Abstract
Within the brain stem, the nucleus tractus solitarii (NTS) serves as a principal central site for sensory afferent integration from the cardiovascular and respiratory reflexes. Neuronal activity and synaptic transmission in the NTS are highly pliable and subject to neuromodulation. In the central nervous system, hydrogen sulfide (H2S) is a gasotransmitter generated primarily by the enzyme cystathionine-β-synthase (CBS). We sought to determine the role of H2S, and its generation by CBS, in NTS excitability. Real-time RT-PCR, immunoblot, and immunohistochemistry analysis identified the presence of CBS in the NTS. Patch-clamp electrophysiology in brain stem slices examined excitatory postsynaptic currents (EPSCs) and membrane properties in monosynaptically driven NTS neurons. Confocal imaging of labeled afferent synaptic terminals in NTS slices monitored intracellular calcium. Exogenous H2S significantly increased the amplitude of evoked solitary tract (TS)-EPSCs, frequency of miniature (m)EPSCs, and presynaptic terminal calcium fluorescence in the NTS. H2S did not alter action potential discharge or postsynaptic properties. On the other hand, the CBS inhibitor aminooxyacetate (AOA) significantly reduced the amplitude of TS-EPSCs and presynaptic terminal calcium fluorescence in the NTS without altering postsynaptic properties. Taken together, these data support a presynaptic role for endogenous H2S in modulation of excitatory neurotransmission in the NTS.
Keywords: cystathionine-β-synthase, electrophysiology, aminooxyacetate, immunohistochemistry
the nucleus tractus solitarii (NTS) in the dorsomedial brain stem serves as the first termination and integration site for visceral sensory afferents, including baroreceptor and chemoreceptor fibers (Andresen 2004; Dampney 1994; Loewy 1990; Spyer 1990). It is also the initial site in which glutamate release or its receptor binding may be altered by neuromodulators to modify synaptic, neuronal, and cardiorespiratory reflexes. For instance, the gasotransmitter nitric oxide (NO) modulates synaptic activity within the NTS (Wang et al. 2007), and NO and carbon monoxide (CO, an additional gasotransmitter) alter cardiorespiratory and autonomic function (Krukoff 1999; Lo et al. 2002, 2004; Prabhakar 1998).
Hydrogen sulfide (H2S) is an additional endogenous gasotransmitter (Kimura 2002; Mancuso et al. 2010; Qu et al. 2008). Within the central nervous system, H2S is primarily produced by the enzyme cystathionine-β-synthase (CBS) (Abe and Kimura 1996). This enzyme is located throughout the brain, including the forebrain and brain stem (Bantikyan et al. 2009; Dawe et al. 2008; Enokido et al. 2005; Russo et al. 2000). Similar to NO and CO, H2S diffuses through the plasma membrane to modulate cellular properties (Ritter 2010). In particular, H2S may augment glutamate release (Tan et al. 2010) and excitatory field potentials, as well as inducing synaptic long-term potentiation (Abe and Kimura 1996). CBS-produced H2S may also regulate the cardiorespiratory system. Microinjection of H2S into the posterior hypothalamus reduces mean arterial pressure, whereas blockade of CBS with the competitive inhibitor aminooxyacetate (AOA) increases blood pressure (Dawe et al. 2008). However, the role of H2S in the integration and modulation of NTS function remains unknown.
On the basis of these findings, we sought to determine whether H2S plays a role in NTS activity. Interestingly, H2S modulates peripheral chemoreceptor sensory afferents that project to the NTS, as well as the ventilatory response to a brief hypoxic challenge (Peng et al. 2010), suggesting that H2S may function in the peripheral afferent-NTS reflex arc. The present study examined the localization of the H2S-generating enzyme CBS, along with the function of H2S, in the NTS. CBS messenger RNA and protein were identified in the NTS. Patch-clamp recordings in rat horizontal brain stem slices demonstrated that H2S plays an excitatory role in synaptic transmission, which is mediated by presynaptic mechanisms. Finally, confocal imaging of fluorescent afferent terminals in NTS brain slices demonstrated that H2S augments intracellular calcium. Taken together, these studies suggest a novel role for H2S in modulating synaptic function in the NTS.
METHODS
Animals.
Experiments were performed according to the guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The University of Missouri and Pennington Biomedical Research Center Animal Care and Use Committees approved all procedures and protocols. Male Sprague-Dawley rats (Harlan, Indianapolis, IN; n = 38) aged 3–5 wk were used. Rats were housed within an in-house animal facility on a 12:12-h day-night cycle. Temperature and humidity were maintained at 22°C and 40%, respectively, and food and water were available ad libitum.
Real-time reverse transcriptase-polymerase chain reaction.
The presence of CBS mRNA in the NTS (n = 3) was examined by real-time reverse transcriptase-polymerase chain reaction (RT-PCR). Animals were deeply anesthetized, and the brain was removed. Horizontal NTS sections were cut in a manner to minimize the amount of non-NTS tissue within the sample as previously described (Kline et al. 2007). Briefly, the brain stem was removed and sectioned on a vibratome. The dorsal surface of the brain stem was trimmed to remove the majority of the area postrema, after which a section of NTS (∼400 μm) was rapidly removed. The lateral edges of the NTS were trimmed to the solitary tract, non-NTS tissue was removed, and the tissue was placed in RNAlater (Qiagen). Cerebellar tissue was also removed, placed in RNAlater, and used as a positive control (Enokido et al. 2005). Total RNA was extracted and treated with DNase I with the RNAqueous-Micro Kit (Ambion, Austin, TX). First-strand DNA synthesis used oligo(dT) primers (SuperScript III kit, Invitrogen, Carlsbad, CA), and 1 μl of produced cDNA was used for PCR amplification using rat CBS primers (NM_012522: forward 5′ GCT GAT GGT GTT TGG TGT TG 3′; reverse 5′ GTG GAA ACC AGT CGG TGT CT 3′). For control purposes, PCR was also run on samples containing no template (NT), no primers (NP), or no reverse transcriptase (−RT). RT-PCR cycling (Smartcycler, Cepheid, Sunnyvale, CA) was set at the following parameters: 1 cycle at 95°C for 10 s; 45 cycles at 95°C for 5 s, followed by 60°C for 20 s; and finally a melt curve established from 60°C to 95°C. After cycling, PCR products were separated on a 1.5% agarose gel and imaged.
Immunoblot.
CBS protein in the NTS was confirmed with Western blot analysis modified from Austgen et al. (2008). Animals were deeply anesthetized, and the brain was removed. The NTS (400-μm sections cut horizontally) was rapidly removed, carefully trimmed to remove non-NTS tissue as above, and subsequently snap frozen in liquid nitrogen. NTS was pooled from three animals. Tissue was subsequently suspended, and cells were lysed by sonication in two volumes of RIPA buffer [final concentrations in mM: 1% NP-40, 150 NaCl, 50 Tris, 1 ethylenediaminetetraacetic acid (EDTA), 10 sodium fluoride, 10 sodium orthovanadate, 1 phenylmethylsulfonyl fluoride (PMSF), and 0.25% sodium deoxycholate] with a protease inhibitor cocktail (Roche, Indianapolis, IN) freshly added. Insoluble protein was removed by centrifugation at 14,000 g at 4°C, and the supernatant was collected. Protein concentration of the tissue samples was measured by the Micro BCA Method (Pierce, Thermo Scientific, Rockford, IL). Twenty micrograms of protein was separated on 4–20% Tris·HCl gels (Bio-Rad Laboratories, Hercules, CA) and transferred to polyvinylidene difluoride (PVDF) membranes. As a positive control, immunoblots were also run with lysate from HEK cells transfected with CBS [CBS (h2) lysate, sc-112304, Santa Cruz Biotechnology, Santa Cruz, CA] and total rat brain lysate (sc-2395, Santa Cruz Biotechnology). PVDF membranes were blocked with 5% nonfat dry milk in phosphate-buffered saline (PBS)-0.1% Tween 20 (PBS-T, Fisher Scientific, Pittsburgh, PA) for 2 h at room temperature. Membranes were incubated with primary antibodies against CBS (mouse, 1:1,000, sc-133208, Santa Cruz Biotechnology) overnight at 4°C on a rotary. After washing, membranes were incubated for 1 h at room temperature with anti-mouse horseradish peroxidase (HRP)-linked secondary antibody (1:10,000, 715-035-151, Jackson Immuno, West Grove, PA) in blocking buffer. After washing, blots were developed with the Immuno-Star WesternC Kit (Bio-Rad) and visualized on Hyperfilm-ECL (GE Life Sciences, Little Chalfont, UK).
Immunohistochemistry.
In addition to RT-PCR and immunoblot assays, to confirm CBS localization in the NTS we performed immunohistochemistry. Rats (n = 3) were transcardially perfused with 4% paraformaldehyde-0.1 M phosphate buffer as previously described (Austgen et al. 2008; Kline et al. 2010). Brain stems were cut in the coronal plane at 30 μm on a vibrating microtome (Leica, VT 1000s, Wetzlar, Germany). Immunohistochemistry was performed in every 1 in 6 sections throughout the rostral-caudal extant of the NTS. Sections were rinsed in PBS (pH 7.4) and blocked in 10% normal donkey serum (NDS, Millipore, Billerica, MA) in PBS with 0.3% Triton X-100 (PBS-Tx). Tissue was rinsed and incubated in PBS-Tx for 16 h with a primary antibody raised against CBS (mouse anti-CBS, 1:500, sc-133208, Santa Cruz Biotechnology). In a subset of sections, CBS immunohistochemistry was paired with an antibody against glial fibrillary acidic protein (rabbit anti-GFAP, 1:1,000, AB5804, Millipore), a marker for glia cells. The following day sections were rinsed in PBS and incubated for 2 h in PBS-Tx with a Cy3-conjugated donkey anti-mouse IgG (1:200, 705-165-003, Jackson Immuno) or a Cy2-conjugated donkey anti-rabbit IgG secondary antibody (1:200, 711-225-152, Jackson Immuno). Sections were rinsed a final time in PBS, mounted on gelatin-coated slides, air dried, and coverslipped with ProLong Gold (Invitrogen). Slides were then sealed with nail polish.
In each protocol, the primary antibody was withheld from individual sections for control purposes. Specificity of CBS antibody labeling was also tested in separate tissue sections where the CBS antibody was preabsorbed with a CBS recombinant protein (H00000875-P01, Abnova, Taipei, Taiwan). Primary antibody solutions (1:500) were incubated with the CBS recombinant protein (1:10) for 24 h, followed by tissue processing as described above. Parallel sections were incubated with CBS antibody alone for 24 h. Immunofluorescence did not differ with antibody incubation of 16 to 24 h.
Brain stem sections were examined with an Olympus epifluorescent microscope (BX51) with a three-axis motorized stage (Ludl Electronic Products, Hawthorn, NY). Appropriate filter sets for Cy2 and Cy3 were used to visualize immunofluorescent tissue. Images were acquired with a cooled monochrome digital camera (ORCA-AG, Hamamatsu, Bridgewater, NJ) with each required filter set at the same focal plane. Images were imported into Photoshop (version 11.0.2, Adobe, San Jose, CA), and image brightness and contrast were adjusted for clarity.
In vitro brain slice preparation.
Rats were deeply anesthetized with isoflurane (Vet-One, Meridian, IN) and decapitated, and the brain stem was quickly removed and placed in ice-cold low-Ca2+, high-Mg2+ artificial cerebrospinal fluid (aCSF, in mM: 124 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 d-glucose, 0.4 l-ascorbic acid, 1 CaCl2, and 2 MgCl2, saturated with 95% O2-5% CO2, pH 7.4, 315–325 mosmol/kgH2O). The medulla was trimmed ventrally to yield lengthy segments of tractus solitarius (TS). Horizontal slices (280 μm) were cut with a vibrating microtome (Leica), placed in a superfusion chamber, and secured with a nylon mesh. Slices were superfused at 3–4 ml/min with normal aCSF (in mM: 124 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 d-glucose, 0.4 l-ascorbic acid, and 2 CaCl2, saturated with 95% O2-5%CO2, pH 7.4, 315–325 mosmol/kgH2O) at 33°C.
Electrophysiological recordings.
Neurons were visualized with an Olympus BX51WIF microscope equipped with differential interface contrast (DIC) and an infrared-sensitive camera (Retiga, Q-Imaging, Burnaby, BC, Canada). All recordings were made from caudal (commissural and medial subnuclei) NTS neurons. To record excitatory postsynaptic currents (EPSCs), recording electrodes (8250 glass, 3.5–4.5 MΩ, Garner Glass, Claremont, CA) were filled with (in mM) 10 NaCl, 130 K+ gluconate, 11 EGTA, 1 CaCl2, 10 HEPES, 1 MgCl2, 2 MgATP, 0.2 NaGTP, pH 7.3, 295–300 mosmol/kgH2O. In experiments in which we recorded miniature (m)EPSCs, tetrodotoxin (1 μM) and GABAzine (SR 95531 HBr, 25 μM) were added to the bath. The recording pipette was guided with a piezoelectric micromanipulator (PCS-6000; Burleigh, Victor, NY). Neurons were voltage clamped (−60 mV) in the whole cell configuration. Evoked synaptic currents were generated by placing a concentric bipolar stimulating electrode (F. Haer, Bowdoinham, ME) on the TS, which contains the visceral afferents (Andresen and Kunze 1994), and stimulating at a duration of 0.1 ms with an isolated programmable stimulator (AMPI, Jerusalem, Israel). Stimulation intensity was increased until an EPSC was evoked. Final TS intensity was set at 1.5× threshold and was stimulated to generate a single evoked current (0.5 Hz) or multiple evoked currents (20 Hz). During 0.5-Hz protocols, a 5-mV step (−60 to −65 mV) was used to determine input resistance of the cell. Resting membrane potential (RMP) and action potential discharge (APD) were measured under current clamp. Action potentials were induced by step injection of 10 pA of depolarizing current for 50 ms (10 total steps). Data were recorded with a Multiclamp700B amplifier, filtered at 2 kHz, and sampled at 10 kHz with pClamp10 software (Molecular Devices, Palo Alto, CA).
Synaptic terminal calcium labeling and imaging.
Imaging was performed as previously described (Kline et al. 2009; Rogers et al. 2006a). Briefly, 4-wk-old rats (n = 4) were anesthetized with urethane, and the nodose-petrosal ganglia complex was exposed by a midline incision in the neck. Micropipettes were filled with Calcium Green-1-dextran (Molecular Probes; 15% with 1% Triton X-100 in distilled water). Pressure pulses (2–10 psi; Picospritzer model II, General Valve, Fairfield, NJ) injected the Calcium Green-1-dextran into the ganglion, with a total injected volume of ∼100 nl. Incisions were subsequently closed, animals were returned to their home cage, and anterograde transport of the dye took place over the next 5 days.
After 5 days, coronal brain slices (∼250 μm) containing the NTS were generated similar to those in electrophysiological studies. Brain slices were stored at room temperature until experiments. An individual slice was placed in a submerged chamber and perfused with aCSF at 33°C, and after stabilization of the slice a bipolar electrode (WPI) was situated on the TS. Time-lapse laser confocal calcium imaging used a Nikon F1 upright microscope with a Fast Scan confocal system, equipped with heated water-immersion objectives. Experiments focused on the caudal (medial and commissural subnuclei) NTS. The TS was stimulated with an isolated stimulator (WPI; −0.2 mA, 0.1 ms, 20 Hz). Recordings were made with either one of two protocols. In the first protocol, slices received 1) TS stimulation; 2) 10-min rest; 3) TS stimulation; 4) 10-min exposure to NaHS (10 μM) or AOA (1 mM); 5) TS stimulation. In this case, responses from the first and second TS stimulation periods were compared as time controls and responses from the second and third TS stimulation periods were compared as experimental groups (NaHS or AOA). In the second protocol, slices received 1) TS stimulation; 2) 10-min rest with no drug, NaHS (10 μM), or AOA (1 mM); 3) TS stimulation. Responses of the second TS stimulation period were compared with the first. As there were no differences in the responses in either protocol, data were combined and compared as time control, NaHS, or AOA.
Drugs.
The following drugs were used in electrophysiological and imaging studies: NaHS (H2S donor; Sigma, St. Louis, MO), AOA (CBS inhibitor; Sigma), tetrodotoxin citrate (TTX; Tocris, Ellisville, MO), and SR 95531 HBr (GABAzine, GABAA antagonist; Tocris). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Bath solutions were added by a gravity feed system from individual 60-ml reservoirs. Switching between solution reservoirs was achieved through valve controllers.
Data analysis.
CBS immunohistochemical data were analyzed in every 1 in 6 sections throughout the caudal-rostral NTS as previously described (Austgen et al. 2008; Fong et al. 2005; Kline et al. 2010). Positively labeled CBS neurons in bilateral NTS sections from 540 μm caudal to 900 μm rostral of calamus scriptorius (caudal pole of the area postrema) were counted by two individuals. Criteria used to identify positively labeled CBS cells included a filled cytosolic labeling and a blank nuclear region (Austgen et al. 2008; Fong et al. 2005; Kline et al. 2010). If the cells were out of focus they were excluded from analysis. Labeled processes were not included in the analysis. The two individual counts were averaged, and the caudal-rostral distribution of CBS was compared by one-way repeated-measures ANOVA (Sigma Plot 11, Systat).
Electrophysiological data were analyzed with Clampfit (Molecular Devices) software. Cells with holding currents less than −50 pA and a RMP depolarized more than −45 mV upon initial membrane rupture were not considered for further analysis. Second-order neurons were identified by jitter analysis, defined as the standard deviation of the TS-EPSC latency from the shock artifact (Doyle and Andresen 2001; Kline et al. 2002). Neurons with jitter values of <250 μs were considered monosynaptic and directly connected to sensory TS fibers. The paired pulse ratio (PPR) of two consecutive TS-EPSCs was determined from the ratio of the amplitude of the second TS-EPSC to that of the first TS-EPSC (TS-EPSC2/TS-EPSC1). The failure rate was the percentage of TS stimulations that failed to evoke an EPSC response. mEPSCs were discriminated from noise by thresholding events 2.5 times above the root mean square value of noise. Statistical analyses were performed with Microsoft Excel or SigmaPlot 11 (Systat). EPSC peak amplitude (pA), instantaneous frequency (Hz), decay time τ (τ90–10%, ms), and the total number of action potentials, threshold (mV), peak amplitude (mV), peak afterhyperpolarization from threshold (mV), and half-width (ms) between control and drug periods were compared by a paired t-test. Twenty-hertz TS-EPSC depression and APD by current step were compared by two-way repeated-measures ANOVA.
Terminal calcium labeling experiments examined the change in cytoplasmic calcium through changes in peak fluorescence (%ΔF/F), where F is the baseline fluorescence intensity in the synaptic terminal and ΔF is the change in intensity following TS stimulation (Rogers et al. 2006a). Background fluorescence was subtracted prior to analysis. A responsive terminal was defined as one in which TS stimulation produced a >10% increase in %ΔF/F. For a given experiment, all viable terminals were included for analysis during a drug protocol. Time control and drug periods were compared with one-way ANOVA.
For all analyses, statistical significance was accepted at P < 0.05. All data are presented as means ± SE.
RESULTS
CBS is localized in the NTS.
Real-time RT-PCR analysis demonstrated CBS mRNA (152 bp) in tissue isolated from NTS and cerebellum (used as a positive control; Fig. 1A). PCR product was not observed in the NT, NP, and −RT lanes. Immunoblot analysis confirmed the expression of CBS protein in NTS tissue. CBS protein in NTS was compared with two positive controls, HEK cells transfected with CBS mRNA and rat brain lysate. As shown in Fig. 1B, CBS protein was detected in NTS samples at the expected molecular mass with a prominent band at ∼62 kDa (Eto et al. 2002), matching bands from HEK cells transfected with CBS mRNA and rat brain lysate.
Fig. 1.
Cystathionine-β-synthase (CBS) is located in the nucleus tractus solitarii (NTS). A: real-time RT-PCR analysis demonstrated CBS mRNA (152 bp arrow) in tissue from NTS and cerebellum (CB; used as a positive control; Abe and Kimura 1996). No PCR product was observed in the no template (NT), no primer (NP), and no reverse transcriptase (−RT) lanes. L, 123-bp ladder. NTS and CB samples were run in duplicate. B: immunoblot analysis illustrated that CBS protein was located in the NTS. Twenty micrograms of NTS protein (3rd lane) was matched with HEK cells transfected with CBS (2nd lane) and a rat brain lysate (1st lane). Immunoblots for CBS show bands in each lane at ∼62 kDa arrow. MM, molecular mass.
CBS immunoreactivity is distributed in NTS neurons and glia.
Immunohistochemistry for CBS protein was performed to determine the distribution of CBS within the NTS network. As demonstrated in the representative photomicrographs (Fig. 2A), CBS immunoreactivity (CBS-IR) was localized throughout the NTS in cell bodies as well as the area postrema. To confirm the specificity of the CBS antibody, preabsorption of the CBS antibody with the CBS recombinant protein reduced or eliminated CBS-IR (Fig. 2B). IR in CBS-peptide and nonimmune controls (not shown) was similar and reduced compared with CBS-IR performed with antibody alone. CBS labeling in cell bodies was examined throughout the caudal to rostral extent of the NTS, from 540 μm caudal to 900 μm rostral to calamus scriptorius (the caudal pole of area postrema, denoted as “0” in Fig. 2C). As quantified in the NTS distribution plot (Fig. 2C; n = 3), CBS-IR cells were observed in the caudal (Fig. 2D) and postremal (Fig. 2E) regions yet were reduced in the rostral areas (Fig. 2F).
Fig. 2.
CBS immunoreactivity (IR) in the NTS. Coronal sections (30 μm) were immunohistochemically processed to examine the distribution of CBS within the NTS. As shown in A, CBS-IR was observed throughout the NTS, including in cell bodies. Preabsorption of the primary antibody with the recombinant protein decreased IR (B). Images in A and B were taken at the same exposure time and light histogram characteristics. To determine the caudal to rostral localization of CBS-IR, positively labeled CBS cells were counted bilaterally throughout the NTS (C; n = 3). CBS-IR was found in the caudal (D) and postremal (E) regions of the NTS, with diminished IR in the rostral (F) region. AP, area postrema. Scale bars, 50 μm.
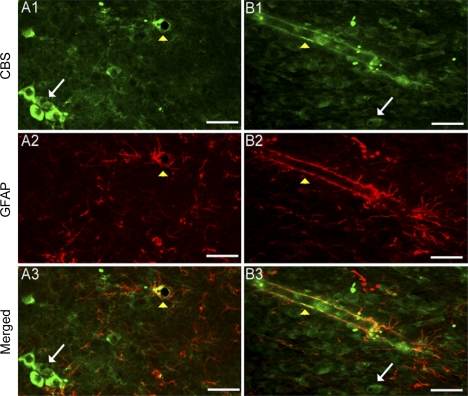
To determine whether CBS localized to glial cells in the NTS, a subset of tissue sections were cotreated with antibodies against a marker for glia and astrocytes, GFAP, and anti-CBS. As shown in Fig. 3 (white arrows), CBS-IR was localized to NTS cells that were negative for GFAP-IR. However, CBS-IR was also colocalized with GFAP-IR surrounding blood vessels throughout the NTS (Fig. 3, yellow arrowheads). Taken together, these results are consistent with our RT-PCR and immunoblot data demonstrating that CBS protein is localized in the NTS.
Fig. 3.
CBS is located in glia and nonglia cells. Pseudocolored photomicrographs of CBS (1, green) and glial fibrillary acid protein (GFAP) (2, red) in the NTS are shown. Subsequent merged images are also shown (3) In representative images of the NTS (A and B), CBS and GFAP do not colabel, suggesting that CBS is localized to neurons (white arrows). Scattered throughout the NTS, CBS and GFAP colabeling was observed near blood vessels (yellow arrowheads). Note the cross section of a blood vessel that contains CBS and GFAP-IR in A. Shown in B is a longitudinal section of an NTS blood vessel containing CBS and GFAP. Scale bars, 50 μm.
H2S modulates NTS synaptic function.
We next asked whether H2S in the NTS plays a functional role. Electrophysiology was performed in the caudal NTS in acute horizontal brain stem slices. Only monosynaptically driven neurons, those that form a direct synapse with afferent sensory fibers in the TS, were evaluated. Overall, monosynaptic neurons had a latency of 5.00 ± 0.21 ms, with a standard deviation of latency (jitter) of 204 ± 0.01 μs. Whole cell recordings were made from 35 monosynaptic neurons from 25 rats.
Exogenous H2S augments solitary tract-evoked excitatory postsynaptic currents.
The dose-response effect of exogenous H2S on TS-EPSC amplitude evoked at 0.5 Hz was determined. Varying doses of H2S were administered through bath application of the H2S donor NaHS. As shown in the representative traces in Fig. 4A, NaHS (10 μM, 5 min) increased the amplitude of TS-EPSCs evoked at 0.5 Hz compared with aCSF baseline. The individual increase in TS-EPSC amplitude during NaHS in the cells tested is shown in Fig. 4B. Quantitatively, 10 μM NaHS increased the average evoked current amplitude from 132 ± 15 pA to 181 ± 27 pA (Fig. 4B, control vs. NaHS, a 35 ± 7% increase; P < 0.05, n = 10). By comparison, 1 μM NaHS produced a variable augmentation among the cells tested that did not reach significance (control 122 ± 21 pA vs. NaHS 173 ± 58, a 40 ± 40% increase; n = 5, P = 0.37). Fifty micromolar NaHS significantly increased TS-EPSCs (control 50 ± 18 pA vs. NaHS 110 ± 28 pA, a 149 ± 56% increase; P < 0.05, n = 5), whereas 200 μM NaHS produced large depolarizations and cellular death (n = 2; data not shown). Subsequent experiments were completed with 10 μM NaHS.
Fig. 4.
Exogenous hydrogen sulfide (H2S) augments evoked synaptic activity. A: representative traces of solitary tract (TS) excitatory postsynaptic currents (EPSCs) (30 sweeps, averaged) that were augmented during bath application of NaHS (10 μM, 5 min) compared with artificial cerebrospinal fluid (aCSF) control. Cells were voltage clamped at −60 mV. B: group and scatterplot comparison (n = 10) shows that bath application of 10 μM NaHS (filled circles) increased the amplitude of monosynaptic TS-EPSCs compared with aCSF control (open circles; *P < 0.05). Small connected symbols indicate data from individual neurons. Large circles indicate means ± SE. C: 20-Hz TS train produced frequency-dependent depression in synaptic currents in aCSF control and after bath application of NaHS (10 μM). NaHS also augmented the amplitude of TS-EPSC1, -2, -3, -7, -10, -12, and -20 (*P < 0.05). D: representative example (average of 5 sweeps) of 2 consecutive TS-EPSCs during control and NaHS (20 Hz). Note that NaHS augmented both TS-EPSCs, yet the increase in TS-EPSC2 was greater than in TS-EPSC1. E: bath application of NaHS (10 μM, n = 10) significantly increased the paired pulse ratio (PPR; TS-EPSC2/TS-EPSC1) compared with control. *P < 0.05.
Increasing TS stimulus frequency to 20 Hz (20 events) was used to mimic in vivo enhancement of sensory afferent activation to the NTS. As typical of this synapse, continued TS stimulation reduced current amplitude following the first evoked event (use- or frequency-dependent depression; Fig. 4C). Bath application of 10 μM NaHS significantly augmented TS-EPSC amplitude throughout the stimulus train compared with control (P < 0.05, n = 10). Thus exogenous H2S can influence evoked synaptic activity during both low- and high-frequency TS stimulation.
The increase in TS-EPSC amplitude following bath application of NaHS was not accompanied by a change in input resistance (control 741 ± 147 MΩ vs. NaHS 598 ± 77 MΩ; n = 10), holding current (control −21.5 ± 14.3 pA vs. NaHS −10.9 ± 16.9 pA; n = 10), TS-EPSC decay time constant τ (τ90–10%, control 3.4 ± 1.0 ms vs. NaHS 3.5 ± 1.0 ms; n = 10), or percentage of failure rate (control 3.7 ± 1.9% vs. NaHS 5.3 ± 2.5%; n = 10).
Exogenous H2S augments TS-EPSCs by a presynaptic mechanism.
The augmentation of TS-EPSC amplitude by NaHS may be due to alterations at the presynaptic terminal or the postsynaptic receptor. Changes in presynaptic neurotransmitter release can be determined by examining the PPR (Regehr and Stevens 2001). A change in the PPR following NaHS that is not accompanied by an alteration in input resistance would suggest a presynaptic mechanism. Figure 4D is a representative example of two consecutive TS-EPSCs (50-ms interval) during baseline aCSF and NaHS. As stated above, bath application of NaHS increased the amplitude of TS-EPSC1 and TS-EPSC2. However, the relative increase of TS-EPSC2 due to NaHS was greater than that of TS-EPSC1. This augmentation in current amplitude following 10 μM NaHS enhanced the PPR from 0.31 ± 0.1 during control to 0.42 ± 0.1 during NaHS (Fig. 4E; P < 0.05, n = 10) without altering the input resistance.
To further examine the pre- vs. postsynaptic mechanism of NaHS on synaptic activity, mEPSCs were examined in a separate set of cells. mEPSCs recorded in the presence of TTX and GABAzine were used as a measure of quantal release of glutamate in the absence of action potential activity within the available NTS network. As illustrated in the example shown in Fig. 5A, NaHS augmented mEPSC frequency but not amplitude. Quantitative analysis confirmed that bath application of NaHS did not alter the amplitude of mEPSCs (Fig. 5B; control 14.1 ± 2.7 pA vs. NaHS 14.3 ± 3.9 pA; n = 5) but significantly enhanced their frequency (Fig. 5C; control 6.7 ± 1.0 Hz vs. NaHS 8.5 ± 1.0 Hz, a 30 ± 11% increase; P < 0.05, n = 5). Taken together, these data suggest that exogenous H2S, through bath application of NaHS, increased synaptic transmission most likely through an increase in presynaptic neurotransmitter release.
Fig. 5.
Exogenous H2S increases miniature (m)EPSC frequency. A: representative example of mEPSCs during aCSF control (top) and bath application of NaHS (10 μM, bottom). Recordings were made with GABAzine (25 μM) and tetrodotoxin (1 μM) present in the bath. Note the increase in current frequency. Group analysis of mEPSCs (n = 6) showed that bath application of NaHS (10 μM) did not change the amplitude of mEPSCs (B). However, NaHS enhanced the frequency of mEPSCs (C; *P < 0.05).
Exogenous H2S does not alter intrinsic membrane properties.
Measurement of membrane potential and action potential properties allows for direct analysis of changes in intrinsic cellular properties by H2S. Bath application of NaHS did not alter RMP (control −62 ± 4 mV vs. NaHS −54 ± 4 mV; n = 10). Microinjecting depolarizing current into the cell evoked APD during aCSF control and NaHS. Compared with control, NaHS did not alter the total number of action potentials to 10 steps (control 47 ± 13 vs. NaHS 40 ± 12; n = 7). Moreover, NaHS did not alter the properties of the first induced action potential: amplitude (control 82.8 ± 6.3 mV vs. NaHS 85.6 ± 4.5 mV; n = 7), peak afterhyperpolarization (control −22.8 ± 1.9 mV vs. NaHS −22.5 ± 0.8 mV; n = 7), half-width (control 2.3 ± 1.0 ms vs. NaHS 1.3 ± 0.1 ms; n = 7), or threshold of activation (control −32.9 ± 3.1 mV vs. NaHS −27.6 ± 1.5 mV; n = 7).
Blockade of endogenous CBS attenuates synaptic transmission in the NTS.
H2S is generated in the central nervous system by CBS (Abe and Kimura 1996), which is localized to the NTS (Figs. 1–3). To determine whether endogenous CBS-generated H2S plays a role in synaptic transmission under baseline slice conditions, the CBS inhibitor AOA (1 mM, 5 min) was bath applied. As shown in the representative example in Fig. 6A, AOA decreased the amplitude of TS-EPSCs evoked from a 0.5-Hz TS stimulation. The individual responses in TS-EPSC amplitude during AOA in the cells tested are shown in Fig. 6B. As demonstrated, AOA reduced the amplitude of evoked EPSCs in the majority of cells (11 of 15). There was a minority of cells in which AOA increased (n = 2) or did not appreciably change (n = 2) TS-EPSC amplitude. However, these latter neurons met the criteria for acceptance and remained in the study. Quantitatively, overall AOA significantly reduced the mean TS-EPSC amplitude from 171 ± 25 pA during aCSF control to 139 ± 27 pA in AOA (a 22 ± 7% decrease, P < 0.05, n = 15; Fig. 6B). During a 20-Hz TS stimulation, synaptic depression occurred in the presence of AOA and control (Fig. 6C), but AOA did not alter the level of depression to a significant extent (n = 15, P = 0.13). However, AOA did significantly reduce the amplitude of the initial current (TS-EPSC1, control 207 ± 27 vs. AOA 178 ± 29; P < 0.05, n = 15) and TS-ESPC4 (control 67.4 ± 9.6 vs. AOA 47.1 ± 7.3; P < 0.05, n = 15). Additionally, AOA did not alter the PPR (control 0.50 ± 0.07 vs. AOA, 0.55 ± 0.03; n = 15).
Fig. 6.
Blockade of endogenous H2S production reduces synaptic activity. A: representative traces of TS-EPSCs (30 sweeps, averaged) illustrating that bath application of aminooxyacetate (AOA; 1 mM, 5 min) reduced the amplitude of TS-EPSCs. B: group and scatterplot data of neurons prior to (open circles) and after (gray circles) bath application of AOA (1 mM). Small connected symbols indicate data from individual neurons. Large circles indicate means ± SE. Blockade of endogenous H2S production reduced the amplitude of TS-EPSCs (n = 15; *P < 0.05). C: stimulating the TS at 20 Hz produced frequency-dependent depression of TS-EPSCs in both control and AOA (1 mM; n = 15). Bath application of AOA significantly depressed the 1st and 4th TS-EPSC amplitudes (*P < 0.05) and reduced the amplitude of TS-EPSC9 (+P = 0.08) and TS-EPSC10 (+P = 0.07).
Input resistance did not change in the presence of AOA (control 679 ± 102 MΩ vs. AOA 682 ± 126 MΩ; n = 15). AOA also did not alter TS-EPSC decay time constant (τ90–10%: control 4.6 ± 1.6 ms vs. AOA 2.8 ± 0.5 ms; n = 15), percentage of failure rate (control 2.4 ± 1.4% vs. AOA 4.2 ± 1.7%; n = 15), or holding current (control −20.4 ± 11.3 pA vs. AOA −11.6 ± 16.1 pA; n = 6).
Blockade of endogenous H2S production does not alter mEPSCs.
Examination of mEPSCs in the presence of TTX and GABAzine revealed that AOA did not alter the amplitude (control −11.3 ± 1.9 pA vs. AOA −12.1 ± 2.6 pA) or frequency (control 9.3 ± 2.3 Hz vs. AOA 10.5 ± 2.7 Hz) of mEPSCs in a separate set of cells (n = 6). This information suggests that endogenous H2S has a role in augmentation of evoked neurotransmitter release but does not alter neurotransmitter release in the absence of NTS neural network activity.
Blockade of CBS does not alter intrinsic membrane properties.
Similar to NaHS, AOA did not alter membrane potential (control −59 ± 3 mV vs. AOA −59 ± 5 mV; n = 15). Furthermore, AOA did not alter the total number of action potentials to 10 current steps (control 42 ± 11 vs. AOA 25 ± 8; n = 9). Action potential amplitude (control 73.1 ± 8.0 mV vs. AOA 66.3 ± 8.4 mV; n = 9), peak afterhyperpolarization (control −19.2 ± 3.9 mV vs. AOA −17.6 ± 2.8 mV; n = 9), threshold (control −31.7 ± 2.1 mV vs. AOA −31.5 ± 2.8 mV; n = 9), and half-width (control 2.1 ± 0.6 ms vs. AOA 2.8 ± 0.7 ms; n = 9) also did not change with AOA. This suggests that blockade of endogenous H2S does not affect intrinsic cellular properties in the NTS.
H2S augments intracellular calcium in presynaptic terminals in NTS.
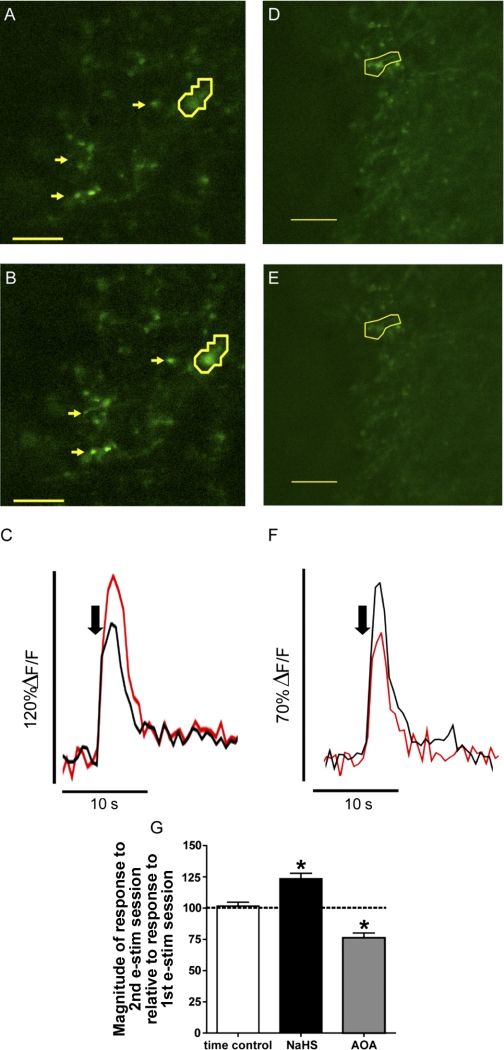
H2S enhances synaptic transmission between sensory afferents and second-order NTS cells by a presynaptic mechanism (Fig. 4). Conversely, AOA decreased synaptic transmission (Fig. 6). We subsequently determined whether the alteration in presynaptic neurotransmitter release by H2S or AOA is due to changes in intracellular calcium concentration at visceral afferent terminals in the NTS. TS stimulation in acute brain slices was used to depolarize Calcium Green-labeled sensory synaptic terminals and invoke an increase in calcium fluorescence (Kline et al. 2009; Rogers et al. 2006a). A representative example of the effect of NaHS and AOA on regions of interest (ROIs, 3 arrows and outlines) is shown in Fig. 7. TS stimulation increased terminal calcium fluorescence (Fig. 7, A and D), indicative of an augmentation of intracellular calcium. In the presence of NaHS (Fig. 7B) the augmentation of terminal fluorescence during TS stimulation was potentiated, whereas AOA reduced terminal fluorescence during TS stimulation (Fig. 7E). Compared with their time controls, NaHS augmented calcium in synaptic terminals [Fig. 7C, representing the circled ROIs in Fig. 7A (control) and Fig. 7B (NaHS)] and AOA decreased calcium in synaptic terminals [Fig. 7F, representing circled ROIs in Fig. 7D (control) and Fig. 7E (AOA)]. Quantitatively, the enhancement in TS-induced fluorescence intensity (%ΔF/F) during NaHS (123 ± 4.4%, 88 ROIs) was greater than time (aCSF) control (102 ± 3% change from 1st response, 130 ROIs; Fig. 7G). Conversely, in the presence of AOA, calcium fluorescence was significantly attenuated compared with the aCSF time controls (76 ± 3.8%, 82 ROIs; Fig. 7G).
Fig. 7.
H2S modulates presynaptic calcium concentration. A and D: example of Calcium Green-labeled afferent varicosities in the NTS during stimulation of the TS during aCSF. B: Varicosities shown in A during TS stimulation in the presence of NaHS (10 μM). Yellow arrows and outlines in A and B highlight sensory afferent varicosities that augment fluorescent intensity after NaHS. The outlined varicosities are further plotted in C and demonstrate a significant increase in change in peak fluorescence from baseline (%ΔF/F) on application of NaHS (red) compared with the control stimulation in aCSF (black). E: varicosities shown in D during TS stimulation in the presence of AOA (1 mM). Yellow outlines in D and E demonstrate sensory afferent varicosities that have reduced fluorescent intensity after AOA. The outlined varicosities are plotted in F and demonstrate a significant reduction in %ΔF/F following application of AOA (red) compared with control stimulation in aCSF (black). Black arrow in C and F denotes point of TS stimulation. G: change in fluorescence intensity during time control [aCSF, 103 regions of interest (ROIs)], NaHS (10 μM, 88 ROIs), and AOA (1 mM, 80 ROIs) compared with the 1st initial event. Note that %ΔF/F did not significantly change over time. However, H2S significantly increased the fluorescence of sensory afferent varicosities, while AOA significantly decreased the fluorescence of sensory afferent varicosities (*P < 0.05). Scale bar, 5 μm.
DISCUSSION
The present study is the first to demonstrate a functional role for H2S in the NTS. We show that the H2S-generating enzyme CBS was located in neurons and glia in NTS. Exogenous H2S, through the donor NaHS, augmented synaptic transmission in TS-driven monosynaptic NTS neurons, primarily by a presynaptic mechanism. Blockade of endogenous H2S with the CBS antagonist AOA depressed synaptic activity. Modulation of synaptic transmission by H2S was accompanied by alterations in presynaptic calcium concentration. H2S had little effect on postsynaptic cellular properties. Taken together, the results suggest that H2S in NTS modulates synaptic transmission by presynaptic alteration in neurotransmitter release.
CBS is located in the NTS.
H2S is primarily generated in the central nervous system by the enzyme CBS, which is distributed throughout the brain (Kimura 2002; Mancuso et al. 2010; Qu et al. 2008) and localized to glial cells and neurons (Abe and Kimura 1996; Enokido et al. 2005; Hu et al. 2008; Ichinohe et al. 2005). We show through immunohistochemistry, immunoblot, and RT-PCR that CBS was located in the NTS, a region critical for modulation of autonomic and respiratory reflexes. CBS was found in cells caudal to and at the level of the area postrema, regions that contain the central terminals of visceral sensory afferents, including those originating from arterial baroreceptors and chemoreceptors (Andresen and Kunze 1994). We did not, however, observe CBS-IR in punctate structures that would be suggestive of synaptic terminals. Rather, CBS was localized to cells, presumably neurons, devoid of GFAP. The phenotypes of these cells or their target are unknown and require further study. CBS-IR also colocalized with GFAP-IR surrounding blood vessels. Because the NTS lacks a true blood-brain barrier (Maolood and Meister 2009), the localization of CBS in neurons and glia surrounding the vasculature raises the possibility that H2S may also act in vascular-neuronal signaling, much like NO (Paton et al. 2002). Regardless of the source of H2S production, the results of the present study demonstrate that H2S functionally modulates synaptic transmission in the NTS.
Exogenous H2S augments synaptic transmission by a presynaptic mechanism.
H2S is a potent neuromodulator in other central nuclei (Abe and Kimura 1996; Dawe et al. 2008; Enokido et al. 2005; Eto et al. 2002; Russo et al. 2000). Endogenous H2S concentrations in central tissue have been estimated to range between 10 nM and 160 μM (Kimura 2002; Mancuso et al. 2010; Qu et al. 2008). In initial studies, the H2S donor NaHS was bath applied between 1 and 200 μM, based on previous studies and within predicted physiological concentrations (Dawe et al. 2008; Hu et al. 2009; Peng et al. 2010). We show that 10–50 μM NaHS increased TS-EPSCs, consistent with previous studies showing that 10–60 μM NaHS modulates long-term potentiation (Abe and Kimura 1996). Several lines of evidence suggest that the increase in TS-EPSC amplitude is due to a presynaptic mechanism. H2S increased the PPR of two consecutive TS-EPSCs without altering TS-EPSC decay time constant or input resistance. H2S also increased mEPSC frequency but not mEPSC amplitude. The augmentation of presynaptic calcium by NaHS further supports a presynaptic action of H2S. These changes further occur without altering RMP or action potential firing in response to depolarizing current injections. Altogether, the results suggest that H2S increases quantal glutamate release from sensory afferents rather than altering postsynaptic receptor sensitivity or excitability.
H2S increases presynaptic terminal calcium in the NTS.
Cytosolic calcium influx is vital to neurotransmitter release, and H2S augments activity of several calcium-permeable channels. Transient receptor potential cation channel subfamily V member 1 (TRPV1) is present in sensory afferents and augments transmitter release in the NTS (Guo et al. 1999; Peters et al. 2010). Additionally, visceral presynaptic L-type calcium currents contribute to TS-EPSCs (Andresen and Mendelowitz 1996) and T-type calcium currents in nodose sensory afferents may play a role in synaptic transmission in the NTS, especially spontaneous release at membrane potentials near their activation range (Kline et al. 2009; Lambert et al. 1998; Pachuau and Martin-Caraballo 2007). H2S augments the activity of TRPV1 (Schicho et al. 2006; Trevisani et al. 2005) and L- and T-type calcium channels (Kawabata et al. 2007; Takahashi et al. 2010; Tang et al. 2010), which is consistent with the observed increase in presynaptic calcium and neurotransmitter release. Finally, H2S modulates calcium release from intracellular stores (Tang et al. 2010), which contribute to neurotransmitter release in NTS (Rogers et al. 2006b). These potential mechanisms are intriguing, and future studies are needed to determine their contribution in the action of H2S in the NTS.
Endogenous H2S modulates synaptic transmission.
CBS is the predominant H2S-generating enzyme in the brain (Gadalla and Snyder 2010). To determine whether endogenous H2S produced from CBS has a role in synaptic transmission, we used the CBS inhibitor AOA at the concentration that blocks H2S production ∼75% (Abe and Kimura 1996). AOA reduced TS-EPSC amplitude in the majority of neurons during a 0.5-Hz TS stimulus, the first and fourth TS-EPSC in a 20-Hz train, and presynaptic calcium entry. This occurred without an alteration in EPSC decay time constant, input resistance, or membrane properties. This suggests a presynaptic mechanism. We did observe a minority of cells in which AOA did not reduce TS-EPSC amplitude. The nature of these latter responses is unclear; however, given the diffuse nature of CBS expression (Fig. 2), it is possible that these cells either do not contain CBS or are not in close proximity to CBS-containing neurons to be effected by AOA's block of H2S production.
In comparison to the effects of NaHS on the PPR and mEPSC, AOA did not significantly alter these parameters. The lack of alterations in mEPSC amplitude or frequency during AOA is of particular interest, as it could be expected that withdrawal of H2S by AOA would have the opposite effect of NaHS on the frequency of mEPSC. These responses may be due to several factors. First, they may be due to low basal H2S generation under in vitro recording conditions. Recent studies in the carotid body demonstrate that H2S production under normoxic conditions is low because of CO inhibition of its production (Peng et al. 2010). The NO- and CO-generating enzymes are localized in the NTS (Lin et al. 2007; Lo et al. 2004), and the binding of NO and CO to CBS heme moiety inhibits H2S production (Mancuso et al. 2010; Szabó 2007). Thus NO and CO may reduce endogenous H2S production within the NTS. Second, AOA did not alter mEPSC properties when recorded in TTX to block neuronal activity. CBS is a Ca2+/calmodulin-dependent enzyme and activity dependent (Abe and Kimura 1996; Enokido et al. 2005; Hu et al. 2008; Ichinohe et al. 2005). Therefore neuronal activity, such as occurs in vivo, may be required to elicit the full effects of endogenous H2S.
The concentration of AOA used in the present experiments was similar to that in previous studies (Abe and Kimura 1996). However, one consideration with the use of AOA is its potential influence on GABAergic activity. AOA may increase GABAergic signaling in neurons through blockade of γ-aminobutyrate aminotransferase (Pagliusi et al. 1983), which breaks down GABA, resulting in increased GABA in the synaptic cleft. Our results are consistent with AOA altering synaptic transmission through blockade of CBS rather than via a GABAergic mechanism. Specifically, AOA did not alter EPSC decay time, confirming that AOA did not increase inhibitory shunting or TS-evoked GABAergic currents. RMP and holding currents were also not changed. Rather, these data suggest that the changes in synaptic activity with AOA are due to blockade of CBS and withdrawal of H2S on glutamatergic signaling.
In summary, the present study demonstrates a novel influence of H2S on synaptic activity in the NTS, during both the application of exogenous H2S and the withdrawal of endogenous H2S, through blockade of CBS activity. The effect of H2S is to increase synaptic transmission between visceral afferents and second-order neurons via mechanisms at the presynaptic terminal, including enhancement in intracellular calcium, rather than altering postsynaptic properties. Thus H2S plays an important role as a gaseous neuromodulator in the NTS. The combination of H2S activity within the NTS at initial synapse of the peripheral afferents within the NTS in addition to H2S activity within the carotid body (Peng et al. 2010) and through carotid afferents (Li et al. 2010) suggests a possible important role in maintaining or modulating autonomic or respiratory function.
GRANTS
This work was funded through National Heart, Lung, and Blood Institute HL-085108 (D. D. Kline).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Brenna Barger for her technical assistance with the real-time RT-PCR preparation and analysis and Dr. Eileen M. Hasser, Dr. Cheryl M. Heesch, and Dr. M. Cathleen Kuehl-Kovarik for critical review of this manuscript.
REFERENCES
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen M. Cardiovascular integration in the nucleus of the solitary tract. In: Neural Mechanisms of Cardiovascular Regulation, edited by Dun N, Machado B, Pilowsky P. Boston, MA: Kluwer, 2004, p. 59–80 [Google Scholar]
- Andresen M, Kunze D. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994 [DOI] [PubMed] [Google Scholar]
- Andresen M, Mendelowitz D. Sensory afferent neurotransmission in caudal nucleus tractus solitarius—common denominators. Chem Senses 21: 387–395, 1996 [DOI] [PubMed] [Google Scholar]
- Austgen J, Fong A, Foley C, Mueller P, Kline D, Heesch C, Hasser E. Expression of group I metabotropic glutamate receptors on phenotypically different cells within the nucleus of the solitary tract in the rat. Neuroscience 159: 701–716, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantikyan A, Song G, Feinberg-Zadek P, Poon CS. Intrinsic and synaptic long-term depression of NTS relay of nociceptin- and capsaicin-sensitive cardiopulmonary afferents hyperactivity. Pflügers Arch 457: 1147–1159, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampney R. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- Dawe G, Han S, Bian J, Moore P. Hydrogen sulphide in the hypothalamus causes an ATP-sensitive K+ channel-dependent decrease in blood pressure in freely moving rats. Neuroscience 152: 169–177, 2008 [DOI] [PubMed] [Google Scholar]
- Doyle M, Andresen M. Reliability of monosynaptic sensory transmission in brain stem neurons in vivo. J Neurophysiol 85: 2213–2223, 2001 [DOI] [PubMed] [Google Scholar]
- Enokido Y, Suzuki E, Iwasawa K, Namekata K, Okazawa H, Kimura H. Cystathionine β-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J 19: 1854–1856, 2005 [DOI] [PubMed] [Google Scholar]
- Eto K, Ogasawara M, Umemura K, Nagai Y, Kimura H. Hydrogen sulfide is produced in response to neuronal excitation. J Neurosci 22: 3386–3391, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fong A, Stornetta R, Foley C, Potts J. Immunohistochemical localization of GAD67-expressing neurons and processes in the rat brainstem: subregional distribution in the nucleus tractus solitarius. J Comp Neurol 493: 274–290, 2005 [DOI] [PubMed] [Google Scholar]
- Gadalla M, Snyder S. Hydrogen sulfide as a gasotransmitter. J Neurochem 113: 14–26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoreceptor and IB4 binding sites. Eur J Neurosci 11: 946–958, 1999 [DOI] [PubMed] [Google Scholar]
- Hu H, Shi Y, Chen Q, Yang W, Zhou H, Chen L, Tang Y, Zheng Y. Endogenous hydrogen sulfide is involved in regulation of respiration in medullary slice of neonatal rats. Neuroscience 156: 1074–1082, 2008 [DOI] [PubMed] [Google Scholar]
- Hu LF, Lu M, Wu ZY, Wong PH, Bian J. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol Pharmacol 75: 27–34, 2009 [DOI] [PubMed] [Google Scholar]
- Ichinohe A, Kanaumi T, Takashima S, Enokido Y, Nagai Y, Kimura H. Cystathionine β-synthase is enriched in the brains of Down's patients. Biochem Biophys Res Commun 338: 1547–1550, 2005 [DOI] [PubMed] [Google Scholar]
- Kawabata A, Ishiki T, Nagasawa K, Yoshida S, Maeda Y, Takahashi T, Sekiguchi F, Wada T, Ichida S, Nishikawa H. Hydrogen sulfide as a novel nociceptive messenger. Pain 132: 74–81, 2007 [DOI] [PubMed] [Google Scholar]
- Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol 26: 13–19, 2002 [DOI] [PubMed] [Google Scholar]
- Kline D, Hendricks G, Hermann G, Rogers R, Kunze D. Dopamine inhibits N-type channels in visceral afferents to reduce synaptic transmitter release under normoxic and chronic intermittent hypoxic conditions. J Neurophysiol 101: 2270–2278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D, King T, Austgen J, Heesch C, Hasser E. Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla. Neuroscience 167: 510–527, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D, Ramirez-Navarro A, Kunze D. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci 27: 4663–4673, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D, Takacs K, Ficker E, Kunze D. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol 88: 2736–2744, 2002 [DOI] [PubMed] [Google Scholar]
- Krukoff T. Central actions of nitric oxide in regulation of autonomic functions. Brain Res Rev 30: 52–65, 1999 [DOI] [PubMed] [Google Scholar]
- Lambert R, McKenna F, Maulet Y, Talley E, Bayliss D, Cribbs L, Lee JH, Perez-Reyes E, Feltz A. Low-voltage-activated Ca2+ currents are generated by members of the CavT subunit family (α1G/H) in rat primary sensory neurons. J Neurosci 18: 8605–8613, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sun B, Wang X, Jin Z, Zhou Y, Dong L, Jiang LH, Rong W. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid Redox Signal 12: 1179–1189, 2010 [DOI] [PubMed] [Google Scholar]
- Lin LH, Taktakishvili O, Talman W. Identification and localization of cell types that express endothelial and neuronal nitric oxide synthase in the rat nucleus tractus solitarii. Brain Res 1171: 42–51, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WC, Chan J, Tung CS, Tseng CJ. Carbon monoxide and metabotropic glutamate receptors in rat nucleus tractus solitarii: participation in cardiovascular effect. Eur J Pharmacol 454: 39–45, 2002 [DOI] [PubMed] [Google Scholar]
- Lo WC, Hsiao M, Tung CS, Tseng CJ. The cardiovascular system effects of nitric oxide and carbon monoxide in the nucleus tractus solitarii of rats. J Hypertens 22: 1183–1190, 2004 [DOI] [PubMed] [Google Scholar]
- Loewy A. Central autonomic pathways. In: Central Regulation of Autonomic Functions, edited by Loewy A, Spyer K. New York: Oxford Univ. Press, 1990, p. 88–103 [Google Scholar]
- Mancuso C, Navarra P, Preziosi P. Roles of nitric oxide, carbon monoxide, and hydrogen sulfide in the regulation of the hypothalamic-pituitary-adrenal axis. J Neurochem 113: 563–575, 2010 [DOI] [PubMed] [Google Scholar]
- Maolood N, Meister B. Protein components of the blood-brain barrier (BBB) in the brainstem area postrema-nucleus tractus solitarius region. J Chem Neuroanat 37: 182–195, 2009 [DOI] [PubMed] [Google Scholar]
- Pachuau J, Martin-Caraballo M. Extrinsic regulation of T-type Ca2+ channel expression in chick nodose ganglion neurons. Dev Neurobiol 67: 1915–1931, 2007 [DOI] [PubMed] [Google Scholar]
- Pagliusi S, Gomes C, Leite J, Trolin G. Aminooxyacetic acid induced accumulation of GABA in the rat brain. Interaction with GABA receptors and distribution in compartments. Naunyn Schmiedebergs Arch Pharmacol 322: 210–215, 1983 [DOI] [PubMed] [Google Scholar]
- Paton J, Kasparov S, Paterson D. Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci 25: 626–631, 2002 [DOI] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla M, Kumar G, Snyder S, Prabhakar N. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA 107: 10719–10724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, McDougall S, Fawley J, Smith S, Andresen M. Primary afferent activation of thermosensitive glutamate release at central neurons. Neuron 65: 657–669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar N. Endogenous carbon monoxide in control of respiration. Respir Physiol 114: 57–64, 1998 [DOI] [PubMed] [Google Scholar]
- Qu K, Lee S, Bian J, Low CM, Wong PH. Hydrogen sulfide: neurochemistry and neurobiology. Neurochem Int 52: 155–165, 2008 [DOI] [PubMed] [Google Scholar]
- Regehr W, Stevens C. Physiology of synaptic transmission and short-term plasticity. In: Synapses, edited by Cowan W, S̈udhof T, Stevens C, Davies K. Baltimore, MD: Johns Hopkins Univ. Press, 2001, p. 135–177 [Google Scholar]
- Ritter J. Human pharmacology of hydrogen sulfide, putative gaseous mediator. Br J Clin Pharmacol 69: 573–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R, Nasse J, Hermann G. Live-cell imaging methods for the study of vagal afferents within the nucleus of the solitary tract. J Neurosci Methods 150: 47–58, 2006a [DOI] [PubMed] [Google Scholar]
- Rogers R, Van Meter M, Hermann G. Tumor necrosis factor potentiates central vagal afferent signaling by modulating ryanodine channels. J Neurosci 26: 12642–12646, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo C, Tringali G, Ragazzoni E, Maggiano N, Menini E, Vairano M, Preziosi P, Navarra P. Evidence that hydrogen sulphide can modulate hypothalamo-pituitary-adrenal axis function: in vitro and in vivo studies in the rat. J Neuroendocrinol 12: 225–233, 2000 [DOI] [PubMed] [Google Scholar]
- Schicho R, Krueger D, Zeller F, Von Weyhern C, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B, Schemann M. Hydrogen sulfide is a novel prosecretory neuromodulator in the guinea-pig and human colon. Gastroenterology 131: 1542–1552, 2006 [DOI] [PubMed] [Google Scholar]
- Spyer K. The central nervous organization of reflex circulatory control. In: Central Regulators of Autonomic Functions, edited by Loewy A, Spyer K. New York: Oxford Univ. Press, 1990, p. 168–188 [Google Scholar]
- Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discovery 6: 917–935, 2007 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Aoki Y, Okubo K, Maeda Y, Sekiguchi F, Mitani K, Nishikawa H, Kawabata A. Upregulation of Cav3.2 T-type calcium channels targeted by endogenous hydrogen sulfide contributes to maintenance of neuropathic pain. Pain 150: 183–191, 2010 [DOI] [PubMed] [Google Scholar]
- Tan B, Wong PH, Bian J. Hydrogen sulfide: a novel signaling molecule in the central nervous system. Neurochem Int 56: 3–10, 2010 [DOI] [PubMed] [Google Scholar]
- Tang G, Wu L, Wang R. Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol 37: 753–763, 2010 [DOI] [PubMed] [Google Scholar]
- Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N, Zagli G, Cremion C, Geppetti P, Harrison S. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol 145: 1123–1131, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Paton J, Kasparov S. Differential sensitivity of excitatory and inhibitory synaptic transmission to modulation of nitric oxide in rat nucleus tractus solitarii. Exp Physiol 92: 371–382, 2007 [DOI] [PubMed] [Google Scholar]