Abstract
Until recently, intensified efforts in China to suppress the transmission of Schistosoma japonicum relied principally on routine praziquantel treatment, extensive use of molluscicides and health education programs. These efforts, now supplemented by a broader range of control measures, have been quite successful in reducing the prevalence and intensity of human infection to very low levels. However, re-emergent transmission has occurred in formerly endemic areas of several provinces, signaling the need for more locally effective, integrated control strategies. We argue that these low but persistent levels of transmission also require important changes in both the tactics and strategy of disease surveillance to move forward towards elimination. Here we present recent data exemplifying the low transmission environment which suggests that we are reaching limits of detection of current diagnostic techniques used for human infection surveillance in these communities. both epidemiological data and theoretical results indicate that i) transmission in the human population can persist at very low infection intensities even in the presence of routine control activities; ii) the parasite can be reintroduced into parasite-free environments by very modest external inputs; and iii) transmission at these low infection intensities exhibits very slow inter-year dynamics. These observations motivate the need for new, sensitive tools to identify low-level infections in mammalian or snail hosts, or the presence of S. japonicum in environmental media. Environmental monitoring offers an alternative, and perhaps more efficient, approach to large-scale surveillance of human infections in low transmission regions.
Keywords: Schistosomiasis, China, Low transmission, Elimination, Environmental monitoring
China’s national schistosomiasis control program, established in the 1950s, has resulted in tremendous reductions in Schistosoma japoncium prevalence and disease morbidity throughout the historically endemic regions of the country (Zhou et al., 2010). During the early years, the program focused on health education and various types of environmental modification including snail control. The introduction of praziquantel in the 1980s, and a subsequent World Bank Loan in the 1990s to promote its widespread use, provided both the necessary economic resources and effective tools to reduce infections and morbidity in the endemic areas where schistosomiasis disease burden has been highest (Utzinger et al., 2010). More recently, China’s remarkable economic development has resulted in increased internal resources for control programs and a comprehensive national policy aimed at sustainably interrupting schistosomiasis transmission (Wang et al., 2009). Current thinking is that the necessary elements to move towards elimination will focus on improved disease surveillance systems, better integrated intervention approaches, and a more rigorous and quantitative evaluation of program effectiveness (Utzinger et al., 2010).
The history of schistosomiasis control in the hilly and mountainous transmission areas of Sichuan Province, China, where most of our work has been concentrated, follows a similar historical pattern. Recent efforts have been quite successful in reducing prevalence and intensity of human and cattle infections to very low levels, but re-emergent transmission has occurred in formerly endemic areas of Sichuan as well as in other provinces (Li et al., 2004; Wang et al., 2004; Liang et al., 2006). The potential for persistent or re-emergent transmission, which has now been realized, was the motivation for our early focus on environmental determinants of transmission intensity (Spear et al., 2004). Our environmental focus evolved into studies of locally effective integrated intervention strategies (Liang et al., 2007) and in development of indices to guide these interventions (Spear, 2011).
The nature and effectiveness of integrated intervention strategies are likely to be quite different for the hilly and mountainous ecology of Sichuan versus the lakes and plains ecology of the lower Yangtze. For example, the development of a bovine vaccine, while useful in both settings, would clearly be a major advance towards elimination in the lower Yangtze environment (Da’Dara et al., 2008; Rudge et al., 2009; Gray et al., 2010). However, the challenges associated with disease surveillance in the low transmission environment are very similar. Here, we focus on the surveillance challenge and present recent data from the irrigated agricultural environments of Sichuan Province to illustrate the nature of the challenge in low transmission environments. We then offer our opinion as to the key technical advances that would contribute to the surveillance element of the program necessary to achieve the 2015 goal of eliminating schistosomiasis transmission in the Province and the 2020 goal of elimination in all endemic areas of China.
During the last decade the World Health Organization (WHO) convened two expert panels to consider the elimination of schistosomiasis from low transmission areas (WHO, 2001, 2009). The earlier report summarized the recommended strategic approach:
“As the parasite reservoir decreases, focusing on sustainable transmission control using hygiene and sanitation improvements and environmental snail control should become the major consideration.”
And, a challenge:
“Where elimination is aimed for, case detection may be a problem, as the commonly used clinical and parasitological diagnostic procedures may lack sensitivity in these instances.”
The recommendations of the most recent group appeared in 2009 and were generally consistent with the earlier report although two new emphases emerged: a caution that drug treatment alone may have little impact in low transmission environments, and an expanded discussion of molecular methods of diagnosis and environmental monitoring likely to be particularly important in these environments. As noted above, current national policy in China supports the importance of these themes. However, it is useful to bring some specificity as to what constitutes a low transmission environment and, in particular, to illustrate its challenging characteristics. A stringent definition of a low transmission environment is offered by Chinese public health authorities who consider transmission ‘control’ as transmission suppressed to below 1% prevalence in humans and domestic bovines (specifically cattle and water buffalo) and with the detection of no infected snails for at least two consecutive years. Re-emergent transmission is often defined as re-emergence from controlled status. For example, in Sichuan in 2004 it was found that transmission had re-emerged in eight counties between two and 15 years after having reached that status (Liang et al., 2006).
In 2007, we conducted baseline studies in 53 villages that originally attained transmission control status between 1985 and 1995, 25 of which had previous evidence of re-emergent transmission and 28 without. The details are presented in Carlton et al. (2011), but the essential findings for present purposes are that targeted infection surveys were conducted in humans and bovines with the results that:
Of the 3,009 humans tested, 6.5% were infected. Infections were found in 18 of the 25 villages that were previously known to have re-emergent transmission and 17 of the 28 where re-emergence had not previously been detected. However, the mean intensity in the villages with transmission was only 1.6 eggs per gram of feces (epg).
At least one infected bovine was detected in 23 villages, with an average village prevalence of 13.4% and a village maximum of 65.4%. However, the highest mean village infection intensity among infected animals was 0.11 epg.
In this survey, average egg excretion among those tested using the Kato-Katz procedure was 1.6 epg and the highest village average infection intensity was 10.6 epg. Only seven individuals had infection intensities greater than 100 epg. While such low infection intensities indicate the success of efforts to curb morbidity, the sensitivity of the two coprological diagnostic methods, the miracidial hatch test and the Kato-Katz thick smear procedure, declines with infection intensity (Yu et al., 2007). In 2007, of the 157 people testing positive using three hatch tests (each conducted using a stool sample from a different day), we found only 52 (33%) tested Kato-Katz positive by examining a single stool sample using three slides. Of the people testing positive based on at least one of their three hatch tests producing miracidia, 81% had at least one of their hatch tests showing negative results. Further, the standard snail surveys conducted as part of these studies found only one infected snail among the 7,515 collected in 2007 despite the human and animal infections rates.
Despite treatment of those found infected in 2007, a follow-up survey conducted in 2010 in 36 of the villages found human infections in 21 and bovine infections in 10. Village prevalence ranged from 0 to a rare prevalence as high as 30%, but infection intensities remain low, as observed in 2007. Clearly, ongoing low-level transmission continues in this region and another survey in Xichang County, which is approximately 400 kilometers southwest of the provincial capital of Chengdu, shows a very similar picture among the human population, based on targeted surveys in 2009, with average village infection intensity levels of approximately 1–2 epg. These data illustrate that the diagnostic capabilities of public health agencies for routine surveillance are reaching their limit in this region and there is reason to believe these low-level transmission dynamics will be persistent.
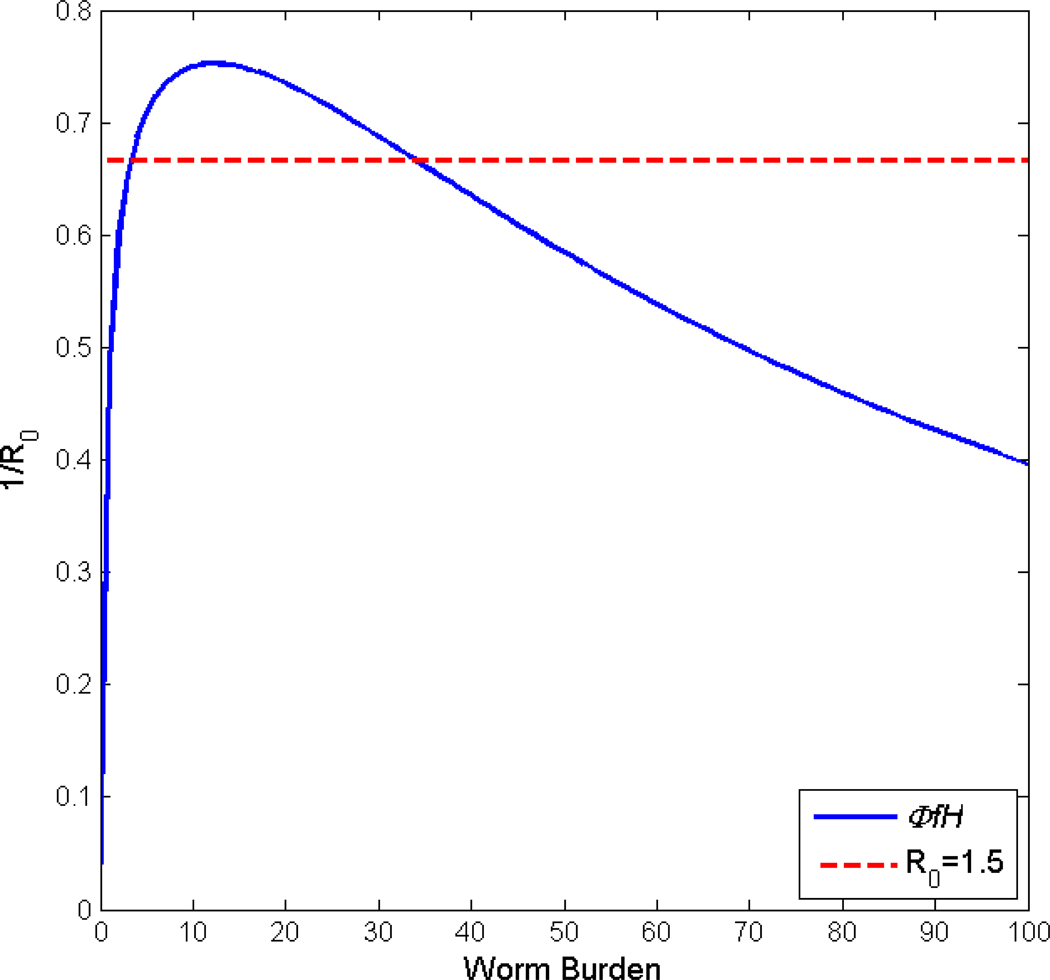
Viewing the issue from a theoretical perspective, it is widely recognized that for parasites which involve sexual pairing in the definitive host the transmission system has two stable equilibrium states, the zero state where infection is not present in either snails or mammalian hosts and the endemic state where medium to high transmission persists. These two states are separated by a third unstable equilibrium point, often called the breakpoint, which controls whether the infection level goes to zero or to the endemic state (May, 1977). This behavior is also called the ‘strong Allee effect’, a concept originating in conservation biology (Hilker et al., 2009). Fig. 1 illustrates the situation for a deterministic mathematical model of transmission in an isolated village based on our earlier work which tracks mean worm burden in humans and the density of infected snails in the village irrigation system (Liang et al., 2007; Spear, 2011). The horizontal lines are the inverse of the basic reproductive number, R0, and the curved line a function of the system non-linearities. The intersection of the horizontal and curved lines occurs at w̄ = 0, the breakpoint at 2–3 epg, and the endemic level at approximately 35 epg in this case. Clearly, as R0 increases, the breakpoint decreases and the endemic level increases. For schistosomiasis generally, this breakpoint is very near the zero state which implies that even where no residual infection is present in the community, re-emergent transmission can be initiated by a very modest input of cercariae or miricida from external sources.
Fig. 1.
Equilibrium states of a deterministic mathematical model of schistosomiasis transmission showing the interaction of the basic reproductive number (R0) of the system and its non-linear functions describing mating in the definitive host, , the worm establishment limitation in vivo, f, and effects of acquired immunity in humans, H.
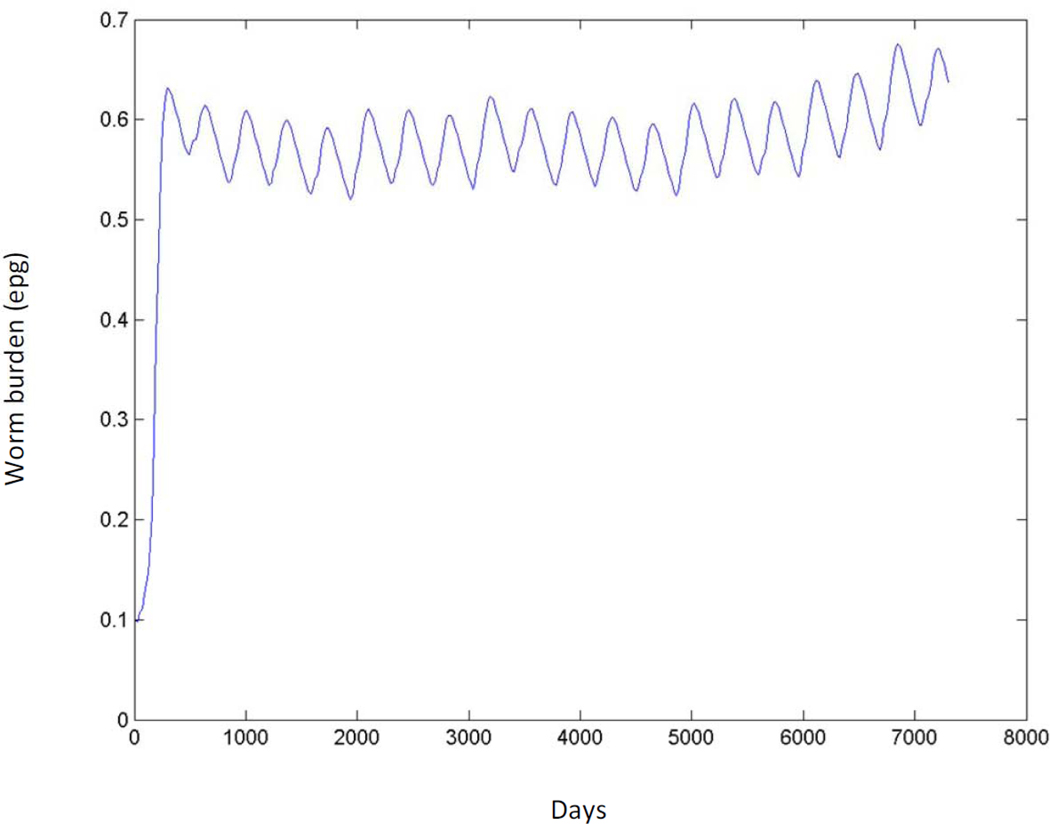
Equally important for maintaining residual infection is the behavior of the system in the neighborhood of this breakpoint. Fig. 2 shows the behavior of the model at very low transmission levels over a 20 year period. This fictitious village is assumed to be without infection until a wandering infected bovine spends a month in residence beginning at time (t) = 0 (Fig. 2). The results demonstrate that i) transmission in an uninfected human population can be initiated by a very modest parasite introduction and can persist at very low infection intensity; and ii) transmission dynamics in the neighborhood of the breakpoint exhibit very slow inter-year dynamics. Thus, while the breakpoint is unstable, system dynamics are sufficiently slow near the breakpoint so as to make the system resistant to state change. This implies that, from a practical control perspective, bringing low-level transmission to no transmission is more challenging than bringing high transmission to medium or even low transmission given the constraints of currently used surveillance tools. Gambhir and Michael (2008) have found similar effects in their model of lymphatic filariasis and suggestive evidence of slow dynamics based on field data. In both cases, the use of deterministic models can be questioned at these low infection levels but we have conducted preliminary investigations with stochastic models that have also shown this effect. We suspect that system dynamics at low levels, near the breakpoint, describe a good deal of what is being seen in our epidemiological data and this picture is consistent with the high rates of re-infection following praziquantel treatment that have been observed for all three major schistosome species (Zhou et al., 2007; Garba et al., 2009).
Fig. 2.
Mean worm burden in humans in a fictitious village expressed as eggs per gram of feces (epg) over a 20 year period (expressed in days) as a result of one infected bovine in residence for 1 month following time (t) = 0.
In the low transmission regions described above, three issues arise with respect to the elimination of transmission. First, the egg excretion of reservoir hosts other than bovines, of which there are many in China, almost certainly become much more significant than at endemic levels. That is, in a low transmission environment, parasite input from human and bovine sources is likely to be well below 10% of that at endemic levels due mainly to praziquantel treatment, while the input from untreated reservoir hosts may be largely unaltered, at least in the short term. Second, while the foregoing example pertains to a single village isolated from its neighbors except for the wandering bovine, villages are seldom so isolated, being connected hydrologically and from the intra-village movement of humans, animals and reservoir hosts. Guararie and Seto (2009) have shown theoretically that endemic transmission can exist among networks of villages where those villages would not sustain transmission individually. It seems likely that any of these forms of connectivity could exacerbate the persistence of low-level transmission.
Third, low-level transmission is likely to persist in the presence of continued selective treatments. Combinations of praziquantel and the molluscicide niclosamide have historically been successful in achieving transmission control and, no doubt, the elimination of schistosomiasis transmission in some environments. Clearly, the situation underscores the WHO committee’s caution that elimination will likely be difficult to achieve using drug treatment and molluscicide-based snail control and strengthens the case for integrated control. Moreover, it is questionable whether villagers will comply with continued treatments without obvious signs of morbidity (Guo et al., 2005), and continued treatments raise the specter of selective pressure and resulting parasite resistance to praziquantel, although to date there is little evidence for epidemiologically relevant levels of drug resistance in China (Seto et al., 2011). Targeting treatments to those infected is crucial, yet as we noted above, current diagnostic tools lack adequate specificity or sensitivity and are logistically challenging due to the processing of stool samples. Mass treatment is unlikely to be a feasible long-term strategy for maintaining control even if the elimination of transmission was not the goal (Gray et al., 2010).
New diagnostic tools are being developed that should provide the sensitivity needed to better identify low-level infections in human hosts. Immunoassays are widely used in China as testing is relatively inexpensive and technically simple, however the specificity of immunoassays is low enough to require a second, confirmatory test (Xu et al., 2010, 2011). In contrast, nucleic acid-based techniques using PCR and loop-mediated isothermal amplification (LAMP) show promise (Xia et al., 2009; Kumagai et al., 2010: Xu et al., 2010), but issues of cost, sampling design, villager acceptance of continued surveys involving fecal samples, laboratory capacity and real-world performance in detecting early signs of re-emergence need to be evaluated in the field. In China, where resources no longer constrain the development and deployment of new methods to the degree that remains the case in many other parts of the world, the rate of development of these new methods is likely to be rapid, but the rate of deployment will be slower and probably controlled by needs for training of personnel at the county level.
An alternative, and perhaps more efficient, solution to large-scale surveillance of human infections in low transmission regions is to acknowledge and exploit the environmental phases of the parasite’s lifecycle. The same methods being developed to provide better diagnostics in the human host can be employed for environmental monitoring. Monitoring for presence of the parasite in the village environment is based on the premise that human diagnostics are, by their nature, reactive public health instruments, while environmental monitoring allows for early warning and preventative measures. This benefit has been recognized in the design of surveillance systems to monitor emerging arboviruses and waterborne pathogens such as poliovirus (Manor et al., 1999; Eidson et al., 2001). Yet the development of environmental diagnostics for the infective stage of parasites such as schistosomes is stunningly behind those of other parasites present in water. Populations at risk of infection by Cryptosporidium and Giardia, for example, have benefited tremendously from recent standardization of reliable environmental monitoring techniques (Zarlenga and Trout, 2004), while the much larger populations at risk of contracting schistosome parasites have no standardized, direct measure of cercarial or miracidial numbers in water—although new approaches are under development (Hung et al., 2008; Worrell et al., 2011). Environmental monitoring could be carried out on surface water samples, specimens from human and animal wastes collected for recycling as fertilizer or composited snail samples as shown by Kumagai et al. (2010).
The need for environmental monitoring is particularly important to define the scale of the transmission process in the low transmission environment. Where villages are connected to neighbors via hydrological connections and by mobile human and animal hosts, a very modest external input may be adequate to re-initiate transmission (Fig. 2), but very little is known of the scale at which infected hosts may travel (Seto et al., 2007) or parasites move in irrigation systems (Lowe et al., 2004). There is mounting indirect evidence that these connections are important, particularly in the low transmission environment (Xu, 2006; Spear, 2011). Clearly, an ability to detect the parasite in water or in other environmental media would greatly aid in identifying the scale of transmission and in tracking infection sources.
In summary, we advocate an approach to eliminating the transmission of S. japonicum in China that rests on the development of new tools for the detection of infections in humans, snails and environmental samples, and the use of these tools to guide integrated and sustainable control efforts. However, we believe that effective integrated and sustainable efforts extend well beyond those that can be managed or funded by public health authorities. In China, there is considerable ongoing change in rural villages in endemic areas, often unmotivated by schistosomiasis control considerations, that effectively and sustainably reduce human or snail exposure to parasites. In Sichuan, for example, the provision of piped water into villages lessens water contact in irrigation ditches for food preparation or clothes washing. Biogas digesters installed by rural energy improvement programs provide both gas for cooking as well as waste treatment that greatly diminishes the parasite egg content of the waste used for crop fertilization. Concrete lining of irrigation ditches for improved system performance reduces snail habitat as does the replacement of intensively fertilized row crops with fruit trees, which reduce surface water demand and human exposures. The costs of these rural development projects generally transcend the levels available to public health authorities. As a consequence, an important role for public health officials is to identify and advocate for high priority sites for such improvements based on their importance to disease control objectives. Hence, new tools to identify and prioritize sites for opportunistic interventions are an essential first step.
Highlights.
In many areas of China, schistosomiasis transmission has been eliminated or suppressed to very low levels.
Semi-stable transmission at low levels in humans, bovines and snails can persist for long periods.
Current diagnostic methods are insufficient to move from low transmission to disease elimination.
Extending new diagnostic methods to environmental monitoring may be a significant step towards disease elimination.
Acknowledgements
The research underlying this article was supported by grants R01AI068854 and K01AI091864 (JVR) from the National Institute of Allergy and Infectious Diseases, U.S.A., by the Ministry of Science and Technology, P.R. China, and the Sichuan Provincial Government, P.R. China.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carlton E, Bates M, Zhong B, Seto E, Spear R. Evaluation of mammalian and intermediate host surveillance methods for detecting schistosomiasis reemergence in southwest China. PLoS Negl Trop Dis. 2011;5:e987. doi: 10.1371/journal.pntd.0000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da'Dara AA, Li YS, Xiong T, Zhou J, Williams GM, McManus DP, Feng Z, Yu XL, Gray DJ, Harn DA. DNA-based vaccine protects against zoonotic schistosomiasis in water buffalo. Vaccine. 2008;26:3617–3625. doi: 10.1016/j.vaccine.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson M, Kramer, Stone W, Hagiwara Y, Schmit K. Dead bird surveillance as an early warning system for West Nile virus. Emerg Infect Dis. 2001;7:631–635. doi: 10.3201/eid0704.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhir M, Michael E. Complex ecological dynamics and eradicability of the vector borne macroparasitic disease, lymphatic filariasis. PLoS ONE. 2008;3:e2874. doi: 10.1371/journal.pone.0002874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garba A, Toure S, Dembele R, Boisier P, Tohon Z, Bosque-Oliva E, Koukounari A, Fenwick A. Present and future schistosomiasis control activities with support from the Schistosomiasis Control Initiative in West Africa. Parasitology. 2009;136:1731–1737. doi: 10.1017/S0031182009990369. [DOI] [PubMed] [Google Scholar]
- Gray DJ, McManus DP, Li Y, Williams GM, Bergquist R, Ross AG. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis. 2010;10:733–736. doi: 10.1016/S1473-3099(10)70099-2. [DOI] [PubMed] [Google Scholar]
- Guo JG, Cao CL, Hu GH, Lin H, Li D, Zhu R, Xu J. The role of 'passive chemotherapy' plus health education for schistosomiasis control in China during maintenance and consolidation phase. Acta Trop. 2005;96:177–183. doi: 10.1016/j.actatropica.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Gurarie D, Seto EY. Connectivity sustains disease transmission in environments with low potential for endemicity: modelling schistosomiasis with hydrologic and social connectivities. J R Soc Interface. 2009;6:495–508. doi: 10.1098/rsif.2008.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilker F, Langlais M, Malchow H. The Allee effect and infectious diseases: extinction, multistability, and the (dis-) appearance of oscillations. Am. Nat. 2009;173:72–88. doi: 10.1086/593357. [DOI] [PubMed] [Google Scholar]
- Hung YW, Remais J. Quantitative detection of Schistosoma japonicum cercariae in water by real-time PCR. PLoS Negl Trop Dis. 2008;2:e337. doi: 10.1371/journal.pntd.0000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai T, Furushima-Shimogawara R, Ohmae H, Wang TP, Lu S, Chen R, Wen L, Ohta N. Detection of early and single infections of Schistosoma japonicum in the intermediate host snail, Oncomelania hupensis, by PCR and loop-mediated isothermal amplification (LAMP) assay. Am J Trop Med Hyg. 2010;83:542–548. doi: 10.4269/ajtmh.2010.10-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YS, Cai KP. The epidemic trend and challenges for schistosomiasis in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:572–574. [PubMed] [Google Scholar]
- Liang S, Spear RC, Seto E, Hubbard A, Qiu DC. A multi-group model of Schistosoma japonicum transmission dynamics and control: model calibration and control prediction. Trop Med Int Health. 2005;10:263–278. doi: 10.1111/j.1365-3156.2005.01386.x. [DOI] [PubMed] [Google Scholar]
- Liang S, Yang C, Zhong B, Qiu D. Re-emerging schistosomiasis in hilly and mountainous areas of Sichuan, China. Bull World Health Organ. 2006;84:139–144. doi: 10.2471/blt.05.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Seto EY, Remais JV, Zhong B, Yang C, Hubbard A, Davis GM, Gu X, Qiu D, Spear RC. Environmental effects on parasitic disease transmission exemplified by schistosomiasis in western China. Proc Natl Acad Sci U S A. 2007;104:7110–7115. doi: 10.1073/pnas.0701878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D, Xi J, Meng X, Wu Z, Qiu D, Spear R. Transport of Schistosoma japonicum cercariae and the feasibility of niclosamide for cercariae control. Parasitol Int. 2005;54:83–89. doi: 10.1016/j.parint.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Manor Y, Handsheer R, Halmut T, Neuman M, Bobrov A, Rudich H, Vonsover A, Shulman L, Kew O, Mendelson E. Detection of poliovirus circulation by environmental surveillance in the absence of clinical cases in Israel and the Palestinian Authority. J Clin Micro. 1999;37:1670–1675. doi: 10.1128/jcm.37.6.1670-1675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R. Thresholds and breakpoints in ecosystems with a multiplicity of stable states. Nature. 1977;269:471–477. [Google Scholar]
- Rudge JW, Lu DB, Fang GR, Wang TP, Basanez MG, Webster JP. Parasite genetic differentiation by habitat type and host species: molecular epidemiology of Schistosoma japonicum in hilly and marshland areas of Anhui Province, China. Mol Ecol. 2009;18:2134–2147. doi: 10.1111/j.1365-294X.2009.04181.x. [DOI] [PubMed] [Google Scholar]
- Seto E, Lee Y, Liang S, Zhong B. Individual and village-level study of water contact patterns and Schistosoma japonicum infection in mountainous rural China. Trop Med Int Health. 2007;12:1199–1209. doi: 10.1111/j.1365-3156.2007.01903.x. [DOI] [PubMed] [Google Scholar]
- Seto E, Wong B, Lu D, Zhong B. Praziquantel resistance in China: should we be worried? Am J Trop Med Hyg. 2011;85:74–82. doi: 10.4269/ajtmh.2011.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear RC, Seto E, Liang S, Birkner M, Hubbard A, Qiu D, Yang C, Zhong B, Gu X, Davis GM. Factors influencing the transmission of Schistosoma japonicum in the mountains of Sichuan Province of China. Am J Trop Med Hyg. 2004;70:48–56. [PubMed] [Google Scholar]
- Spear R. Internal versus external determinants of S. japonicum transmission in irrigated agricultural villages. J. R. Soc. Interface. 2011 doi: 10.1098/rsif.2011.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzinger J, Bergquist R, Olveda R, Zhou XN. Important helminth infections in Southeast Asia diversity, potential for control and prospects for elimination. Adv Parasitol. 2010;72:1–30. doi: 10.1016/S0065-308X(10)72001-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Utzinger J, Zhou XN. Schistosomiasis control: experiences and lessons from China. Lancet. 2008;372:1793–1795. doi: 10.1016/S0140-6736(08)61358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LD, Guo JG, Wu XH, Chen HG, Wang TP, Zhu SP, Zhang ZH, Steinmann P, Yang GJ, Wang SP, Wu ZD, Wang LY, Hao Y, Berquist R, Utzinger J, Zhou XN. China's new strategy to block Schistosoma japonicum transmission: experiences and impact beyond schistosomiasis. Trop Med Int Health. 2009;14:1475–1483. doi: 10.1111/j.1365-3156.2009.02403.x. [DOI] [PubMed] [Google Scholar]
- Wang RB, Wang TP, Wang LY, Guo JG, Yu Q, Xu J, Gao FH, Ying ZC, Zhou XN. Study on the re-emerging situation of schistosomiasis epidemics in areas already under control and interruption. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:564–567. [PubMed] [Google Scholar]
- World Health Organization. Report of the WHO informal consultation on schistosomiasis in low transmission areas: control strategies and criteria for elimination. 2001 WHO/CDS/CPE/SIP/2001.1.
- World Health Organization. Elimination of schistosomiasis from low-transmission areas; Report of a WHO informal consultation. 2009 WHO/HTM/NTD/PCT/2009.2.
- Worrell C, Xiao N, Vidal JE, Chen L, Zhong B, Remais J. Field detection of Schistosoma japonicum cercariae in environmental water samples by quantitative PCR. Appl Environ Microbiol. 2011;77:2192–2195. doi: 10.1128/AEM.01561-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CM, Rong R, Lu ZX, Shi CJ, Xu J, Zhang HQ, Gong W, Luo W. Schistosoma japonicum: a PCR assay for the early detection and evaluation of treatment in a rabbit model. Exp Parasitol. 2009;121:175–179. doi: 10.1016/j.exppara.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Xu B, Gong P, Seto E, Liang S, Yang C, Wen S, Qiu D, Gu X, Spear R. A Spatial-Temporal Model for Assessing the Effects of Inter-Village Connectivity in Schistosomiasis Transmission. Annals Am. Assoc. Geographers. 2006;96:31–46. [Google Scholar]
- Xu J, Rong R, Zhang HQ, Shi CJ, Zhu XQ, Xia CM. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP) Int J Parasitol. 2010;40:327–331. doi: 10.1016/j.ijpara.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Xu J, Peeling RW, Chen JX, Wu XH, Wu ZD, Wang SP, Feng T, Chen SH, Li H, Guo JG, Zhou XN. Evaluation of Immunoassays for the Diagnosis of Schistosoma japonicum Infection Using Archived Sera. PLoS Negl Trop Dis. 2011;5:e949. doi: 10.1371/journal.pntd.0000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, de Vlas S, Jiang Q, Gryseels B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol Int. 2007;56:45–49. doi: 10.1016/j.parint.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Zarlenga D, Trout J. Concentrating, purifying and detecting waterborne parasites. Vet Parasitol. 2004;126:195–217. doi: 10.1016/j.vetpar.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Zhou XN, Guo JG, Wu XH, Jiang QW, Zheng J, Dang H, Wang XH, Xu J, Zhu HQ, Wu GL, Li YS, Xu XJ, Chen HG, Wang TP, Zhu YC, Qiu DC, Dong XQ, Zhao GM, Zhang SJ, Zhao NQ, Xia G, Wang LY, Zhang SQ, Lin DD, Chen MG, Hao Y. Epidemiology of schistosomiasis in the People's Republic of China, 2004. Emerg Infect Dis. 2007;13:1470–1476. doi: 10.3201/eid1310.061423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, Chen XY, Zheng J, Utzinger J. The public health significance and control of schistosomiasis in China--then and now. Acta Trop. 2005;96:97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Zhou XN, Bergquist R, Leonardo L, Yang GJ, Yang K, Sudomo M, Olveda R. Schistosomiasis japonica control and research needs. Adv Parasitol. 2010;72:145–178. doi: 10.1016/S0065-308X(10)72006-6. [DOI] [PubMed] [Google Scholar]