Abstract
Spinal reflexes are modified by spinal cord injury (SCI) due the loss of excitatory inputs from supraspinal structures and changes within the spinal cord. The stretch reflex is one of the simplest pathways of the central nervous system and was used presently to evaluate how inputs from primary and secondary muscle spindles interact with spinal circuits before and after spinal transection (i.e., spinalization) in 12 adult decerebrate cats. Seven cats were spinalized and allowed to recover for 1 mo (i.e., chronic spinal state), whereas 5 cats were evaluated before (i.e., intact state) and after acute spinalization (i.e., acute spinal state). Stretch reflexes were evoked by stretching the left triceps surae (TS) muscles. The force evoked by TS muscles was recorded along with the activity of several hindlimb muscles. Stretch reflexes were abolished in the acute spinal state due to an inability to activate TS muscles, such as soleus (Sol) and lateral gastrocnemius (LG). In chronic spinal cats, reflex force had partly recovered but Sol and LG activity remained considerably depressed, despite the fact that injecting clonidine could recruit these muscles during locomotor-like activity. In contrast, other muscles not recruited in the intact state, most notably semitendinosus and sartorius, were strongly activated by stretching TS muscles in chronic spinal cats. Therefore, stretch reflex pathways from TS muscles to multiple hindlimb muscles undergo functional reorganization following spinalization, both acute and chronic. Altered activation patterns by stretch reflex pathways could explain some sensorimotor deficits observed during locomotion and postural corrections after SCI.
Keywords: functional recovery, muscle length, force
sensorimotor interactions within the spinal cord, such as spinal reflexes, can be modified following complete spinal cord injury (SCI), due the loss of excitatory inputs from supraspinal structures and changes within the spinal cord (reviewed in Frigon and Rossignol 2006; Rossignol and Frigon 2011). Despite numerous studies on spinal reflex changes after SCI, the role played by various sensory inputs interacting with intrinsic spinal circuits in the recovery of motor functions after SCI remains rudimentary. For instance, the stretch reflex, considered one of the simplest circuits within the nervous system, has received surprisingly little attention after complete SCI (i.e., spinalization).
Studies have shown that homonymous stretch reflexes in triceps surae (TS) muscles are abolished following acute spinalization in cats (Ahlman et al. 1971; Ellaway and Trott 1975; Granit 1950; Miller et al. 1996) and in the acute stages of SCI in humans (Leis et al. 1996). Similarly, in decerebrate rabbits, postural limb reflexes evoked by tilting a platform, which are thought to be mediated primarily by muscle spindles, are lost after acute spinalization (Musienko et al. 2010). What is unclear is the recovery of stretch reflexes over the weeks that follow spinalization. Do sensory inputs from primary (group Ia) and/or secondary (group II) muscle spindles recover the ability to activate TS extensor motoneurons over time?
Moreover, group Ia and II inputs from TS muscles can activate interneuronal circuits through various polysynaptic pathways, such as those involved in generating locomotion (Conway et al. 1987; Guertin et al. 1995; Jankowska 1992). Descending pathways are potent regulators of spinal reflex pathways (Holmqvist and Lundberg 1961), and their loss results in immediate functional changes in how peripheral sensory inputs interact with spinal neurons. Not surprisingly, reflex responses not observed in the intact state can appear after SCI (Dietz et al. 2009; Frigon et al. 2009; Frigon and Rossignol 2008; Roby-Brami and Bussel 1987), although some of these responses only appear in the chronic stages of SCI. As a result, new patterns of activity evoked by stretching TS muscles could also occur in the acutely and chronically transected spinal cord. Detailed studies of TS stretch reflexes before and after spinalization have focused so far exclusively on homonymous and heteronymous pathways to TS muscles.
Length feedback regulates the mechanical properties of joints in a directionally specific manner and the mechanical coupling between joints (Nichols and Cope 2001). A change in the functional organization of stretch reflex pathways to homonymous and heteronymous muscles would disrupt this control system. Therefore, the effect of spinalization (acute and chronic) on stretch reflexes requires further investigation. The goal of the present study was to evaluate reflex force production from TS muscles and the activity of hindlimb muscles that cross hip, knee, and ankle joints with ramp-and-hold stretches of TS muscles in intact (i.e., intact spinal cord), acute spinal, and 1-mo chronic spinal cats during terminal experiments. Such information is required to guide future investigations at the cellular level of abnormal reflex mechanisms that develop after SCI and also studies using chronically implanted animals.
MATERIALS AND METHODS
Ethical Information
All procedures were approved by the Institutional Animal Care and Use Committee of Northwestern University. All animals were obtained from a designated breeding establishment for scientific research. Before the experiments, animals were housed and fed within designated areas, which were monitored daily by veterinarians and trained personnel. The current data set was compiled from 12 adult cats weighing between 2.5 and 5.0 kg.
Terminal experiment.
During the terminal experiment, cats were first placed in a clear plastic cylinder and anesthetized with 1.5–3% isoflurane in a 1:3 mixture of O2 and NO2. After ∼15 min, anesthesia was continued with a mask. Once the animal was deeply anesthetized, a tracheotomy was performed and the cat was intubated to deliver the anesthesia. The right common carotid artery and right jugular vein were cannulated to monitor blood pressure and for fluid administration, respectively. The level of anesthesia was confirmed and adjusted by monitoring blood pressure, applying pressure to the paw to detect limb withdrawal, and verifying the size and reactivity of the pupils. The animal was then transferred to a stereotaxic frame for further surgery. After a craniotomy, the cortex was removed and all tissue rostral to the colliculi was removed (i.e., a precollicular decerebration). At this point, animals are considered to have complete lack of sentience and anesthesia was discontinued. A lethal injection of potassium chloride (2 mg/kg) was administered at the end of the experiment through the right jugular vein.
Survival surgery.
In 7 cats, the spinal cord was completely transected at low thoracic levels ∼1 mo before the terminal experiment. The spinalization was performed under aseptic conditions in an operating room with sterilized equipment. Before surgery, cats were sedated (butorphanol, 0.4 mg/kg im; acepromazine, 0.05–0.1 mg/kg im; glycopyrrolate, 0.01 mg/kg sc). Induction was done with propofol (2–3 mg/kg iv) or ketamine-diazepam (0.11 ml/kg iv in 1:1 ratio). Once anesthetized, the cat was quickly intubated with a flexible endotracheal tube, and anesthesia was maintained by adjusting isoflurane concentration as needed (1.5–3%). The fur overlying the back was shaved with electric clippers, and loose hair was vacuumed. A forelimb was shaved, and an intravenous line was placed in a cephalic vein. The level of anesthesia was confirmed and adjusted throughout the surgery by monitoring cardiac rate and respiratory rate, by applying pressure to the paw to detect limb withdrawal, and by evaluating jaw tone. Body temperature was monitored using a rectal thermometer. Monitoring of the parameters was done on a continuous basis (i.e., cardiac and respiratory rates) and recorded every 15 min. Cats received fluids intravenously (warmed lactated Ringer solution or saline + 2.5% dextrose) at a rate of 5–10 ml · kg−1 · h−1 throughout the surgery for cardiovascular support. Ophthalmic ointment was also applied to the eyes.
A laminectomy was performed at T12–T13, the dura was removed, and, after local lidocaine application (Xylocaine, 2%), the spinal cord was completely transected with surgical scissors. Hemostatic material (Surgicel) was inserted within the gap, and muscles and skin were sewn back to close the opening in anatomic layers. A transdermal fentanyl patch (25 μg/h) was taped to the back of the animal 2–3 cm from the base of the tail. During surgery and ∼7 h later, an analgesic (buprenorphine, 0.01 mg/kg) was administered subcutaneously. An oral antibiotic (Baytril, 5 mg/kg) was given once a day for 5 days after spinalization to prevent urinary infection. The bladder was manually emptied one to two times each day up to the acute experiment. The animals were monitored daily by experienced personnel and veterinarians. The hindlimbs of the cats were frequently cleaned by placing the lower half of the body in a warm soapy bath.
Experimental Design
Experiments lasted 8–12 h. Stretch reflexes were evoked in the left hindlimb in all cats. The Achilles tendon was freed from surrounding tissue, leaving only a small piece of the calcaneal bone attached. The tendon was connected through an in-series force transducer to a linear motor (Copley Controls), which was controlled by custom-made software to alter muscle length. Force signals were low-pass filtered at 1,000 Hz and sampled at 10,000 Hz. Both hind feet were held with a clamp. The left knee joint was also fixed with a custom-made clamp attached to the femoral epicondyles. Hip, knee, and ankle joint angles were ∼120°, 160°, and 90° for both hindlimbs. The hind paws were not contacting the surface.
Bipolar wire electrodes were inserted into the soleus (Sol; ankle extensor), lateral gastrocnemius (LG; ankle extensor/knee flexor), semitendinosus (St; knee flexor/hip extensor), anterior sartorius (Srt; hip flexor/knee extensor), and tibialis anterior (TA; ankle flexor) muscles of the left hindlimb and in the right St for electromyography (EMG). EMG signals were amplified (×1,000) with a multichannel amplifier (AM Systems model 3500), band-pass filtered (300–3,000 Hz), and sampled at 10,000 Hz simultaneously with force data.
In the five cats not spinalized 1 mo before the terminal experiment, a laminectomy was made at T12–T13. After data were obtained with the spinal cord intact, gaseous anesthesia was reinduced. The dura was removed, and after local lidocaine application (Xylocaine, 2%), the spinal cord was completely transected with surgical scissors. Hemostatic material (Surgicel) was inserted within the gap. In all cats, the sciatic nerve was transected near the end of the experiment at the level of the greater trochanter for passive force measurements.
Experimental Protocol
Several ramp-hold-return stretches of 4-s duration of the left TS muscles were performed for each cat. The stretch consisted of a 5-mm ramp in 0.25 s, a 3.5-s hold, and a return to the initial length in 0.25 s, for a total of 4 s (see Fig. 1). Such stretches are similar in terms of time course and amplitude to postural perturbations used in rabbits (Musienko et al. 2010). The left TS muscles were stretched at two starting lengths. The “short” muscle length was the length that corresponded to a 90° ankle joint angle. The “long” muscle length was +5 mm longer than the short length. Muscle stretches were performed at intervals of no less than 1 min.
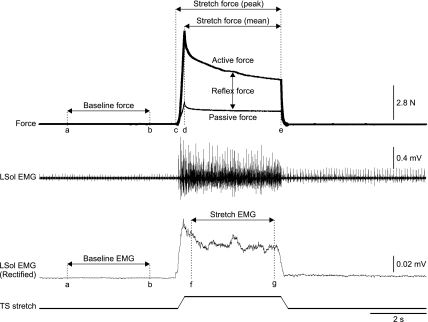
Fig. 1.
Measurements of force and muscle activity (cat 9). Data were collected during a stretch reflex trial that generally lasted 20 s. The stretch was evoked 5 s after data collection started. A single stretch lasted 4 s (5-mm ramp up in 0.25 s, followed by a 3.5-s hold and a 5-mm ramp down in 0.25 s). A window from 1 to 4 s (cursors a and b) was used to measure baseline force and EMG from the rectified and smoothed (time constant of 0.1 s) EMG channels. Peak force was measured as the maximal force value from 5 to 9 s (cursors c and e) minus the mean baseline force. The mean force was measured as the average force during the hold period of the stretch (i.e., from 5.25 to 8.75 s, cursors d and e) minus the mean baseline force. The EMG during stretch was measured as the area under the curve (modulus area function in Spike2 6.0) during the 3-s middle portion of the hold (cursors f and g) minus the baseline EMG using the same calculation. The 3-s middle portion of the hold, instead of the entire hold, was used to avoid movement artifacts during the ramps of the stretch. LSol, left soleus muscle; TS, triceps surae.
Measurements
The windows used for measurements of force and EMG are shown in Fig. 1. The peak force evoked during stretch (PFstretch) was calculated as the maximal value between cursors c and e minus the mean baseline force between cursors a and b. The mean force evoked during the 3.5-s hold portion of the stretch (MFstretch) was calculated as the mean value between cursors d and e minus the mean baseline force between cursors a and b. The peak (PFpassive) and mean (MFpassive) passive forces were measured after sciatic nerve transection using the same windows. Peak and mean passive forces were subtracted from the trials obtained before sciatic nerve transection to determine peak and mean reflex force, respectively:
For EMG measurements, the EMG during stretch was measured as the area under the curve (modulus area function in Spike2 6.0) during the 3-s middle portion of the hold (cursors f and g) minus the baseline EMG using the same calculation. The 3-s middle portion of the hold, instead of the entire hold, was used to avoid movement artifacts during the ramps of the stretch. We refer to this measurement as the stretch-induced EMG, which is very sensitive to any changes in activity evoked by the TS muscle stretch. It also takes into account whether or not the muscle was tonically active before the stretch and if the signal was inherently noisy. It assumes that a similar level of noise, when present, persists during the stretch.
Statistical Analysis
A two-factor ANOVA was used to evaluate the effect of state (i.e., intact, acute spinal, chronic spinal) and muscle length (i.e., short and long) on the following independent variables: peak reflex force, mean reflex force, and the stretch-induced EMG for left Sol, LG, St, TA, and Srt. Bonferroni corrections were used for multiple tests, and statistical significance was set at P < 0.01. If the ANOVA revealed a significant effect of state, Tukey's post hoc test was used to evaluate differences between states (P < 0.01). All values are means ± SE.
RESULTS
TS stretch reflexes were altered by a spinalization performed minutes (i.e., acute spinal) or 1 mo (i.e., chronic spinal) before testing. The following sections describe changes in the force produced by TS muscles and patterns of activity of several hindlimb muscles evoked by stretching TS muscles following acute or chronic spinalization.
Stretch Reflexes Before and After Acute Spinalization
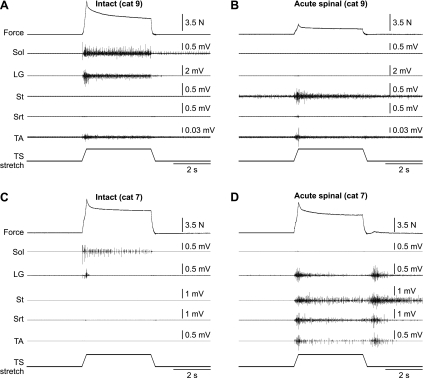
Stretch reflexes were evoked before and after an acute spinalization in five cats at two muscle lengths (2–4 stretches per state). Figure 2 shows examples in two cats at a muscle length of +5 mm. In cat 9, stretching TS muscles in the intact state evoked strong activity in Sol and LG, as well as weak activity in TA (Fig. 2A). Peak and mean reflex forces were 5.68 ± 0.14 and 3.16 ± 0.11 N, respectively, in the intact state for this cat at a muscle length of +5 mm (n = 3 trials). After spinalization, the reflex force evoked during the stretch was almost completely abolished (Fig. 2B). Peak and mean reflex forces were 0.39 ± 0.07 and 0.13 ± 0.03 N, respectively, in the acute spinal state for this cat at a muscle length of +5 mm (n = 3 trials). Thus the peak and mean reflex forces after acute spinalization were 6.8 and 4.1% of values obtained in the intact state, respectively. The reduction in reflex force can be attributed to the loss of activity in TS muscles (e.g., Sol and LG). After acute spinalization, the appearance of activity in muscles not recruited in the intact state, such as the St (Fig. 2B), could be observed. The loss of stretch-evoked activity in Sol and LG after acute spinalization was the pattern observed in three of five cats.
Fig. 2.
Force and EMG responses evoked by stretching the TS muscles in the intact (A and C) and acute spinal states (B and D) in 2 decerebrate cats at a starting muscle length of +5 mm. Stretch of the left TS muscles lasted 4 s and consisted of a 5-mm ramp up in 0.25 s, followed by a 3.5-s hold and a 5-mm ramp down in 0.25 s. EMG activity is shown below the force trace for 5 muscles of the left hindlimb: soleus (Sol), lateral gastrocnemius (LG), semitendinosus (St), anterior sartorius (Srt), and tibialis anterior (TA).
In the other two cats, stretch-evoked activity was present in Sol but absent in LG in the intact state. An example is shown in Fig. 2C. In cat 7, stretch of the TS muscles evoked activity in Sol but only a short burst of activity in LG. Peak and mean reflex forces were 4.41 ± 0.46 and 3.09 ± 0.25 N, respectively, in the intact state for this cat at a muscle length of +5 mm (n = 4 trials). After spinalization, reflex force evoked during the stretch was reduced but not abolished (Fig. 2D). Peak and mean reflex forces were 3.08 ± 0.15 and 1.82 ± 0.11 N, respectively, in the acute spinal state for this cat at a muscle length of +5 mm (n = 2 trials). Thus the peak and mean reflex forces after acute spinalization were 69.7 and 59.1% of values obtained in the intact state, respectively. Activity in Sol was abolished. However, stretch elicited activity in LG, St, Srt, and TA muscles. Activity in LG could be involved in producing a reflex force that is similar to the intact state. In all cats, activity in Sol was lost after acute spinalization, whereas other muscles not recruited in the intact state became active.
Stretch Reflexes in Chronic Spinal Cats
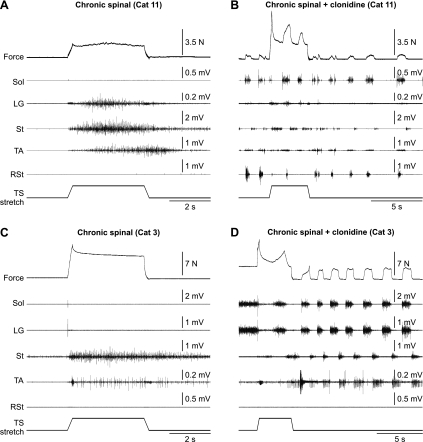
Seven cats were spinalized at T12–T13 and allowed to recover for 1 mo before the terminal experiment. One month postspinalization (i.e., at the time of the terminal experiment), all cats had vigorous reflex responses, air-stepping ability, and paw-shake responses when the hind feet were placed in water (not shown). Stretch reflexes were evoked and evaluated at two muscle lengths in these seven cats. Figure 3 shows examples in two cats at a muscle length of +5 mm. In all chronic spinal cats, stretching the TS muscles did not activate the Sol muscle (Fig. 3, A and C). In some cats, TS stretch could activate the LG muscle (e.g., Fig. 3A). However, in all cats, muscles that perform no ankle extensor torque, such as St, Srt (not shown), and TA, could be activated by stretching TS muscles (Fig. 3, A and C). In all chronic spinal cats, the St muscle became strongly active by stretching TS muscles, and this activity could persist long after the stretch (Fig. 3C). The inability of stretch-related inputs from TS muscles to activate Sol is not due to a general depression in the excitability of its motor pool, because after injection of clonidine (250–500 μg/kg iv), the Sol muscle became active during locomotor-like activity along with other muscles (Fig. 3, B and D).
Fig. 3.
Force and EMG responses evoked by stretching the TS muscles in 1-mo chronic spinal cats before (A and C) and after (B and D) intravenous injection of clonidine (0.25 mg/kg) in 2 decerebrate cats at a starting muscle length of +5 mm. Stretch of the left TS muscles lasted 4 s and consisted of a 5-mm ramp up in 0.25 s, followed by a 3.5-s hold and a 5-mm ramp down in 0.25 s. EMG activity is shown below the force trace for 4 muscles of the left hindlimb and 1 muscle of the right hindlimb (RSt).
State-Dependent Changes in Reflex Force
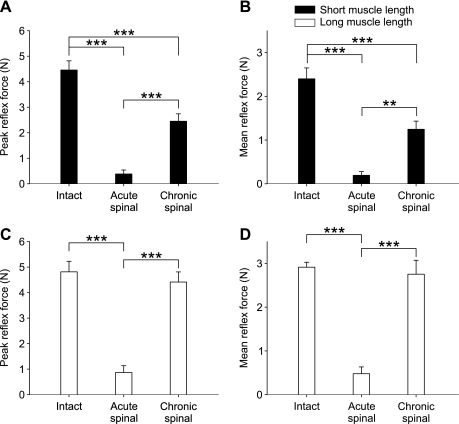
Figures 2 and 3 show that the force evoked by stretching the TS muscles was strongly dependent on the state (i.e., intact, acute spinal, chronic spinal). Group data for reflex force are shown in Fig. 4. For peak and mean reflex force there was a significant effect of state and muscle length (2-factor ANOVA, P < 0.005) As a result, one-factor ANOVAs were performed to determine state-dependent differences for peak and mean reflex forces at the two muscle lengths independently.
Fig. 4.
State-dependent changes in reflex force for the group at short and long muscle lengths. Measures of peak (A and C) and mean reflex force (B and D) in the intact (n = 28 stretches in 5 cats; 15 and 13 stretches at short and long muscle lengths, respectively), acute spinal (n = 27 stretches in 5 cats; 13 and 14 stretches at short and long muscle lengths, respectively), and chronic spinal states (n = 54 stretches in 7 cats; 27 and 27 stretches at short and long muscle lengths, respectively) at long and short muscle lengths. Values are means ± SE. **P < 0.01; ***P < 0.001, significant differences between states (Tukey's post hoc test).
Peak and mean reflex forces were considerably reduced following acute spinalization at long and short muscle lengths (Fig. 4, A–D). At the short muscle length, peak and mean reflex forces were larger in the chronic spinal state than in acute spinal cats, although still significantly smaller than in the intact state (Fig. 4, A and B). On the other hand, at the long muscle length, peak and mean reflex forces were not significantly different than in the intact state (Fig. 4, C and D). Thus there appears to be “recovery” of reflex force 1-mo postspinalization, particularly when the muscle is stretched at a longer starting muscle length. This recovery cannot be attributed to a reduction in passive force, because it accounted for 31.7 ± 3.2 and 32.0 ± 2.4% of total peak and mean force, respectively, in the intact state (both muscle lengths combined). In chronic spinal cats, passive force accounted for 42.3 ± 2.5 and 48.4 ± 2.3% of total peak and mean forces, respectively (both muscle lengths combined).
State-Dependent Changes in Muscle Activity
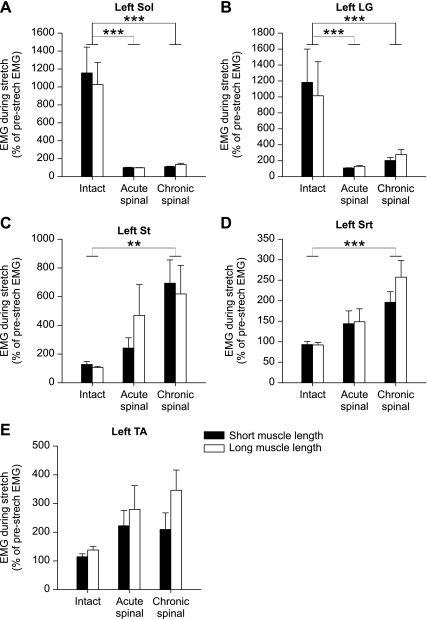
Figures 2 and 3 also showed that the activation of various hindlimb muscles with stretch of TS muscles was strongly dependent on the state (i.e., intact, acute spinal, chronic spinal). There was a significant effect of state on stretch-induced EMG for all muscles except TA (2-factor ANOVA, P < 0.01) but no significant effect of muscle length for any of the recorded muscles.
Not surprisingly, in the intact state, stretching TS muscles produced strong activity in Sol and LG muscles (Fig. 5, A and B; Tukey's post hoc test, P < 0.01). However, after acute spinalization, the same stretch did not elicit activity in Sol or LG muscles across the group. The activity in these muscles remained considerably reduced in chronic spinal cats. In contrast, muscles that were not recruited in the intact state by stretching TS muscles, such as St and Srt, became active in chronic spinal cats (Fig. 5, C and D; Tukey's post hoc test, P < 0.01). The activity in Srt even became significantly larger after acute spinalization (Fig. 5D). Although there was a trend for an increase in TA, this was not significant.
Fig. 5.
State-dependent changes in stretch-induced muscle activity for the group. EMG activity during TS muscle stretch expressed as a percentage of baseline EMG in the intact (n = 28 stretches in 5 cats; 15 and 13 stretches at short and long muscle lengths, respectively), acute spinal (n = 27 stretches in 5 cats; 13 and 14 stretches at short and long muscle lengths, respectively), and chronic spinal states (n = 54 stretches in 7 cats; 27 and 27 stretches at short and long muscle lengths, respectively) at long and short muscle lengths in left Sol (A), LG (B), St (C), Srt (D), and TA muscles (E). For some muscles, a few trials were removed from the analysis because of noise in the signal. Values are means ± SE. **P < 0.01; ***P < 0.001, significant differences between states (Tukey's post hoc test, both muscle lengths combined).
DISCUSSION
In acute and chronic spinal cats, stretching TS muscles failed to activate Sol and, in most instances, the LG muscle as well, which reduced the reflex contribution to force production. Failure to activate ankle extensors was not due to an inability of spinal neurons to activate Sol and LG, because after injection of clonidine, these muscles were recruited during locomotor-like activity. Moreover, some muscles not recruited in the intact state displayed strong activity with stretch of TS muscles after spinalization, particularly in the chronic spinal state. Therefore, results demonstrate that TS stretch reflex pathways to multiple hindlimb muscles are functionally altered after spinalization and continue to change over time, which has important implications for the control of movement and posture after SCI.
Recovery of Reflex Contribution to Force Production
Responses to muscle stretch are determined by numerous factors occurring within the central nervous system and muscle. However, changes in activation patterns across several muscles following spinalization must involve functional changes within the spinal cord. After acute spinalization, the reflex contribution to force production was considerably reduced but showed signs of partial recovery 1 mo postspinalization (Fig. 4). Recovery cannot be attributed to changes at the muscle because passive force accounted for a greater percentage of total peak and mean forces in chronic spinal cats compared with the intact state, in keeping with studies reporting that passive muscle force was unchanged or increased after SCI in humans (Mirbagheri et al. 2001).
The recovery of reflex force cannot be attributed to recruitment of Sol and LG because these muscles were not, or weakly, activated in chronic spinal cats. The activation of other ankle extensors by TS stretch reflex pathways, such as medial gastrocnemius (MG), plantaris, flexor hallucis longus, and the peroneals, might participate in the recovery of reflex force production after spinalization. Indeed, the MG muscle can compensate for the loss of synergists following denervation or chemical paralysis of Sol and LG (Bouyer et al. 2001; Frigon and Rossignol 2007; Pearson et al. 1999, 2003). However, it remains unclear whether MG compensates for reduced activity of LG and Sol after spinal transection, because it is also affected by the loss of descending inputs. Moreover, muscles that cross other joints, such as semimembranosus and biceps femoris, can exert ankle extensor force through transarticular attachments (Sherrington 1910), a phenomenon that is difficult to evaluate because isolating structures through lesions induces compensatory changes in spared pathways/structures. Further work from a larger subset of recorded muscles in the different states is required to more thoroughly evaluate the structures contributing to the recovery of reflex force several weeks after spinalization.
Reorganization of Stretch Reflex Pathways After Spinalization
Muscle stretch activates afferents from primary (group Ia) and secondary (group II) muscle spindles. Studies have shown that electrically stimulating TS muscle afferents at group I (i.e., Ia and Ib) strength evokes similar or larger homonymous and heteronymous excitatory postsynaptic potentials (EPSPs) in chronic spinal cats (>6 wk) compared with cats with intact spinal cords (Hochman and McCrea 1994; Nelson and Mendell 1979). Also, immediately after spinalization, group I-evoked EPSPs are increased in TS motoneurons (Nelson et al. 1979). Similarly, in rats (Valero-Cabre et al. 2004) and humans (Hiersemenzel et al. 2000; Leis et al. 1996), H-reflex amplitude is increased or unaltered after SCI. Although differences in the regulation of responses evoked by natural (e.g., stretch) and electrical stimuli exist (Burke et al. 1984; Enriquez-Denton et al. 2002; Morita et al. 1998), an alternative explanation is that responses in TS muscles evoked by ramp-and-hold stretches, which were greatly reduced after spinalization in the present study (see also Ahlman et al. 1971; Ellaway and Trott 1975; Granit 1950; Miller et al. 1996), are mediated by other inputs. However, Ia and Ib afferents still need to be stimulated naturally (e.g., muscle vibration, stretches at various speeds and force levels) to clearly ascertain their role in the observed stretch reflex changes. The involvement of group II inputs from secondary muscle spindles in cat and human stretch reflexes are discussed elsewhere (Grey et al. 2001; Jankowska 1992; Jankowska and Hammar 2002; Schieppati and Nardone 1999) and could be key in the observed stretch reflex changes. Contributions from group II, III, and/or IV afferents from free nerve endings, which can be excited by muscle stretch (Cleland et al. 1990; Cleland and Rymer 1990; Mense and Meyer 1985), also cannot be excluded.
The clasp-knife response, consisting of brief excitation followed by long-lasting inhibition of agonists and close synergists in response to large muscle stretches, appears after spinal lesions (Burke et al. 1972; Cleland et al. 1990; Cleland and Rymer 1990). Clasp-knife inhibition is thought to be mediated by muscle afferents from free nerve endings (Cleland et al. 1990). Cleland and Rymer (1990) also showed that stretching TS muscles after an acute dorsal hemisection in a decerebrate cat evoked inhibition in ankle and knee extensors (i.e., the clasp knife response) while eliciting activity in other muscles, such as St, TA, and iliopsoas (hip flexor). Nichols and Cope (2001) also showed activation of TA with TS muscle stretch in a decerebrate cat spinalized 28 days before the terminal experiment. We extend these findings by showing that the activation of muscles throughout the hindlimb with TS muscle stretch is consistently observed after spinalization, particularly in animals spinalized 1 mo before testing. Thus mechanisms underlying clasp-knife responses in dorsal hemisected cats could explain several of the stretch reflex changes presently observed.
Loss of serotonergic drive is probably responsible for depressed stretch reflexes after acute spinalization, because selective activation of 5-HT2 receptors restores TS excitability during MG muscle stretch in acute spinal cats (Miller et al. 1996). Although actions of monoaminergic neuromodulators are thought to be diffuse (Heckman et al. 2008; Johnson and Heckman 2010), there might be differential regulation of the intrinsic properties of extensor and flexor motoneurons by descending pathways (see Cotel et al. 2009). However, functional reorganization of stretch reflex pathways after spinalization likely occurs at premotoneuronal sites, because stretching TS muscles fails to recruit Sol and LG even when these muscles are rhythmically or tonically active following clonidine administration (Fig. 3). In decerebrate cats with intact spinal cords, stretching TS muscles or electrically stimulating their afferents during spontaneous fictive locomotion produces sustained activity in extensors (Frigon and Gossard 2010). Altered fusimotor activity is probably not responsible for changes in stretch reflexes after spinalization, because hyperreflexia is observed in chronic spinal cats despite normal or depressed spindle activity (Bailey et al. 1980). Therefore, functional changes in spinal interneuron circuits that regulate or are integral components of TS stretch reflex pathways must have occurred.
It was proposed that alternate reflex pathways within the spinal cord are regulated by descending pathways and other segmental inputs (Jankowska and Hammar 2002; Lundberg et al. 1987), which enables the same muscle afferent to mediate excitatory and inhibitory reflex actions on extensor and flexor motoneurons depending on the state of interposed interneuronal circuitry. We can posit that, in the intact state, interneurons interposed in reflex pathways from TS group II inputs are excitable and readily transmit signals to ankle extensor motoneurons, whereas those that project to St and Srt motoneurons are tonically inhibited. After spinalization, there is a reversal in the excitability of interneurons regulating motoneuron activity such that interneurons receiving group II inputs from TS motoneurons projecting to ankle extensor motoneurons are inhibited while those projecting to St and Srt motoneurons are disinhibited.
Studies have demonstrated that disynaptic reciprocal inhibition between ankle flexors and ankle extensors can be altered following SCI in humans (Crone et al. 2003; Xia and Rymer 2005), which was demonstrated by conditioning the H-reflex or with tendon taps. Although there was a trend for increased activity in TA during TS stretch reflexes after spinalization (Fig. 5), increased activity in St and Srt suggests more widespread changes in spinal interneuronal circuits. Inhibitory mechanisms within the spinal cord are particularly affected by SCI (Boulenguez et al. 2010; Vinay and Jean-Xavier 2008).
Spinalization-induced changes in presynaptic inhibition also could be involved, because descending pathways regulate presynaptic inhibition of afferent terminals (Quevedo et al. 1993; Rudomin et al. 2004b; Rudomin and Schmidt 1999). After spinalization, collaterals from the same muscle afferent can be differentially regulated by other segmental inputs (Rudomin et al. 2004a). Changes in presynaptic regulation of TS muscle afferents could explain why the same muscle stretch fails to activate some muscles after spinalization that were strongly activated in the intact state (e.g., Sol and LG) while recruiting muscles that were inactive before spinalization (e.g., St and Srt).
Whatever the mechanism, the loss of descending pathways alters the balance of excitatory and inhibitory inputs within spinal neuronal circuits, modifying the activation pattern of various muscles by TS afferent inputs. Musienko et al. (2010) also showed changes in activation patterns with postural perturbations following spinalization in decerebrate rabbits. Results from the present study strongly indicate that such changes could be due to functional reorganization of stretch reflex pathways from a small group of synergists, potentially even from single muscles.
Functional Considerations
The indication that TS muscle afferents no longer produces proper activation patterns after spinalization could explain some motor deficits. Bennett et al. (1996) attributed 50 and 23% of TS force during postural corrections and locomotion, respectively, to stretch reflexes in cats. In humans, TS stretch reflexes were suggested to contribute 30–60% of soleus EMG during stance (Yang et al. 1991), but this was probably an overestimation due to nonlinear properties of human ankle reflexes (Bennett et al. 1996; Stein and Kearney 1995). However, even if TS stretch-related inputs contribute closer to 25% of the ankle extensor force during locomotion, this could partly explain reduced hindlimb extensor activity during treadmill walking in chronic spinal cats (Belanger et al. 1996; Frigon and Rossignol 2008). Moreover, altered stretch reflex pathways after spinalization could factor in impaired postural control along with the loss of descending signals, because stretch signals are important for coordinating activation patterns across joints (Nichols and Cope 2001).
At present, it is unknown whether locomotor training after spinalization restores proper recruitment patterns when TS muscles are stretched. Locomotor training can normalize reflex pathways from load and cutaneous mechanoreceptors in chronic spinal cats (Cote and Gossard 2004; Cote et al. 2003) and also maintain normal inhibitory influences on α- and γ-motoneurons after spinalization in neonatal rats (Ichiyama et al. 2011). It is probable that responses to TS muscle stretch continue to evolve over time and are influenced by task-specific training (e.g., stand or locomotor training) and various drugs. Reflex testing in chronically implanted animals receiving various training regimens should answer some of these questions.
GRANTS
The present work was supported by an individual grant from the Wings for Life Foundation and a postdoctoral fellowship from the Canadian Institutes of Health Research to A. Frigon, as well as a National Institute of Neurological Disorders and Stroke Grant NS034382 to C. J. Heckman.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Marin Manuel and Dr. Jack Miller for technical assistance.
REFERENCES
- Ahlman H, Grillner S, Udo M. The effect of 5-HTP on the static fusimotor activity and the tonic stretch reflex of an extensor muscle. Brain Res 27: 393–396, 1971 [DOI] [PubMed] [Google Scholar]
- Bailey CS, Lieberman JS, Kitchell RL. Response of muscle spindle primary endings to static stretch in acute and chronic spinal cats. Am J Vet Res 41: 2030–2036, 1980 [PubMed] [Google Scholar]
- Belanger M, Drew T, Provencher J, Rossignol S. A comparison of treadmill locomotion in adult cats before and after spinal transection. J Neurophysiol 76: 471–491, 1996 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, De Serres SJ, Stein RB. Gain of the triceps surae stretch reflex in decerebrate and spinal cats during postural and locomotor activities. J Physiol 496: 837–850, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med 16: 302–307, 2010 [DOI] [PubMed] [Google Scholar]
- Bouyer LJ, Whelan PJ, Pearson KG, Rossignol S. Adaptive locomotor plasticity in chronic spinal cats after ankle extensors neurectomy. J Neurosci 21: 3531–3541, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol 52: 435–448, 1984 [DOI] [PubMed] [Google Scholar]
- Burke D, Knowles L, Andrews C, Ashby P. Spasticity, decerebrate rigidity and the clasp-knife phenomenon: an experimental study in the cat. Brain 95: 31–48, 1972 [DOI] [PubMed] [Google Scholar]
- Cleland CL, Hayward L, Rymer WZ. Neural mechanisms underlying the clasp-knife reflex in the cat. II. Stretch-sensitive muscular-free nerve endings. J Neurophysiol 64: 1319–1330, 1990 [DOI] [PubMed] [Google Scholar]
- Cleland CL, Rymer WZ. Neural mechanisms underlying the clasp-knife reflex in the cat. I. Characteristics of the reflex. J Neurophysiol 64: 1303–1318, 1990 [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res 68: 643–656, 1987 [DOI] [PubMed] [Google Scholar]
- Cote MP, Gossard JP. Step training-dependent plasticity in spinal cutaneous pathways. J Neurosci 24: 11317–11327, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Menard A, Gossard JP. Spinal cats on the treadmill: changes in load pathways. J Neurosci 23: 2789–2796, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotel F, Antri M, Barthe JY, Orsal D. Identified ankle extensor and flexor motoneurons display different firing profiles in the neonatal rat. J Neurosci 29: 2748–2753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 126: 495–507, 2003 [DOI] [PubMed] [Google Scholar]
- Dietz V, Grillner S, Trepp A, Hubli M, Bolliger M. Changes in spinal reflex and locomotor activity after a complete spinal cord injury: a common mechanism? Brain 132: 2196–2205, 2009 [DOI] [PubMed] [Google Scholar]
- Ellaway PH, Trott JR. The mode of action of 5-hydroxytryptophan in facilitating a stretch reflex in the spinal cat. Exp Brain Res 22: 145–162, 1975 [DOI] [PubMed] [Google Scholar]
- Enriquez-Denton M, Morita H, Christensen LO, Petersen N, Sinkjaer T, Nielsen JB. Interaction between peripheral afferent activity and presynaptic inhibition of Ia afferents in the cat. J Neurophysiol 88: 1664–1674, 2002 [DOI] [PubMed] [Google Scholar]
- Frigon A, Barriere G, Leblond H, Rossignol S. Asymmetric changes in cutaneous reflexes after a partial spinal lesion and retention following spinalization during locomotion in the cat. J Neurophysiol 102: 2667–2680, 2009 [DOI] [PubMed] [Google Scholar]
- Frigon A, Gossard JP. Evidence for specialized rhythm-generating mechanisms in the adult mammalian spinal cord. J Neurosci 30: 7061–7071, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Adaptive changes of the locomotor pattern and cutaneous reflexes during locomotion studied in the same cats before and after spinalization. J Physiol 586: 2927–2945, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Functional plasticity following spinal cord lesions. Prog Brain Res 157: 231–260, 2006 [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Plasticity of reflexes from the foot during locomotion after denervating ankle extensors in intact cats. J Neurophysiol 98: 2122–2132, 2007 [DOI] [PubMed] [Google Scholar]
- Granit R. Reflex self-regulation of muscle contraction and autogenetic inhibition. J Neurophysiol 13: 351–372, 1950 [DOI] [PubMed] [Google Scholar]
- Grey MJ, Ladouceur M, Andersen JB, Nielsen JB, Sinkjaer T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol 534: 925–933, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault MC, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol 487: 197–209, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Hyngstrom AS, Johnson MD. Active properties of motoneurone dendrites: diffuse descending neuromodulation, focused local inhibition. J Physiol 586: 1225–1231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiersemenzel LP, Curt A, Dietz V. From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology 54: 1574–1582, 2000 [DOI] [PubMed] [Google Scholar]
- Hochman S, McCrea DA. Effects of chronic spinalization on ankle extensor motoneurons. I. Composite monosynaptic Ia EPSPs in four motoneuron pools. J Neurophysiol 71: 1452–1467, 1994 [DOI] [PubMed] [Google Scholar]
- Holmqvist B, Lundberg A. Differential supraspinal control of synaptic actions evoked by volleys in the flexion reflex afferents in alpha motoneurones. Acta Physiol Scand Suppl 186: 1–15, 1961 [PubMed] [Google Scholar]
- Ichiyama RM, Broman J, Roy RR, Zhong H, Edgerton VR, Havton LA. Locomotor training maintains normal inhibitory influence on both alpha- and gamma-motoneurons after neonatal spinal cord transection. J Neurosci 31: 26–33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38: 335–378, 1992 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I. Spinal interneurones; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Res Brain Res Rev 40: 19–28, 2002 [DOI] [PubMed] [Google Scholar]
- Johnson MD, Heckman CJ. Interactions between focused synaptic inputs and diffuse neuromodulation in the spinal cord. Ann NY Acad Sci 1198: 35–41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis AA, Kronenberg MF, Stetkarova I, Paske WC, Stokic DS. Spinal motoneuron excitability after acute spinal cord injury in humans. Neurology 47: 231–237, 1996 [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Exp Brain Res 65: 271–281, 1987 [DOI] [PubMed] [Google Scholar]
- Mense S, Meyer H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. J Physiol 363: 403–417, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JF, Paul KD, Lee RH, Rymer WZ, Heckman CJ. Restoration of extensor excitability in the acute spinal cat by the 5-HT2 agonist DOI. J Neurophysiol 75: 620–628, 1996 [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Barbeau H, Ladouceur M, Kearney RE. Intrinsic and reflex stiffness in normal and spastic, spinal cord injured subjects. Exp Brain Res 141: 446–459, 2001 [DOI] [PubMed] [Google Scholar]
- Morita H, Petersen N, Christensen LO, Sinkjaer T, Nielsen J. Sensitivity of H-reflexes and stretch reflexes to presynaptic inhibition in humans. J Neurophysiol 80: 610–620, 1998 [DOI] [PubMed] [Google Scholar]
- Musienko PE, Zelenin PV, Orlovsky GN, Deliagina TG. Facilitation of postural limb reflexes with epidural stimulation in spinal rabbits. J Neurophysiol 103: 1080–1092, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SG, Collatos TC, Niechaj A, Mendell LM. Immediate increase in Ia-motoneuron synaptic transmission caudal to spinal cord transection. J Neurophysiol 42: 655–664, 1979 [DOI] [PubMed] [Google Scholar]
- Nelson SG, Mendell LM. Enhancement in Ia-motoneuron synaptic transmission caudal to chronic spinal cord transection. J Neurophysiol 42: 642–654, 1979 [DOI] [PubMed] [Google Scholar]
- Nichols TR, Cope TC. The organization of distributed proprioceptive feedback in the chronic spinal cat. In: Motor Neurobiology of the Spinal Cord, edited by Cope TC. Boca Raton, FL: CRC, 2001 [Google Scholar]
- Pearson KG, Fouad K, Misiaszek JE. Adaptive changes in motor activity associated with functional recovery following muscle denervation in walking cats. J Neurophysiol 82: 370–381, 1999 [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE, Hulliger M. Chemical ablation of sensory afferents in the walking system of the cat abolishes the capacity for functional recovery after peripheral nerve lesions. Exp Brain Res 150: 50–60, 2003 [DOI] [PubMed] [Google Scholar]
- Quevedo J, Eguibar JR, Jimenez I, Schmidt RF, Rudomin P. Primary afferent depolarization of muscle afferents elicited by stimulation of joint afferents in cats with intact neuraxis and during reversible spinalization. J Neurophysiol 70: 1899–1910, 1993 [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B. Long-latency spinal reflex in man after flexor reflex afferent stimulation. Brain 110: 707–725, 1987 [DOI] [PubMed] [Google Scholar]
- Rossignol S, Frigon A. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu Rev Neurosci 34: 413–440, 2011 [DOI] [PubMed] [Google Scholar]
- Rudomin P, Lomeli J, Quevedo J. Differential modulation of primary afferent depolarization of segmental and ascending intraspinal collaterals of single muscle afferents in the cat spinal cord. Exp Brain Res 156: 377–391, 2004a [DOI] [PubMed] [Google Scholar]
- Rudomin P, Lomeli J, Quevedo J. Tonic differential supraspinal modulation of PAD and PAH of segmental and ascending intraspinal collaterals of single group I muscle afferents in the cat spinal cord. Exp Brain Res 159: 239–250, 2004b [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129: 1–37, 1999 [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A. Group II spindle afferent fibers in humans: their possible role in the reflex control of stance. Prog Brain Res 123: 461–472, 1999 [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol 40: 28–121, 1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Kearney RE. Nonlinear behavior of muscle reflexes at the human ankle joint. J Neurophysiol 73: 65–72, 1995 [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Fores J, Navarro X. Reorganization of reflex responses mediated by different afferent sensory fibers after spinal cord transection. J Neurophysiol 91: 2838–2848, 2004 [DOI] [PubMed] [Google Scholar]
- Vinay L, Jean-Xavier C. Plasticity of spinal cord locomotor networks and contribution of cation-chloride cotransporters. Brain Res Rev 57: 103–110, 2008 [DOI] [PubMed] [Google Scholar]
- Xia R, Rymer WZ. Reflex reciprocal facilitation of antagonist muscles in spinal cord injury. Spinal Cord 43: 14–21, 2005 [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Exp Brain Res 87: 679–687, 1991 [DOI] [PubMed] [Google Scholar]