhibition of M-current in cultured rat superior cervical ganglia by linopirdine: mechanism of action studies. Neuropharmacology 36: 1747–1753, 1997 [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci 24: 1236–1244, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmedyb P, Calloe K, Schmitt N, Hansen RS, Grunnet M, Olesen SP. Modulation of ERG channels by XE991. Basic Clin Pharmacol Toxicol 100: 316–322, 2007 [DOI] [PubMed] [Google Scholar]
- Dodt HU, Zieglgänsberger W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res 537: 333–336, 1990 [DOI] [PubMed] [Google Scholar]
- Geiger J, Weber YG, Landwehrmeyer B, Sommer C, Lerche H. Immunohistochemical analysis of KCNQ3 potassium channels in mouse brain. Neurosci Lett 400: 101–104, 2006 [DOI] [PubMed] [Google Scholar]
- Golomb D, Yue C, Yaari Y. Contribution of persistent Na+ current and M-type K+ current to somatic bursting in CA1 pyramidal cells: combined experimental and modeling study. J Neurophysiol 96: 1912–1926, 2006 [DOI] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol 566: 689–715, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D, Tkatch T, Surmeier DJ, Armstrong WE, Foehring RC. Kv2 subunits underlie slowly inactivating potassium current in rat neocortical pyramidal neurons. J Physiol 581: 941–960, 2007a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D, Lee JCF, Higgs M, Spain WJ, Foehring RC. Functional roles of Kv1 channels in neocortical pyramidal neurons. J Neurophysiol 97: 1931–1940, 2007b [DOI] [PubMed] [Google Scholar]
- Guan D, Lee JCF, Tkatch T, Surmeier DJ, Armstrong WE, Foehring RC. Expression and function of Kv1 channels in supragranular neocortical pyramidal neurons. J Physiol 571: 371–389, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutfreund Y, Yarom Y, Segev I. Subthreshold oscillations and resonant frequency in guinea-pig cortical neurons: physiology and modelling. J Physiol 483: 621–640, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, Covarriubias M, Desir GV, Furuichi K, Ganetzky B, Garcia ML, Grissmer S, Jan LY, Karschin A, Kim D, Kuperschmidt S, Kurachi Y, Lazdunski M, Lesage F, Lester HA, McKinnon D, Nichols CG, O'Kelly I, Robbins J, Robertson GA, Rudy B, Sanguinetti M, Seino S, Stuehmer W, Tamkun MM, Vandenberg CA, Wei A, Wulff H, Wymore RS; International Union of Pharmacology International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev 55: 583–586, 1995 [DOI] [PubMed] [Google Scholar]
- Halliwell JV. M-current in human neocortical neurons. Neurosci Lett 67: 1–6, 1986 [DOI] [PubMed] [Google Scholar]
- Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res 250: 71–92, 1982 [DOI] [PubMed] [Google Scholar]
- Hetka R, Rundfeldt C, Heinemann U, Schmitz D. Retigabine strongly reduces repetitive firing in rat entorhinal cortex. Eur J Pharmacol 386: 165–171, 1999 [DOI] [PubMed] [Google Scholar]
- Higgs MH, Foehring RC, Spain WJ. Conditional burst firing controlled by Kv7 and SK channels in neocortical layer 2/3 pyramidal neurons (Abstract). Soc Neurosci Abstr 33: 587.6, 2007 [Google Scholar]
- Higgs MH, Spain WJ. Conditional bursting enhances resonant firing in neocortical layer 2–3 pyramidal neurons. J Neurosci 29: 1285–1299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region. J Neurosci 27: 1853–1867, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol 545: 783–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen HS, Callo K, Jespersen T, Jensen BS, Olesen SP. The KCNQ5 potassium channel from mouse: a broadly expressed M-current like potassium channel modulated by zinc, pH, and volume changes. Mol Brain Res 139: 52–62, 2005 [DOI] [PubMed] [Google Scholar]
- Jentsch T. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 1: 21–30, 2000 [DOI] [PubMed] [Google Scholar]
- Koyama S, Appel SB. Characterization of M-current in ventral tegmental area dopamine neurons. J Neurophysiol 96: 535–543, 2006 [DOI] [PubMed] [Google Scholar]
- Koyama S, Brodie MS, Appel SB. Ethanol inhibition of M-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons. J Neurophysiol 97: 1977–1985, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas JA, Brown DA. Inhibition of M-type potassium current IK(M) in rat sympathetic neurons by linopirdine (DuP996). Br J Pharmacol 116: 123P, 1995 [Google Scholar]
- Lamas JA, Selyanko AA, Brown DA. Effects of a cognitive enhancer, linopirdine (DuP 996), on M-type potassium currents (IK(M)) and some other voltage-and ligand-gated currents in rat sympathetic neurons. Eur J Neurosci 9: 605–616, 1997 [DOI] [PubMed] [Google Scholar]
- Lampe BW, Brown BS. Electrophysiological effects of DuP996 on hippocampal CA1 neurons (Abstract). Soc Neurosci Abstr 17: 1588, 1991 [Google Scholar]
- Lawrence JJ, Saraga F, Churchill JF, Statland JM, Travis KE, Skinner FK, McBain CJ. Somatodendritic Kv7/KCNQ/M channels control interspike interval in hippocampal interneurons. J Neurosci 26: 12325–12338, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche C, Scherer CR, Seebohm G, Derst C, Wei AD, Busch AE, Steinmeyer K. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J Biol Chem 275: 22395–22400, 2000 [DOI] [PubMed] [Google Scholar]
- Marrion NV. Control of M-current. Annu Rev Physiol 59: 483–504, 1997 [DOI] [PubMed] [Google Scholar]
- Mazurek ME, Shadlen MN. Limits to the temporal fidelity of cortical spike rate signals. Nat Neurosci 5: 463–471, 2002 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Mechanisms of action of acetylcholine in the guinea-pig cerebral cortex in vitro. J Physiol 375: 169–194, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci USA 86: 8098–8102, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SD, Madamba SG, Schweitzer P, Siggins GR. Voltage-dependent effects of opioid peptides on hippocampal CA3 pyramidal neurons in vitro. J Neurosci 14: 809–820, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SD, Madamba SG, Siggins GR. Ethanol diminishes a voltage-dependent K+ current, the M-current, in CA1 hippocampal pyramidal neurons in vitro. Brain Res 516: 222–228, 1990 [DOI] [PubMed] [Google Scholar]
- Nickolson VJ, Tam SW, Meyers MJ, Cook L. DuP 996 (3,3-bis(4-pyrindylmethyl)-1-phenylindolin-2-one) enhances the stimulus-induced release of acetylcholine from rat brain in vitro and in vivo. Drug Dev Res 19: 285–300, 1990 [Google Scholar]
- Noda M, Obana M, Akaike N. Inhibition of M-type K+ current by linopirdine, a neurotransmitter-release enhancer, in NG108–15 cells and rat cerebral neurons in culture. Brain Res 794: 274–280, 1998 [DOI] [PubMed] [Google Scholar]
- Oiu C, Johnson BN, Tallent MK. K+ M-current regulates the transition to seizures in immature and adult hippocampus. Epilepsia 48: 2047–2058, 2007 [DOI] [PubMed] [Google Scholar]
- Otto JF, Kimball MM, Wilcox KS. Effects of the anticonvulsant retigabine on cultured cortical neurons: changes in electroresponsive properties and synaptic transmission. Mol Pharmacol 61: 921–927, 2002 [DOI] [PubMed] [Google Scholar]
- Otto JF, Yang Y, Frankel WN, White HS, Wilcox KS. A spontaneous mutation involving Kcnq2 (Kv7.2) reduces M-current density and spike frequency adaptation in mouse CA1 neurons. J Neurosci 26: 2053–2059, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto JF, Yang Y, Frankel WN, Wilcox KS, White HS. Mice carrying the szt1 mutation exhibit increased seizure susceptibility and altered sensitivity to compounds acting at the m-channel. Epilepsia 45: 1009–1016, 2004 [DOI] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci 26: 2599–2613, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore GM, Selyanko AA, Mistry M, Al-Qatari M, Marsh SJ, Matthews EA, Dickenson AH, Brown TA, Burbidge SA, Main M, Brown DA. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci 23: 7227–7236, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Alavez-Perez N. Epileptiform activity induced by pharmacologic reduction of M.-current in the developing hippocampus in vitro. Epilepsia 47: 47–54, 2006 [DOI] [PubMed] [Google Scholar]
- Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci 8: 51–60, 2005 [DOI] [PubMed] [Google Scholar]
- Prescott SA, Ratte S, De Konnick Y, Sejnowski TJ. Nonlinear interaction between shunting and adaptation controls a switch between integration and coincidence detection in pyramidal neurons. J Neurosci 26: 9084–9097, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, Sejnowski TJ. Spike-rate coding and spike-time coding are affected oppositely by different adaptation mechanisms. J Neurosci 28: 13649–13661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie KJ, Weng T, Correia MJ. Effects of KCNQ channel blockers on K+ currents in vestibular hair cells. Am J Physiol Cell Physiol 280: C473–C480, 2001 [DOI] [PubMed] [Google Scholar]
- Richter A, Sander SE, Rundfeldt C. Antidystonic effects of Kv7 (KCNQ) channel openers in the dt sz mutant, an animal model of primary paroxysmal dystonia. Br J Pharmacol 149: 747–753, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche JP, Westenbroek R, Sorom AJ, Hille B, Mackie K, Shapiro MS. Antibodies and a cysteine-modifying reagent show correspondence of M current in neurons to KCNQ2 and KCNQ3 K+ channels. Br J Pharmacol 137: 1173–1186, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saganich MJ, Machado E, Rudy B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J Neurosci 21: 4609–4624, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee ME, Brown BS. Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons. J Pharmacol Exp Ther 286: 709–717, 1998 [PubMed] [Google Scholar]
- Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem 275: 24089–24095, 2000 [DOI] [PubMed] [Google Scholar]
- Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, Kaji R, Bostock H. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol 573: 17–34, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer P. Cannabinoids decrease the K+ M-current in hippocampal CA1 neurons. J Neurosci 20: 51–58, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyanko AA, Delmas P, Hadley JK, Tatulian L, Wood IC, Mistry M, London B, Brown DA. Dominant-negative subunits reveal potassium channel families that contribute to M-like potassium currents. J Neurosci 22: RC212, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyanko AA, Hadley JK, Brown DA. Properties of single M-type KCNQ2/KCNQ3 potassium channels expressed in mammalian cells. J Physiol 534: 15–24, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyanko AA, Hadley JK, Wood IC, Abogadie FC, Jentsch TJ, Brown DA. Inhibition of KCNQ1–4 potassium channels expressed in mammalian cells via M1 muscarinic acetylcholine receptors. J Physiol 522: 349–355, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci 18: 3870–3896, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Mistry M, Marsh SJ, Brown DA, Delmas P. Molecular correlates of the M-current in cultured rat hippocampal neurons. J Physiol 544: 29–37, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MS, Roche JP, Kaftan El Cruzblanca H, Mackie K, Hille B. Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3 K+ channels that underlie the neuronal M-current. J Neurosci 20: 1710–1721, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci 25: 7449–7458, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol 409: 171–190, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res 83: 161–187, 1990 [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Arch 423: 511–518, 1993 [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory Data Analysis. Reading, PA: Addison-Wesley, 1977 [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282: 1890–1893, 1998 [DOI] [PubMed] [Google Scholar]
- Wang Z, McCormick DA. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S,3R-ACPD. J Neurosci 13: 2199–2216, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber YG, Geiger J, Kämpchen K, Landwehrmeyer B, Sommer C, Lerche H. Immunohistochemical analysis of KCNQ2 potassium channels in adult and developing mouse brain. Brain Res 1077: 1–6, 2006 [DOI] [PubMed] [Google Scholar]
- Wickenden AD, Zou A, Wagoner PK, Jegla T. Characterization of KCNQ5/Q3 potassium channels expressed in mammalian cells. Br J Pharmacol 132: 381–384, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wladyka CL, Kunze DL. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J Physiol 575: 175–189, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung SYM, Greenwood IA. Electrophysiological and functional effects of the KCNQ channel blocker XE991 on murine portal vein smooth muscle cells. Br J Pharmacol 146: 585–595, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Alonso A. Cell-type-specific modulation of intrinsic firing properties and subthreshold membrane oscillations by the M(Kv7)-current in neurons of the entorhinal cortex. J Neurophysiol 98: 2779–27994, 2007 [DOI] [PubMed] [Google Scholar]
- Yue C, Yaari Y. Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells. J Neurophysiol 95: 3480–3495, 2006 [DOI] [PubMed] [Google Scholar]
- Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci 24: 4614–4624, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yus-Najera E, Munoz A, Salvador N, Jensen BS, Rasmussen HB, Defelipe J, Villarroel A. Localization of KCNQ5 in the normal and epileptic human temporal neocortex and hippocampal formation. Neuroscience 120: 353–364, 2003 [DOI] [PubMed] [Google Scholar]
- Zaczek R, Chorvat RJ, Saye JA, Pierdomenico ME, Maciag CM, Logue AR, Fisher BN, Rominger DH, Earl RA. Two new potent neurotransmitter release enhancers, 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone and 10,10-bis(2-fluoro-4-pyridinylmethyl)-9(10H)-anthracenone: comparison to linopirdine. J Pharmacol Exp Ther 285: 724–730, 1998 [PubMed] [Google Scholar]