Abstract
Extrasynaptic GABAA receptors (eGABARs) allow ambient GABA to tonically regulate neuronal excitability and are implicated as targets for ethanol and anesthetics. These receptors are thought to be heteropentameric proteins made up of two α subunits—either α4 or α6—two β2 or β3 subunits, and one δ subunit. The GABA analog 4,5,6,7-tetrahydroisoxazolo (5,4-c)pyridin-3(-ol) (THIP) has been proposed as a selective ligand for eGABARs. Behavioral and in vitro studies suggest that eGABARs have nanomolar affinity for THIP; however, all published studies on recombinant versions of eGABARs report micromolar affinities. Here, we examine THIP sensitivity of native eGABARs on cerebellar neurons and on reconstituted GABARs in heterologous systems. Concentration-response data for THIP, obtained from cerebellar granule cells and molecular layer interneurons in wild-type and δ subunit knockout slices, confirm that submicromolar THIP sensitivity requires δ subunits. In recombinant experiments, we find that δ subunit coexpression leads to receptors activated by nanomolar THIP concentrations (EC50 of 30–50 nM for α4β3δ and α6β3δ), a sensitivity almost 1,000-fold higher than receptors formed by α4/6 and β3 subunits. In contrast, γ2 subunit expression significantly reduces THIP sensitivity. Even when δ subunit cDNA or cRNA was supplied in excess, high- and low-sensitivity THIP responses were often apparent, indicative of variable mixtures of low-affinity αβ and high-affinity αβδ receptors. We conclude that δ subunit incorporation into GABARs leads to a dramatic increase in THIP sensitivity, a defining feature that accounts for the unique behavioral and neurophysiological properties of THIP.
Keywords: δ subunit, cerebellar granule cell, tonic inhibition
fast signaling by gaba in the brain is mediated by GABAA receptors (GABARs), heteropentameric proteins that form agonist-gated chloride channels and can be classified into two main groups (Farrant and Nusser 2005). Synaptic GABARs (sGABARs) have a relatively low sensitivity to GABA, appropriate for fast phasic inhibition, and are thought to consist of two α subunits, two β subunits, and one γ subunit (usually, γ2). By contrast, extrasynaptic GABARs (eGABARs) have a high GABA sensitivity, are tonically activated by low concentrations of ambient GABA, and have a distinct molecular composition, with the most abundant subtypes typically consisting of α6 or α4, together with β and δ subunits. Although not as abundant as sGABARs, eGABARs are persistently activated by ambient GABA, allowing them to exert a powerful, inhibitory influence over excitability (Farrant and Nusser 2005). These receptors have been suggested as important targets for alcohol (Sundstrom-Poromaa et al. 2002; Wallner et al. 2003) and as possible mediators of physiological sleep (Winsky-Sommerer et al. 2007).
Due to the heteromeric nature of eGABARs, discrimination of their molecular composition is challenging in both native and recombinant systems. For γ2 subunit-containing sGABARs, sensitivity to classical benzodiazepines (BZ) provides a definitive means to identify receptor subtype, since γ subunits are required for most high-affinity BZ binding (Pritchett et al. 1989). Unfortunately, no equivalent pharmacological tools exist for eGABARs. Although it has been argued that differential Zn2+ sensitivity, sensitivity to anesthetics, or sensitivity to low-concentration ethanol can discriminate δ subunit-containing from δ subunit-lacking (i.e., αxβx) receptors, these features remain controversial, particularly in recombinant systems (Baur et al. 2009; Meera et al. 2009, 2010; Santhakumar et al. 2007; Storustovu and Ebert 2006; Sundstrom-Poromaa et al. 2002; Wallner et al. 2003).
We reasoned that some of this variability and controversy over basic pharmacological properties may have arisen from difficulties in reconstituting heteropentameric, δ subunit-containing GABARs in recombinant systems (Meera et al. 2010). To evaluate this issue in more detail, we turned to the conformationally constrained GABA analog 4,5,6,7-tetrahydroisoxazolo (5,4-c)pyridin-3(-ol) (THIP) (Krogsgaard-Larsen et al. 1977). THIP has been suggested as a selective ligand for eGABARs (Belelli et al. 2005; Cope et al. 2005; Drasbek and Jensen 2006; Jia et al. 2005; Winsky-Sommerer et al. 2007), yet the reported THIP EC50 of recombinant δ subunit-containing receptors range from 3 to 53 μM (Adkins et al. 2001; Brown et al. 2002; Jia et al. 2005; Mortensen et al. 2010; Saarelainen et al. 2008; Storustovu and Ebert 2006), which are far too high to account for this selectivity. We therefore decided to systematically compare the activity of THIP at recombinant and native GABARs. We find that coexpression of δ subunits in recombinant systems leads to the formation of GABARs, which possess an almost three orders of magnitude higher THIP sensitivity than their αβ counterparts, whereas γ2 subunit coexpression reduces the apparent THIP sensitivity. Our findings thus confirm THIP as a valuable tool for discriminating eGABARs in native and recombinant settings. They also imply that inclusion of δ and γ subunits allosterically influences ligand sensitivity. This improved understanding of the basis for selectivity of the GABA-site ligand THIP should facilitate the development of subtype-specific GABAR drugs.
MATERIALS AND METHODS
Animal care and all experimental procedures were approved by the Chancellors Animal Research Committee (the Institutional Animal Care and Use Committee) at the University of California, Los Angeles.
Xenopus oocyte electrophysiology.
Standard methods have been used for isolation, injection, and recordings from Xenopus oocytes and for preparation of cRNA (Wallner et al. 2003). Oocytes were injected with 0.4 ng of α and β subunit cRNA and 2 ng of δ subunit or γ2 subunit cRNA. Currents were measured 1–10 days after injection using a two-electrode voltage clamp mode at −80 mV. As indicated, GABA and THIP were added to the perfusion medium (ND96; composition in mM: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, pH 7.5).
To fit concentration-response curves (see Figs. 3–6), we used the Hill equation: I/Imax = 1/(1 + (EC50/[agonist])h), where I is the agonist-evoked current, Imax is the maximum current, EC50 is the concentration of drug eliciting a one-half-maximal response, “[agonist]” is the agonist concentration, and h is the Hill coefficient. Double-component Hill equation fits (see Fig. 4B) consisted of an expansion of the above equation, such that I/Imax = Plow/(1 + (EC50, low/[agonist])h, low) + Phigh/(1 + (EC50, high/[agonist])h, high), where Plow and Phigh sum to 1 and, respectively, represent the low- and high-affinity components of the curve. The χ2 goodness of fit test was used to determine the appropriateness of single- vs. double-component Hill equations. Current values elicited by saturating GABA have been normalized to the same values as the THIP responses in these figures, and thus are a measure of the relative efficacy of GABA vs. THIP.
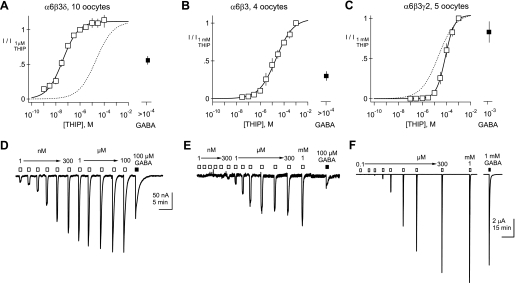
Fig. 3.
Inclusion of a δ subunit confers nanomolar THIP sensitivity. Normalized mean concentration-response curves for Xenopus oocytes injected with cRNAs encoding α6, β3, and δ (A); α6 and β3 (B); or α6, β3, and γ2 (C). EC50 and Hill slopes (h) of the solid line-fitted curves are 37 nM and 0.68, 21 μM and 0.61, and 80 μM and 1.15 for A–C, respectively. Dotted lines in panels A and C reproduce the fitted curve for α6β3. D–F: representative examples of THIP-evoked currents obtained from single Xenopus oocytes injected with α6β3δ (D); α6β3 (E); or α6β3γ2 (F). THIP and GABA applications are indicated by open and filled boxes above the traces, respectively. THIP concentrations ranging from 1 nM to 1 mM were applied as indicated, followed by a saturating concentration of GABA (100 μM or 1 mM). Scale bar in D applies to both D and E. I/I1 μM and I/I1 mM represent current at each concentration normalized to the current at 1 μM and 1 mM, repectively.
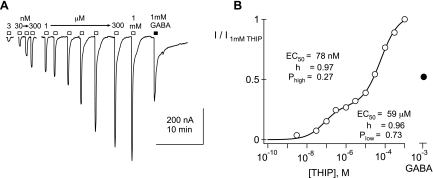
Fig. 4.
Biphasic THIP concentration-response curve from an α4β3δ-injected oocyte. A: representative THIP-evoked currents obtained from a Xenopus oocyte, injected with cRNA, encoding α4, β3, and δ. Responses to THIP concentrations of 3 nM, 30 nM, 100 nM, 300 nM, and 1–1,000 μM are shown, along with a response to a saturating concentration of GABA (1 mM). B: concentration-response plot of the THIP data in A is indicated with open circles. The filled circle shows the relative size of the current elicited by 1 mM GABA. Fit of a double-component Hill equation to the THIP data is displayed as a solid line; the fit yields the indicated parameters.
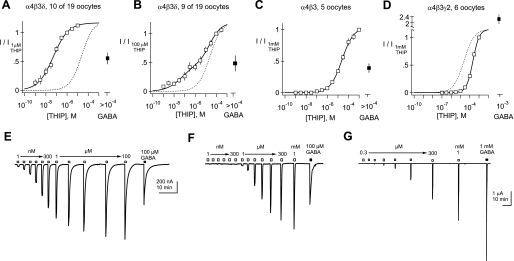
Fig. 5.
Summary of concentration-response data for THIP obtained from α4β3δ-, α4β3-, and α4β3γ2-injected oocytes. A–D: normalized mean concentration-response curves for Xenopus oocytes injected with cRNAs encoding α4, β3, and δ (A and B); α4 and β3 (C); or α4, β3, and γ2 (D). EC50 and Hill slopes of the solid line-fitted curves are A: 51 nM and 0.61; C: 24 μM and 0.79; and D: 219 μM and 1.3. A subset of oocytes injected with α4, β3, and δ was analyzed separately based on the threshold concentration generating a response and the shallowness of the concentration-response curve. B: fit with the sum of 2 Hill equations constrained to have EC50 (51 nM and 24 μM) and Hill slopes (0.61 and 0.79) obtained from the fits to A and C, with the only free parameter being the ratio of these 2 Hill components (Phigh = 0.39 and Plow = 0.61). The dotted lines (A, B, and D) are the curve from C. E–G: representative examples of THIP-evoked currents obtained from single Xenopus oocytes injected with cRNA encoding α4, β3, and δ (E); α4 and β3 (F); or α4, β3, and γ2 (G). THIP concentrations ranging from 1 nM to 1 mM, followed by a saturating concentration of GABA (100 μM or 1 mM), were tested on each oocyte.
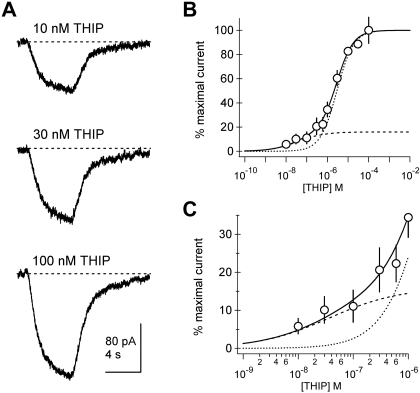
Fig. 6.
Human embryonic kidney (HEK) cell recordings also indicate a high-affinity pool of α6β3δ receptors. A: example currents elicited from a HEK-293 cell transfected with α6, β3, and δ cDNA. Currents were easily discriminated at concentrations of THIP as low as 10 nM. B: population concentration-response data indicate a high-affinity component. Each point represents mean ± SEM normalized current amplitude from 8 to 19 cells. The solid line is a fit of a double-component Hill with EC50 of 37 nM and 2.9 μM and slopes (h) of 0.68 and 1.08, respectively. The high-affinity component represents 16% of the population. Curves representing the high-affinity (dashed) and low-affinity (dotted) Hill components are superimposed. C: an expansion of the submicromolar concentration range indicates that responses to the lowest THIP concentrations are systematically larger than those predicted by a single-component Hill relationship.
Human embryonic kidney cell electrophysiology.
Human α6, β3, and δ cDNAs were either cloned by RT-PCR using human total brain mRNA (Invitrogen, Carlsbad, CA), as described previously (Wallner et al. 2003) or were from cDNA repositories. Clones were sequenced to ensure that the protein sequences conform to consensus human protein sequences found in the RefSeq public database (http://www.ncbi.nlm.nih.gov/RefSeq/). For functional expression in mammalian cells, human GABAR cDNAs were subcloned into a eukaryotic expression vector containing a cytomegalovirus promoter, as well as a T7 RNA polymerase promoter. Human embryonic kidney (HEK)-293 T cells (American Type Culture Collection, Manassas, VA) were transfected using a dextran-transfection method as described (Meera et al. 2010). Cotransfections with the δ subunit contained a fivefold excess of δ over α6 and β3 subunits and a limiting amount of enhanced green fluorescent protein (eGFP) cDNA to identify successfully transfected cells by GFP epifluorescence. Total amounts of plasmid DNA were 4 μg α6, 4 μg β3, and 20 μg δ, together with 0.4 μg of eGFP-plasmid DNA for each 10-cm diameter plate. Whole cell electrophysiological recordings were performed between 70 and 150 h post-transfection. Recordings were made from individual cells plated on polylysine-coated coverslips at 22–24°C. Voltage was clamped using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) at a holding potential of −60 mV and with data acquisition parameters as presented below. The external solution was (in mM): 142 NaCl, 1 CaCl2, 6 MgCl2, 8 KCl, 10 glucose, and 10 HEPES, pH 7.4 (327–330 mOsm). The pipette internal solution was (in mM): 140 CsCl, 4 NaCl, 0.5 CaCl2, 10 HEPES, 5 EGTA, 2 Mg2+ ATP, and 0.2 GTP. Drug solutions were applied using a multibarrel pipette driven by a stepper motor (SF-77B; Warner Instruments, Hamden, CT) with an approximate onset exchange time of 10 ms. Recording pipettes had a bath resistance of ∼4 MΩ.
Brain-slice preparation and electrophysiology.
Parasagittal cerebellar slices were prepared using standard techniques (Hanchar et al. 2005; Santhakumar et al. 2006). The cerebellum was removed from the cranium of 25- to 48-day-old mice, submerged in cold (<4°C) artificial cerebrospinal fluid (aCSF), and sectioned parasagitally using a vibratome (VT1000; Leica Microsystems, Buffalo Grove, IL). Following sectioning, the 300-μm slices were stored in 35°C aCSF for 30 min and brought to room temperature for subsequent electrophysiological experiments. The aCSF used during sectioning, storage, and electrophysiological recordings was saturated with 95% O2 and 5% CO2 and consisted of the following (in mM): 119 NaCl, 26 NaHCO3, 11 glucose, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, and 1 NaH2PO4. Whole cell pipette solution consisted of (in mM): 100 KCl, 5 NaCl, 40 HEPES, 4 MgCl2, 4 ATP, and 0.4 GTP, titrated to pH 7.4 with KOH. Cerebellar granule cells (CGCs) and molecular layer interneurons (MLIs) were visualized using an upright microscope with a 40× water immersion lens and equipped with an infrared differential interference contrast enhancement. Pipette resistances were 7–12 MΩ. Recordings from CGCs and MLIs were performed using an Axopatch 200B or Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) and were filtered at 2 kHz and digitized at 10 kHz. Neurons were voltage clamped at −70 mV, and recordings were performed at 22–24°C with glutamate receptor-mediated transmission blocked by 10 μM dinitroquinoxalinedione and action potentials blocked by 0.3 μM TTX. All drugs were purchased from Tocris Cookson (Ballwin, MO), with the exception of picrotoxin (Sigma-Aldrich, St Louis, MO).
Analysis.
For clarity, current traces recorded from neurons have been analyzed using a variance discrimination method to remove spontaneous inhibitory postsynaptic currents (Nusser and Mody 2002). Analysis was performed with custom routines written in IGOR Pro 6.04. Statistical analyses of neuronal THIP currents in Figs. 1 and 2 involved paired comparisons of average current in a 10- to 20-s time window during a control period (i.e., no drug perfusion) with a similar measurement during THIP perfusion in each neuron. For the statistical comparisons of tonic GABA current and THIP-induced current, P values are reported for one-tailed t-tests using the Bonferroni correction due to the use of control-value distributions in multiple comparisons. Comparisons of Hill parameters in the oocyte experiments used two-tailed t-tests.
Fig. 1.
Nanomolar 4,5,6,7-tetrahydroisoxazolo (5,4-c)pyridin-3(-ol) (THIP) currents are observed in cerebellar granule cells (CGCs) but not molecular layer interneurons (MLIs). A and C: currents elicited by the indicated concentrations of THIP in a granule cell and in a MLI. Note that 1 μM THIP evokes a >50-pA current from the granule cell but is ineffective on the interneuron. GBZ, gabazine. B and D: summary histograms indicating mean concentration dependence of THIP currents in granule cells and in MLIs. Numbers above bars indicate n values; *significant current compared with sham perfusion (P < 0.005).
Fig. 2.
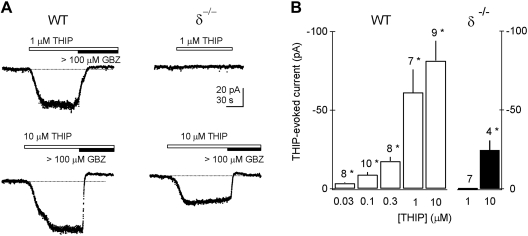
Nanomolar THIP sensitivity is absent in CGCs of δ−/− mice. A: currents elicited by the indicated concentrations of THIP in wild-type (WT) and δ−/− granule cells. Responses to ≤1 μM THIP are absent in the δ−/− neuron. Current blockade by the antagonist gabazine confirms that the responses are GABAR mediated. B: summary histogram indicating mean concentration dependence of THIP currents in wild-type (open bars) and knockout (filled bars) granule cells at the indicated concentrations. Numbers above bars indicate n values; *significant current compared with sham perfusion (P < 0.005).
RESULTS
THIP sensitivity of native eGABARs on cerebellar neurons.
To establish whether native cerebellar eGABARs composed of α6 and δ subunits are responsive to submicromolar THIP concentrations, we conducted whole-cell voltage clamp recordings from cerebellar neurons in brain slices. Tonic GABA currents (10.8 ± 1.3 pA; range 0–35 pA; n = 40) were observed in all wild-type CGCs. Such currents are generated by endogenous submicromolar concentrations of GABA acting on α6/δ subunit-containing GABARs (Brickley et al. 2001; Santhakumar et al. 2006; Stell et al. 2003), which are expressed exclusively in CGCs (Fritschy and Möhler 1995; Nusser et al. 1998). Bath perfusion of submicromolar concentrations of THIP elicited robust currents in CGCs, which were superimposed on tonic GABA currents; threshold THIP currents could be detected with concentrations of THIP as low as 30 nM (Fig. 1). THIP-elicited currents as well as tonic GABA currents were abolished by the GABAR antagonist gabazine (GBZ) (Figs. 1 and 2).
Experiments on δ−/− mice demonstrated that δ subunits are required for low-concentration THIP actions in these neurons. As has been reported previously (Stell et al. 2003), tonic currents in δ−/− CGCs were reduced significantly from wild-type (−0.3 ± 0.7 pA; n = 7; P = 0.00026). Figure 2 demonstrates that submicromolar THIP currents are also lost in δ−/− CGCs, whereas currents at higher THIP concentrations are reduced. These results are consistent with THIP acting on both δ subunit-containing GABARs with submicromolar THIP affinity as well as the δ subunit-lacking GABARs with lower THIP affinity.
We further examined submicromolar THIP responsiveness on other neuronal subtypes in the cerebellar cortex. MLIs do not express δ subunits (Fritschy and Möhler 1995), and the tonic GABA current was absent from these neurons (−1.2 ± 0.6 pA; n = 7; P = 0.0024 compared with wild-type CGCs). THIP concentrations of 1 μM and below did not activate significant current in MLIs (Fig. 1). Together, these findings demonstrate that submicromolar concentrations of THIP activate α6/δ subunit-containing eGABARs on CGCs but not on MLIs and are in agreement with recent reports showing that α4/δ subunit-expressing neurons in the thalamus and cortex exhibit responsiveness to submicromolar THIP (Belelli et al. 2005; Chandra et al. 2006; Cope et al. 2005; Drasbek et al. 2007; Jia et al. 2005).
Although the data described above strongly imply that δ subunit-containing GABARs have selectively high affinity for THIP, there is a remote possibility that inactivation of the δ subunit gene in the δ−/− mice leads to a general loss of GABAR species with low affinity for THIP, resulting in a proportional loss of responses at threshold THIP concentrations. CGCs are known to express α1, α6, β2, β3, γ2, and δ subunits (Nusser et al. 1998), and responses to a saturating concentration of THIP will thus be generated by a mixture of receptor types. To rule out such a general downregulation of all GABARs, we compared in wild-type and δ−/− granule cells responses to 1 mM THIP, a concentration expected to maximally activate α1/γ2 subunit-containing as well as α6/δ subunit-containing receptor subtypes on granule cells. Currents elicited by 1 mM THIP in wild-type (263 ± 42 pA; n = 8) and δ−/− (184 ± 25 pA; n = 6) CGCs were not significantly different (P = 0.17). On the basis of these observations, we conclude that δ subunit-containing GABARs give rise to a fraction of THIP-evoked current on CGCs and that these receptors exhibit high affinity for THIP, allowing them to detect nanomolar concentrations of this agonist.
THIP sensitivity of recombinant isoforms of eGABARs.
The loss of submicromolar THIP sensitivity coupled with the lack of significant reduction in responsiveness to millimolar THIP in δ−/− CGCs suggest that deletion of the δ subunit leads to the loss of a GABAR species with high apparent affinity for THIP. To assess THIP sensitivity in reconstituted versions of eGABARs, we measured THIP- or GABA-activated currents in Xenopus oocytes after injection of cRNAs encoding different combinations of GABAR subunits. As expected for GABAR currents, all THIP- and GABA-evoked currents were abolished by 100 μM picrotoxin (data not shown). Comparison of THIP concentration-response curves in α6β3-, α6β3δ-, and α6β3γ2-injected oocytes indicated that incorporation of δ subunits leads to >500-fold increases in THIP sensitivity compared with GABARs lacking δ subunits (compare Fig. 3, A with B), whereas inclusion of a γ2 subunit shifted the THIP concentration-response curve to the right, relative to the curve for α6β3 (compare Fig. 3, C with B, and see Table 1). Single-component Hill equation fits to THIP concentration-response relationships suggested that under our experimental conditions, α6β3δ-injected oocytes express a homogenous pool of high-sensitivity receptors (Fig. 3A). Table 1 summarizes, for the various subunit combinations tested, the EC50, Hill slopes, maximal current amplitudes, and efficacies of GABA relative to THIP at a saturating concentration. In all cases, the mean Hill equation parameters obtained with fits to individual oocytes (Table 1) closely matched the parameters from fits to the mean concentration-response curves (Fig. 3, and see Fig. 5), with the exception of the Hill slopes, which were consistently larger based on fits to individual oocytes. We attribute the differences in Hill slope estimates between the table (estimates from average of fits) and legends for Fig. 3 (and see Fig. 5; estimates from fits to average) to the fact that averaging normalized, concentration-response curves with slightly different EC50 leads to a shallowing effect on the slope of the mean concentration-response relationship.
Table 1.
Summary of THIP concentration response data from oocyte experiments
| mRNA Injected | Maximal Current (nA) | EC50 (μM) | Hill Slope | IGABA/ITHIP | n |
|---|---|---|---|---|---|
| α6 + β3 | 429 ± 126 | 18 ± 6.3 | 0.78 ± 0.12 | 0.37 ± 0.07 | 5 |
| α6 + β3 + δ | 155 ± 31* | 0.031 ± 0.01† | 1.0 ± 0.32 | 0.51 ± 0.04 | 10 |
| α6 + β3 + γ2 | 7091 ± 1936* | 92 ± 20* | 1.3 ± 0.16* | 0.69 ± 0.06* | 5 |
| α4 + β3 | 639 ± 173 | 22 ± 2.6 | 0.89 ± 0.10 | 0.39 ± 0.11 | 5 |
| α4 + β3+ δ | 351 ± 111 | 0.035 ± 0.009‡ | 0.99 ± 0.09* | 0.55 ± 0.10 | 10 |
| α4 + β3 + γ2 | 2972 ± 426§ | 196 ± 18¶ | 1.4 ± 0.09* | 2.2 ± 0.21† | 6 |
| α1+ β2 + γ2 | 4464 ± 1473 | 423 ± 58* | 1.3 ± 0.07 | 1.8 ± 0.17 | 7 |
Each value represents the mean ± SEM based on THIP concentration-response measurements from n oocytes injected with the indicated mRNAs. IGABA/ITHIP is the mean response to 1 mM GABA relative to the saturating current elicited by THIP. The α4β3δ values are from the pure pool (see Fig. 5A). Statistically significant differences with the respective α4β3 or α6β3 values are shown, where
P < 0.05;
P < 0.005;
P < 0.0005;
P < 5 × 10−5; and
P < 5 × 10−9. For α1β2γ2, statistical comparisons were made to the respective values for α4β3γ2.
Similar experiments conducted in α4β3δ-injected oocytes show a more complicated situation, reflective of multiple pools of receptors. Figure 4 shows a representative oocyte recording, in which two components can be discerned from the THIP concentration-response relationship. We found that approximately one-half of the 19 oocytes injected with cRNA for α4, β3, and δ showed a broadening of the THIP concentration-response curve, and many displayed two components as in Fig. 4. In these oocytes, the high-affinity fraction estimated by two-component Hill equation fitting ranged from 11% to 87%. For constructing the population curves in Fig. 5, we thus divided the α4β3δ-injected oocytes into two groups—those that expressed a “pure”, high-affinity pool and those that expressed mixtures of receptors with different apparent affinities for THIP (Fig. 5, A and B, respectively; note that in Table 1, we summarize only the pure, high-affinity pool). Concentration-response data from the pure, high-affinity pool yielded an EC50 of 39 ± 7 nM, similar to the 30 ± 6 nM EC50 observed in the α6β3δ-injected oocytes (Table 1). In contrast, population data from mixed pool oocytes required a two-component Hill equation to achieve an acceptable fit. Figure 5B shows that an excellent fit to the mean population could be obtained under conditions in which the affinities and Hill slopes were constrained to those from the fit to pure α4β3δ and α4β3 mean population conditions (51 nM and 24 μM, respectively), with a single parameter—the relative size of the two pools (Phigh)—allowed to vary. These data and the fitting analyses are consistent with a mixture of two pools of GABARs—one high-affinity, δ subunit-containing pool and another low-affinity, δ subunit-lacking pool.
Similar to α6-containing receptors, coinjection of γ2 cRNA, along with α4 and β3, led to receptors with decreased sensitivity to THIP relative to the α4β3 combination (Fig. 5D). Also illustrated in Fig. 5, D and G, γ2 inclusion decreased the relative efficacy of THIP compared with GABA and dramatically increased the maximal amplitudes of currents (Table 1). Altogether, these findings demonstrate that for both α4- and α6-containing GABARs, the apparent affinity for THIP shifts to the left with inclusion of δ subunits and to the right with γ2 subunits.
To determine whether high sensitivity of δ subunit-containing GABARs is also observed in a mammalian expression system, we conducted concentration-response experiments in HEK-293 cells transfected with cDNAs encoding α6, β3, and δ. Figure 6 displays example responses showing robust currents elicited by concentrations of THIP as low as 10 nM. Mean concentration-response curves showed a clear “foot” in the submicromolar concentration range apparent in the expanded panel in Fig. 6C. This region of the mean concentration-response curve made it such that single Hill equations produced inferior fits. Figure 6, B and C, represents a fit with a two-component Hill equation, and each individual Hill equation component is displayed. This yields EC50 of 37 nM and 2.9 μM with the high-affinity component representing 16% of the peak.
DISCUSSION
It has been strongly suggested, based on data from native eGABARs, that the constrained GABA analogue THIP is a selective, nanomolar affinity ligand; however, recombinant studies of δ subunit-containing GABARs have led to the conclusion that there are uniform populations of receptors with much higher THIP EC50 ranging from 3 to 53 μM (Adkins et al. 2001; Brown et al. 2002; Jia et al. 2005; Mortensen et al. 2010; Saarelainen et al. 2008; Storustovu and Ebert 2006). Here, we reconcile this discrepant THIP sensitivity between native eGABARs and recombinant forms of eGABARs. We find that like their native α4/δ subunit-containing counterparts on thalamic neurons (Chandra et al. 2006; Herd et al. 2009), native α6/δ subunit-containing GABARs are also responsive to submicromolar concentrations of THIP and that such THIP sensitivity requires δ subunits. Our experiments on recombinant GABARs in Xenopus oocytes demonstrate that coinjection of δ subunit cRNAs shifts the EC50 for THIP almost three orders of magnitude compared with functional receptors expressed from α and β subunits alone. This is true for both α6/δ- and α4/δ-containing GABARs, although the fraction of high-affinity receptors in the α4/δ-injected oocytes was typically lower. Similar results were obtained for α6β3δ receptors in a mammalian expression system, suggesting an incomplete population of high-affinity sites.
Implications for the study of recombinant versions of eGABARs.
Prior studies have used single-component Hill equations to derive EC50 for THIP, and these estimates range from 6 to 53 μM for α4β2/3δ receptors (Adkins et al. 2001; Brown et al. 2002; Jia et al. 2005; Storustovu and Ebert 2006) and 3.1 to 8.2 μM for α6β3δ receptors (Saarelainen et al. 2008; Storustovu and Ebert 2006). This work suggested that the eGABAR dependence of the behavioral and cellular actions of THIP could not be accounted for by subunit-specific differences in sensitivity. With this in mind, efficacy differences have been proposed to account for selectivity. Maximal THIP currents in δ subunit-containing receptors are 1.5 to two times larger than maximal GABA currents (Adkins et al. 2001; Brown et al. 2002; Jia et al. 2005; Mortensen et al. 2010; Saarelainen et al. 2008; Storustovu and Ebert 2006); however, we find that this THIP superagonism is also observed for α4β3 and α6β3 receptors (Figs. 3–5), and thus it is not a feature that distinguishes δ subunit-containing GABARs from functional receptors without a δ subunit.
Our data imply that mixed populations of αβδ and αβ receptors are common in heterologous expression systems and that αβδ components with high apparent affinity for THIP may be obscured by low-affinity, αβ-mediated components. Consistent with this view, most reported EC50 values for THIP on recombinant αβδ receptors are similar to those observed for their αβ counterparts (Adkins et al. 2001; Brown et al. 2002; Jia et al. 2005; Mortensen et al. 2010; Saarelainen et al. 2008; Storustovu and Ebert 2006). Examination of THIP concentration-response relationships for α4β2/3δ in published papers on mammalian cell lines (Fig. 2b from Brown et al. 2002 and Fig. 5, d and e, from Jia et al. 2005) shows the presence of a small but obvious high-affinity component. Notably, our HEK cell data for α6β3δ (Fig. 6) closely resemble those described in the study by Jia et al. (2005) for α4β2δ in exhibiting a small fraction, high-affinity “tail” in the concentration-response relationship. Our data imply that the predominating components of the concentration-response curve in Fig. 6 derive from α6β3 receptors, a view consistent with the 11 μM EC50 of α6β3 receptors measured in prior work on HEK cells (Saarelainen et al. 2008).
The dual-component, concentration-response curves observed for “mixed pool” α4β3δ oocytes and α6β3δ HEK cells could, in principle, reflect two binding sites of different affinity on the same receptor molecule. However, this explanation fails to account for one-half of the α4β3δ oocytes and all of the α6β3δ oocytes that appear to express a uniform high-affinity pool. Moreover, it cannot explain the variable fraction of high- and low-affinity pools in the α4β3δ mixed pool oocytes. Dual-component Hill equation fits, such as that displayed in Fig. 4, yielded estimates of the contribution of the low-affinity pool. In the nine mixed pool oocytes, such fits indicated that the mean contribution of the low-affinity pool was 59 ± 9% with a range of 13–89% (n = 9). Taken together, these data seem most consistent with a mixture of α4β3 and α4β3δ receptors rather than a uniform population of receptors, each possessing two binding sites of differing affinity.
Implications for THIP pharmacology in situ.
Our findings provide a straightforward explanation for the behavioral effects of THIP, which have been shown to be markedly reduced in both α4 and δ knockout mice (Boehm et al. 2006; Chandra et al. 2006; Herd et al. 2009). Highly water-soluble drugs, such as THIP, usually do not readily permeate the blood-brain barrier, and nanomolar affinity THIP targets reported here provide a plausible explanation for how rather low doses of THIP (10–30 mg/kg), applied peripherally in behavioral studies, can have pronounced behavioral effects. In this context, it is worth noting that the THIP EC50 reported here for α6β3δ receptors of 30 nM is in excellent agreement with the THIP equilibrium KD of 38 nM reported in radioligand-binding studies of cerebellar membranes (Friemel et al. 2007). Moreover, such high, δ subunit-dependent THIP affinity accounts for the concordance between autoradiography with brain sections using 250 nM [3H]THIP (Friemel et al. 2007) and the distribution of δ subunit protein expression in the brain (Pirker et al. 2000).
The data also provide insight into residual THIP behavioral actions in δ subunit knockout mice. Considering that the principal sGABAR isoform α1β2γ2 is insensitive to behaviorally relevant concentrations of THIP (Table 1), it seems unlikely that γ2 subunit-containing receptors could explain residual THIP actions in these mice. An interesting possibility is that residual THIP actions might be mediated by α4β3 receptors, which have been suggested to be abundant based on biochemical assays (Bencsits et al. 1999) and have an intermediate THIP sensitivity (Figs. 3 and 5) and in many respects, a similar pharmacology to their αβδ counterparts (Meera et al. 2009). Although such a mechanism is possible for α4β2/3 receptors, it is unlikely to be the case for α6β3 receptors, as indicated in Fig. 2 and in prior studies (Jechlinger et al. 1998; Stell et al. 2003).
In this study, recombinant receptors appear to be even more sensitive than the corresponding native receptors studied in brain slices (compare Figs. 1 with 3). A likely explanation is that in slice preparations, eGABARs are partially occupied by ambient GABA, which has been estimated to be ∼160 nM (Santhakumar et al. 2006). Indeed, in the slice experiments of Figs. 1 and 2, tonic currents could be measured prior to THIP application, and with the use of the assumptions outlined in Santhakumar et al. (2006), these can be used to estimate an endogenous GABA concentration. Analyzed in this way, the range of tonic current amplitudes (0–35 pA) predicts that the THIP applications occurred in a background of 0–302 nM endogenous GABA (mean ± SEM: 145 ± 11 nM; n = 40). Given that THIP and GABA compete for the same binding sites, this would lower the apparent THIP sensitivity compared with the GABA-free environment in recombinant expression.
We conclude that nanomolar THIP sensitivity of native neurons and δ subunit-dependent behavioral responses arises because of extremely high THIP potency at δ subunit-containing GABARs. These findings validate THIP as a highly selective tool for the study of eGABARs in both recombinant expression systems and native neurons.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS35985 to T. S. Otis and AA017891 to M. Wallner and T. S. Otis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. R. Olsen for support, Drs. S. Dellal, K. Olofsdotter Otis, and J. Wondolowski for comments on the manuscript, Drs. C. Gundersen and R. Olcese for oocytes, and E. Fitzmorris for technical support.
REFERENCES
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem 276: 38934–38939, 2001 [DOI] [PubMed] [Google Scholar]
- Baur R, Kaur KH, Sigel E. Structure of α6β3δ GABAA receptors and their lack of ethanol sensitivity. J Neurochem 111: 1172–1181, 2009 [DOI] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J Neurosci 25: 11513–11520, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencsits E, Ebert V, Tretter V, Sieghart W. A significant part of native GABAA receptors containing α4 subunits do not contain γ or δ subunits. J Biol Chem 274: 19613–19616, 1999 [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Homanics GE, Blednov YA, Harris RA. δ Subunit containing GABAA receptor knockout mice are less sensitive to the actions of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridin-3-ol. Eur J Pharmacol 541: 158–162, 2006 [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409: 88–92, 2001 [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol 136: 965–974, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA 103: 15230–15235, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci 25: 11553–11563, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasbek KR, Hoestgaard-Jensen K, Jensen K. Modulation of extrasynaptic THIP conductances by GABAA-receptor modulators in mouse neocortex. J Neurophysiol 97: 2293–2300, 2007 [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex 16: 1134–1141, 2006 [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 6: 215–229, 2005 [DOI] [PubMed] [Google Scholar]
- Friemel A, Ebert B, Hutson PH, Brust P, Nieber K, Deuther-Conrad W. Postnatal development and kinetics of [3H]gaboxadol binding in rat brain: in vitro homogenate binding and quantitative autoradiography. Brain Res 1170: 39–47, 2007 [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Möhler H. GABAA receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359: 154–194, 1995 [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat Neurosci 8: 339–345, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd MB, Foister N, Chandra D, Peden DR, Homanics GE, Brown VJ, Balfour DJ, Lambert JJ, Belelli D. Inhibition of thalamic excitability by 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridine-3-ol: a selective role for δ GABAA receptors. Eur J Neurosci 29: 1177–1187, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jechlinger M, Pelz R, Tretter V, Klausberger T, Sieghart W. Subunit composition and quantitative importance of hetero-oligomeric receptors: GABAA receptors containing α6 subunits. J Neurosci 18: 2449–2457, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol 94: 4491–4501, 2005 [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Johnston GA, Lodge D, Curtis DR. A new class of GABA agonist. Nature 268: 53–55, 1977 [DOI] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M. Alcohol- and alcohol antagonist-sensitive human GABAA receptors: tracking δ subunit incorporation into functional receptors. Mol Pharmacol 78: 918–924, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M. Etomidate, propofol and the neurosteroid THDOC increase the GABA efficacy of recombinant α4β3δ and α4β3 GABAA receptors expressed in HEK cells. Neuropharmacology 56: 155–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Ebert B, Wafford K, Smart TG. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol 588: 1251–1268, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 87: 2624–2628, 2002 [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18: 1693–1703, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815–850, 2000 [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature 338: 582–585, 1989 [DOI] [PubMed] [Google Scholar]
- Saarelainen KS, Ranna M, Rabe H, Sinkkonen ST, Moykkynen T, Uusi-Oukari M, Linden AM, Luddens H, Korpi ER. Enhanced behavioral sensitivity to the competitive GABA agonist, gaboxadol, in transgenic mice over-expressing hippocampal extrasynaptic α6β GABAA receptors. J Neurochem 105: 338–350, 2008 [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Hanchar HJ, Wallner M, Olsen RW, Otis TS. Contributions of the GABAA receptor α6 subunit to phasic and tonic inhibition revealed by a naturally occurring polymorphism in the α6 gene. J Neurosci 26: 3357–3364, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Wallner M, Otis TS. Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol 41: 211–221, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA 100: 14439–14444, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther 316: 1351–1359, 2006 [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci 5: 721–722, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ GABAA receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA 100: 15218–15223, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Vyazovskiy VV, Homanics GE, Tobler I. The EEG effects of THIP (gaboxadol) on sleep and waking are mediated by the GABAA δ subunit-containing receptors. Eur J Neurosci 25: 1893–1899, 2007 [DOI] [PubMed] [Google Scholar]