Abstract
Recognition of relatives is important for dispersing animals to avoid inbreeding and possibly for developing cooperative, reciprocal relationships between individuals after dispersal. We demonstrate under controlled captive conditions that cotton-top tamarins (Saguinus oedipus) have a long-term memory for long calls of relatives from which they had been separated for periods ranging from 4 to 55 months. Tamarins responded with lower levels of arousal behavior to playbacks of long calls from current mates and from separated relatives compared to calls of unfamiliar, unrelated tamarins. Four animals had been out of contact with relatives for more than 4 years and still showed recognition as evidenced by low levels of arousal. Results could not be explained in terms of proximity to former relatives. Long-term memory for vocal signatures of relatives is adaptive and may be much more common than has been demonstrated.
Keywords: cotton-top tamarins, vocalizations, long-term memory, relatives
Long-term memory for cues indicating socially important individuals should be of great importance for maintaining social relationships and avoidance of inbreeding after dispersal in migratory species. Long-term social memory has been shown in various species. For example, migratory hooded warblers (Wilsonia citrina) remembered the songs of the territory neighbors 8 months later when returning from migration (Godard, 1991). Belding’s ground squirrels (Spermophilis beldingi) remembered the odors of littermates following approximately 6 months of hibernation, although they did not respond differentially to odors from familiar versus unfamiliar individuals (Mateo & Johnston, 2000). Insley (2000) reported that 5 yearling Northern fur seal (Callorhinus ursinus) mother and infant pairs recognized each other’s calls after 1 year of separation and 4 pups returning after 4 years oriented to playback of their mothers’ calls.
Long-term memory for learning task in nonhuman primates has been frequently assessed over a period of days or a few weeks (Tomasello & Call, 1998) with a few notable exceptions. Burdyn, Noble, Shreves and Thomas (1984) reported long term retention in squirrel monkeys (Saimiri sp.) of oddity over 2.33 years, and of conditional discrimination of simultaneous and successive cues over 1.33 years and in one monkey numerosity discrimination over 5 years. Recently, Fagot and Cook (2006) demonstrated that baboons (Papio papio) could memorize 3,500–5,000 different items over a three year period with retention of specific items lasting for several months. Tamarins (Saguinus sp.) also appear able to store information over a long period. Menzel & Menzel (1979) tested saddleback tamarins (Saguinus fuscicollis) with a novel object each day for 100 days and when they presented the first stimulus again after 100 days, the tamarins appeared to be habituated to the object. Moscovice and Snowdon (2006) reported that cotton-top tamarins (Saguinus oedipus) retained a spatial foraging task over a period of 17 months with little decrement in performance. In the only study to date of memory for vocal signals in primates, Lemasson, Hausberger and Zuberbuhler (2005) found that Campbell’s monkeys (Cercopithecus campbelli) changed the structure of harmonic calls over a four year period, but responded to playbacks of former familiar harmonic calls with a cessation of vocal behavior indicating recognition.
Wild cotton-top tamarins (Saguinus oedipus) of both sexes are equally likely to disperse and may remain out of contact with natal group members for years before coming into contact again (Savage, Giraldo, Soto & Snowdon, 1996). We do not know the lifespan of wild tamarins but captive tamarins can live more than 20 years. If these animals come into contact with former group members, it would be important to recognize these individuals in order to maintain social relationships and avoid inbreeding. Adult and subadult tamarins of both sexes produce long calls in contexts of territorial defense and separation from familiar group members (Cleveland & Snowdon 1982). Based on the analyses and playback of long calls, captive cotton-top tamarins have discriminated between familiar and unfamiliar individuals by showing higher levels of arousal to unfamiliar individuals (Snowdon, Cleveland & French, 1983, Weiss, Garibaldi & Hauser, 2001), suggesting that long calls provide a cue for recognition of familiar callers.
As normal husbandry practice, we housed cotton-top tamarins in natal families until they had experience caring for at least two sets of younger siblings (18–24 months). As postpubertal adults tamarins are than removed from their natal families and paired with unrelated animals to establish breeding or non-reproductive pairs. Based on tamarin natural history, we hypothesized that both sexes should show similar responses to both calls of their familiar mates and calls of relatives from which they had been separated for varying lengths of time. Furthermore, we hypothesized that responses to both mates and separated relatives would differ from responses to calls of animals unrelated and unfamiliar to both the subject and its mate. The colony contained related animals that had been separated from each other for periods ranging from 4 to 65 months so we also tested whether duration of separation affected responses.
Methods
Subjects and Housing
We studied 22 adult cotton-top tamarins. We housed tamarins as pairs in cages measuring 1.8 × 1.0 X 2.3 m or as family groups in cages measuring 1.8 × 3.0 × 2.3 m. Cages were constructed of anodized aluminum with a urethane coated mesh and contained tree branches, ropes and other climbing structures. We presented food and water on platforms greater than 1 m above the floor. Rooms were maintained at 26 C°and were on a 12:12 light dark cycle. In rooms with multiple cages, cloth barriers prevented animals from seeing each other but they had auditory and olfactory contact. We fed monkeys a high protein snack with vitamins at lights on, a main feed of PNI Nutrition International New World Primate Diet and Zupreem Marmoset diet supplemented with fresh fruits and vegetables at mid-day and a high protein supplemental snack in late afternoon. Further details of husbandry are in Ginther, Ziegler and Snowdon (2001).
The colony was housed in 9 separate rooms that were located up to 29 m from each other. All subjects had been separated from the relative whose calls were used in testing for periods ranging from 4 to 65 months (mean 25.6 ± 4.16 months) and had never been housed in the same room as the relative throughout that time.
Procedure
We collected samples of Normal Long Calls (see Cleveland & Snowdon, 1982). Long calls are typically two to three tonal calls of ascending frequency that are slightly more than 1 s in duration. We recorded long calls with a Sennheiser ME66 directional microphone and a Marantz PMD 221 cassette recorder. The system had a flat frequency response to 14kHz. We created a library of calls, which included for each subject a familiar individual (its current mate) a separated relative (parent, offspring or sibling) and an animal that was unfamiliar and unrelated to both the subject and its mate.
We constructed test tapes for each stimulus consisting of 5 min of silence (to record baseline data) followed by 1 min presentation of a three note long call repeated 6 times from the stimulus animal which was followed by another 5 min of silence to record responses. The experimenter entered the room and placed the speaker (which had a flat frequency response across the frequency range of tamarin long calls) behind a screen approximately 2 m from the corner of the cage to be tested and turned on the recorder while gathering data from a position directly in front of the cage. We defined behaviors indicative of alerting and arousal as frequency of piloerection, scanning, approaching the speaker and giving antiphonal long call vocalizations. Two experimenters gathered data (one observer focusing on the subject and the other on its mate) and had an interobserver reliability of >85%. Stimulus type was counter-balanced across subjects. Animals were tested in pairs since social separation was stressful and disrupted natural behavior in our tamarins. No pair was tested more than once a day, and in rooms housing multiple groups, no room was tested more than once a day. Because calls presented to each pair were from different individuals, each stimulus set was from different recording sessions. Each pair was tested once with each stimulus to prevent habituation and at least a week elapsed between successive tests.
Data Analyses
To determine if call structure differed among individuals we analyzed long call structure in a subset of 8 unrelated animals for which we had at least 8 samples of long calls using SIGNAL/RTS software. Calls were band pass filtered between 1 and 10 kHz and were sampled at 44 kHz. Spectrograms were created using a 1024 point fast Fourier transform with a frequency resolution of 97.7 Hz and a temporal resolution of 10.2 ms. We made measurements of total number of notes, total duration, start, peak and end frequency, duration and inflection point of each of the first three notes of the long call and the duration of intervals between notes 1 and 2 and notes 2 and 3. These measurements were analyzed with a Discriminant Analysis using SPSS 14.0. A leave one out validation analysis was also run whereby each call was classified based on all cases other than the call being evaluated.
The four categories of arousal behavior varied greatly across individuals and so were summed for pre-stimulus baseline and post-stimulus intervals and the value for the pre-stimulus interval was subtracted from the post-stimulus value to give a measure of change in alerting following the playback. Given the short duration of the playback relative to the baseline and post-baseline periods and the fact that playback stimuli might mask antiphonal vocal responses, we did not analyze behavior during the playback itself. Measures of skewness using SPSS showed no significant deviations from normality and so parametric t-tests were used. The alpha level was set at 0.025 because the same data were used in two comparisons. Correlations were calculated between mean change in alerting response to the relative’s calls and the time since the subject and its relative were separated and also with the distance between the rooms housing the subject and its relative. All tests are two-tailed.
Results
The discriminant analysis indicated that 95.3% of long calls could be correctly classified by the initial analysis and 90.6% correctly classified using the leave one out procedure. The chance value for classification is 12.5%.
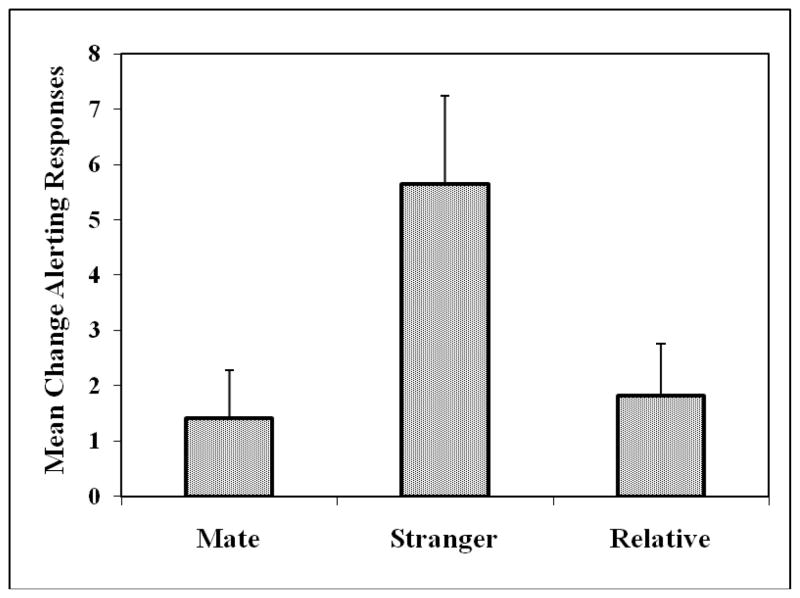
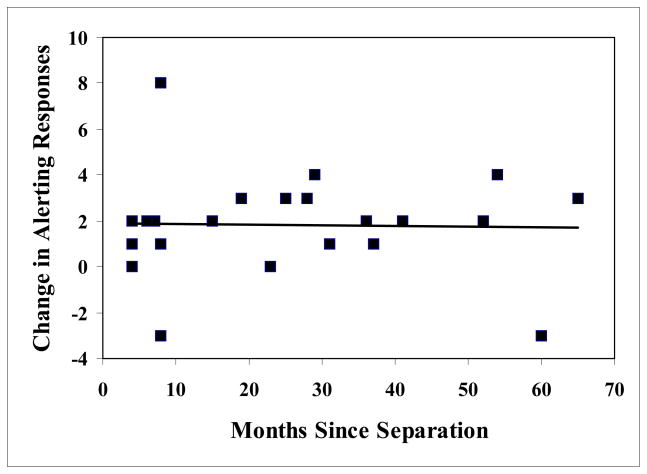
Figure 1 shows the behavioral results. There was a significant increase in alerting responses to the calls of unfamiliar, unrelated animals compared to the mate’s call (t (21) = 6.70, p = 0.000001) and compared to the call of the relative (t (21) = 4.26, p= 0.00035). In contrast there was no difference in alerting behavior between the calls of the mate and the calls of a relative (t (21) = 0.683, p = 0.501). There was no effect of duration of separation between relatives on the amount of alerting behavior shown (r2 (20) = 0.00048, p = 0.923, fig. 2) or the distance in the colony between the subject and the relative (r2 (20) = 0.0047, p = 0.761). Thus, whether the subject had been separated from its relative by 4 or 65 months there was no difference in response nor was there any difference in how far apart in the colony animals had been housed during the period of separation.
Fig. 1.
Mean (+ 95% CI) of differences in alerting responses before and after playback of long calls from the mate, an unfamiliar, unrelated animal or a separated relative. Alerting responses are significantly greater to an unfamiliar, unrelated animal than to either mate or relative (p’s < 0.0005).
Fig. 2.
Scatter plot showing change in pre-stimulus versus post-stimulus alerting response to playback of long calls from a relative as a function of months since the subject was last in contact with that relative.
Discussion
Cotton-top tamarins displayed a long-term memory for some features of calls from relatives with whom they had previously lived with as indicated by the lack of difference in response to mate’s versus relative’s calls and the robust responses they gave to calls of an unfamiliar, unrelated animal. This study was not designed to show that tamarins recognized a specific individual but rather that there was a differential response to calls of separated relatives compared with calls of unfamiliar, unrelated animals, similar to results on Campbell’s monkeys shown by Lemasson, Hausberger, and Zuberbuhler (2005). The long calls of the subset of tamarins that we analyzed were clearly distinct from one another with a discriminative function accuracy of 95% (or 90% using the leave one out method). This confirms previous reports (Snowdon Cleveland & French 1983; Weiss, Garibaldi & Hauser,. 2001) that cotton-top tamarins have distinctive individual structures in their long calls. None of our sample for vocal analysis was related so we could not examine whether “family” signatures might facilitate long-term memory.
There was no difference in response rate as a function of how long animals had been separated from their mates, ranging from 4–65 months. The mean duration of separation of 25 months is a longer time period than most previous studies of memory for social stimuli except the 4 fur seals studied by Insley (2000) and the 4 Campbell’s monkeys studied by Lemasson, Hausberger, and Zuberbuhler (2005). In this study, 4 tamarins had been separated from relatives for more than 4 years and had a mean increase in alerting responses of 1.5, well within the range of response to calls from the mate.
Epple and Niblick (1997) tested male saddleback tamarins (Saguinus fuscicollis) with a choice between a separated relative (mean separation 21.8 months, range 4–65 months) and an unfamiliar male and found more non-aggressive contacts and fewer mildly aggressive tongue flicks to separated relatives than to the stranger with no effect of separation duration. However, all their animals were housed in the same colony room and, thus, had continuous access to vocal and chemical cues. In this study, the tamarins did not have continuous access to vocal and chemical cues from separated relatives and still showed no difference in alerting behavior between the calls of their current mate and the calls of a separated relative.
In field studies of migratory species it is assumed, but not known, that the animals have no contact with one another during migration. With a controlled captive environment we can be sure that the tamarins we tested had no direct contact with one another during the period between separation and testing. Although it is possible that long calls from relatives could have been heard between colony rooms, three arguments suggest this explanation is unlikely. Animals were separated in colony rooms ranging from adjacent rooms to 29 m away with up to 9 solid concrete walls intervening, but there was no difference in response to calls of relatives housed nearby versus those housed at a distance. Walls attenuate the higher frequency components of calls, making it unlikely that individual features of calls would be transmitted through several walls. Second, even if animals might have heard the calls of former relatives, they also would hear as often the calls of unrelated, unfamiliar animals and yet all tamarins showed increased alerting to the calls of unfamiliar, unrelated animals. Third some calls were from animals that had died hence there would be no recent cues to the calls of those individuals and the mean responses to the calls of animals that had died did not differ from the confidence interval of responses to still living relatives.
The acoustic environments in which wild tamarins live are more complex than those of captive animals and it may be that long lasting memory for calls may be less robust in wild populations, but vocal signals are but one means to identify relatives and olfactory cues in particular could also provide cues for recognition in wild tamarins.
Although long-term memory for calls in tamarins is impressive these results should not be surprising. In nature dispersing animals may meet other individuals with whom they have lived previously and this has been documented to occur in long-term studies of cotton-top tamarins (Savage et al. 1996). It is adaptive to remember vocal cues (as in seals, warblers, Campbell’s monkeys or tamarins) or olfactory cues (as in ground squirrels) to avoid mating with relatives. Memory for signals of relatives can also lead to cooperative or altruistic behavior if dispersing relatives encounter one another. Other species should display long-term memory for socially relevant signals and the duration of tamarin long-term memory is likely to be even greater than the 5.5 years reported here.
Acknowledgments
Supported by USPHS Grant MH029775, a University of Wisconsin Hilldale Student Faculty Research Grant, and the McNair Scholars Program. We thank Mackenzie Risch for assistance in data collection and Kirsten Carlson for assistance in measuring long calls. This research adhered to the Association for the Study of Animal Behaviour/Animal Behavior Society Guidelines for the Use of Animals in Research, and all applicable federal state and institutional requirements for animal research and was approved by the University of Wisconsin, College of Letters and Science Animal Care and Use Committee.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/com.
References
- Burdyn LE, Jr, Noble LM, Shreves LE, Thomas RK. Long term memory for concepts by squirrel monkeys. Physiological Psychology. 1984;12:97–102. [Google Scholar]
- Cleveland J, Snowdon CT. The complex vocal repertoire of the adult cotton-top tamarin (Saguinus oedipus) Zeitschrift fur Tierpsychologie. 1982;58:231–270. [Google Scholar]
- Epple G, Niblick H. Social memory in the saddleback tamarin (Saguinus fuscicollis) Folia Primatologica. 1997;68:265–271. doi: 10.1159/000157252. [DOI] [PubMed] [Google Scholar]
- Fagot J, Cook RG. Evidence or large long-term memory capacities in baboons and pigeons and its implications for learning and the evolution of cognition. Proceedings of the National Academy of Science, USA. 2006;103:17564–17567. doi: 10.1073/pnas.0605184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard R. Long term memory of individual neighbours in a migratory songbird. Nature. 1991;350:228–229. [Google Scholar]
- Ginther AJ, Ziegler TE, Snowdon CT. Reproductive biology of captive male cotton-top tamarin monkeys as a function of social environment. Animal Behaviour. 2001;61:65–78. doi: 10.1006/anbe.2000.1587. [DOI] [PubMed] [Google Scholar]
- Insley SJ. Long-term vocal recognition in the northern fur seal. Nature. 2000;406:404–405. doi: 10.1038/35019064. [DOI] [PubMed] [Google Scholar]
- Lemasson A, Hausberger M, Zuberbuhler K. Socially meaningful vocal plasticity in adult Campbell’s monkeys (Cercopithecus campbelli) Journal of Comparative Psychology. 2005;119:220–229. doi: 10.1037/0735-7036.119.2.220. [DOI] [PubMed] [Google Scholar]
- Mateo JM, Johnston RE. Retention of social recognition after hibernation in Belding’s ground squirrels. Animal Behaviour. 2000;59:491–499. doi: 10.1006/anbe.1999.1363. [DOI] [PubMed] [Google Scholar]
- Menzel EW, Jr, Menzel CR. Cognitive, developmental and social aspects of responsiveness to novel objects in a family group of marmosets Saguinus fuscicollis. Behaviour. 1979;70:251–279. [Google Scholar]
- Moscovice LR, Snowdon CT. The role of social context and individual experience in novel task acquisition in cotton-top tamarins (Saguinus oedipus) Animal Behaviour. 2006;71:933–943. doi: 10.1016/j.anbehav.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage A, Giraldo LH, Soto LH, Snowdon CT. Demography, group composition and dispersal in wild cotton-top tamarin (Saguinus oedipus) groups. American Journal of Primatology. 1996;38:85–100. doi: 10.1002/(SICI)1098-2345(1996)38:1<85::AID-AJP7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Snowdon CT, Cleveland J, French JA. Responses to context- and individual-specific cues in cotton-top tamarin long calls. Animal Behaviour. 1983;31:92–101. [Google Scholar]
- Tomasello M, Call J. Primate Cognition. Oxford: Oxford University Press; 1998. [Google Scholar]
- Weiss DJ, Garibaldi DT, Hauser MD. The production and perception of long calls by cotton-top tamarins (Saguinus oedipus): acoustic analyses and playback experiments. Journal of Comparative Psychology. 2001;115:258–271. doi: 10.1037/0735-7036.115.3.258. [DOI] [PubMed] [Google Scholar]