Abstract
Approximately 20% of the adult population suffers from migraine. This debilitating pain disorder is three times more prevalent in women than in men. To begin to evaluate the underlying mechanisms that may contribute to this sex difference, we tested the hypothesis that there is a sex difference in the inflammatory mediator (IM)-induced sensitization of dural afferents. Acutely dissociated retrogradely labeled dural afferents from adult Sprague-Dawley rats were examined with whole cell patch-clamp recordings. Baseline passive and active electrophysiological properties of dural afferents from both sexes were comparable. However, while IM-induced increases in the excitability of dural afferents from male and female rats were also comparable, the proportion of dural afferents from female rats sensitized by IM (∼100%) was significantly greater than that of dural afferents from male rats (∼50%). This appeared to be due to differences downstream of IM receptors, as tetrodotoxin-resistant sodium current was increased by IM in a majority of male dural afferents (13/14). These data indicate that there are both quantitative and qualitative differences in the IM-induced sensitization of dural afferents that may contribute to the sex difference in the manifestation of migraine.
Keywords: trigeminal ganglion neuron, current clamp, primary afferent, chloride current, pain
conservative estimates indicate that over 20% of the adult population suffers from migraine. Interestingly, this debilitating pain disorder is far more common in women than in men, with a prevalence roughly three times higher in women than in men [18% vs. 6% (Stewart et al. 1992)] and an incidence more than two times higher [43% vs. 18% (Stewart et al. 2008)]. The underlying mechanisms of this sex difference have yet to be identified.
While there is still debate over the mechanisms underlying the initiation of a migraine attack, compelling evidence indicates that activation of primary afferents innervating the dural vasculature mediates migraine pain. Electrical stimulation of the dura and associated vasculature is associated with migraine-like pain (Wolff 1946). The majority of dural afferents are nociceptive in that they are sensitized by inflammatory mediators (IM) and are activated by algogenic substances (Levy and Strassman 2002; Oshinsky and Luo 2006; Strassman et al. 1996). A majority of these afferents express serotonin type 1D receptor (5-HT1DR) (Harriott and Gold 2008; Longmore et al. 1997), a primary target for triptans, one of the most effective classes of abortive medications for migraine. Afferents innervating the dura may also contain the neuropeptide calcitonin gene-related peptide (CGRP) (Keller and Marfurt 1991; McIlvried et al. 2010; O'Connor and van der Kooy 1988), a transmitter implicated in migraine pain based on its appearance in the jugular venous blood during the ictal phase of migraine (Moreno et al. 2002; Sarchielli et al. 2000) and the apparent efficacy of CGRP receptor antagonists for the treatment of migraine (Villalon and Olesen 2009). Importantly, the sensitization and subsequent activation of dural afferents is thought to be responsible not only for the initiation of migraine pain but for driving the central sensitization that serves to amplify afferent input as well as mediate the emergence of allodynia in cutaneous dermatomes (Burstein et al. 2004; Oshinsky and Luo 2006).
On the basis of the compelling evidence in support of a role for dural afferents in migraine, we hypothesized that the sex difference in the manifestation of migraine may be due, at least in part, to differences in the passive and active electrophysiological properties of dural afferents and/or their response to IM. To begin to test this hypothesis, whole cell patch-clamp techniques were used to study retrogradely labeled dural afferents from male and female rats. Results indicate that the magnitude of excitability once sensitized by IM is comparable in both sexes. However, sex differences were detected in the proportion of neurons sensitized by IM and in the underlying mechanisms of sensitization.
MATERIALS AND METHODS
Intact adult female and male Sprague-Dawley rats (Harlan, 200–290 g) were used for all experiments. Animals were housed one per cage in a temperature- and humidity-controlled animal facility on a 12:12-h light-dark schedule with food and water freely available. Prior to all procedures, animals were deeply anesthetized with an intraperitoneal injection containing ketamine (55 mg/kg), xylazine (5.5 mg/kg), and acepromazine (1.1 mg/kg). All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with National Institutes of Health guidelines for the use of laboratory animals in research.
Afferents innervating the dura were identified after application of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) to the dura as previously described (Harriott and Gold 2008). After at least 10 days post afferent labeling, the trigeminal ganglia from intact male and female rats were removed bilaterally, enzymatically treated, and mechanically dissociated as previously described (Harriott et al. 2006). Intact adult male and female rats were used in the present study based on the recommendations detailed in the consensus statement on the study of sex differences in pain and analgesia (Greenspan et al. 2007) in which the authors weighed the strengths and weaknesses of initiating such a study with intact animals versus one in which gonadal hormones were surgically or chemically manipulated. In light of further recommendations in the same document, and our previous results with neurons from intact female rats, in which ∼100% of dural afferents were sensitized by IM (Harriott and Gold 2009), we did not attempt to determine the stage of the female rats studied in their estrous cycle. Neurons were studied within 8 h after plating.
Electrophysiology: voltage and current clamp.
All whole cell patch-clamp experiments were carried out with a HEKA EPC9 amplifier (HEKA Elektronik, Lambrecht/Rhineland-Pfalz, Germany). Glass electrodes (1–4 MΩ) were filled with (in mM) 110 K-methanesulfonate, 30 KCl, 5 NaCl, 1 CaCl2, 2 MgCl2, 10 HEPES, 11 EGTA, 2 Mg-ATP, and 1 Li-GTP, and the pH was adjusted with Tris-base to 7.2. Osmolality was adjusted to 320 mosmol/kgH2O with sucrose. The bath solution contained (in mM) 3 KCl, 130 NaCl, 2.5 CaCl2, 0.6 MgCl2, 10 HEPES, and 10 glucose. The pH was adjusted with Tris-base to 7.4, and osmolality was adjusted with sucrose to 320 mosmol/kgH2O.
IM-induced changes in excitability were assessed in current-clamp mode by five distinct measures: the emergence of spontaneous activity, action potential (AP) threshold, rheobase, current threshold, and accommodation. Spontaneous activity was assessed at resting membrane potential (Vrest) for 30 s before and up to 90 s after the application of IM. The second two measures were determined with a 750-ms depolarizing square-pulse current injection. AP threshold was defined as the greatest depolarization reached before spike generation in response to depolarizing current injections. Rheobase was defined as the smallest amount of current needed to evoke a single AP. Because rheobase is positively correlated with cell size, values were normalized with respect to membrane capacitance to facilitate comparisons between neurons. A ramp and hold protocol consisting of a 250-ms ramp followed by 500-ms sustained current injection was used to monitor the impact of IM on accommodation. Accommodation was determined by counting the number of APs evoked during this protocol. The magnitude of current injection was adjusted so as to evoke an AP during the ramp phase of the stimulation protocol. This protocol was then used to stimulate neurons every 30 s before and after application of IM, where at least three stimuli were used to establish the stability of neuronal excitability prior to the application of IM. IM were applied for 90 s in the majority of experiments based on our previous results (Gold et al. 1996a) as well as those of others (Hargreaves et al. 1994; Momin and McNaughton 2009) indicating that the effects of a wide variety of IM on isolated neurons are rapid, with peak responses detected in milliseconds to seconds. To assess the presence of more slowly developing changes in excitability, IM were applied for up to 10 min to a subpopulation of dural afferents from male rats. As no spontaneous activity was detected in any neuron studied, a neuron was considered “sensitized” if the application of IM resulted in a significant increase in the number of APs evoked during the ramp and hold protocol, relative to baseline. To facilitate comparisons between neurons, IM-induced changes were analyzed as a percent change from baseline.
Passive and active electrophysiological properties were assessed for each neuron studied. Passive properties included input resistance (RI), capacitance, and Vrest. After whole cell access was gained, RI and capacitance were determined in voltage-clamp mode with amplifier circuitry. RI was determined before and 90 s after application of IM and/or niflumic acid (NFA). Active electrophysiological properties consisted of parameters describing the AP waveform and included magnitude of AP overshoot, AP duration, magnitude of afterhyperpolarization (AHP), and AHP decay. Single APs were evoked with a 4-ms depolarizing current injection. The magnitude of AP overshoot was measured from 0 mV to the peak depolarization. AP duration was measured at 0 mV. The magnitude of AHP was measured from the Vrest to the maximum point of hyperpolarization. The decay of the AHP was estimated with a single exponential function fitted to the decay phase of the waveform.
The electrode solution used to record Na+ currents in relative isolation was composed of (in mM) 100 cesium methanesulfonate (CsMs), 5 NaCl, 40 TEA-Cl, 1 CaCl2, 2 MgCl2, 11 EGTA, 10 HEPES, 2 ATP-Mg, and 1 GTP-Li. The bath solution was composed of (in mM) 35 NaCl, 65 choline-Cl, 30 TEA-Cl, 0.1 CaCl2, 5 MgCl2, 10 HEPES, and 10 glucose. Voltage-clamp protocols were used to assess the impact of IM on the tetrodotoxin-resistant (TTX-R) Na+ current. A steady-state inactivation protocol consisting of 500-ms by 5-mV voltage steps from −110 mV was used to determine the prepulse potential to inactivate fast-inactivating tetrodotoxin-sensitive (TTX-S) Na+ current, leaving slowly activating TTX-R Na+ current in relative isolation. A voltage protocol was used to isolate TTX-R from TTX-S, to facilitate the assessment of changes in both currents in the same neuron. Importantly, we have previously demonstrated that the currents isolated with voltage steps are identical to those isolated with TTX (Gold et al. 2002). The impact of IM on Na+ current was monitored by evoking Na+ current with a step to 0 mV every 5s. IM was applied after confirmation of the stability of evoked current. A neuron was considered responsive to IM if peak current was increased by 20% of baseline.
Drugs.
All salts and test compounds were obtained from Sigma-Aldrich (St. Louis, MO). The IM solution consisted of bradykinin (10 μM), histamine (1 μM), and prostaglandin (PG)E2 (1 μM). Bradykinin was dissolved in 1% acetic acid, PGE2 was dissolved in 100% EtOH, and histamine was dissolved in water at stock concentrations of 100 mM, 100 mM, and 10 mM, respectively; stocks were divided into aliquots and stored at −20°C until the day of use. A normal bath solution containing final concentrations of 0.01% EtOH and 0.001% acetic acid was used as the vehicle control. NFA was dissolved in 100% EtOH, which was then diluted to 0.20%, yielding a final NFA concentration of 10 μM. All drugs were administered through a gravity-fed perfusion system.
Data analysis.
Data were analyzed with PulseFit (HEKA), SigmaPlot, and Prism software. Differences in baseline, rheobase, and threshold between neurons from males and females were compared with an unpaired Student's t-test. Changes in excitability and passive and active properties, before and after IM, were determined with a paired t-test. Data are expressed as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
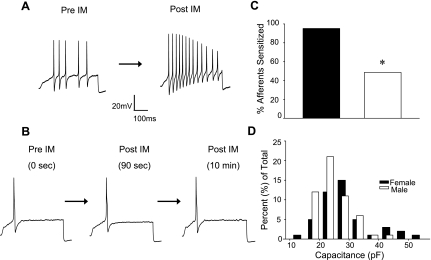
Data were collected from 120 dural afferents acutely dissociated from 38 (16 female, 22 male) Sprague-Dawley rats. The size distribution of these neurons was similar to that of our previous study (Vaughn and Gold 2010), with a median cell body capacitance of 24.91 pF (with 20.06 pF and 27.79 pF as 25th and 75th percentiles, respectively). The size of dural afferents from male and female rats was comparable (P > 0.05) (Fig. 1).
Fig. 1.
Sex difference in the proportion of dural afferents sensitized by inflammatory mediators (IM). A: action potentials evoked from a representative dural afferent from a female rat with a ramp and hold current injection protocol before (Pre-IM) and 90 s after (Post-IM) IM application. Resting membrane potential was −66 mV before IM application and −53 mV after IM. B: voltage traces evoked as in A from a dural afferent from a male rat unresponsive to IM application. Resting membrane potential was −68 mV before, −67 mV after 90-s IM application, and −68 mV after 10-min IM application. C: the proportion (20/21) of female dural afferents sensitized by IM was significantly (*P < 0.05) greater than that (19/37) of male afferents. D: frequency distribution of membrane capacitance, used as a measure of cell body size, in the total population of dural afferents studied.
Passive and active electrophysiological properties.
Conventional whole cell patch configuration was used for the initial characterization of passive and active electrophysiological properties. These data were collected from dural afferents acutely dissociated from 12 female and 14 male rats. There were no significant differences between dural afferents from male and female rats with respect to baseline passive or active electrophysiological properties or baseline excitability with the whole cell patch-clamp technique (Table 1).
Table 1.
IM-induced changes in passive and active electrophysiological properties of dural afferents
| Capacitance, pF | Vrest, mV | RI, MΩ | Magnitude of AHP, mV | τ of Decay, ms | AP Overshoot, mV | AP Duration, ms | |
|---|---|---|---|---|---|---|---|
| Female (n = 37) | |||||||
| Pre-IM | 25.12 ± 7.61 | −65 ± 5.22 | 589 ± 438.04 | −15.59 ± 3.93 | 45.66 ± 17.15 | 41.07 ± 12.15 | 3.18 ± 0.99 |
| Post-IM | −57 ± 7.85† | 416 ± 272.86* | −16.55 ± 3.36 | 38.59 ± 12.36 | 48.41 ± 4.52† | 3.58 ± 0.81 | |
| Male (n = 39) | |||||||
| Pre-IM | 22.07 ± 4.62 | −69 ± 8.06 | 712 ± 606.81 | −15.14 ± 3.73 | 66.74 ± 27.23 | 41.97 ± 14.58 | 3.36 ± 1.47 |
| Post-IM | −65 ± 8.62† | 477 ± 353.38* | −19.14 ± 7.87* | 47.63 ± 23.47 | 49.8 ± 9.17† | 4.18 ± 2.43* |
Values are expressed as means ± SE Application of inflammatory mediators (IM) resulted in significant changes in passive and active electrophysiological properties of dural afferents from both males and females. Pre-IM, values obtained prior to application of IM; Post-IM, values obtained after the application of IM; Vrest, resting membrane potential; RI, input resistance; AHP, afterhyperpolarization; τ, time constant; AP, action potential. Significant difference between Pre-IM and Post-IM:
P < 0.05,
P < 0.01.
Males versus females: difference in the response to inflammatory mediators.
To determine whether there is a sex difference in the magnitude of IM-induced sensitization (see materials and methods for definition) of dural afferents, excitability was assessed before and after application of an IM solution containing PGE2, bradykinin, and histamine in 21 female and 37 male dural afferents. Neither male nor female afferents displayed spontaneous activity in the absence or presence of IM. Consistent with previous observations, almost all dural afferents (20/21) from females were sensitized after the application of IM. In contrast, nearly half of the dural afferents (18/37) from males demonstrated no change in excitability following 90-s IM application and thus were considered unresponsive (Fig. 1). The difference in proportion of dural afferents from male and female rats sensitized by IM was significant (P < 0.01, Fisher's exact test). To determine whether the smaller fraction of dural afferents from male rats sensitized by IM could be due to the presence of slower second messenger signaling processes, a subgroup (17) of the afferents from male rats were studied with a 10-min IM application. An increase in excitability was detected in seven of these neurons, and this increase was detectable within 90 s. In the remaining 10 neurons, no change in excitability was detected throughout the 10-min IM application period.
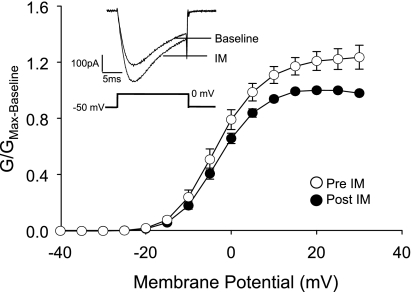
To begin to assess the basis for the sex difference in the proportion of dural afferents sensitized by IM, we assessed the impact of IM on TTX-R voltage-gated Na+ currents (INa) in male dural afferents. This is a stable current shown to be a common target for the second messenger pathways initiated by an array of IM (Gold 1999) in a variety of different afferents including dural afferents (Vaughn and Gold 2010) and therefore serves as a sensitive bioassay for the presence of IM-induced receptor activation in primary afferents. TTX-R INa was measured before and after application of IM to 14 dural afferents from 3 male rats. Thirteen of these demonstrated an IM-induced increase in TTX-R INa, indicating that functional IM receptors are present on a larger percentage of male dural afferents than were sensitized by the same combination of mediators (P = 0.01) (Fig. 2). Consistent with our previous results from female rats (Vaughn and Gold 2010), we detected no significant influence of IM on TTX-S currents in the same group of neurons: pre- and post-IM maximal conductance (Gmax) were 9.33 ± 2.52 nS and 8.26 ± 1.63 nS, respectively (P > 0.05).
Fig. 2.
IM increase tetrodotoxin-resistant voltage-gated Na+ current (TTX-R INa) in dural afferents from male rats. Na+ current was evoked in dural afferents before (Pre-IM) and after (Post-IM) IM application. A voltage protocol was used to isolate TTX-R INa from tetrodotoxin-sensitive (TTX-S) INa as described in materials and methods. A significant increase in current was detected in 13 of 14 afferents studied and was associated with a ∼25% increase in conductance (G). Conductance-voltage (G-V) data from each neuron tested were normalized to the maximal conductance determined before IM application (Gmax-Baseline) and pooled. Data points between −60 and −45 mV are omitted to facilitate comparisons between Pre- and Post-IM. Pooled data are fitted with a Boltzmann equation with values for potential for half-maximal activation (V0.5) of −2.9 ± 0.3 and −5.8 ± 1.3 and slope of 4.5 ± 0.6 and 3.8 ± 0.7 for Pre- and Post-IM, respectively. Inset, typical TTX-R INa evoked in dural afferents from male rats before (Baseline) and 90 s after (IM) the IM application.
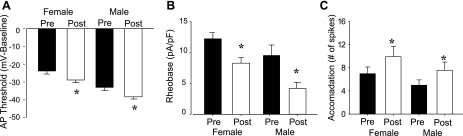
Of the dural afferents sensitized by IM, the magnitude of change in all measures of excitability was comparable in males and females (Fig. 3). Interestingly, there were both similarities and differences between male and female dural afferents with respect to the changes in passive and active electrophysiological properties associated with the increase in excitability. A significant (P < 0.01) IM-induced decrease in RI was detected in both groups of responsive neurons. This was associated with a significant (P < 0.01) depolarization of the Vrest. An increase in the AP overshoot was also observed in afferents from both males and females. The magnitude of all three changes was comparable. In contrast, significant increases in AP duration and AHP magnitude were only observed for male dural afferents (Table 1).
Fig. 3.
The magnitude of changes in excitability associated with IM-induced sensitization was comparable in sensitized dural afferents from males and females. As no spontaneous activity was detected in response to IM application, parameters used to quantify changes in excitability included action potential (AP) threshold (A), rheobase (B), and the number of APs evoked during the ramp and hold stimulation protocol (C). Data assessed before (Pre-IM) and after (Post-IM) IM application to dural afferents from males (n = 19) and females (n = 20) were pooled and plotted as means ± SE. There was no difference between male and female dural afferents with respect to either baseline excitability or the IM-induced increase in excitability. All IM-induced changes in excitability were statistically significant (*P < 0.05, Fisher's post hoc after 2-way repeated-measures ANOVA).
Role of IIM-Cl in IM-induced changes in passive and active electrophysiological properties.
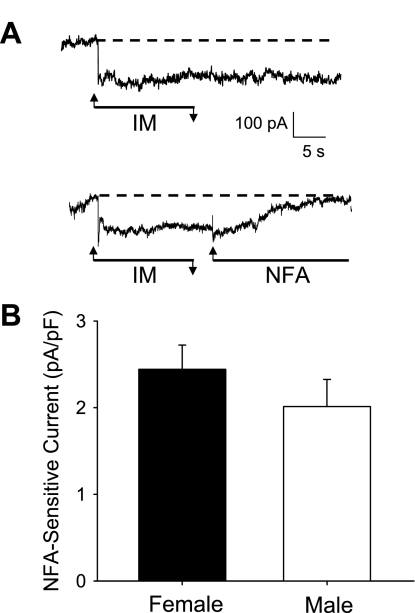
NFA, originally identified and subsequently used clinically as an inhibitor of COX-2, is commonly used experimentally as a nonselective Cl− channel blocker (Danko et al. 2011; Forrest et al. 2010; Sagheddu et al. 2010). There are at least some data to suggest NFA has efficacy for the treatment of migraine (1975 U.S. Patent 4,024,279). We have previously demonstrated that the IM-induced activation of a NFA-sensitive Cl− current (IIM-Cl) plays a dominant role in the sensitization of female dural afferents (Vaughn and Gold 2010). Consistent with the suggestion that IIM-Cl plays a critical role in the sensitization of male dural afferents, there was no decrease in RI in neurons from male rats that did not demonstrate an IM-induced increase in excitability. Furthermore, IIM-Cl activation appeared comparable in sensitized male and female dural afferents based on the magnitude of the IM-induced changes in RI and Vrest (Table 1). Nevertheless, to confirm this prediction, we assessed the magnitude of the NFA-sensitive current in sensitized male and female dural afferents from six female and four male rats. The NFA-sensitive current in male and female dural afferents (2.01 ± 0.89 pA/pF and 2.44 ± 0.97 pA/pF, respectively) was comparable between groups (P > 0.05; Fig. 4).
Fig. 4.
Magnitude of niflumic acid (NFA)-sensitive Cl− current (IIM-Cl) was comparable in sensitized dural afferents from male and female rats. A: IM application resulted in a persistent increase in inward current (top). The current was completely blocked after application of NFA (10 μM; bottom) in dural afferents from both males and females. B: IIM-Cl was assessed from a holding potential of −60 mV, as illustrated in A, and was quantified by assessing the magnitude of the current blocked by NFA in 12 and 16 dural afferents from males and females. Current was normalized to capacitance to minimize the impact of cell size. There is no significant difference in the magnitude of NFA-current activated between the sexes (P > 0.05).
DISCUSSION
The purpose of this study was to begin to test the hypothesis that the sex difference in the manifestation of migraine is due to differences in the baseline excitability of dural afferents and/or the influence of IM on dural afferent excitability. While we observed no difference between afferents from male and female rats with respect to baseline excitability or the magnitude of IM-induced sensitization of dural afferents, our results suggest at least two major differences that may contribute to the sex difference in the manifestation of migraine. First, a significantly greater proportion of dural afferents (20/21) from female rats were sensitized after IM application than in males (19/37). This difference in the proportion of IM-sensitized neurons was present despite the observation that IM-induced activity was detectable in the majority (13/14) of dural afferents from male rats, as indicated by an IM-induced increase in TTX-R INa. Second, changes in the active electrophysiological properties of afferents sensitized by IM were different in males and females, suggesting that the mechanisms underlying IM-induced sensitization of male dural afferents are different from those underlying sensitization of female dural afferents.
There are at least five major features of our experimental design that impact on the conclusions drawn from the data. First, we utilized the acutely dissociated sensory neuron as a model for the terminals of dural afferents in vivo. This necessarily involves injuring the afferents studied, which may influence the subsequent response to IM (Gold et al. 1998). Dissociation also disrupts the normal anatomic constraints and relative distribution of proteins that may influence the response to IM and/or excitability. Second, we assessed the impact of acute administration of a combination of IM rather than a single compound and/or manipulations performed in vivo. A combination of IM was used to facilitate comparisons between the results of the present study and our previous results (Harriott and Gold 2009; Vaughn and Gold 2010) as well as those of other investigators (Edelmayer et al. 2009; Oshinsky and Luo 2006; Strassman et al. 1996). More importantly, unlike other pain syndromes, which may be associated with persistent changes in the affected tissue and/or the nervous system, migraine is characterized by transient attacks that are associated with increases in a combination of IM (Levy et al. 2007; Sarchielli et al. 2000). However, despite the pronounced impact of the combination employed, a sex difference in the proportion of neurons sensitized may have been due to the specific combination of mediators employed. Third, and related to the previous issue, IM were applied acutely, and while more slowly developing processes such as those that depend on changes in gene expression may contribute to the manifestation of migraine, acute application of mediators precluded the engagement of such processes. Fourth, the use of depolarizing current injection to assess changes in excitability precluded our ability to detect changes mediated by transduction mechanisms. Finally, we studied dural afferents from rats. While several animal models of migraine have recently been developed, it has yet to be determined whether there is a sex difference in the behavioral changes associated with these models. Nevertheless, as with the use of rats in the present study, the use of such preclinical models of migraine is predicated on the assumption that even nonmigraineurs could become migraineurs under the right conditions.
There are at least three explanations for the absence of a detectable IM-induced increase in the excitability of roughly half of the male dural afferents: 1) male afferents lack functional receptors for the mediators employed, 2) the wrong combination of mediators was employed, and 3) there are differences in downstream mechanisms of excitability. Our data argue against the absence of functional receptors for the mediators employed based on the significant increase in TTX-R INa in 13 of 14 male dural afferents. Similarly, while a different combination of mediators might have sensitized a larger proportion of male dural afferents, the failure to detect an increase in excitability despite the increase in TTX-R INa in the majority of these afferents argues that the combination of mediators used was not the primary reason for the observation. Thus we suggest that the third possibility is the most likely to account for the absence of a detectable increase in excitability in half of the male dural afferents. Consistent with this suggestion, there is evidence for sex differences in the second messenger pathways underlying inflammatory hypersensitivity, which could influence the pattern of ion channel changes and subsequently the changes in excitability of dural afferents (Dina et al. 2003; Levine et al. 2001). For example, an IM-induced increase in K+ conductance may have masked the impact of an increase in TTX-R INa in unresponsive male dural afferents. We and others have described an IM-induced increase in intracellular Ca2+ in dural afferents as well as evidence of several different Ca2+-modulated/dependent K+ currents in sensory neurons (Gold et al. 1996b; Vaughn and Gold 2010; Zhang et al. 2010), suggesting that a substrate for such an increase in K+ current is present in dural afferents. Given the absence of a detectable decrease in RI and/or depolarization of membrane potential in unresponsive neurons, a more likely explanation for the absence of sensitization of a subpopulation of male dural afferents is a lack of IIM-Cl activation in these neurons, underscoring the importance of this conductance to the IM-induced sensitization of dural afferents.
Features of the IM-induced changes in passive and active electrophysiological properties reported here point to specific ion channels as underlying the increase in dural afferent excitability. Sensitization of female dural afferents was associated with an increase in AP overshoot, a decrease in RI, and depolarization of Vrest. These changes are consistent with both an increase in TTX-R INa and the activation of IIM-Cl as we have described previously in female rats (Vaughn and Gold 2010). Both currents appear to contribute to the sensitization of male dural afferents. Interestingly, the magnitude of IIM-Cl was also comparable in dural afferents from males and females sensitized by IM, supporting the suggestion that IIM-Cl is a common target that could have efficacy for the treatment of migraine in males and females. However, in male afferents, there was also an increase in AHP and AP duration. An increase in AHP magnitude is generally reflective of a relative increase in K+ conductance during the falling phase of the AP. However, such an increase in K+ conductance should also result in a decrease in AP duration, while the opposite was observed in the present study. Because Ca2+ influx via voltage-gated Ca2+ channels also contributes to the AP duration (Blair and Bean 2002), an explanation for these apparently discrepant changes in AP waveform would be an IM-induced increase in Ca2+ influx that subsequently results in the increased activation of a Ca2+-dependent K+ current. Such an explanation would be inconsistent with previous data indicating that PGE2 results in the suppression of Ca2+ current (ICa) in trigeminal sensory neurons (Borgland et al. 2002) and in marked contrast to our previous description of IM-induced changes in ICa and Ca2+-dependent K+ currents in female dural afferents (Vaughn and Gold 2010). Nevertheless, there is evidence, albeit from avian sensory neurons, suggesting that PGE2 can increase ICa (Nicol et al. 1992), raising the possibility that the underlying mechanisms of dural afferent sensitization in males and females are truly distinct.
The epidemiology of migraine, with onset at menarche and resolution with menopause, suggests that gonadal hormones contribute significantly to the prevalence of migraine in women. Interestingly, a growing body of evidence suggests that it is not just the levels of gonadal hormones that are the problem, but fluctuations in hormone levels, in particular the drop in 17β-estradiol that occurs with ovulation that may be the most effective trigger for migraine (Shuster et al. 2011). These clinical data raise the intriguing possibility that acute deprivation of estrogens associated with the harvest and dissociation of dural afferents from female rats may contribute to the higher proportion of afferents sensitized by IM from females compared with males. It is also tempting to suggest that such a mechanism contributes to the sex difference in the manifestation of migraine given evidence that gonadal hormones, in particular estrogens, can directly influence the properties [i.e., L-type Ca2+ channels (Mermelstein et al. 1996)] and/or expression pattern [i.e., splice variants of the BK Ca2+-modulated K+ channels (Poulsen et al. 2009)] of ion channels that influence neuronal excitability. However, increasing awareness of childhood migraine (Bigal and Arruda 2010) and the persistence of migraine in a significant proportion of postmenopausal women (MacGregor 2009) suggests that other mechanisms are also likely to contribute to the sex difference in migraine. Whether the sex differences observed in the present study are due to genetics (SRY, X-inactivation) or organizational and/or activational influences of gonadal hormones has yet to be determined (Arnold and Chen 2009; Huang et al. 2008). Nevertheless, the fact that we have identified a sex difference in intact animals provides compelling support for subsequent mechanistic analysis.
In summary, we have identified a sex difference in the proportion of afferents sensitized by IM as well as potential differences underlying mechanisms of excitability. Further exploration into the underlying mechanisms of excitability in responsive neurons will be necessary to more clearly define the role of IIM-Cl in vivo and to identify the basis for observed sex differences. If differences in the relative contribution of different ion channels in dural afferents from males and females are substantiated, the difference suggests that it may ultimately be possible, if not necessary, to develop different approaches for the treatment of migraine tailored specifically to men and women.
GRANTS
This work was supported by research funds from the Department of Anesthesiology at the University of Pittsburgh, as well as National Institute of Dental and Craniofacial Research Grant 1R01-DE-018252 (M. S. Gold).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Drs. Gebhart and Albers for the valuable input during the preparation of this manuscript.
REFERENCES
- Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 30: 1–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Arruda MA. Migraine in the pediatric population—evolving concepts. Headache 50: 1130–1143, 2010 [DOI] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci 22: 10277–10290, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Ryan RM, Ball HJ, Christie MJ. Prostaglandin E2 inhibits calcium current in two sub-populations of acutely isolated mouse trigeminal sensory neurons. J Physiol 539: 433–444, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol 55: 19–26, 2004 [DOI] [PubMed] [Google Scholar]
- Danko T, Hargitai D, Pataki A, Hakim H, Molnar M, Zsembery A. Extracellular alkalinization stimulates calcium-activated chloride conductance in cystic fibrosis human airway epithelial cells. Cell Physiol Biochem 27: 401–410, 2011 [DOI] [PubMed] [Google Scholar]
- Dina OA, McCarter GC, de Coupade C, Levine JD. Role of the sensory neuron cytoskeleton in second messenger signaling for inflammatory pain. Neuron 39: 613–624, 2003 [DOI] [PubMed] [Google Scholar]
- Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol 65: 184–193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest AS, Angermann JE, Raghunathan R, Lachendro C, Greenwood IA, Leblanc N. Intricate interaction between store-operated calcium entry and calcium-activated chloride channels in pulmonary artery smooth muscle cells. Adv Exp Med Biol 661: 31–55, 2010 [DOI] [PubMed] [Google Scholar]
- Gold MS. Tetrodotoxin-resistant Na+ currents and inflammatory hyperalgesia. Proc Natl Acad Sci USA 96: 7645–7649, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Hampl KF, Drasner K, Levine JD. Lidocaine toxicity in primary afferent neurons from the rat. J Pharmacol Exp Ther 285: 413–421, 1998 [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci USA 93: 1108–1112, 1996a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Role of a Ca2+-dependent slow afterhyperpolarization in prostaglandin E2-induced sensitization of cultured rat sensory neurons. Neurosci Lett 205: 161–164, 1996b [DOI] [PubMed] [Google Scholar]
- Gold MS, Zhang L, Wrigley DL, Traub RJ. Prostaglandin E2 modulates TTX-R INa in rat colonic sensory neurons. J Neurophysiol 88: 1512–1522, 2002 [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 132, Suppl 1: S26–S45, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves KM, Swift JQ, Roszkowski MT, Bowles W, Garry MG, Jackson DL. Pharmacology of peripheral neuropeptide and inflammatory mediator release. Oral Surg Oral Med Oral Pathol 78: 503–510, 1994 [DOI] [PubMed] [Google Scholar]
- Harriott AM, Dessem D, Gold MS. Inflammation increases the excitability of masseter muscle afferents. Neuroscience 141: 433–442, 2006 [DOI] [PubMed] [Google Scholar]
- Harriott AM, Gold MS. Electrophysiological properties of dural afferents in the absence and presence of inflammatory mediators. J Neurophysiol 101: 3126–3134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harriott AM, Gold MS. Serotonin type 1D receptors (5HTR) are differentially distributed in nerve fibres innervating craniofacial tissues. Cephalalgia 28: 933–944, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Acosta-Martinez M, Levine JE. Ovarian steroids stimulate adenosine triphosphate-sensitive potassium (KATP) channel subunit gene expression and confer responsiveness of the gonadotropin-releasing hormone pulse generator to KATP channel modulation. Endocrinology 149: 2423–2432, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JT, Marfurt CF. Peptidergic and serotoninergic innervation of the rat dura mater. J Comp Neurol 309: 515–534, 1991 [DOI] [PubMed] [Google Scholar]
- Levine JE, Chappell PE, Schneider JS, Sleiter NC, Szabo M. Progesterone receptors as neuroendocrine integrators. Front Neuroendocrinol 22: 69–106, 2001 [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain 130: 166–176, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Strassman AM. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J Neurophysiol 88: 3021–3031, 2002 [DOI] [PubMed] [Google Scholar]
- Longmore J, Shaw D, Smith D, Hopkins R, McAllister G, Pickard JD, Sirinathsinghji DJ, Butler AJ, Hill RG. Differential distribution of 5HT1D- and 5HT1B-immunoreactivity within the human trigemino-cerebrovascular system: implications for the discovery of new antimigraine drugs. Cephalalgia 17: 833–842, 1997 [DOI] [PubMed] [Google Scholar]
- MacGregor EA. Migraine headache in perimenopausal and menopausal women. Curr Pain Headache Rep 13: 399–403, 2009 [DOI] [PubMed] [Google Scholar]
- McIlvried LA, Albers K, Gold MS. Distribution of artemin and GFRalpha3 labeled nerve fibers in the dura mater of rat: artemin and GFRalpha3 in the dura. Headache 50: 442–450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci 16: 595–604, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momin A, McNaughton PA. Regulation of firing frequency in nociceptive neurons by pro-inflammatory mediators. Exp Brain Res 196: 45–52, 2009 [DOI] [PubMed] [Google Scholar]
- Moreno MJ, Abounader R, Hebert E, Doods H, Hamel E. Efficacy of the non-peptide CGRP receptor antagonist BIBN4096BS in blocking CGRP-induced dilations in human and bovine cerebral arteries: potential implications in acute migraine treatment. Neuropharmacology 42: 568–576, 2002 [DOI] [PubMed] [Google Scholar]
- Nicol GD, Klingberg DK, Vasko MR. Prostaglandin E2 increases calcium conductance and stimulates release of substance P in avian sensory neurons. J Neurosci 12: 1917–1927, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TP, van der Kooy D. Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in the trigeminal sensory projection to the intracranial arteries. J Neurosci 8: 2468–2476, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshinsky ML, Luo J. Neurochemistry of trigeminal activation in an animal model of migraine. Headache 46, Suppl 1: S39–S44, 2006 [DOI] [PubMed] [Google Scholar]
- Poulsen AN, Wulf H, Hay-Schmidt A, Jansen-Olesen I, Olesen J, Klaerke DA. Differential expression of BK channel isoforms and beta-subunits in rat neuro-vascular tissues. Biochim Biophys Acta 1788: 380–389, 2009 [DOI] [PubMed] [Google Scholar]
- Sagheddu C, Boccaccio A, Dibattista M, Montani G, Tirindelli R, Menini A. Calcium concentration jumps reveal dynamic ion selectivity of calcium-activated chloride currents in mouse olfactory sensory neurons and TMEM16b-transfected HEK 293T cells. J Physiol 588: 4189–4204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia 20: 907–918, 2000 [DOI] [PubMed] [Google Scholar]
- Shuster LT, Faubion SS, Sood R, Casey PM. Hormonal manipulation strategies in the management of menstrual migraine and other hormonally related headaches. Curr Neurol Neurosci Rep 11: 131–138, 2011 [DOI] [PubMed] [Google Scholar]
- Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA 267: 64–69, 1992 [PubMed] [Google Scholar]
- Stewart WF, Wood C, Reed ML, Roy J, Lipton RB. Cumulative lifetime migraine incidence in women and men. Cephalalgia 28: 1170–1178, 2008 [DOI] [PubMed] [Google Scholar]
- Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 384: 560–564, 1996 [DOI] [PubMed] [Google Scholar]
- Vaughn AH, Gold MS. Ionic mechanisms underlying inflammatory mediator-induced sensitization of dural afferents. J Neurosci 30: 7878–7888, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalon CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther 124: 309–323, 2009 [DOI] [PubMed] [Google Scholar]
- Wolff HG. Headache mechanisms. McGill Med J 15: 127–169, 1946 [PubMed] [Google Scholar]
- Zhang XL, Mok LP, Katz EJ, Gold MS. BKCa currents are enriched in a subpopulation of adult rat cutaneous nociceptive dorsal root ganglion neurons. Eur J Neurosci 31: 450–462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]