Abstract
Wood formation requires a continuous supply of carbohydrates for structural growth and metabolism. In the montane belt of the central Austrian Alps we monitored the temporal dynamics of xylem growth and non-structural carbohydrates (NSC) in stem sapwood of Pinus sylvestris L. during the growing season 2009, which was characterized by exceptional soil dryness within the study area. Soil water content dropped below 10 % at the time of maximum xylem growth end of May. Histological analyses have been used to describe cambial activity and xylem growth. Determination of NSC was performed using specific enzymatic assays revealing that total NSC ranged from 0.8 to 1.7 % dry matter throughout the year. Significant variations (P < 0.05) of the size of the NSC pool were observed during the growing season. Starch showed persistent abundance throughout the year reaching a maximum shortly before onset of late wood formation in mid-July. Seasonal dynamics of NSC and xylem growth suggest that (i) high sink activity occurred at start of the growing season in spring and during late wood formation in summer and (ii) there was no particular shortage in NSC, which caused P. sylvestris to draw upon stem reserves more heavily during drought in 2009.
Introduction
In dry inner Alpine valleys of the European Alps several tree ring studies revealed that drought occurring during the growing season has a strong impact on radial stem growth and tree mortality of Scots pine (Pinus sylvestris L.) (e.g., Oberhuber 2001; Rebetez and Dobbertin 2004). Recently, wood formation dynamics in P. sylvestris exposed to drought was analysed by repeatedly taking small punched cores of the outermost tree rings during short time intervals (cf. Rossi et al. 2006a, Gruber et al. 2010). Authors concluded that the dynamics and duration of cell differentiation processes in P. sylvestris was strongly influenced by drought, which is in agreement with the general assumption that cell division and cell enlargement are more sensitive to drought, than are photosynthesis and stomatal closure (Larcher 2003). In this regard, the assessment of the size and seasonal variability of the mobile (or non-structural) carbon pool during the growing season is a straightforward approach to analyse the balance between carbon supply by photosynthesis and demand by growth and metabolism (Hoch et al. 2002; Körner 2003). Non-structural carbohydrates (NSC) and lipids are the most important carbon compounds for storage (Chapin et al. 1990), whereby the lipid pool (in P. sylvestris mostly triacylglycerols, Fischer and Höll 1992) is assumed to act mainly as long-term store and was found to be far more stable throughout the growing season than is the NSC pool (Hoch et al. 2002; Hoch and Körner 2003). Hence, the pool of NSC is a measure of the carbon source-sink relationship within a plant (Chapin et al. 1990; Hoch 2007). Recently, several studies found that the NSC pool, especially starch and low molecular weight sugars like glucose, fructose and sucrose, increased in conifers in response to growth-limiting low temperatures at tree line sites (e.g., Hoch and Körner 2003; Solfjeld and Johnsen 2006) indicating that sink activities (i.e., structural growth) were more restricted by low temperatures during the growing season than source activities (i.e., carbon assimilation). In mature temperate conifers at low altitude where favorable growing conditions prevail, NSC did not vary significantly throughout the growing season (Hoch et al. 2003). On the other hand, occurrence of abiotic stresses during the growing season, such as drought, might cause an imbalance between carbon availability and carbon loss, which can lead to a depletion of carbon reserves (McDowell et al. 2008; Sala et al. 2010). Within the study area the phase of radial stem growth in P. sylvestris in spring coincides with frequent drought periods (cf. Oberhuber and Gruber 2010) and therefore might be a period of potential carbon shortage. To our knowledge assessment of seasonal variations of NSC in stem sapwood concurrently with determination of temporal dynamics of cambial activity and xylem cell differentiation under drought stress is still lacking. Therefore, we analyzed seasonal variations in NSC in comparison to crucial phenological stages of xylem growth, i.e., onset, maximum and ending of early wood and late wood formation in stem sapwood of P. sylvestris exposed to a dry inner Alpine environment. We hypothesized that drought during the growing season causes a depletion of stored carbohydrates in stem sapwood.
Materials and methods
Site description
The study site (for the geographical location, see Oberhuber et al. 1998) is part of a postglacial rock-slide area situated in the montane belt (c. 750 m asl) within the inner Alpine dry valley of the Inn River (Tyrol, Austria, 47° 14′ 00″ N, 10° 50′ 20″ E) and has a relatively continental climate with mean annual precipitation and temperature of 716 mm and 7.3 °C, respectively (long-term mean during 1911-2008 at Ötz, 812 m asl, 5 km from the study area). Pinus sylvestris forms widespread forest ecosystems in the lower montane region within dry inner Alpine valleys in the central Austrian and Swiss Alps (Ellenberg and Leuschner 2010).
A dry-mesic site in a hollow (partly facing north, slope < 10°) was selected. Stand height was 10-12 m with a canopy-coverage of about 66 %. Spring heath (Erica carnea L.), crowberry (Vaccinium vitis-idaea L.) and a thick moss layer dominate the understory, which indicates slightly moist conditions. Soils of protorendzina type–i.e. rendzic leptosols according to the FAO classification system (FAO 1998)–are developed and consist of unconsolidated, coarse-textured materials with low water holding capacity (soil depth 20–30 cm). All measurements were carried out on dominant mature trees with mean tree age of c. 165 yr at sampling height (Table 1).
Table 1.
Characteristics of Pinus sylvestris trees selected for monitoring dynamics of xylem growth and non-structural carbohydrate content (RW = ring width, SD = standard deviation).
| Method | Tree age1 (yr) mean ± SD |
Stem diameter2 (cm) mean ± SD |
RW3 (μm) mean ± SD |
|---|---|---|---|
| Xylem growth (micro-cores) | 166 ± 23 | 30.6 ± 3.0 | 534 ± 99 |
| Non-structural carbohydrate content | 162 ± 28 | 29.9 ± 4.6 | 583 ± 183 |
Cambial age at sampling height
Mean tree diameter measured at 1m stem height
Mean values for the period 2004–2008
Xylem sampling and determination of wood formation
Seasonal wood formation dynamics was monitored during the growing season 2009 by taking small punched cores from five trees of the outermost tree rings (micro-cores) with a diameter and length of 2.5 mm and c. 2 cm, respectively (Rossi et al. 2006b; Gruber et al. 2010). Micro-cores were taken from March to October in weekly to 10-day intervals to include the whole dynamics of xylem formation. 19 samples were taken throughout the growing season 2009 from each selected tree. Samples were taken on the slope-parallel side of the stem following a spiral trajectory up the stem starting at c. 1 m stem height. A distance of c. 2 cm in tangential and longitudinal direction was kept to avoid lateral influence of wound reactions on adjacent sampling positions.
Immediately after extraction, cores were placed in a solution of 70% ethanol, propionic acid, and 40% formaldehyde (mixing ratio: 90/5/5), subsequently embedded in glycolmethacrylate (Technovit 7100) and polymerized after adding an accelerator. Transverse sections of c. 12 μm were cut with a rotary microtome, stained with a water solution of 0.05 % cresyl fast violet and observed under a light microscope with polarized light to differentiate the development of xylem cells–i.e. the discrimination between tracheids in enlarging and cell-wall thickening phase (Deslauriers et al. 2003; Rossi et al. 2006a). The number of cambial cells (i.e., fusiform cells lacking radial enlargement), radial enlarging cells, cells undergoing secondary wall thickening and lignification, and mature xylem cells were counted on all sampled cores in three radial rows. Because dynamics of wood formation may vary within the tree circumference, number of cells were standardized based on the total cell numbers of the previous three tree rings (cf. Rossi et al. 2003, Gruber et al. 2010). The increase in tree-ring width over time was determined to the nearest 0.001 mm by image analysis (ProgRes CapturePro 2.5, Jenoptik, Germany) and included cells in radial enlargement, cell-wall thickening and mature xylem cells (means of three radial rows and five trees per date).
Short-term variation in intra-annual increase in ring width was modelled with a Gompertz function (cf. Rossi et al. 2003) using the nonlinear regression procedure included in the Origin software package (OriginLab Corporation, Northampton, MA, USA). Based on the finding that the beginning and end of cambial activity is closely related to the beginning and end of the enlargement phase (Gričar et al. 2009) and in accordance with suggestions made by Rathgeber et al. (2011), onset of cambial activity and wood formation were defined as the date at which more than 50 % of the observed radial files had at least one first enlarging cell. Cambial activity and wood formation were considered to have ended when less than 50 % of the observed radial files showed at most one last enlarging cell and cell wall thickening and lignification were completed, respectively. The early wood – late wood transition within an annual ring is generally abrupt in P. sylvestris and was defined as two times double wall thickness equal or greater than the width of the lumen (Denne 1988). Accordingly, the date of onset of late wood formation was determined when this criterion was detected in more than 50 % of the observed radial files.
Phenology of shoot growth in the upper crown was recorded at the same trees selected for micro-coring. Onset of shoot elongation was assessed weekly and defined as readily identifiable swelling of buds and extension of the terminal shoot by > 5 mm. At this time, bud scales were still covering the new needles, which broke free about one week later.
Analysis of non-structural carbohydrates (NSC)
In accordance with Hoch and Körner (2003) NSC are defined as the sum of free, low-molecular-weight sugars (glucose, fructose and sucrose) and starch. Since Fischer and Höll (1992) and Hoch et al. (2003) reported only weak seasonal variations of lipids in P. sylvestris throughout the growing season, we concentrated on determination of seasonal variability in NSC. From the sapwood fraction of stem xylem, samples were taken at breast height from March through December 2009 at eight dates from 6 to 7 trees using an increment corer (5 mm diameter). All samples were collected around noon to minimise effects of diurnal NSC changes (Li et al. 2008) and were stored in a cool box immediately after collection. Within three hours enzymes in the samples were denatured by heating the samples in a microwave at 600 W for 90 seconds (Hoch et al 2002; Hoch and Körner 2003). Afterwards the samples were dried to weight constancy at 60°C, grained to powder and stored dry until they were analysed.
For binding plant phenols 0.1 mg Polyvinylpyrrolidon was added to approximately 10 mg of fine ground plant material. Soluble carbohydrates were extracted from the weight in samples twice in 80 % acetone (v/v) for 15 minutes at 50 °C. After vaporizing the acetone, the residual of the soluble fraction was resolved in distilled water and the concentration of glucose was determined photometrical at 340 nm, as NADPH+ H+ formation during enzymatic conversion of glucose-6-phosphate to gluconate-6-phosphate. Aliquots of the resolved extract were treated with hexocinae and isomerase as well as invertase to convert fructose and sucrose into glucose, which was subsequently measured as described above. Starch extraction was carried out by incubating the insoluble fraction with hydrochloric acid for 2 h at 60 °C. After pH adjustment starch was hydrolyzed enzymatically to glucose and subsequently measured. The photometric analyses were conducted using enzymatic BioAnalysis kits for starch and sucrose/D-glucose/D-fructose from Boehringer Mannheim (Mannheim, Germany). To avoid influence of frequent wounding on tree physiology, trees selected for monitoring dynamics of xylem growth and NSC within the study plot were different but had similar age, stem diameter and annual increments (Table 1). Differences in NSC and its components (soluble carbohydrates and starch) between sampling dates were tested for significance by applying one-way repeated measures ANOVA using SPSS18 (IBM, NY, USA). Kendall’s rank correlation coefficient (τ; Sheskin 2007) was calculated to analyse the relationship between NSC and wood formation.
Microclimate records
During the study period, daily precipitation and air temperature were collected automatically at 2 m height (ONSET, Pocasset, MA, USA). Long-term records (LTM) of total monthly precipitation and mean monthly temperatures since 1911 were available from a nearby meteorological station (Ötz, 812 m asl, 5 km from the study area). Soil moisture dynamics (volumetric water content) in the upper 10 cm of the soil layer were continuously monitored. Moisture sensors are based on a capacitive method (Cyclobios, proprietary development at University of Innsbruck, Austria). Due to small-scale variability of soil structure and soil depth, records of three soil moisture and temperature sensors were averaged. Measuring intervals for all sensors were 30 min. Mean daily air temperature and soil water content (vol %) were calculated by averaging all measurements (48 values/day).
Environmental variables during growing season 2009
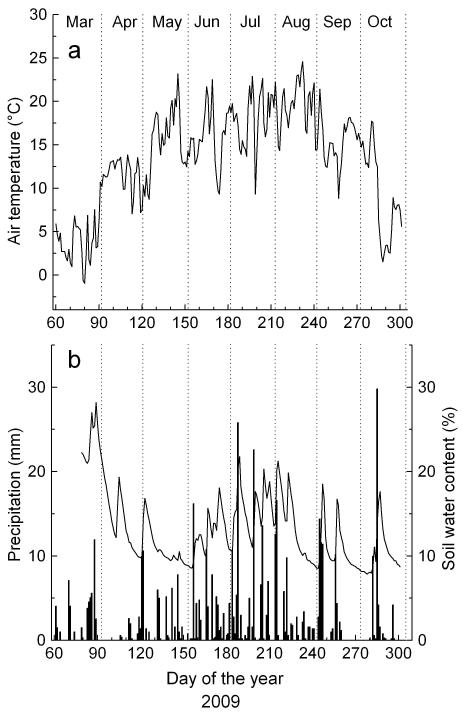
Climate in 2009 was characterized by mild temperatures and dry conditions in spring (Fig.1). Only scattered rainfall was recorded in April and total monthly precipitation reached 11 mm, i.e. about one-third of LTM in April amounting to 39 ± 20 mm (mean ± standard deviation). While in May precipitation sum of 60 mm corresponded to LTM (64 ± 26 mm), precipitation during summer (June-August; 258 mm) was about 20 % below LTM (317 ± 65 mm). Mean air temperature in April (11.5 ± 1.9 °C), May (15.5 ± 3.7 °C) and during summer (17.8 ± 3.4 °C) exceeded LTM by 3.9 °C in April (LTM 7.6 ± 1.8 °C), 3.5 °C in May (LTM 12.0 ± 1.8 °C) and 2 °C during summer (LTM 15.8 ± 1.0 °C). During periods with scattered low rainfall events in April and May soil water content dropped to less than 10 vol% for several weeks. Soil moisture temporarily reached 20 vol% in the selected stand after more extensive rainfall events occurred in June (Fig. 1).
Fig. 1.
Climate variables recorded during the growing season 2009 within the study area. a Mean daily air temperature b Daily precipitation sum (bars) and soil water content.
Results
Dynamics of tree-ring growth
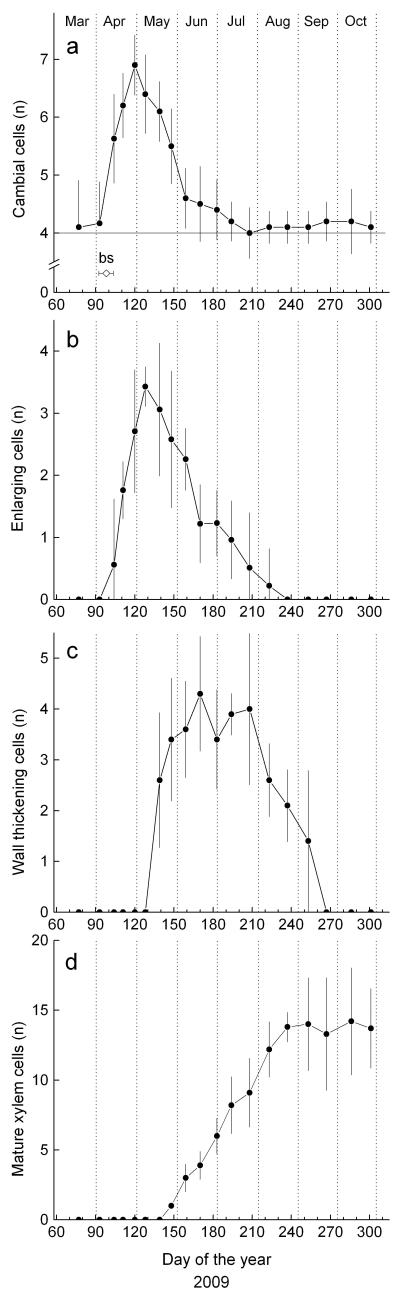
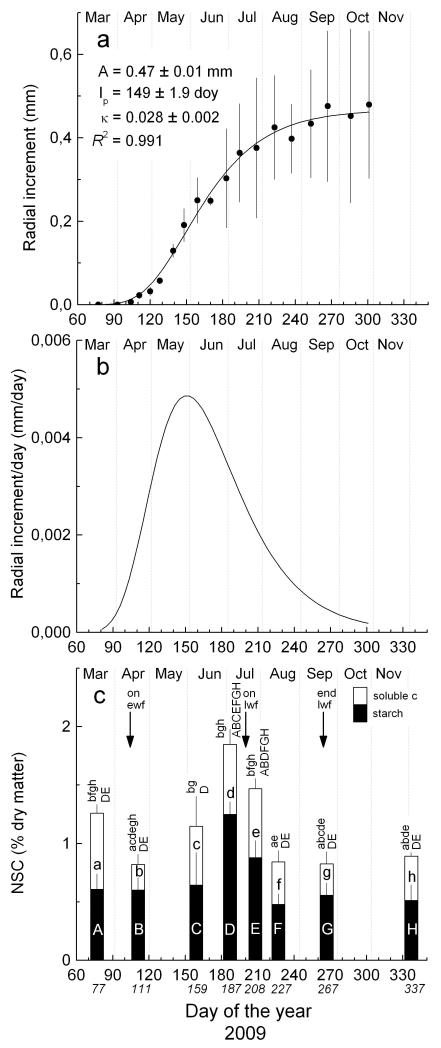
The dormant cambium consisted of c. four cells (Fig. 2a). In mid-April the number of cells in the cambial zone rapidly increased, whereby maximum values were reached in late April through early May. In mid July cambial cell number and shape appeared to be the same as before reactivation after winter dormancy. Based on number of enlarging cells cambial activity lasted from mid-April (107 ± 2 day of the year [doy]) to early August (214 ± 4 doy). Hence, in 2009 cambial cells divided throughout c. 107 d (Table 2). Bud swelling and onset of shoot elongation in the upper crown occurred in early April (98 ± 6 doy). Delayed bell-shaped curves (enlarging and wall-thickening cells) and a growing S-shaped curve (mature xylem cells) characterize the dynamic of cell differentiation (Figure 2b-d). First tracheids were undergoing wall thickening on 19 May (139 doy) and cell maturation, i.e. wall-thickening and lignification was completed in mid-September (261 ± 3 doy). First mature early wood tracheids were detected on 28 May, while mean onset of late wood formation (i.e., formation of radially flattened tracheids) started on 17 July (198 ± 2 doy). In 2009 mean duration of wood formation, including all developmental phases from cell enlargement to the end of wall-thickening and lignification lasted for 155 ± 3 days. Measurement of the developing xylem (including cells in enlarging and wall-thickening phase and mature xylem cells) during the growing season 2009 is depicted in Fig. 3a. The intra-annual dynamic of wood formation followed a sigmoid-shaped curve. Based on modeled xylem growth by applying the Gompertz function, the maximum radial growth (mm d−1) occurred at the end of May and annual increment in 2009 amounted to 0.47 ± 0.011 mm (Table 2, Fig. 3).
Fig. 2.
Number of cells a in the cambial zone, b in radial enlargement, c in secondary wall thickening and lignification and d mature xylem cells during 2009. Bars represent confidence intervals at P < 0.05. Horizontal thin line in a indicates the number of dormant cambial cells (bs = bud swelling and onset shoot elongation).
Table 2.
Phenological dates and duration of cambial activity and wood formation in 2009 (doy = day of the year; EW/LWt = early wood –late wood transition; Mx = maximum daily growth rate. Mean values ± standard error are shown.
| Cambial activity | Wood formation | ||||||
|---|---|---|---|---|---|---|---|
| onset (doy) |
end (doy) |
duration (days) |
onset (doy) |
end (doy) |
duration (days) |
EW/LWt (doy) |
Mx (doy) |
| 107 ± 2 | 214 ± 4 | 107 ± 4 | 107 ± 2 | 261 ± 3 | 155 ± 3 | 198 ± 2 | 149 ± 2 |
Fig. 3.
a Dynamics of xylem growth modelled by applying the Gompertz function, b daily xylem growth rates calculated on the basis of modelled growth and c dynamics of non-structural carbohydrate content (NSC) and its components starch (filled bars) and soluble carbohydrates (open bars; soluble c) in stem sapwood. Ewf and lwf indicate time of onset/end of early wood and late wood formation, respectively (cf. Table 2). In a parameters of the Gompertz function for xylem growth and R2 of the model are indicated (A = upper asymptote, Ip = inflection point, κ = rate of change parameter, mean values ± standard deviation).Bars in a represent standard deviations and in c confidence intervals at P < 0.05. Lowercase and uppercase lettering above bars in c denote significant differences (P < 0.05) among sampling dates for soluble carbohydrate (a-h) and starch content (A-H), respectively. Sampling dates for determination of NSC are indicated in italics.
NSC in stem sapwood amounted to 1.2 % dry matter in March before onset of cambial activity occurred in mid-April (Table 2, Fig. 3c). The largest NSC fraction within the non-structural carbon pool was starch throughout the growing season (April – September). Soluble carbohydrate content decreased significantly after onset of early wood formation, which was followed by a significant increase in NSC and its components (soluble carbohydrates and starch) in June. Starch content was quite stable from March through June and peaked in early July shortly before onset of late wood formation. Cambial activity ceased about 2 weeks later (Table 2, Figs. 2 and 3). During the period of late wood formation a statistically significant drop in both, soluble carbohydrates and starch was detected. After completion of late wood formation in mid-September starch content remained stable, while content of soluble carbohydrates increased slightly. Concentrations of soluble carbohydrates were significantly higher before onset of shoot elongation than after growth cessation in mid-September and during dormancy in December. Correlation coefficients (Kendall τ) between daily radial increment and NSC and its components starch and soluble carbohydrates amounted to 0.733 (P < 0.05), 0.348 (P > 0.05) and 0.733 (P < 0.05), respectively, when a time lag of 16 days in NSC content was considered.
Discussion
Temporal dynamics of cambial activity and xylem growth in 2009 are comparable to recently published data for 2007 and 2008 at the same dry-mesic site (see Gruber et al. 2010). While several findings indicate that onset of cambial activity is highly responsive to temperature (e.g., Oribe et al. 2001; Gričar et al. 2006), the early peak in daily growth rate in late spring (end of May) led to the suggestion that a strong sink competition for carbon to mycorrhizal root and shoot growth exists (cf. Oberhuber and Gruber 2010) as an adaptation to cope with extreme environmental conditions, i.e., recurring drought periods in spring combined with limited water holding capacity and nutrient deficiency of shallow, stony soils prevailing within the study area. In agreement with our results maximum growth rates around end of May were also found for several deciduous tree species, e.g., Acer platanoides (Marion et al. 2007), Fagus sylvatica (Čufar et al. 2008) and Populus sp. (Deslauriers et al. 2009). Latter authors, however, related early achievement of maximum growth rates to high transpiration of developing leaves, which requires rapid production of xylem elements in spring to adequately sustain water transport.
Carbon is involved in radial stem growth as structural material (i.e., cell wall thickening and lignification) and as source for metabolic energy. Hansen et al. (1996) reported that in P. sylvestris photosynthetic activity during winter causes minimal loss of NSC and stored C-compounds peak at bud break. In our study, concentrations of soluble carbohydrates were also found to be significantly higher in March (before bud break) than after growth cessation in mid-September and during dormancy in December. Significant depletion of soluble carbohydrates after onset of xylem growth in April might indicate the use of stored carbohydrates for early wood formation. Using 14C pulse-labeling Hansen and Beck (1994) demonstrated that in P. sylvestris stored carbohydrates of the previous year contribute to tree-ring growth of the current year. Carry-over effects of stored products in tree ring formation from one year to the next were also detected by Helle and Schleser (2004) and Keel et al. (2007). However, as demonstrated by several authors (Maunoury-Danger et al. 2010; Maier et al. 2010) a seasonal shift in substrate use from reserves to recently fixed carbohydrate during active radial growth might occur.
At our study site, mean NSC concentration in stem sapwood throughout the year amounted to c. 1.13 % dry matter, which is similar to findings reported by Hoch and Körner (2003) for P. sylvestris stands at their northernmost distribution in Sweden, whereas slightly higher NSC values were found in a mixed forest stand with a mean canopy height of approximately 30 m at low altitude in Switzerland (Hoch et al. 2003). Quite favorable environmental conditions might reasonably explain the higher NSC pool at the latter site. Accumulation of NSC was detected in our study in stem sapwood in early July at the time when cambial activity decreased. This finding might indicate that growth declined faster than photosynthesis, i.e. during drought periods in spring, when soil water content dropped to c. 10 % for several weeks, sink activity (structural growth) was more impaired than source activity (carbon assimilation). Correspondingly, the time lag found between daily radial increment and soluble carbohydrate content of c. two weeks might be due to primary use of currently produced photoassimilates for wood formation (cf. Eilmann et al. 2010) and successive accumulation of carbohydrates in stem sapwood after cambial activity decreased. Several studies support this explanation by demonstrating that cell division is generally more sensitive to drought than is the rate of photosynthesis (e.g., Frensch 1997; Abe and Nakai 1999). Furthermore, it is unlikely that the accumulation of NSC during the growing season reflects osmotic adjustment in response to drought stress (for a review see Morgan 1984), because not only osmotically active low molecular weight carbohydrates, but also starch concentration increased significantly in early July. The subsequent decrease in NSC from July through September indicates an increased demand of NSC for late wood formation, which is supported by results of Eilmann et al. (2010), who found a tight coupling between wood formation and currently produced photoassimilates. However, a high demand of cell wall material as a sink for carbohydrates is questionable due to rather low numbers of differentiating xylem cells found throughout the growing season. Rather, in addition to respiration losses and/or carbon storage in other tissues, importing tissues below ground (roots and mycorrhiza) have to be considered, because carbon allocation to roots in response to drought is a well known phenomenon (for a review see McDowell et al. 2008; Brunner et al. 2009) and the allocation of current photosynthate-carbon to below ground is known to be at maximum in late summer and autumn (e.g., Hansen and Beck 1990; Bhupinderpal-Singh et al. 2003). Small-scale variability of environmental conditions (e.g., soil structure and depth) might have differently influenced carbon allocation patterns and dynamics of wood formation of trees selected for monitoring NSC and xylem growth throughout the year. However, we regard this possibility to be negligible, because all trees were located within the same stand and showed similar age and growth characteristics.
Starch is considered the most important reserve carbohydrate and accumulates as a storage compound during times of surplus carbon supply (Chapin et al. 1990; Pallardy 2008). Hoch et al. (2003) reported that the largest NSC fraction in woody tissues of mature temperate forest trees was starch throughout the growing season. Because we also detected persistent high starch concentrations throughout the growing season and the size of a plant’s mobile carbon pool is assumed to reflect its carbon supply status (Chapin et al. 1990; Körner 2003), we conclude that carbon was not a limiting resource for radial stem growth in P. sylvestris at the dry-mesic inner Alpine study site in 2009, which possibly indicates that photosynthesis was less affected by moisture shortage than xylem growth. Hence, we reject our hypothesis that drought during the growing season 2009 caused a depletion of stored carbohydrates in stem sapwood of P. sylvestris. However, a prolonged drought will constrain both, xylem growth and photosynthesis (Eilmann et al. 2010) and Galiano et al. (2011) reported that in dying P. sylvestris trees a depletion of carbon reserves occurred, which was most likely the result of drought-induced leaf shedding. Hence, whether carbon starvation within the study area is restricted to more xeric sites with higher canopy transparency and/or is associated with the occurrence of multi-year drought events needs to be elucidated in future studies lasting over several successive growing seasons and including different stands along a soil moisture gradient.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF Project Nos. P19563-B16 “Dynamics of cambial activity and wood formation of Scots pine (Pinus sylvestris L.) exposed to soil dryness” and P22280-B16 “Conifer radial stem growth in response to drought”). We greatly acknowledge Hydrographischer Dienst, Innsbruck, for providing us the climate data. We also thank anonymous reviewers for their valuable suggestions and comments to improve the manuscript.
References
- Abe H, Nakai T. Effect of the water status within a tree on tracheid morphogenesis in Cryptomeria japonica D. Don. Trees. 1999;14:124–129. [Google Scholar]
- Bhupinderpal-Singh, Nordgren A, Ottosson Löfvenius M, Högberg MN, Mellander PE, Högberg P. Tree root and soil heterotrophic respiration as revealed by girdling of boreal Scots pine forest: extending observations beyond the first year. Plant Cell. Environ. 2003;26:1287–1296. [Google Scholar]
- Brunner I, Pannatier EG, Frey B, Rigling A, Landolt W, Zimmermann S, Dobbertin M. Morphological and physiological responses of Scots pine fine roots to water supply in a dry climatic region in Switzerland. Tree Physiol. 2009;29:541–550. doi: 10.1093/treephys/tpn046. [DOI] [PubMed] [Google Scholar]
- Chapin FS, Schulze ED, Mooney HA. The ecology and economics of storage in plants. Ann. Rev. Ecol. Syst. 1990;21:423–447. [Google Scholar]
- Čufar K, Prislan P, de Luis M, Gričar J. Tree-ring variation, wood formation and phenology of beech (Fagus sylvatica) from a representative site in Slovenia, SE Central Europe. Trees. 2008;22:749–758. [Google Scholar]
- Denne MP. Definition of latewood according to Mork (1928) IAWA Bull. 1988;10:59–62. [Google Scholar]
- Deslauriers A, Morin H, Begin Y. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada) Can. J. For. Res. 2003;33:190–200. [Google Scholar]
- Deslauriers A, Giovannelli A, Rossi S, Castro G, Fragnelli G, Traversi L. Intra-annual cambial activity and carbon availability in stem of poplar. Tree Physiol. 2009;29:1223–1235. doi: 10.1093/treephys/tpp061. [DOI] [PubMed] [Google Scholar]
- Eilmann B, Buchmann N, Siegwolf R, Saurer M, Cherubini P, Rigling A. Fast response of Scots pine to improved water availability reflected in tree-ring width and δ13C. Plant Cell Environ. 2010;33:1351–1360. doi: 10.1111/j.1365-3040.2010.02153.x. [DOI] [PubMed] [Google Scholar]
- Ellenberg H, Leuschner C. Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht. Ulmer; Stuttgart: 2010. [Google Scholar]
- FAO . World reference base for soil resources. FAO; Rome: 1998. [Google Scholar]
- Fischer C, Höll W. Food reserves of scots pine (Pinus sylvestris L.). II. Seasonal changes and radial distribution of carbohydrate and fat reserves in pine wood. Trees. 1992;6:147–155. [Google Scholar]
- Frensch J. Primary responses of root and shoot elongation to water deficits in the atmosphere and soil solution. J. Exp. Bot. 1997;48:985–999. [Google Scholar]
- Galiano L, Martínez-Vilalta J, Lloret F. Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytol. 2011 doi: 10.1111/j.1469-8137.2010.03628.x. doi: 10.1111/j.1469-8137.2010.03628.x. [DOI] [PubMed] [Google Scholar]
- Gričar J, Zupančič M, Čufar K, Primož O. Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Sci. Techn. 2006;41(6):463–475. [Google Scholar]
- Gričar J, Krze L, Čufar K. Number of cells in xylem, phloem and dormant cambium in silver fir (Abies alba), in trees of different vitality. IAWA J. 2009;30:121–133. [Google Scholar]
- Gruber A, Strobl S, Veit B, Oberhuber W. Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Phys. 2010;30:490–501. doi: 10.1093/treephys/tpq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Beck E. The fate and path of assimilation products in the stem of 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees. 1990;4:16–21. [Google Scholar]
- Hansen J, Beck E. Seasonal changes in the utilization and turnover of assimilation products in 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees. 1994;8:172–182. [Google Scholar]
- Hansen J, Vogg G, Beck E. Assimilation, allocation and utilization of carbon by 3-year-old Scots pine (Pinus sylvestris L.) trees during winter and early spring. Trees. 1996;11:83–90. [Google Scholar]
- Helle G, Schleser GH. Beyond CO2-fixation by Rubisco-an interpretation of 13C/12C variations in tree rings from novel intra-seasonal studies on broad-leaf trees. Plant Cell Environ. 2004;27:367–380. [Google Scholar]
- Hoch G, Popp M, Körner C. Altitudinal increase of mobile carbon pools in Pinus cembra suggest sink limitation at the Swiss treeline. Oikos. 2002;98:361–374. [Google Scholar]
- Hoch G, Körner C. The mobile carbon supply of pines at the climatic treeline: a global comparison. Oecologia. 2003;135:10–21. doi: 10.1007/s00442-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Hoch G, Richter A, Körner C. Non-structural carbon compounds in temperate forest trees. Plant Cell Environ. 2003;26:1067–1081. [Google Scholar]
- Hoch G. Cell wall hemicellulose as mobile carbon stores in non-reproductive plant tissues. Funct. Ecol. 2007;21:823–834. [Google Scholar]
- Keel SG, Siegwolf RTW, Jäggi M, Koerner C. Rapid mixing between old and new C pools in the canopy of mature forest trees. Plant Cell Environ. 2007;30:963–972. doi: 10.1111/j.1365-3040.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- Körner C. Carbon limitation in trees. J. Ecol. 2003;91:4–17. [Google Scholar]
- Larcher W. Physiological plant ecology. Ecophysiology and stress physiology of functional groups. Springer; Berlin: 2003. [Google Scholar]
- Li MH, Xiao WF, Wang SG, Cheng G, Cherubini P, Cal XH, Liu XL, Wang XD, Zhu WZ. Mobile carbohydrates in Himalayan treeline trees I. Evidence for carbon gain limitation but not for growth limitation. Tree Physiol. 2008;28:1287–1296. doi: 10.1093/treephys/28.8.1287. [DOI] [PubMed] [Google Scholar]
- Maier CA, Johnsen KH, Clinton BD, Ludovici KH. Relationships between stem CO2 efflux, substrate supply and growth in young loblolly pine trees. New Phytol. 2010;185:502–513. doi: 10.1111/j.1469-8137.2009.03063.x. [DOI] [PubMed] [Google Scholar]
- Marion L, Gričar J, Oven P. Wood formation in urban Norway maple trees studied by the micro-coring method. Dendrochronologia. 2007;25:97–102. [Google Scholar]
- Maunoury-Danger F, Fresneau C, Eglin T, Berveiller D, François C, Lelarge-Trouverie C, Daesin C. Impact of carbohydrate supply on stem growth, wood and respired CO2 δ13C: assesment by experimental girdling. Tree Physiol. 2010;30:818–830. doi: 10.1093/treephys/tpq039. [DOI] [PubMed] [Google Scholar]
- McDowell NG, Pockman W, Allen C, Breshears D, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams D, Yepez EA. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb? New Phytol. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- Morgan JM. Osmoregulation and water stress in higher plants. Annu Rev Plant Physiol. 1984;35:299–319. [Google Scholar]
- Oberhuber W, Stumböck M, Kofler W. Climate-tree-growth relationships of Scots pine stands (Pinus sylvestris L.) exposed to soil dryness. Trees. 1998;13:19–27. [Google Scholar]
- Oberhuber W. The role of climate in the mortality of Scots pine (Pinus sylvestris L.) exposed to soil dryness. Dendrochronologia. 2001;19(1):45–55. [Google Scholar]
- Oberhuber W, Gruber A. Climatic influences on intra-annual stem radial increment of Pinus sylvestris (L.) exposed to drought. Trees. 2010;24:887–898. doi: 10.1007/s00468-010-0458-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oribe Y, Funada R, Shibagaki M, Kubo T. Cambial reactivation in locally heated stems of evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta. 2001;212:684–691. doi: 10.1007/s004250000430. [DOI] [PubMed] [Google Scholar]
- Pallardy SG. Physiology of woody plants. Academic Press; Elsevier, Amsterdam: 2008. [Google Scholar]
- Rathgeber CBK, Longuetaud F, Mothe F, Cuny H, Moguédec GL. Phenology of wood formation: Data processing, analysis and visualisation using R (package CAVIAR) Dendrochronologia. 2011 doi:10.1016/j.dendro.2011.01.004. [Google Scholar]
- Rebetez M, Dobbertin M. Climate change may already threaten Scots pine stands in the Swiss Alps. Theor. Appl. Clim. 2004;79:1–9. [Google Scholar]
- Rossi S, Deslauriers A, Morin H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia. 2003;21:33–39. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T. Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the Alpine timberline. IAWA J. 2006a;27:383–394. [Google Scholar]
- Rossi S, Anfodillo T, Menardi R. Trephor: a new tool for sampling microcores from tree stems. IAWA J. 2006b;27:89–97. [Google Scholar]
- Sala A, Piper F, Hoch G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010;186:274–281. doi: 10.1111/j.1469-8137.2009.03167.x. [DOI] [PubMed] [Google Scholar]
- Sheskin DJ. Handbook of parametric and nonparametric statistical procedures. 4th edn. Chapman & Hall/CRC; Boca Raton: 2007. [Google Scholar]
- Solfjeld I, Johnsen O. The influence of root-zone temperature on growth of Betula pendula Roth. Trees. 2006;20:320–328. [Google Scholar]