Abstract
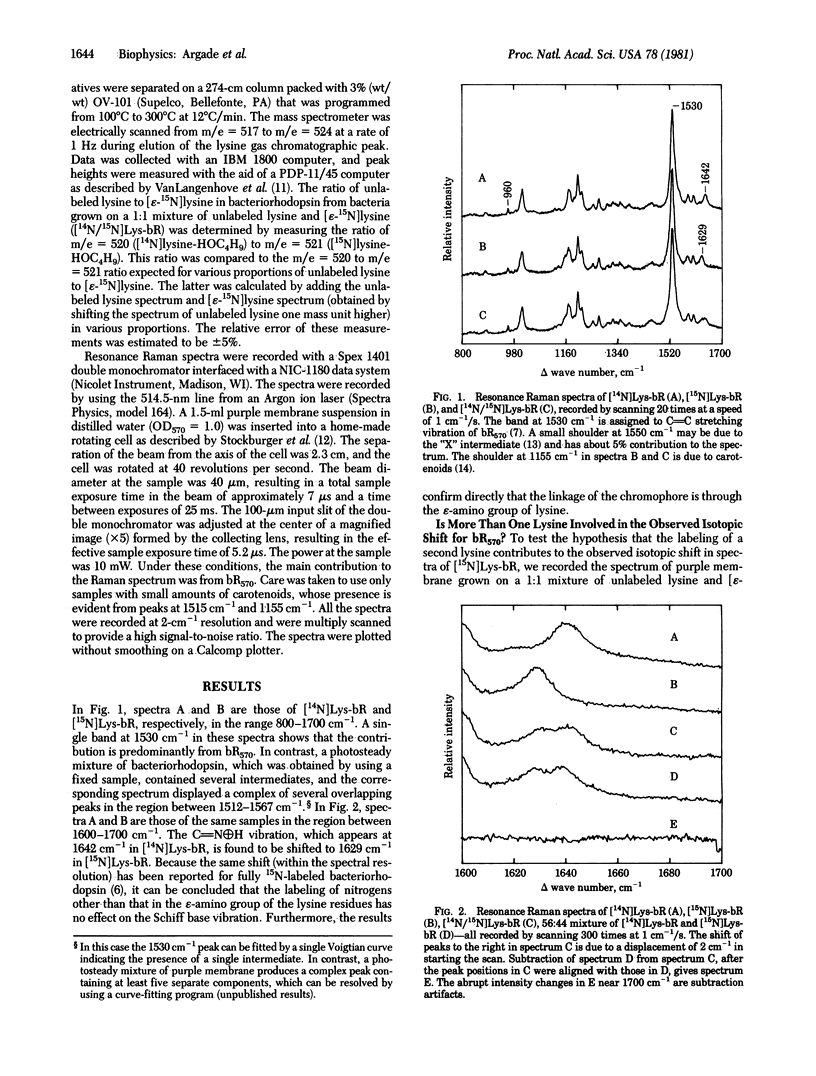
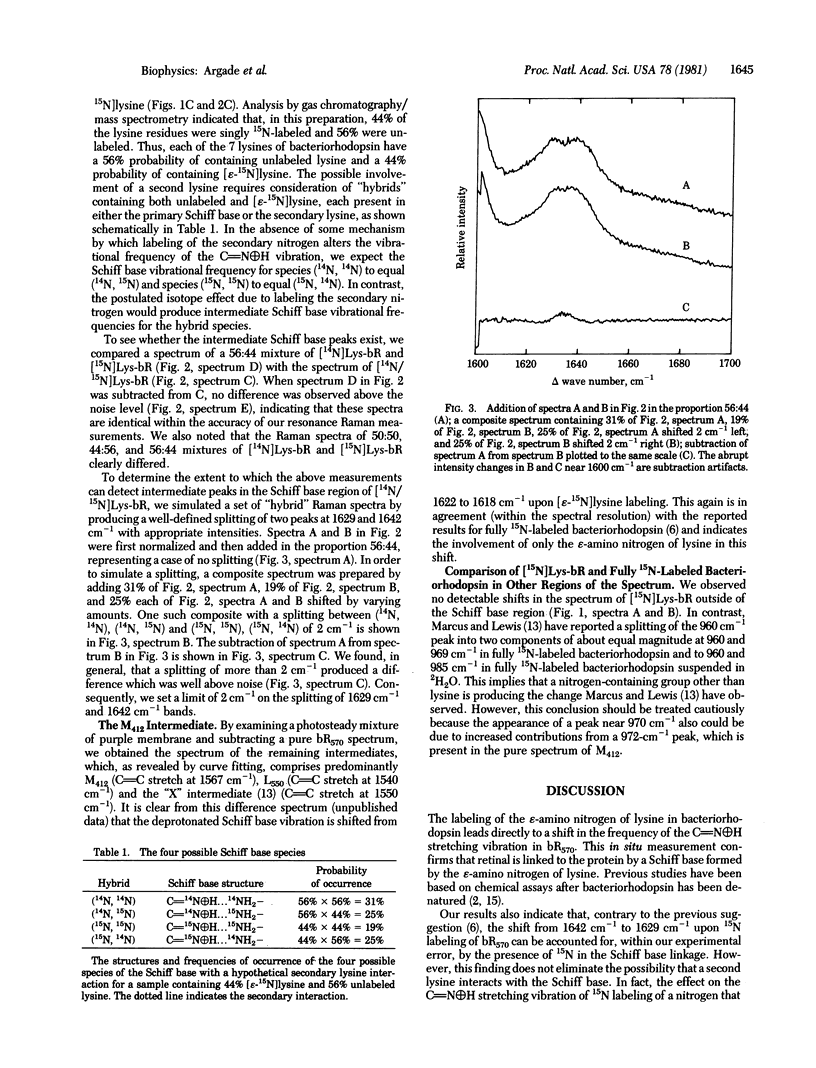
The possible interaction of a second lysine with the retinylidene Schiff base of bacteriorhodopsin (Lewis, A., Marcus, M. A., Ehrenberg, B. & Crespi, H. (1978) Proc. Natl. Acad. Sci. USA 75, 4642-4646) has been investigated by specific incorporation of 15N into the epsilon-amino groups of the lysine residues. Comparison of resonance Raman spectra of bacteriorhodopsin grown on 100%, 0%, and 50% labeled lysine demonstrates that 15N isotope effects on the Schiff base vibration can be accounted for by 15N labeling only at the Schiff base nitrogen. Our data also provide in situ confirmation of the linkage of the retinal chromophore with the epsilon-amino nitrogen of lysine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aton B., Doukas A. G., Narva D., Callender R. H., Dinur U., Honig B. Resonance Raman studies of the primary photochemical event in visual pigments. Biophys J. 1980 Jan;29(1):79–94. doi: 10.1016/S0006-3495(80)85119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgen J., Walker I. D. Photoreceptor protein from the purple membrane of Halobacterium halobium. Molecular weight and retinal binding site. Biochemistry. 1976 Feb 24;15(4):792–798. doi: 10.1021/bi00649a010. [DOI] [PubMed] [Google Scholar]
- Gerber G. E., Anderegg R. J., Herlihy W. C., Gray C. P., Biemann K., Khorana H. G. Partial primary structure of bacteriorhodopsin: sequencing methods for membrane proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):227–231. doi: 10.1073/pnas.76.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochnauer M. B., Kushner D. J. Growth and nutrition of extremely halophilic bacteria. Can J Microbiol. 1969 Oct;15(10):1157–1165. doi: 10.1139/m69-211. [DOI] [PubMed] [Google Scholar]
- Honig B., Ebrey T. G. The structure and spectra of the chromophore of the visual pigments. Annu Rev Biophys Bioeng. 1974;3(0):151–177. doi: 10.1146/annurev.bb.03.060174.001055. [DOI] [PubMed] [Google Scholar]
- Lewis A., Marcus M. A., Ehrenberg B., Crespi H. Experimental evidence for secondary protein-chromophore interactions at the Schiff base linkage in bacteriorhodopsin: Molecular mechanism for proton pumping. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4642–4646. doi: 10.1073/pnas.75.10.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M. A., Lewis A. Resonance Raman spectroscopy of the retinylidene chromophore in bacteriorhodopsin (bR570), bR560, M421, and other intermediates: structural conclusions based on kinetics, analogues, models, and isotopically labeled membranes. Biochemistry. 1978 Oct 31;17(22):4722–4735. doi: 10.1021/bi00615a019. [DOI] [PubMed] [Google Scholar]
- Pearce R. J. Amino acid analysis by gas-liquid chromatography of N-heptafluorobutyryl isobutyl esters. Complete resolution using a support-coated open-tubular capillary column. J Chromatogr. 1977 Jun 1;136(1):113–126. doi: 10.1016/s0021-9673(00)83000-9. [DOI] [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Studies on the retinal-protein interaction in bacteriorhodopsin. Eur J Biochem. 1977 Jun 15;76(2):499–511. doi: 10.1111/j.1432-1033.1977.tb11620.x. [DOI] [PubMed] [Google Scholar]
- Stockburger M., Klusmann W., Gattermann H., Massig G., Peters R. Photochemical cycle of bacteriorhodopsin studied by resonance Raman spectroscopy. Biochemistry. 1979 Oct 30;18(22):4886–4900. doi: 10.1021/bi00589a017. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]



