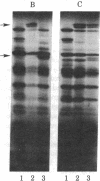
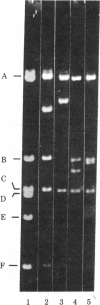
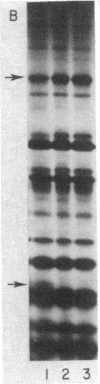
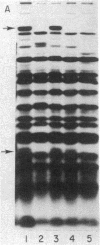
Abstract
Previous work has uncovered the existence of an Escherichia coli locus, groE, that is essential for bacterial growth, lambda phage and T4 phage head morphogenesis, and T5 phage tail assembly. Our genetic and biochemical analyses of lambda groE+ transducing phages and their deletion and point mutant derivatives show that the groE locus consists of two closely linked genes. One groE gene, groEL, has been shown to encode the synthesis of a 65,000 Mr polypeptide, whereas the second, groES, codes for the synthesis of a 15,000 Mr polypeptide. About half of the groE- bacterial isolates fall into the groES complementation group. GroE mutations in either gene cause similar phenotypes, with respect to lambda phage head morphogenesis and bacterial growth at nonpermissive temperatures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carroll D., Ajioka R. S., Georgopoulos C. Bacteriophage lambda cloning vehicles for studies of genetic recombination. Gene. 1980 Aug;10(3):261–271. doi: 10.1016/0378-1119(80)90055-4. [DOI] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F., Takahashi H. Abortive bacteriophage T4 head assembly in mutants of Escherichia coli. J Mol Biol. 1973 May 5;76(1):61–87. doi: 10.1016/0022-2836(73)90081-8. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Madden M. J., Schiop-Stanley P., Vande Woude G. F. Cloning of herpes simplex type 1 DNA fragments in a bacteriophage lambda vector. Science. 1979 Feb 9;203(4380):541–544. doi: 10.1126/science.216076. [DOI] [PubMed] [Google Scholar]
- Fowler R. G., Degnen G. E., Cox E. C. Mutational specificity of a conditional Escherichia coli mutator, mutD5. Mol Gen Genet. 1974;133(3):179–191. doi: 10.1007/BF00267667. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Eisen H. Bacterial mutants which block phage assembly. J Supramol Struct. 1974;2(2-4):349–359. doi: 10.1002/jss.400020224. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Casjens S. R., Kaiser A. D. Host participation in bacteriophage lambda head assembly. J Mol Biol. 1973 May 5;76(1):45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Kaiser A. D., Wood W. B. Role of the host cell in bacteriophage morphogenesis: effects of a bacterial mutation on T4 head assembly. Nat New Biol. 1972 Sep 13;239(89):38–41. doi: 10.1038/newbio239038a0. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hohn B. Identification of a host protein necessary for bacteriophage morphogenesis (the groE gene product). Proc Natl Acad Sci U S A. 1978 Jan;75(1):131–135. doi: 10.1073/pnas.75.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Assembly of bacteriophage lambda heads: protein processing and its genetic control in petit lambda assembly. J Mol Biol. 1975 Jan 15;91(2):187–199. doi: 10.1016/0022-2836(75)90159-x. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979 Apr 15;129(3):375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Tsui L. Role of the host in virus assembly: cloning of the Escherichia coli groE gene and identification of its protein product. Proc Natl Acad Sci U S A. 1978 Jan;75(1):136–139. doi: 10.1073/pnas.75.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn T., Hohn B., Engel A., Wurtz M., Smith P. R. Isolation and characterization of the host protein groE involved in bacteriophage lambda assembly. J Mol Biol. 1979 Apr 15;129(3):359–373. doi: 10.1016/0022-2836(79)90501-1. [DOI] [PubMed] [Google Scholar]
- Murialdo H. Early intermediates in bacteriophage lambda prohead assembly. Virology. 1979 Jul 30;96(2):341–367. doi: 10.1016/0042-6822(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Huskey R. J. Deletion mutants of bacteriophage lambda. I. Isolation and initial characterization. J Mol Biol. 1971 Mar 14;56(2):369–384. doi: 10.1016/0022-2836(71)90471-2. [DOI] [PubMed] [Google Scholar]
- Revel H. R., Stitt B. L., Lielausis I., Wood W. B. Role of the host cell in bacteriophage T4 development. I. Characterization of host mutants that block T4 head assembly. J Virol. 1980 Jan;33(1):366–376. doi: 10.1128/jvi.33.1.366-376.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N. Properties of a mutant of Escherichia coli defective in bacteriophage lambda head formation (groE). II. The propagation of phage lambda. J Mol Biol. 1973 May 5;76(1):25–44. doi: 10.1016/0022-2836(73)90079-x. [DOI] [PubMed] [Google Scholar]
- Takano T., Kakefuda T. Involvement of a bacterial factor in morphogenesis of bacteriophage capsid. Nat New Biol. 1972 Sep 13;239(89):34–37. doi: 10.1038/newbio239034a0. [DOI] [PubMed] [Google Scholar]
- Thomas M., Cameron J. R., Davis R. W. Viable molecular hybrids of bacteriophage lambda and eukaryotic DNA. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4579–4583. doi: 10.1073/pnas.71.11.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]