Abstract
We have studied eye-head coordination in nonhuman primates with acoustic targets after finding that they are unable to make accurate saccadic eye movements to targets of this type with the head restrained. Three male macaque monkeys with experience in localizing sounds for rewards by pointing their gaze to the perceived location of sources served as subjects. Visual targets were used as controls. The experimental sessions were configured to minimize the chances that the subject would be able to predict the modality of the target as well as its location and time of presentation. The data show that eye and head movements are coordinated differently to generate gaze shifts to acoustic targets. Chiefly, the head invariably started to move before the eye and contributed more to the gaze shift. These differences were more striking for gaze shifts of <20–25° in amplitude, to which the head contributes very little or not at all when the target is visual. Thus acoustic and visual targets trigger gaze shifts with different eye-head coordination. This, coupled to the fact that anatomic evidence involves the superior colliculus as the link between auditory spatial processing and the motor system, suggests that separate signals are likely generated within this midbrain structure.
Keywords: gaze kinematics, head movements, monkey, sound localization
primates can change their line of sight by moving their eyes either alone or in coordination with the head. The mechanisms underlying the coordination of these complex movements, called gaze shifts, have been studied extensively with the use of visual targets under various experimental conditions (e.g., Bizzi et al. 1971, 1972; Freedman and Sparks 1997a, 1997b; Gandhi and Sparks 2007; Phillips et al. 1995; Tomlinson 1990; Tomlinson and Bahra 1986a, 1986b). Accordingly, with few exceptions (e.g., Goossens and van Opstal 1999), most models of gaze control are built upon a retinal input (e.g., Galiana and Guitton 1992; Guitton 1992; Laurutis and Robinson 1986; Phillips et al. 1995).
In the horizontal direction gaze shifts of sufficient amplitude typically start with a saccadic eye movement followed by a head movement 20–40 ms later (Bizzi et al. 1971). At the end of the gaze shift, when the line of sight is on the visual target, the head has moved only a small fraction of the total amplitude of the gaze shift but continues to move in the direction of the target, causing the eyes to counterrotate in the orbit to maintain gaze stationary in space, on the target, because of the vestibuloocular reflex (VOR). For gaze shifts >20–25° the contribution of the head, defined as the amplitude of the head movement at the time of gaze offset, increases as a function of the shift amplitude (Freedman and Sparks 1997a). The magnitude of the contribution of the head is also a function of the position of the eyes in the orbit at the time the gaze shift is initiated. When the eyes are deviated in the direction of the target the contribution of the head is larger, whereas when the eyes are away from the target its contribution is smaller (Freedman and Sparks 1997a; Stahl 1999). For gaze shifts smaller than 20–25° the contribution of the head is small, practically nil.
The sequence of events described above in which the movement of the eyes leads the movement of the head, despite electromyographic (EMG) activity in neck muscles preceding EMG activity in eye muscles by 20 ms (Bizzi et al. 1971), is observed in gaze shifts directed to unpredictable visual targets (Bizzi et al. 1972; Uemura et al. 1980; Warabi 1977; Zangemeister and Stark 1982). The order in which the eyes and head start to move to produce a gaze shift to a visual target in primates can vary, however, depending on the experimental conditions (Phillips et al. 1995; Tomlinson and Bahra 1986a; Tweed et al. 1995). For instance, when the position and time of presentation of visual targets are predictable, the latency of eye and head movements is reversed. Under the conditions of Bizzi et al.'s (1972) experiment, for example, which required rhesus monkeys to make gaze shifts to targets presented at regular intervals from ±30° on the horizontal plane, the head moved 150–200 ms before the eye.
Gaze shifts in the vertical direction have not been studied as extensively as those in the horizontal direction. Nevertheless, despite specific characteristics of head biomechanics for movements in the vertical direction, the kinematics of gaze shifts to visual targets are quantitatively similar to gaze shifts in the horizontal direction (Freedman 2005).
Primates can also shift their gaze to targets defined in nonretinal coordinates such as acoustic targets (Populin 2006, 2008), which are encoded in head-centered coordinates in the periphery. The horizontal location of such targets is computed from interaural differences of time and level (Stevens and Newman 1934), i.e., in reference to the head given that the cochleae, containing linear arrays of frequency-sensitive not spatially sensitive epithelia, are anchored to each side of the head. With some exceptions (e.g., Goossens and van Opstal 1999; Vliegen et al. 2004; Whittington et al. 1981), much less is known about how gaze shifts are generated and controlled when the target is encoded in head-centered compared with retinocentric coordinates.
Unlike overt orienting to visual targets, which rhesus monkeys readily accomplish with the head either restrained or unrestrained, albeit with some effects on accuracy (Phillips et al. 1995), orienting to the perceived location of acoustic targets requires free movement of the head in this species (Populin 2006). It is unclear why monkeys require free movement of the head to shift their gaze accurately and consistently to acoustic targets; cats exhibit a similar requirement (Tollin et al. 2005), and no data from humans comparing head-restrained versus head-unrestrained localization are available. The fact that the triggering stimuli are encoded in head-centered coordinates suggests, however, that gaze shifts to targets of this type are led by movement of the head.
For this hypothesis to be substantiated one should find that 1) gaze shifts to unpredictable acoustic targets are consistently initiated with the head, not the eyes as in gaze shifts to unpredictable visual targets, and 2) the early initiation of the head movement results in greater contribution of the head to the gaze shift compared with the contribution of the head to gaze shifts to visual targets of similar amplitude; this should be particularly distinct in gaze shifts to targets of <20–25° of eccentricity, to which the head does not normally contribute if the target is visual.
The results of this study, obtained under psychophysical conditions that required the monkeys to orient accurately to unpredictable visual and acoustic targets presented randomly, are consistent with this assertion. In both horizontal and vertical gaze shifts to randomly presented acoustic targets, movement of the head preceded movement of the eyes.
MATERIALS AND METHODS
Subjects and surgery.
Three adult male macaque monkeys (Macaca mulatta) took part in the experiments. These monkeys had participated in a previous study of sound localization that used gaze as a pointer to indicate perceived sound location. Details about the preparation and procedures have been published (Populin 2006). Briefly, each monkey underwent a sterile surgical procedure to implant 1) a lightweight titanium head post used to hold a spout in front of their lips for the delivery of water rewards regardless of head position and to clean the implant area and 2) eye coils to measure eye position (Judge et al. 1980). The eye coils were made of Teflon-coated stainless steel wire (SA632; Cooner Wire, Chatsworth, CA). An additional coil of similar construction was attached to the front of the acrylic head cap that held the head post to measure head movements. An effort was made during the surgical procedure to restore the external ears to their preimplant position to minimize changes known to affect the acoustic input to the eardrum (Young et al. 1996) and auditory receptive fields in the superior colliculus (SC) (Middlebrooks and Knudsen 1987). All experimental procedures were approved by the University of Wisconsin Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental setup, eye movement recordings, and stimuli presentation.
The experiments were done in a 3 × 2.8 × 2-m double-walled sound-attenuating chamber (Acoustic Systems, Austin, TX). The interior of the chamber and the major pieces of equipment in it were covered with acoustic foam (Ilbruck, Minneapolis, MN) to attenuate reflections. Gaze and head movements were recorded with the search coil technique (Robinson 1963) using a phase angle system (CNC Engineering, Seattle, WA). The horizontal and vertical components of the gaze and head position signals were low-pass filtered at 250 Hz (Krohn-Hite, Brockton, MA), digitally sampled at 500 Hz (Tucker Davis Technologies, Alachua, FL), and stored for off-line analysis.
Head position signals were calibrated by attaching a laser pointer to the head post and moving the head of the monkey manually to align the light of the laser pointer with light-emitting diodes (LEDs) at known positions in the frontal hemifield. Gaze position signals were calibrated with a behavioral procedure that relied on the animals' tendency to look at spots of light presented in a dimly lit environment (Populin and Yin 1998). Linear functions were fit to the horizontal and vertical data separately, and the coefficients were used to convert the voltage output of the coil system into degrees of visual angle.
Visual stimuli were presented with red LEDs positioned in front of speakers. Each LED subtended a visual angle of 0.2°. The LEDs were powered and controlled with a custom-built logic circuit. The acoustic signals used as targets, 50-ms broadband noise bursts with 10-ms rise/fall linear windows, were generated with Tucker Davis Technologies System 3 and were presented at 50–53 dB SPL with either Radio Shack (Fort Worth, TX) supertweeters modified to transduce low frequencies or Morel MDT-20 28-mm (Morel America, Elmont, NY) soft dome speakers. The sound level was roved over a 3-dB range in 1-dB steps to mask physical differences among the speakers that could provide unwanted cues for localization (Populin 2008). The fronts of the speakers were positioned 84 cm from the center of the subject's head. A hardware signal coinciding with the onset of stimuli used as targets was recorded in every trial and used to compute eye, gaze, and head latencies.
Behavioral training and experimental tasks.
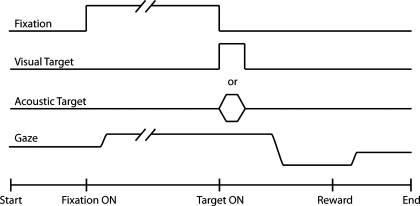
Behavioral training was based on positive reinforcement. First, monkeys were taught to accept handling with a pole and to enter a primate chair (Crist Instrument, Hagerstown, MD) for a fruit reward. Second, with operant conditioning, they were rewarded with water for orienting to the sources of acoustic and visual stimuli based on spatial and temporal criteria using the saccade task (Fig. 1). In each trial a fixation LED was presented at the straight-ahead position ∼750 ms after the defined onset of a trial. The duration of this event was variable, ranging from 1,000 to 2,000 ms. Coinciding with its offset, either an acoustic or a visual target, 50 ms in duration, was turned on elsewhere in the frontal hemifield. The animal was required to first direct his gaze to the LED straight ahead and to maintain fixation on it until it was turned off. Upon the presentation of the target the animal was required to shift his gaze from the straight-ahead position to the perceived location of the target. If temporal and spatial criteria were observed, a reward was delivered. The temporal criterion involved maintaining fixation at the straight-ahead position until the LED was turned off, after which the subject had 500 ms to orient to the target. After the target was acquired, fixation for 100–300 ms was required. The spatial criterion, implemented with an error window surrounding the sources of stimuli, was used to determine whether the monkey's eyes were aligned at the straight-ahead position before the presentation of the targets, and whether final gaze position met the minimum accuracy requirement for reward. The acceptance window was 2–5° for visual targets and 8° for acoustic targets. By requiring fixation for a long enough period, which was determined empirically, we ensured that the animal aligned his head with the straight-ahead position during the initial fixation. No efforts were made to improve the accuracy or precision with which the subjects oriented to the targets.
Fig. 1.
Schematic representation of the experimental task. Subjects were first required to acquire the fixation light-emitting diode (LED) at the straight-ahead position and to maintain fixation for 1,000–2,000 ms. Coinciding with the offset of the fixation LED, a target, visual or acoustic, was presented elsewhere. The subject was required to shift his gaze from the straight-ahead position to the location of the target to receive a reward.
Data analysis.
Analyses were done off-line with custom graphics software written in Matlab (Mathworks, Natick, MA) that displayed the horizontal and vertical components of gaze, head, and eye (in head) position and time derivatives of those signals and other parameters used for analysis and stored the results in a relational database. The specifics of the method have been presented previously (Populin and Yin 1998, 1999). Briefly, for gaze, the start and end of a movement were defined as the end of, and return to, fixation. The end of fixation was defined as the time at which the amplitude of the velocity trace first exceeded 2 SDs of the mean baseline; the velocity of horizontal and vertical signals was computed independently with a first-order polynomial fit on 11 successive data points. For each component of a movement, horizontal and vertical, the mean baseline was computed independently from a portion of the velocity record starting 100 ms before to 10 ms after the onset of the stimulus, during which time the eyes and the head were expected to be stationary; trials with movement during this period were discarded from analysis. The return to fixation was defined as the time at which gaze velocity returned to within 2 SDs of the mean baseline. Peak velocity was defined as the point at which gaze, head, and eye reached maximal velocity between the end of and return to fixation. The same criteria for analysis were applied to head and eye in head movements. Only trials that had suitable gaze, head, and eye movement records were considered for analysis. Trials with gaze shifts smaller than 5° were excluded. A total of 3,011 auditory and 1,434 visual trials in the horizontal direction and 596 auditory and 393 visual trials in the vertical direction were analyzed.
By convention, horizontal movements to the right of the midline are depicted as upward deflections and movements to the left as downward deflections. For vertical movements the up and down directions were preserved in the plots. The contribution of the head to the gaze shift was defined as the amplitude of the head movement between head movement onset and the end of gaze movement (Freedman and Sparks 1997a). Finally, movements of the eye relative to space are referred to as gaze shifts, whereas movements of the eyes relative to the head are referred to as saccades. Movements of the head are called head movements.
RESULTS
All gaze shifts studied were centrifugal, with eyes and head starting aligned to the straight-ahead position. Data from three animals were averaged and presented for both target conditions. Gaze shifts from the leftward and rightward directions are presented together. Gaze shifts from the upward and downward directions were also pooled despite biomechanical differences in head movements because the main observations, that on average the head moved first in the acoustic target condition and the eye moved first in the visual target condition, were consistent.
Basic observations: gaze shifts to acoustic and visual targets.
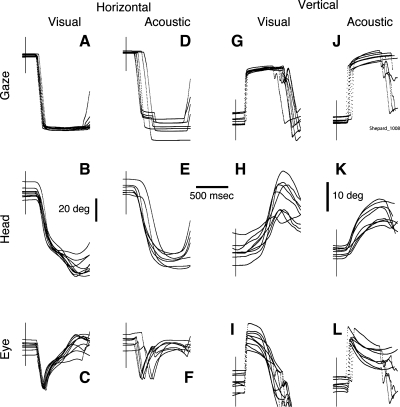
Shown in Fig. 2 are typical examples of gaze shifts to horizontal and vertical visual and acoustic targets and their corresponding head and eye movement components. As expected, the end position of gaze shifts to visual targets exhibited less variability than those directed to acoustic targets (Populin 2006). The latency of the gaze shifts was also more consistent in the visual compared with the acoustic target condition, reflecting a greater level of difficulty in the latter. In the horizontal direction, several of the gaze shifts to the acoustic targets included a corrective saccade (Fig. 2D), which were executed while the head was moving in the direction of the target and the eye was counterrotating because of the action of the VOR (Fig. 2F).
Fig. 2.
Gaze shifts to visual (A–C, G–I) and acoustic (D–F, J–L) targets in horizontal (A–F) and vertical (G–L) directions performed during a single experimental session. All traces are plotted synchronized to the onset of the target, illustrated by a thin vertical line intersecting the traces. Note the differences in variability between gaze shifts to visual and acoustic targets, particularly as it concerns the onset of the eye trace showing the effect of the vestibuloocular reflex. The horizontal scale bar is 500 ms, and the vertical bars are 20° for the horizontal data and 10° for the vertical data.
Some of the head movements in the visual target condition were larger than those in the acoustic target condition in both horizontal and vertical directions (Fig. 2, B, E, H, and K). Note that the horizontal head movement traces of this subject exhibited two movements in the visual, but not the acoustic, target condition (see also Fig. 3 with data from the same subject and session); two movements in the acoustic target condition were observed less frequently. While this feature was prominent in some of this subject's experimental sessions, we have only observed it occasionally in the other two subjects. In addition, note that the two movements were only observed in the head but not in the gaze records, as reported by Freedman and Sparks (2000). Finally, the eye movement traces in the acoustic target condition, in both the horizontal and vertical directions (Fig. 2, F and L), show the effects of the VOR at the onset of the movement, indicating that gaze was stabilized in response to the abrupt onset of the head movement.
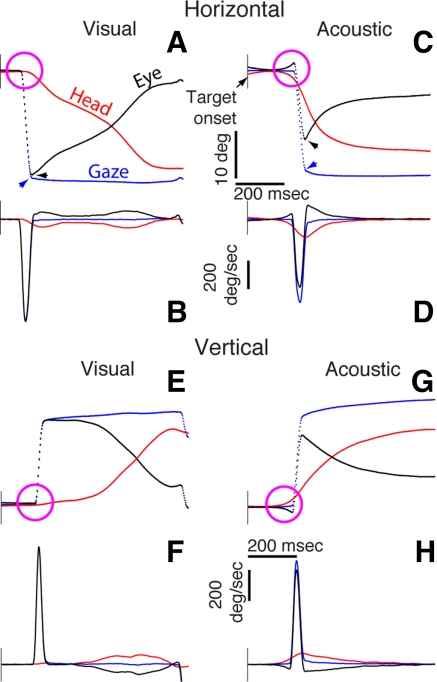
Fig. 3.
Single trial data illustrating gaze, eye, and head movements to visual and acoustic targets in the horizontal and vertical directions (A, C, E, G) and their corresponding first derivatives (B, D, F, H). All data are plotted synchronized to the onset of the target. The vertical scale bar is 10° for the position traces and 200°/s for the velocity traces. The time scale bar for all traces is 200 ms. Arrowheads in A and C indicate the maximal amplitude of gaze and eye traces. Magenta circles highlight the distinct onset of the head and eye movement components: In the acoustic target conditions (C and G), the movement of the head preceded movement of the eye, which counterrotated because of the action of the vestibuloocular reflex.
Despite the consistency of the movements illustrated in Fig. 2, specific details are difficult to discern. Accordingly, Fig. 3 shows four single trial examples, including their velocity profiles, selected on the basis of their similar amplitude. These representative data, which are plotted as a function of time and synchronized to the onset of the stimuli, were selected from the third head-unrestrained session of this animal's psychophysical testing for sound localization (Populin 2006). They illustrate the salient characteristics of gaze shifts to each type of target.
The gaze shift to the visual target in the horizontal direction was primarily accomplished with a saccadic eye movement that reached a velocity of ∼725°/s, as revealed by the nearly equal amplitude of the saccadic eye movement (black) and gaze (blue) traces in Fig. 3, A and B. The start of the head movement, highlighted by the magenta circle, followed the onset of the saccadic eye movement by 18 ms. At this point the gaze shift had nearly ended; therefore the contribution of the head was negligible, and, importantly, it took place after the gaze shift had been initiated. From this point until the end of the gaze shift, the eye counterrotated in the orbit as the head moved in the direction of the target, thereby maintaining the line of sight stationary in space. Note also that the velocity profile of the head movement exhibits two peaks, indicating that it reaccelerated near the end of the response. This sequence of events matches previous descriptions of gaze shifts directed at unpredictable horizontal visual targets (Bizzi et al. 1971, 1972; Freedman and Sparks 1997a; Phillips et al. 1995).
The sample horizontal gaze shift to the broadband acoustic target was different (Fig. 3, C and D). First, movement of the head preceded the saccadic eye movement by 46 ms. The abrupt onset of the head movement in the direction of the target caused the eyes to counterrotate because of the stabilizing action of the VOR. Second, the saccadic eye movement was smaller compared with the saccade in the equivalent gaze shift to the visual target (Fig. 3, A and B), revealing a larger contribution of the head. Third, the maximal velocities reached by both the gaze shift and the saccadic eye movement were slower than in the visual target condition (black and blue traces in Fig. 3, B and D). Fourth, the head movement reached a higher maximal velocity than in the visual target condition, only exhibited a single peak, and was of smaller amplitude (red traces in Fig. 3, B and D).
Vertical gaze shifts to visual and acoustic targets above the horizontal plane are shown in Fig. 3, E–H. Differences in the onset time of the eye and head movements are also highlighted with magenta circles. The gaze shift to the visual target was accomplished solely with a saccadic eye movement (Fig. 3, E and F); the head did not contribute to the gaze shift. During the remainder of the gaze shift, after the eye had reached the target, the head moved toward the target and the eye counterrotated. In this example the head moved slowly and the velocity profile exhibited two peaks. The gaze shift to the acoustic target, on the other hand, was initiated with a head movement, which, in this example, preceded the movement of the eyes by 96 ms (Fig. 3, G and H). As in the horizontal direction, the eye counterrotated at the start of the gaze shift because of the action of the VOR compensating for the abrupt movement of the head. In contrast to the visual target condition, the early movement of the head resulted in a larger contribution to the gaze shift.
Thus gaze shifts of similar amplitudes exhibited different characteristics as a function of target modality. In both the horizontal and vertical directions, gaze shifts to visual targets were initiated with a saccadic eye movement and the head contributed very little, if at all. In contrast, gaze shifts of similar amplitude to acoustic targets were initiated with a head movement, and in both directions the head contributed. These are single examples, however. Proper evaluation of differences in eye-head coordination between gaze shifts to visual and acoustic targets requires the examination of specific measures in a larger number of trials.
Head contribution to gaze shifts.
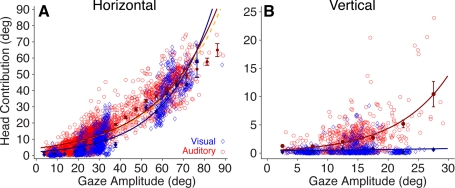
Inspection of the gaze, head, and eye movement traces in Figs. 2 and 3 reveals a potential difference in the magnitude of the contribution of the head to the gaze shifts in the visual and acoustic target conditions. Data from this measure from all three subjects are plotted for each type of target and movement direction in Fig. 4.
Fig. 4.
Comparison of head contribution to gaze shifts to visual and acoustic horizontal (A) and vertical (B) targets. Blue symbols and fitted curve represent visual data, and red symbols and curve represent auditory data. Filled symbols represent binned means (5°), and standard bars represent 95% confidence intervals. The yellow dashed curve represents a fit to the auditory data without points comprising 35–55° gaze shifts. The function is Head Contribution = a × exp(b × x), where a and b are constants, the offset and exponential rise. Values in parentheses represent the coefficients' 95% confidence intervals. A, visual: a = 2.146 (1.98, 2.31), b = 0.0446 (0.0434, 0.0458). A, auditory: a = 4.387 (4.232, 4.541), b = 0.0352 (0.0346, 0.0358). A, yellow: a = 4.37 (4.215, 4.524), b = 0.0345 (0.0339, 0.0351). B, visual: a = 0.408 (0.252, 0.565), b = 0.0228 (−0.0024, 0.0481). B, auditory: a = 2.101 (1.915, 2.288), b = 0.5479 (0.491, 0.605).
In the horizontal direction the head contributed significantly more in the acoustic than in the visual target condition for gaze shifts extending up to 60°. This is relevant for gaze shifts smaller than 20–25°, which for visual targets are accomplished primarily with eye movements (Freedman and Sparks 1997a). An exponential function of the form head contribution = a × exp(b × x) was fit to the data, and the a coefficient, representing the offset of the fitted function, was significantly larger in the auditory condition (P < 0.05), as were the binned means computed at 5° intervals (Fig. 4A, filled symbols).
Because of the layout of the experimental setup, no targets (LEDs or speakers) were located between 35° and 55° of eccentricity and no visual data for this range of gaze amplitudes were recorded as illustrated by the lack of blue symbols in this range of gaze shift eccentricities in Fig. 4A. Auditory data were recorded, on the other hand, because of mislocalization of the targets located at the edges of this area. It is important to note that the auditory data points in this range of gaze shift amplitudes could have pulled the resulting function upward, away from the function resulting from the fit of the visual data (Fig. 4A) and inflating, therefore, the actual contribution of the head to the gaze shift. Accordingly, as a control, an additional fit for the auditory data was computed after removing all data points comprising gaze shifts ranging from 35–55° of amplitude. The results, shown in Fig. 4A with a yellow dashed line, demonstrate that excluding this portion of the auditory data set did not affect the fit. Note that the a coefficient is very similar to the coefficient obtained with the entire data set, confirming, therefore, the difference in head contribution to the gaze shifts between the visual and auditory target modalities (see Fig. 4 legend).
In the vertical direction the difference in the contribution of the head to gaze shifts between the acoustic and visual target conditions was larger than in the horizontal direction (Fig. 4B). For acoustic targets, the contribution of the head increased exponentially as a function of gaze amplitude, albeit at a slower rate than in the horizontal direction for gaze shifts of similar amplitude. For visual targets, on the other hand, the head did not contribute at all to the gaze shift in most trials. In a small number of trials the head did move during the gaze shift, resulting in a small contribution, but not necessarily for the largest gaze shifts.
The larger contribution of the head to gaze shifts in the acoustic target condition could have resulted from several factors. The head might have 1) moved faster, reaching higher peak velocities, 2) reached peak velocity in a shorter time, or 3) as suggested by the data in Figs. 2 and 3, started to move before the eyes. These three possibilities are considered below.
Dynamics of head movements.
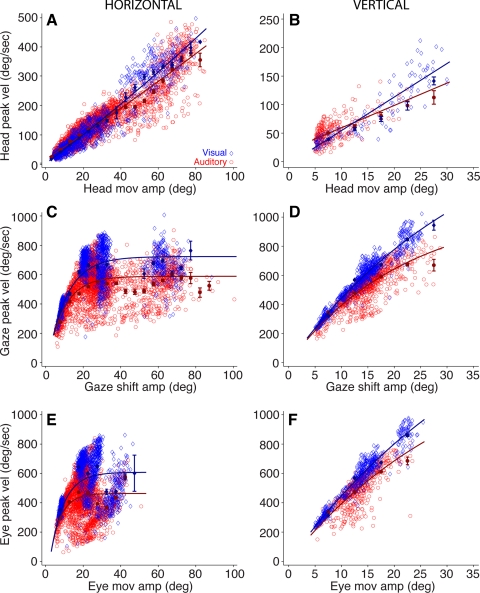
Plots of peak velocity as a function of movement amplitude, the main sequence (Bahill et al. 1975), were constructed for the head components of gaze shifts to visual and acoustic targets in both the horizontal and vertical directions (Fig. 5, A and B). For comparison, main sequence plots for the gaze shifts and the corresponding saccades are also shown (Fig. 5, C–F). Those data revealed that both gaze and eye movements were faster and less variable in the visual target condition (Fig. 5, C–F), as reported previously (Jay and Sparks 1989).
Fig. 5.
Comparison of main sequence plots, peak velocity vs. movement amplitude for head (A and B), gaze (C and D), and eye (E and F) movements; details as in Fig. 4. The function for A is Peak Vel = a × x2 + b × x + c. A, visual: a = 0.00656 (0.0024, 0.0107), b = 4.77 (4.486, 5.055), c = 2.76 (−0.542, 6.063). A, auditory: a = 0.0038 (0.00098, 0.00665), b = 4.157 (3.94, 4.374), c = 17.54 (14.34, 20.73). The function for B is Peak Vel = a × x + b. B, visual: a = 4.629 (4.273, 4.985), b = 2.208 (−5.454, 9.869). B, auditory: a = 3.084 (2.653, 3.515), b = 24.6 (20.28, 28.97). For C–F the function is Peak Vel = a × [1 − exp(−x/b)]. C, visual: a = 723.1 (713.9, 832.3), b = 10.25 (9.786, 10.72). C, auditory: a = 588.4 (582.1, 594.6), b = 8.887 (8.464, 9.31). D, visual: a = 1,824 (1,696, 1,951), b = 35.99 (32.82, 39.16). D, auditory: a = 1,069 (984, 1,154), b = 21.75 (19.21, 24.29). E, visual: a = 607.6 (595.5, 619.7), b = 7.268 (6.653, 7.884). E, auditory: a = 463.1 (455, 471.3), b = 5.56 (4.976, 6.143). F, visual: a = 2,121 (1,893, 2,349), b = 42.18 (36.66, 47.69). F, auditory: a = 1,521 (1,175, 1,866), b = 33.38 (23.92, 42.84).
In the horizontal direction head movements reached up to 80–85° of eccentricity; the most eccentric acoustic and visual targets were located at (±90, 0). Movements of up to 25–30° of eccentricity reached faster peak velocities in the acoustic target condition, as revealed by the averaged binned data (Fig. 5A, filled symbols), but for larger movements the head reached higher velocities in the visual condition, as indicated by the steeper slope of the function and averaged binned data in blue (Fig. 5A).
In the vertical direction the slope of the linear functions indicates that the head moved faster for visual than acoustic targets (Fig. 5B), but the averaged binned data show that the difference was only significant for gaze shift amplitudes between 25° and 30°. Also note that at small head movement amplitudes the head reached faster peak velocities in the auditory condition, and that the lack of auditory data for larger movement amplitudes may have resulted in underestimation of the slope. In the vertical direction, on the other hand, a larger data set would be needed to draw a similar conclusion.
Although we found some differences in the dynamics of head movements of gaze shifts to acoustic and visual targets, they are small for gaze shift amplitudes between 5° and 20° and inconsistent across the entire range of amplitudes and therefore cannot fully explain the larger contribution of the head in the acoustic condition.
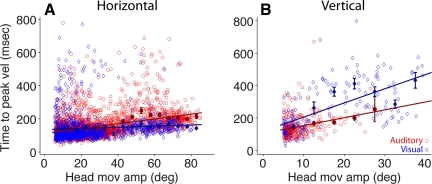
Time to peak velocity of head movements.
The second explanation we considered for the larger contribution of the head to gaze shifts to acoustic targets (Fig. 4) was that the head reached maximal velocity in a shorter period of time compared with the visual target condition. Figure 6 shows the time elapsed between movement onset and peak velocity, i.e., the duration of the acceleration phase, of head movements associated with gaze shifts to visual and acoustic targets in the horizontal and vertical directions. For horizontal movements the data from the visual target condition indicate that time to peak velocity increased as a function of movement amplitude (the coefficients describing the line are listed in the legend of Fig. 6), albeit at a slower rate than in the auditory target condition, which is characterized by a significantly steeper positive slope in this modality. Inspection of the averaged binned data revealed no differences between the two conditions for movement amplitudes of <40° but significant differences, i.e., longer time to peak velocity, in the acoustic target condition for movements >40°. Thus the larger contribution of the head to horizontal gaze shifts to acoustic targets cannot be explained by the head reaching peak velocity more quickly.
Fig. 6.
Comparison of time to peak velocity of head movements of gaze shifts to visual (blue) and acoustic (red) targets in the horizontal (A) and vertical (B) directions. Details as in Fig. 4. The coefficients of the fitted linear functions are A, visual: a = 0.351 (0.09, 0.61), b = 135 (126.5, 143.5); A, auditory: a = 1.46 (1.32, 1.6), b = 112 (106.9, 117); B, visual: a = 8.74 (6.94, 10.55), b = 118.9 (82.76, 155.1); B, auditory: a = 4.85 (3.01, 6.695), b = 105.4 (88.18, 122.6).
In the vertical direction the pattern was reversed. In the acoustic target condition the head achieved peak velocity in a shorter time compared with the visual target condition. However, it must be noted that the head moved little or not at all in conjunction with gaze shifts to visual targets located along the midline. Inspection of the averaged binned data for movements of <10° in amplitude revealed no significant differences.
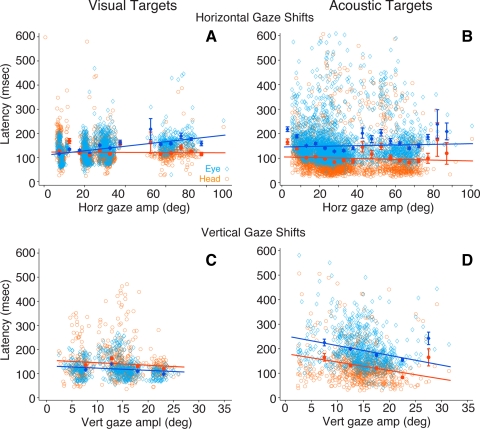
Eye and head latency.
The third explanation we considered to account for the larger contribution of the head to gaze shifts to acoustic targets is a shorter latency of the head movement compared with the latency of the eye movement in visual trials. Because the latency of eye and head movements is reversed under predictable target conditions (Bizzi et al. 1972), it is essential to point out that the data presented here were acquired in experimental sessions configured to minimize the chances that the subject would be able to predict the modality of the target as well as its location and time of presentation. Specifically, for each trial the target was specified by random selection among the following: sensory modality (visual or auditory), time of presentation after fixation (1,000–2,000 ms in 50-ms intervals), and location (16 visual and 24 auditory positions); each of the conditions was assigned a weight of 1 and was drawn without replacement. In experimental sessions in which the number of trials exceeded the number allocated in this arrangement, drawing without replacement was restarted as many times as needed.
The latency of eye and head movements of horizontal and vertical gaze shifts to visual and acoustic targets is shown in Fig. 7. Eye latency is plotted with light blue symbols and head latency with orange symbols. Each data set was fitted with a linear function, the coefficients of which are presented in the figure legend. As in previous figures, the averaged binned data are shown with filled symbols.
Fig. 7.
Comparison of eye and head movement latency from gaze shifts to horizontal (A and B) and vertical (C and D) visual and acoustic targets. Latency data from eye movements are plotted in light blue, and latency data from head movements are plotted in orange. Filled symbols represent binned means (5°), and standard bars represent 95% confidence intervals. The coefficients of the fitted linear functions are A, eye: a = 0.94 (0.83, 1.05), b = 108.9 (105.1, 112.8); A, head: a = −0.035 (−0.16, 0.09), b = 122.5 (118.3, 126.7); B, eye: a = 0.134 (−0.022, 0.29), b = 145.9 (140.5, 151.3); B, head: a = −0.164 (−0.304, −0.024), b = 105.6 (100.7, 110.4); C, eye: a = −1.29 (−1.89, −0.69), b = 138 (129.1, 147); C, head: a = −1.257 (−2.31, −0.21), b = 159.4 (143.8, 175); D, eye: a = −3.98 (−5.286, −2.67), b = 252.4 (231.9, 272.9); D, head: a = −3.45 (−4.699, −2.21), b = 180.2 (160.6, 199.8).
In horizontal gaze shifts to visual targets eye latency increased as a function of gaze amplitude, whereas head latency did not change (Fig. 7A). Note that the linear functions intersect at a gaze amplitude of ∼15°, revealing two modes of eye-head coordination. For gaze shifts smaller than ∼15° the eye moved first, whereas for larger gaze shifts the head moved first.
In gaze shifts to acoustic targets, in contrast, the head moved before the eyes at all gaze amplitudes studied, including gaze shifts of <20°. Furthermore, the slope of the linear functions fitted to the eye and head latency data were not different (Fig. 7B), suggesting that acoustic inputs were processed uniformly for targets at all eccentricities.
On the vertical plane the latency of both eye and head movement components of gaze shifts to visual targets remained constant as a function of gaze amplitude, with the eye, as expected, starting to move with significantly shorter latency than the head (Fig. 7C). In the acoustic target condition, on the other hand, the latencies of eye and head movements were reversed. At all gaze shift amplitudes the head moved with a significantly shorter latency than the eye (Fig. 7D). Thus far the data have shown that the head contributes significantly more to gaze shifts directed at acoustic than visual targets, particularly for gaze shifts in the horizontal direction of <30° in amplitude (Fig. 4), and that it does so not by moving faster (Figs. 5, 6) but by starting to move earlier (Fig. 7), suggesting, therefore, that the commands to move the eyes and the head may be generated independently.
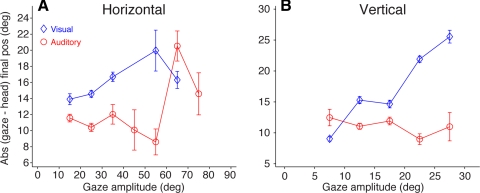
Differences between gaze and head movement end points.
In addition to the latency of the movements of the eyes and head that result in gaze shifts, it is important to examine the end points of those movements, for they may reveal important information about the nature of the commands that generate them. To address this issue we calculated the difference between final gaze and head positions, the absolute value of which is plotted in Fig. 8. As in previous figures, data from the visual target condition are plotted in blue and data from the acoustic target condition are plotted in red.
Fig. 8.
Absolute values of difference in final gaze and head position as a function of gaze amplitude from gaze shifts to horizontal (A) and vertical (B) visual (blue) and acoustic (red) targets. Standard bars represent 95% confidence intervals. Data from all 3 subjects are averaged.
In the horizontal direction the difference between final gaze and head position in the visual target condition increased as a function of gaze shift amplitude, reaching ∼20° for gaze shifts of ∼55° (Fig. 8A). At the smallest gaze amplitudes studied, the difference was approximately the amplitude of the gaze shift itself because in most trials the head moved very little or not at all. For gaze shifts larger than 55° the difference between the gaze and head end position decreased slightly. In the acoustic target condition, in contrast, the difference between final gaze and head position showed a decreasing trend as a function of gaze amplitude up to 55°, beyond which it increased, sharply matching the magnitude of the difference in the visual target condition (Fig. 8A). This is likely the result of similar head contributions in the visual and acoustic target conditions at large gaze amplitudes. And, as in the visual target condition, a decrease was also observed at the largest amplitudes studied.
In the vertical direction the magnitude of the discrepancy between final gaze and head position for visual targets was approximately the magnitude of the former, indicating not only that the head did not contribute to the gaze shifts, as documented in Fig. 4, but that in the trials in which it did move it moved very little (Fig. 8B). For acoustic targets, on the other hand, the magnitude of the difference between final gaze and head position was relatively constant for all gaze amplitudes, just over 10° (Fig. 8B). The discrepancy for the smaller gaze amplitudes resulted in some instances from larger head movements in the direction of the targets.
These results, from both the horizontal and vertical directions, clearly demonstrate that the eyes and head achieved different final position at the end of gaze shifts to visual and acoustic targets.
DISCUSSION
The present behavioral data show that eye and head movements are coordinated very differently to generate gaze shifts to visual and acoustic targets. Unlike gaze shifts to visual targets, in which the eyes move before the head (Bizzi et al. 1971), gaze shifts to acoustic targets typically start with a head movement, which in turn results in greater contribution of the head to the gaze shift, even for movement amplitudes of <20–25° to which the head contributes very little or not at all in the visual target modality (Freedman and Sparks 1997a). These differences suggest that the modality of the targets, which are encoded in different coordinate systems in the periphery, may trigger gaze shifts with different eye-head coordination by activating distinct neural mechanisms. The data suggest, therefore, that despite the great degree of interaction that has already been demonstrated between the eye and head during the execution of gaze shifts (Gandhi and Sparks 2007; Phillips et al. 1995), either gaze and head, or eye and head, can be controlled independently.
In interpreting these results it is essential to keep in mind the experimental conditions in which the data were collected because they are likely to have played a significant role in the outcome of the study. First, acoustic stimuli were used as targets for gaze shifts. Subjects were not simply prompted to orient to uncontrolled acoustic stimuli (e.g., Tomlinson and Bahra 1986b); rather they were presented with acoustic stimuli in a psychophysical context and were rewarded for pointing with their gaze to the perceived location of the sound sources (Populin 2006). It must be noted that the monkeys in this study were shown to base their responses on actual localization of the sound sources and not on remembering the spatial locations that led to rewards (Populin 2006). In short, the conditions of this study prompted the generation of what Phillips et al. (1995) called targeting gaze shifts, but with targets defined in nonretinocentric coordinates.
Second, all data were collected under conditions that produced alignment of the eyes and the head to the straight-ahead position at the time of stimulus presentation. This was actually a requirement, because deviations of the head from the straight-ahead position would have affected interaural differences of time and level, the cues used for localizing sound sources on the horizontal plane (Stevens and Newman 1934), and the contribution of the head to gaze shifts, which is affected by the starting position of the eyes in the orbit (Freedman and Sparks 1997a).
Third, aside from a head plate that prevented the monkeys from leaving the primate chair, the subjects were completely unrestrained during the present experiments. The lack of physical restraint resulted in very different experimental conditions compared with those of studies in which the monkey's body was restrained with a jacket (e.g., Bizzi et al. 1971) or a vest (e.g., Freedman and Sparks 1997a) or the head was attached to a mechanism that allowed movement only about the vertical axis (e.g., Phillips et al. 1995; Tomlinson and Bahra 1986a, 1986b). Under such conditions the sensorimotor integration underlying the processing of sensory feedback and the generation of motor commands is expected to be altered.
Fourth, the standard auditory (and visual) saccade task (Populin and Yin 1998) was used to acquire the present data. In this experimental task gaze shifts are triggered by the presentation of the target, which coincides with the offset of the fixation light, essentially simulating the target stepping from straight ahead to another position, albeit across sensory modalities in the acoustic target condition; Phillips et al. (1995) used this task with only visual stimuli. In a variant of the delay task, on the other hand, that is used extensively to separate in time sensory from motor responses during physiological recordings (e.g., Freedman and Sparks 1997b), the signal that commands the execution of the gaze shift is the offset of the fixation light, not the presentation of the target. Under such conditions of delayed response with the eyes and head aligned at the straight-ahead position, differences in latency between eye and head movements are greatly minimized (pilot data not shown).
Fifth, because predictive conditions, in which the subject expects visual targets to be presented from specific locations at specific times, produce gaze shifts that are led by the head and not the eye (Bizzi et al. 1972), acoustic and visual targets in this study were presented in random order at different locations and at various times after the onset of fixation of the visual stimulus straight ahead to prevent anticipation. Furthermore, to minimize the likelihood of presenting unwanted cues arising from switching artifacts during the process of selecting a speaker, which would have allowed the subjects to prepare their responses, all speakers were selected and deselected in random order at the start of every auditory and visual trial, leaving selected only the speaker from which sound was to be presented in that trial (Populin 2006). In addition, the animals were not able to orient in control trials in which a speaker was selected as indicated above, but the sound was attenuated to the maximum allowed by our equipment (120 dB). That is, while fixating the light stimulus straight ahead in any particular trial, the subject could not have predicted the location, modality, or time of presentation of the upcoming target.
The study of the neural mechanisms underlying the coordination of eye and head movements that result in gaze shifts has a very long and rich history. Hundreds of studies comprising numerous areas of the brain, e.g., the paramedian pontine reticular formation (Gandhi and Sparks 2007; Sparks et al. 2002), the central mesencephalic reticular formation (Pathmanathan et al. 2006a, 2006b), the pontomedullary region (Cowie and Robinson 1994; Cowie et al. 1994), the inhibitory burst neuron area (Cullen and Guitton 1997), the nucleus raphe interpositus (Bergeron and Guitton 2002; Paré and Guitton 1990; Phillips et al. 1999), the nucleus reticularis gigantocellularis (Freedman and Quessy 2004; Quessy and Freedman 2004), the caudal fastigial nucleus (Fuchs et al. 2010; Quinet and Goffart 2005), the frontal eye fields (Bizzi and Schiller 1970; Chen 2006; Guitton and Mandl 1978a, 1978b; Knight and Fuchs 2007; Tu and Keating 2000), the supplementary eye fields (Martinez-Trujillo et al. 2003), and the SC (e.g., Corneil et al. 2002a, 2002b; Freedman and Sparks 1997a; Klier et al. 2001; Munoz et al. 1991; Rezvani and Corneil 2008; Walton et al. 2007, 2008), have been published on the subject. Although there is ample evidence that the eyes and head are moved by separate neural commands (Bizzi et al. 1971; Corneil et al. 2002b; Freedman and Sparks 2000), a persistent and most controversial question concerns the origin of those signals: Are they generated separately or are they decomposed from a gaze signal, and if so where? The present behavioral data showing that gaze shifts to visual and acoustic targets are accomplished with different eye-head coordination may shed much needed light on this controversial issue.
Both single-unit recordings from the intermediate layers of the SC, taken while animals performed gaze shifts to visual targets (Freeman and Sparks 1997a; Munoz and Guitton 1985), and electrical stimulation of this structure, which produced coordinated movements of the eyes and head (Freedman et al. 1996; Klier et al. 2001; Paré et al. 1994), indicate that the SC encodes a gaze signal that in turn must be decomposed downstream into distinct motor commands to move the eyes and the head (Freedman and Sparks 1997b, 2000), the exact location of which remains unknown.
There is, however, anatomic evidence that two distinct descending pathways originate from different layers in the stratum griseum intermediale: an indirect pathway reaching oculomotor centers and a direct pathway reaching the spinal cord (Harting 1977; Huerta and Harting 1982; May and Porter 1992). Importantly, the model proposed by May and Porter (1992) includes a collateral input from the direct pathway to oculomotor centers in the reticular formation. Evidence corroborating the existence of two pathways is found in electrical stimulation studies (Corneil et al. 2002b; Cowie and Robinson 1994).
Corneil et al. (2002b), based on EMG activity recorded from neck muscles and evoked movements, proposed a model in which the area of the SC map activated by a stimulus representing a target determines the relative strength with which the two pathways are activated. Specifically, the most caudal locations produce stronger activation of an independent pathway, not gated by omnipause neurons (OPNs), while the most rostral locations activate more strongly a dependent, OPN-contingent pathway, producing, therefore, eye movements only for locations encoding gaze shifts of <20–25° in amplitude. Consistent with earlier models (Galiana and Guiton 1992; Goossens and van Opstal 1997; Guitton et al. 1990), Corneil et al. (2002a) proposed that the output of the SC is a gaze signal. They further hypothesized that the independent pathway might be selectively activated by the low-frequency activity exhibited by some saccade burst neurons that start to discharge well before the onset of gaze shifts when the location of the target is predictable (Basso and Wurtz 1997, 1998; Dorris and Munoz 1998; Dorris et al. 1997; Glimcher and Sparks 1992; Munoz and Wurtz 1995), thus serving as a mechanism to move the head before the eyes under such conditions (Bizzi et al. 1972).
Although this scheme is consistent with the time course of the single trials shown in Fig. 1 of Bizzi et al. (1972), in which the head movement performed in the anticipatory condition is much slower than the head movement performed in the triggered condition, it cannot explain the short-latency head movements recorded during gaze shifts to acoustic targets in the present study across all gaze amplitudes (Fig. 7), most importantly those of <20–25° in amplitude, for which the head does not contribute in the visual target condition (Freedman and Sparks 1997a).
Single-unit recordings in the SC of the monkey performed by Walton et al. (2007) suggest that the SC might independently control head movements. Using an experimental task designed to maintain gaze position constant while the subject moved its head to align a laser pointer mounted on it with a visual target, they showed that there are neurons in the SC whose firing rate is predictive of head displacement, demonstrating, therefore, that the SC may play a role in controlling head movements and gaze shifts independently. Such neurons could potentially directly drive motoneurons in the cervical spinal cord.
However, the same authors, Walton et al. (2008), have also shown that reversible inactivation of areas of the SC produces only a small effect on the latency of head movements, while affecting significantly the execution of gaze shifts, as shown previously in head-restrained preparations (Hikosaka and Wurtz 1985, 1986; Lee et al. 1988; McPeek and Keeler 2004; Quaia et al. 1998). It is clear, therefore, that movements of the head, contributing or not to gaze shifts, can be produced without contribution of the SC. It is important to note, nevertheless, that none of those studies tested the functional significance of deactivating areas of the SC by using acoustic stimuli as targets for gaze shifts, which, as shown above, are led by movements of the head.
The SC is a site of motor convergence for visual and auditory information (Jay and Sparks 1987a) and the only known link between circuits processing spatial auditory information and circuits responsible for initiating short-latency movements of the eyes and head. To our knowledge, no direct auditory inputs to head movement areas downstream from the SC, such as the pontomedullary head movement region (Cowie and Robinson 1994; Cowie et al. 1994) or the nucleus reticularis gigantocellularis (Quessy and Freedman 2004), that could mediate head movements to acoustic targets with latencies as short as those reported here have been demonstrated. Although cortical involvement cannot be strictly ruled out, it would most likely result in much longer head movement latencies, a result of the increased processing, than those observed in the present study.
In addition, numerous auditory inputs converge on the SC (Appell and Behan 1990; Bajo et al. 1993; Covey et al. 1987; Druga and Syka 1984; Edwards et al. 1979; Henkel and Shneiderman 1988; King et al. 1998; Kudo 1981; Kudo et al. 1984), including a very large one from the rostral pole of the inferior colliculus (Harting and van Lieshout 2000), where they form a detailed representation of contralateral space in its intermediate layers (Gordon 1973; Hirsch et al. 1985; King and Palmer 1983; Middlebrooks and Knudsen 1984; Wise and Irvine 1983). This map of auditory space is thought to encode the location of acoustic stimuli in motor, not sensory, coordinates (Jay and Sparks 1987b; Populin et al. 2004); thus its exact mechanism of action remains to be elucidated because it has only been studied in head-restrained preparations, a condition known to hinder accurate orienting to the sources of acoustic stimuli (Populin 2006; Tollin et al. 2005).
Thus, in light of the fact that to date no auditory inputs are known to project to head movement areas downstream from the SC (additional inputs are needed to decompose the gaze signal differently as a function of target modality), we hypothesize that the direct pathway is a preferential conduit for command signals from the SC to reach motoneurons innervating muscles of the neck. Collaterals from the direct pathway to oculomotor centers in the reticular formation (May and Porter 1992) would be responsible for the generation of the eye movement component of gaze shifts led by the head. This view, unlike that of Rezvani and Corneil (2008), would rely not on discharge frequency but on a different population of SC neurons receiving auditory inputs and projecting directly to the spinal cord.
The differences in eye and head movement latencies and head contribution that characterized gaze shifts to unexpected visual and acoustic targets recorded in the present study, coupled to the fact that anatomic evidence suggests the SC as the link between auditory spatial processing and the motor system, led us to conclude that the present data are consistent with the notion that the SC might encode gaze (or eye) and head signals separately. Other views that also postulate separate control of gaze and head incorporate the cerebellum (Daye et al. 2009; Optican et al. 2009). It is difficult to conceive how the alternative, a mechanism driven by a gaze signal downstream from the SC, could account for the present results without auditory inputs distinguishing between visual and acoustic targets.
Testing of this hypothesis will require physiological recordings from the SC while animals perform gaze shifts to acoustic targets under triggered (Bizzi et al. 1972) conditions and/or anatomic demonstration of auditory inputs to head movement areas downstream from the SC.
Because it has been clearly demonstrated in both monkeys (Populin 2006) and cats (Tollin et al. 2005) that the execution of accurate gaze shifts to acoustic targets requires a free-moving head, the process that leads to the generation of motor commands to initiate the eye movement component of gaze shifts to such targets via the indirect pathway might depend on the proper activation of the direct head movement pathway that is specifically activated by auditory inputs.
GRANTS
This work was supported by National Science Foundation Grant IOB-0517458 and National Institutes of Health Grants DC-03693 and T32-GM-007507.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We are thankful to John K. Harting for comments on an earlier version of this manuscript and to Jane Sekulski and Yonghe Yan for computer programming.
REFERENCES
- Appell PP, Behan M. Sources of subcortical GABAergic projections to the superior colliculus in the cat. J Comp Neurol 302: 143–158, 1990 [DOI] [PubMed] [Google Scholar]
- Bahill TA, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math Biosci 24: 191–204, 1975 [Google Scholar]
- Bajo VM, Merchan MA, Lopez DE, Rouiller EM. Neuronal morphology and efferent projections of the dorsal nucleus of the lateral lemniscus in the rat. J Comp Neurol 334: 241–262, 1993 [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity by target uncertainty. Nature 389: 66–69, 1997 [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18: 7519–7534, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron A, Guitton D. In multiple-step gaze shifts: omnipause (OPNs) and collicular fixation neurons encode gaze position error; OPNs gate saccades. J Neurophysiol 88: 1726–1742, 2002 [DOI] [PubMed] [Google Scholar]
- Bizzi E, Schiller PH. Single unit activity in the frontal eye fields of unanesthetized monkeys during eye and head movement. Exp Brain Res 10: 150–158, 1970 [DOI] [PubMed] [Google Scholar]
- Bizzi E, Kalil RE, Morasso P. Eye-head coordination in monkeys: evidence for centrally patterned organization. Science 173: 452–454, 1971 [DOI] [PubMed] [Google Scholar]
- Bizzi E, Kalil RE, Morasso P. Two modes of active eye-head coordination in monkeys. Brain Res 40: 45–48, 1972 [DOI] [PubMed] [Google Scholar]
- Chen LL. Head movements evoked by electrical stimulation in the frontal eye field of the monkey: evidence for independent eye and head control. J Neurophysiol 95: 3528–3542, 2006 [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulus parameters. J Neurophysiol 88: 1980–1999, 2002a [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. II. Gaze shift initiation and volitional head movements. J Neurophysiol 88: 2000–2018, 2002b [DOI] [PubMed] [Google Scholar]
- Covey E, Hall WC, Kobler JB. Subcortical projections of the superior colliculus in the mustache bat Pteronotus parnellii. J Comp Neurol 263: 179–197, 1987 [DOI] [PubMed] [Google Scholar]
- Cowie RJ, Robinson DL. Subcortical contributions to head movements in macaques. I. Contrasting effects of electrical stimulation of a medial pontomedullary region and the superior colliculus. J Neurophysiol 72: 2648–2664, 1994 [DOI] [PubMed] [Google Scholar]
- Cowie RJ, Smith MK, Robinson DL. Subcortical contributions to head movements in macaques. II. Connections of a medial pontomedullary head-movement region. J Neurophysiol 72: 2665–2682, 1994 [DOI] [PubMed] [Google Scholar]
- Cullen KE, Guitton D. Analysis of primate IBN spike trains using system identification techniques. II. Relationship to gaze, eye, and head movement dynamics during head-free gaze shifts. J Neurophysiol 78: 3283–3306, 1997 [DOI] [PubMed] [Google Scholar]
- Daye PM, Optican LM, Blohm G, Lefevre P. New model of gaze tracking in 2D. I. Novel architecture with independent gaze and head controllers. Soc Neurosci Abstr 2009: 851.1/V10, 2009 [Google Scholar]
- Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci 18: 7015–7026, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Paré M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 17: 8566–8579, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druga R, Syka R. Projections from auditory structures to the superior colliculus in the rat. Neurosci Lett 45: 247–252, 1984 [DOI] [PubMed] [Google Scholar]
- Edwards SB, Ginsburgh CL, Henkel CK, Stein BE. Sources of subcortical projections to the superior colliculus in the cat. J Comp Neurol 184: 309–330, 1979 [DOI] [PubMed] [Google Scholar]
- Freedman EG. Head-eye interactions during vertical gaze shifts made by rhesus monkeys. Exp Brain Res 167: 557–570, 2005 [DOI] [PubMed] [Google Scholar]
- Freedman EG, Quessy S. Electrical stimulation of rhesus monkey nucleus reticularis gigantocellularis. II. Effects on metrics and kinematics of ongoing gaze shifts to visual targets. Exp Brain Res 156: 357–376, 2004 [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. J Neurophysiol 77: 2328–2348, 1997a [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Activity of cells in the deep layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J Neurophysiol 78: 1669–1690, 1997b [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Coordination of the eyes and head: movement kinematics. Exp Brain Res 131: 22–32, 2000 [DOI] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol 76: 927–952, 1996 [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Brettler S, Ling L. Head-free gaze shifts provide further insights into the role of the medial cerebellum in the control of primate saccadic eye movements. J Neurophysiol 103: 2158–2173, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiana HL, Guitton D. Central organization and modeling of eye-head coordination during orienting gaze shifts. Ann NY Acad Sci 656: 452–471, 1992 [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Sparks DL. Dissociation of eye and head components of gaze shifts by stimulation of the omnipause neuron region. J Neurophysiol 98: 360–373, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature 355: 542–545, 1992 [DOI] [PubMed] [Google Scholar]
- Goossens HH, van Opstal AJ. Human eye-head coordination in two dimensions under different sensorimotor conditions. Exp Brain Res 114: 542–560, 1997 [DOI] [PubMed] [Google Scholar]
- Goossens HHLM, van Opstal AJ. Influence of head position on the spatial representation of acoustic targets. J Neurophysiol 81: 2700–2736, 1999 [DOI] [PubMed] [Google Scholar]
- Gordon B. Receptive fields in deep layers of the cat superior colliculus. J Neurophysiol 36: 157–178, 1973 [DOI] [PubMed] [Google Scholar]
- Guitton D. Control of eye-head coordination during orienting gaze shifts. Trends Neurosci 15: 174–179, 1992 [DOI] [PubMed] [Google Scholar]
- Guitton D, Mandl G. Frontal “oculomotor” area in alert cat. I. Eye movements and neck activity evoked by stimulation. Brain Res 149: 295–312, 1978a [DOI] [PubMed] [Google Scholar]
- Guitton D, Mandl G. Frontal “oculomotor” area in alert cat. II. Unit discharges associated with eye movements and neck muscle activity. Brain Res 149: 313–327, 1978b [DOI] [PubMed] [Google Scholar]
- Guitton D, Munoz DP, Galiana HL. Gaze control in the cat: studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. J Neurophysiol 64: 509–531, 1990 [DOI] [PubMed] [Google Scholar]
- Harting JK. Descending pathways from the superior colliculus: an autoradiographic analysis in the rhesus monkey (Macaca mulatta). J Comp Neurol 173: 583–612, 1977 [DOI] [PubMed] [Google Scholar]
- Harting JK, van Lieshout DP. Projections from the rostral pole of the inferior colliculus to the cat superior colliculus. Brain Res 881: 244–247, 2000 [DOI] [PubMed] [Google Scholar]
- Henkel CK, Shneiderman A. Nucleus sagulum: projections of a lateral tegmental area to the inferior colliculus in the cat. J Comp Neurol 271: 577–588, 1988 [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscumol and bicuculline in monkey superior colliculus. J Neurophysiol 53: 266–291, 1985 [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Saccadic eye movements following injection of lidocaine into the superior colliculus. Exp Brain Res 61: 531–539, 1986 [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Chan JCK, Yin TCT. Responses of neurons in the cat's superior colliculus to acoustic stimuli. I. Monaural and binaural response properties. J Neurophysiol 53: 726–745, 1985 [DOI] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. Tectal control of spinal cord activity: neuroanatomical demonstration of pathways connecting the superior colliculus with the cervical spinal cord grey. Prog Brain Res 57: 293–328, 1982 [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Sensorimotor integration in the primate superior colliculus. I. Motor convergence. J Neurophysiol 57: 22–34, 1987a [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Sensorimotor integration in the primate superior colliculus. II Coordinates of auditory signals. J Neurophysiol 57: 35–55, 1987b [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Localization of auditory and visual targets for the initiation of saccadic eye movements. In: Comparative Perception, edited by Berkley MA, Stebbins WC. New York: Wiley, 1989, vol. 1, p. 351–374 [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980 [DOI] [PubMed] [Google Scholar]
- King AJ, Palmer AR. Cells responsive to free-field auditory stimuli in guinea-pig superior colliculus: distribution and response properties. J Physiol 342: 361–381, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Jiang ZD, Moore DR. Auditory brainstem projections to the ferret superior colliculus: anatomical contribution to the neural coding of sound azimuth. J Comp Neurol 390: 342–365, 1998 [PubMed] [Google Scholar]
- Klier EM, Wang H, Crawford JD. The superior colliculus encodes gaze commands in retinal coordinates. Nat Neurosci 4: 627–632, 2001 [DOI] [PubMed] [Google Scholar]
- Knight TA, Fuchs AF. Contribution of the frontal eye field to gaze shifts in the head-unrestrained monkey: effects of microstimulation. J Neurophysiol 97: 618–634, 2007 [DOI] [PubMed] [Google Scholar]
- Kudo M. Projections of the nuclei of the lateral lemniscus in the cat: an autoradiographic study. Brain Res 221: 57–69, 1981 [DOI] [PubMed] [Google Scholar]
- Kudo M, Tashiro T, Higo S, Matsuyama T, Kawamura K. Ascending projections from the nucleus of the brachium of the inferior colliculus in the cat. Exp Brain Res 54: 203–211, 1984 [DOI] [PubMed] [Google Scholar]
- Laurutis VP, Robinson DA. The vestibulo-ocular reflex during human saccadic eye movements. J Physiol 373: 209–233, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Rohrer WH, Sparks DL. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature 332: 357–360, 1988 [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Wang H, Crawford JD. Electrical stimulation of the supplementary eye fields in the head-free macaque evokes kinematically normal gaze shifts. J Neurophysiol 89: 2961–2974, 2003 [DOI] [PubMed] [Google Scholar]
- May PJ, Porter JD. The laminar distribution of macaque tectobulbar and tectospinal neurons. Vis Neurosci 8: 257–276, 1992 [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7: 757–763, 2004 [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Knudsen EI. A neural code for auditory space in the cat's superior colliculus. J Neurosci 4: 2621–2634, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC, Knudsen EI. Changes in external ear position modify the spatial tuning of auditory units in the cat's superior colliculus. J Neurophysiol 57: 672–687, 1987 [DOI] [PubMed] [Google Scholar]
- Munoz DP, Guitton D. Tectospinal neurons in the cat have discharges coding gaze position error. Brain Res 341: 184–188, 1985 [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol 73: 2313–2333, 1995 [DOI] [PubMed] [Google Scholar]
- Munoz DP, Guitton D, Pelisson D. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. III. Spatiotemporal characteristics of phasic motor discharges. J Neurophysiol 66: 1642–1666, 1991 [DOI] [PubMed] [Google Scholar]
- Optican LM, Daye PM, Blohm G, Lefevre P. New model of gaze tracking in 2D. II. Compensation for perturbations of the head. Soc Neurosci Abstr 2009: 851.2/V11, 2009 [Google Scholar]
- Paré M, Guitton D. Gaze-related activity of brainstem omnipause neurons during combined eye-head gaze shifts in the alert cat. Exp Brain Res 83: 210–214, 1990 [DOI] [PubMed] [Google Scholar]
- Paré M, Crommelinck M, Guitton D. Gaze shifts evoked by stimulation of the superior colliculus in the head-free cat conform to the motor map but also depend on stimulus strength and fixation activity. Exp Brain Res 101: 123–139, 1994 [DOI] [PubMed] [Google Scholar]
- Pathmanathan JS, Cromer JA, Cullen KE, Waitzman DM. Temporal characteristics of neurons in the central mesencephalic reticular formation of head unrestrained monkeys. Exp Brain Res 168: 471–492, 2006a [DOI] [PubMed] [Google Scholar]
- Pathmanathan JS, Presnell R, Cromer JA, Cullen KE, Waitzman DM. Spatial characteristics of neurons in the central mesencephalic reticular formation (cMRF) of head-unrestrained monkeys. Exp Brain Res 168: 455–470, 2006b [DOI] [PubMed] [Google Scholar]
- Phillips JO, Ling L, Fuchs AF, Siebold C, Plorde JJ. Rapid horizontal gaze movement in the monkey. J Neurophysiol 73: 1632–1652, 1995 [DOI] [PubMed] [Google Scholar]
- Phillips JO, Ling L, Fuchs AF. Action of the brain stem saccade generator during horizontal gaze shifts. I. Discharge patterns of omnidirectional pause neurons. J Neurophysiol 81: 1284–1295, 1999 [DOI] [PubMed] [Google Scholar]
- Populin LC. Monkey sound localization: head-restrained vs. head-unrestrained orienting. J Neurosci 26: 9820–9832, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Populin LC. Human sound localization: measurements in untrained, head-unrestrained subjects using gaze as a pointer. Exp Brain Res 190: 11–30, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Populin LC, Yin TCT. Behavioral studies of sound localization in the cat. J Neurosci 18: 2147–2160, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Populin LC, Yin TCT. Kinematics of eye movements of cats to broadband acoustic targets. J Neurophysiol 82: 955–962, 1999 [DOI] [PubMed] [Google Scholar]
- Populin LC, Tollin DJ, Yin TCT. Effect of eye position on saccades and neuronal responses to acoustic stimuli in the superior colliculus of the behaving cat. J Neurophysiol 92: 2151–2167, 2004 [DOI] [PubMed] [Google Scholar]
- Quaia C, Aizawa H, Optican LM, Wurtz RH. Reversible inactivation of monkey superior colliculus. II. Maps of saccadic deficits. J Neurophysiol 79: 2097–2110, 1998 [DOI] [PubMed] [Google Scholar]
- Quessy S, Freedman EG. Electrical stimulation of rhesus monkey nucleus reticularis gigantocellularis. I. Characteristics of evoked head movements. Exp Brain Res 156: 342–356, 2004 [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Saccade dysmetria in head-unrestrained gaze shifts after muscimol inactivation of the caudal fastigial nucleus in the monkey. J Neurophysiol 93: 2343–2349, 2005 [DOI] [PubMed] [Google Scholar]
- Rezvani S, Corneil BD. Recruitment of a head-turning synergy by low-frequency activity in the primate superior colliculus. J Neurophysiol 100: 397–411, 2008 [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10: 137–145, 1963 [DOI] [PubMed] [Google Scholar]
- Sparks DL, Barton EJ, Gandhi NJ, Nelson J. Studies of the role of the paramedian pontine reticular formation in the control of head-restrained and head-unrestrained gaze shifts. Ann NY Acad Sci 956: 85–98, 2002 [DOI] [PubMed] [Google Scholar]
- Stahl J. Amplitude of human head movements associated with horizontal saccades. Exp Brain Res 126: 41–54, 1999 [DOI] [PubMed] [Google Scholar]
- Stevens S, Newman E. The localization of pure tones. Proc Natl Acad Sci USA 20: 593–596, 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollin DJ, Populin LC, Moore JM, Ruhland JL, Yin TCT. Sound localization performance in the cat: the effect of restraining the head. J Neurophysiol 93: 1223–1234, 2005 [DOI] [PubMed] [Google Scholar]
- Tomlinson RD. Combined eye-head gaze shifts in the primate. III. Contributions to the accuracy of gaze saccades. J Neurophysiol 64: 1873–1891, 1990 [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, Bahra PS. Combined eye-head gaze shifts in the primate. I. Metrics. J Neurophysiol 56: 1542–1557, 1986a [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, Bahra PS. Combined eye-head gaze shifts in the primate. II. Interactions between saccades and the vestibuloocular reflex. J Neurophysiol 56: 1558–1570, 1986b [DOI] [PubMed] [Google Scholar]
- Tu TA, Keating EG. Electrical stimulation of the frontal eye field in a monkey produces combined eye and head movements. J Neurophysiol 84: 1103–1106, 2000 [DOI] [PubMed] [Google Scholar]
- Tweed D, Glenn B, Vilis T. Eye-head coordination during large gaze shifts. J Neurophysiol 73: 766–779, 1995 [DOI] [PubMed] [Google Scholar]
- Uemura T, Arai Y, Shimazaki C. Eye-head coordination during lateral gaze in normal subjects. Acta Otolaryngol 90: 191–198, 1980 [DOI] [PubMed] [Google Scholar]
- Vliegen J, Van Grootel TJ, Van Opstal AJ. Dynamic sound localization during rapid eye-head gaze shifts. J Neurosci 24: 9291–9302, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton MMG, Bechara B, Gandhi NJ. Role of the primate superior colliculus in the control of head movements. J Neurophysiol 98: 2022–2037, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton MMG, Bechara B, Gandhi NJ. Effect of reversible inactivation of superior colliculus on head movements. J Neurophysiol 99: 2479–2495, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warabi T. The reaction time of eye-head coordination in man. Neurosci Lett 6: 47–51, 1977 [DOI] [PubMed] [Google Scholar]
- Whittington DA, Hepp-Reymond MC, Flood W. Eye and head movements to auditory targets. Exp Brain Res 41: 358–363, 1981 [DOI] [PubMed] [Google Scholar]
- Wise LZ, Irvine DRF. Auditory response properties of neurons in deep layers of cat superior colliculus. J Neurophysiol 49: 674–685, 1983 [DOI] [PubMed] [Google Scholar]
- Young ED, Rice JJ, Tong SC. Effects of the pinna position on head related transfer functions in the cat. J Acoust Soc Am 99: 3064–3076, 1996 [DOI] [PubMed] [Google Scholar]
- Zangemeister WH, Stark L. Gaze latency: variable interactions of head and eye latency. Exp Neurol 75: 389–406, 1982 [DOI] [PubMed] [Google Scholar]