Abstract
The economic burden of cancer in the US is substantial and expected to increase significantly in the future due to expected growth and aging of the population and improvements in survival as well as trends in treatment patterns and costs of care following cancer diagnosis. In this paper, we describe measures of the economic burden of cancer and present current estimates and projections of the national burden of cancer in the US. We discuss ongoing efforts to characterize the economic burden of cancer in the US and identify key areas for future work, including developing and enhancing research resources, improving estimates and projections of economic burden, evaluating targeted therapies, and assessing the financial burden for patients and their families. This work will inform efforts by health care policy makers, healthcare systems, and employers to improve the cancer survivorship experience in the US.
Keywords: cost of illness, health care expenditures, neoplasms, projections, SEER-Medicare
INTRODUCTION
At the beginning of 2008, approximately 12 million individuals were alive with a history of cancer in the US (1). The prevalence of cancer survivorship is expected to be even larger in the future because of the aging and growth of the US population, and improved survival following diagnosis (2) due to advances in screening, detection, and treatment. This increasing number of cancer survivors will receive medical care throughout the trajectory of their cancer experiences, starting with diagnosis and including short- and long-term lasting side effects of disease and its treatment. Trends towards greater intensity of health care service use (3,4) and increasing costs of cancer care (3,5–11) are expected to result in a greater burden of cancer in the future. Estimating and projecting the economic burden of cancer, including health care expenditures, productivity loss, and morbidity for patients and their families, are increasingly important issues for health care policy makers, healthcare systems, physicians, employers, and society overall. In this paper, we describe the temporal patterns and measures of the economic burden of cancer, present recent national estimates and projections, and identify key areas for future research.
Temporal patterns of the economic burden of cancer
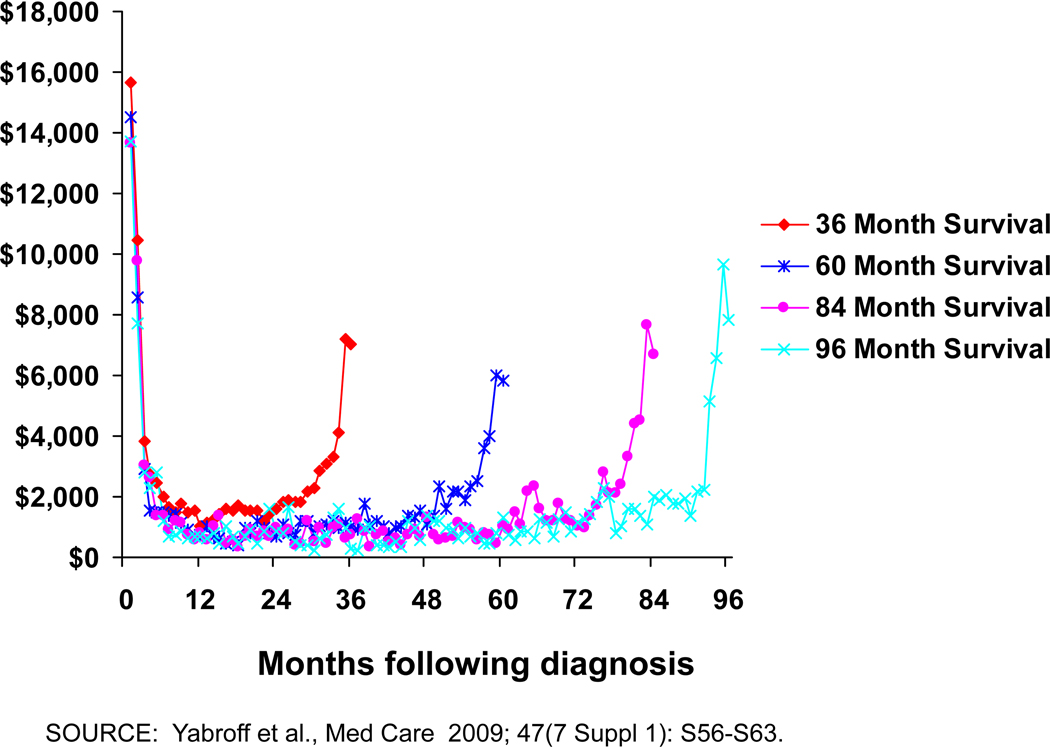
Cancer and its treatment result in the loss of economic resources and opportunities for patients, families, employers, and society overall. These losses include financial loss, morbidity, reduced quality of life, and premature death. When estimating the economic burden of disease, the monetary valuation of resources used to treat disease and the loss of opportunities due to disease is measured as cost. When measured longitudinally starting from cancer diagnosis, the monthly patterns of medical care use and associated costs of cancer change over time. Consistent with the intensity of treatment for initial care, recurrence, and end of life care, cancer costs are highest in the initial period following diagnosis, and among patients who die from their disease, at the end of life (12–18). Costs are lowest in the period between the initial and end of life periods, following a “u-shaped” curve. As shown in Figure 1, this u-shaped medical care cost pattern is consistent across cohorts of colorectal cancer patients with very different survival times (17). The width and height of this u-shaped cost curve varies by cancer site, stage at diagnosis, and patient age (12–14,16,18) and impacts summary measures of incidence and prevalence costs and informs methods used to estimate these costs.
Figure 1.
Monthly Costs of Care for Colorectal Cancer Patients by Length of Survival
Incidence and prevalence cancer costs
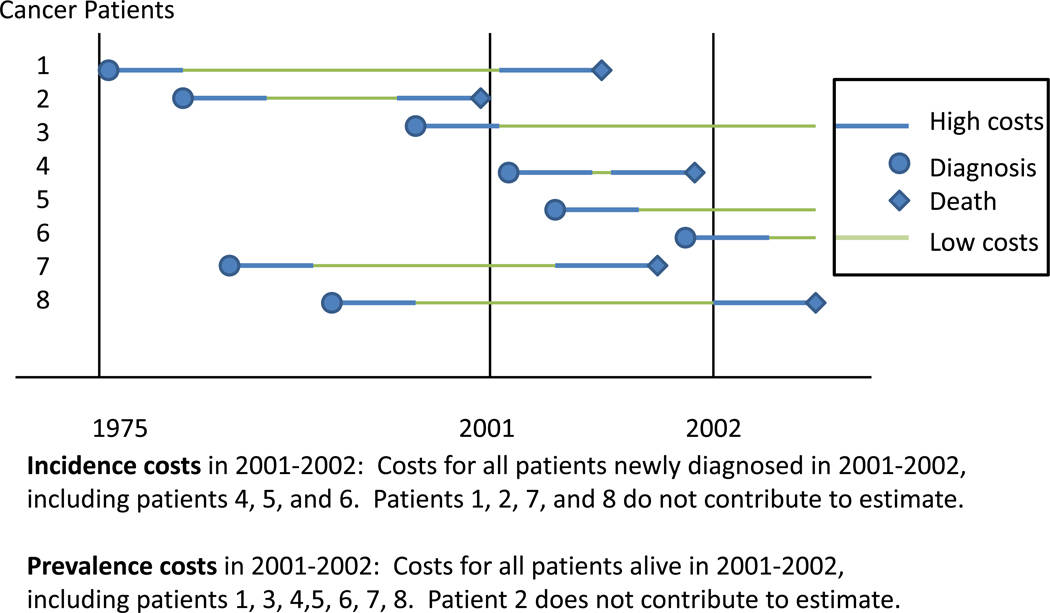
Cancer costs are typically reported starting at diagnosis or the time of a specific event for a group of cancer patients defined by clinical characteristics (incidence costs) or for all cancer survivors alive in a specific year (prevalence costs). Figure 2 shows cost patterns for a series of hypothetical cancer patients, illustrating incidence and prevalence costs. An incidence cost estimate in 2001–2002 will include only those patients diagnosed within this period, and will start at the high cost diagnosis period for patients 4, 5, and 6. Incidence cost estimates can range from periods of less than 1 year to a lifetime. A prevalence cost estimate in 2001–2002 will include all patients alive (i.e., patients 1, 3, 4, 5, 6, 7 and 8), but only the portion of the cost trajectory that occurs during this period. Importantly, the high and low cost portions of the u-shaped curve included in the prevalence cost estimate vary for these patients. As with other measures of disease burden, incidence and prevalence estimates in a given year will be relatively similar when survival is short, because the vast majority of individuals will have high costs in either arm of the “u”, with few individuals with low costs at the bottom of the “u” (i.e., most like patient 4 in 2001–2002). For cancers with long survival, prevalence costs in a given year will be significantly lower than incidence costs in that year, because the prevalence cost estimate will be predominately composed of survivors at the bottom of the “u” where costs are lowest (i.e., most like patient 3 in 2001–2002). Both incidence and prevalence cost measures can be useful for resource allocation and policy and program planning. Incidence costs are commonly used in cost-effectiveness models, for decisions about specific therapies or understanding patient and treatment trajectories, whereas prevalence costs are most commonly used in understanding the overall impact of disease on local, federal or health plan budgets.
Figure 2.
Incidence and Prevalence Costs of Cancer Care
Phase of care approach for estimating cancer costs
One approach for estimating cancer costs, the phase of care approach, uses this u-shaped cost pattern to divide services, costs and observation time into clinically relevant periods or phases in relation to diagnosis and death (i.e., initial, continuing, last year of life phases), which can then be used as an input to estimate either incidence or prevalence costs. Phase of care-specific cost estimates can be combined with survival following diagnosis to yield modeled incidence costs (16) or with phase-of care specific cancer prevalence estimates in a given year to produce prevalence costs for that year (2).
Because the identification of specific services attributable to cancer compared to other conditions is complex, attribution of utilization and costs to cancer is typically done in comparison to individuals without cancer through matching on individual characteristics (e.g., age, sex) where the difference is measured as a “net cost” or in statistical modeling (19). Importantly, costs are generally higher for cancer survivors than similar individuals without cancer in all phases of care (12,16,20,21). It is likely that higher costs of care in the continuing or monitoring phase of care includes costs associated with late effects of treatment and care related to ongoing treatment as well as care for recurrences.
Estimates and projections of the national burden of cancer
Cost domains typically include direct medical costs, the use of resources for medical care; and indirect costs, resulting from the loss of resources and opportunities.
Direct medical costs
Direct medical costs are those associated with services that patients receive, including hospitalizations, surgery, physician visits, radiation therapy and chemotherapy/immunotherapy, and are typically measured by insurance payments and patient out-of-pocket co-payments and deductibles. Within each phase of care, the direct medical costs associated with cancer vary significantly by cancer site. For example, a recent study reported that in the year 2010, mean monthly net costs in the elderly were $1,923 for female breast and $5,074 for female lung cancer patients in the initial phase of care (2). In the continuing phase of care, mean monthly net costs were $184 and $678, respectively. In the last year of life among patients who died of cancer, mean monthly net costs were $5,238 and $7,710. As illustrated in this example, net costs of care were higher in the initial and last year of life phases of care than in the continuing phase of care and higher for lung than breast cancer patients in every phase of care.
Incidence costs
Incidence cost estimates are reported at the person level for many time periods following diagnosis, ranging from several months to patient lifetime (13,16). Incidence costs can also be reported for all newly diagnosed cancer patients at the aggregate national level (16).
Prevalence costs
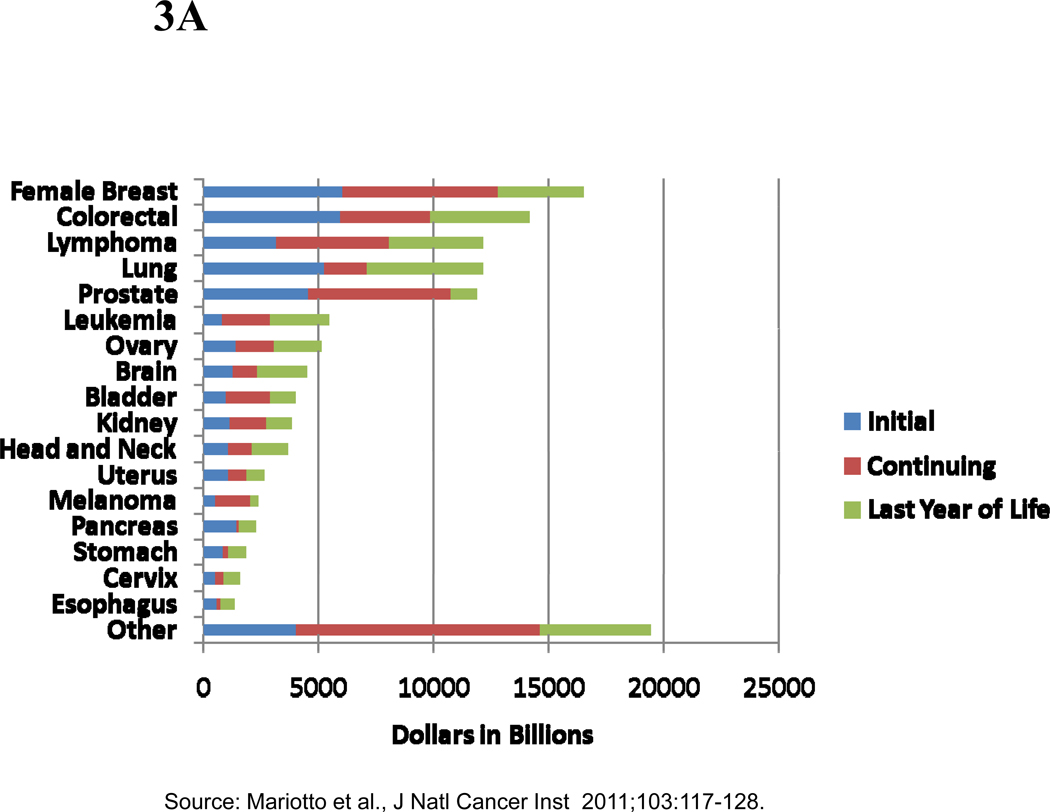
Phase-specific cost estimates can also be combined with phase-specific prevalence estimates obtained from cancer incidence and survival data to estimate the prevalence costs of cancer care by year. The prevalence costs of cancer care in the US in 2010 were estimated to be $124.5 billion dollars(2) with highest costs for breast ($16.5 billion), colorectal ($14.1 billion), lymphoma ($12.1 billion), lung ($12.1 billion) and prostate ($11.9 billion) cancers (Figures 3A and 3B), reflecting the absolute number of cancer survivors by phases of care and annualized phase-specific cost estimates by cancer site. A larger proportion of prevalence costs were in the continuing phase of care for cancers with longer survival, such as breast and prostate cancers, than with short survival, such as lung and pancreas cancers.
Figure 3.
A. Estimates of the National Expenditures for Cancer Care in the US in 2010 (in billions of dollars), by Cancer Site and Phase of Care
B. Estimates of the Proportion of National Expenditures for Cancer Care in the US in 2010 (in billions of dollars), by Cancer Site and Phase of Care
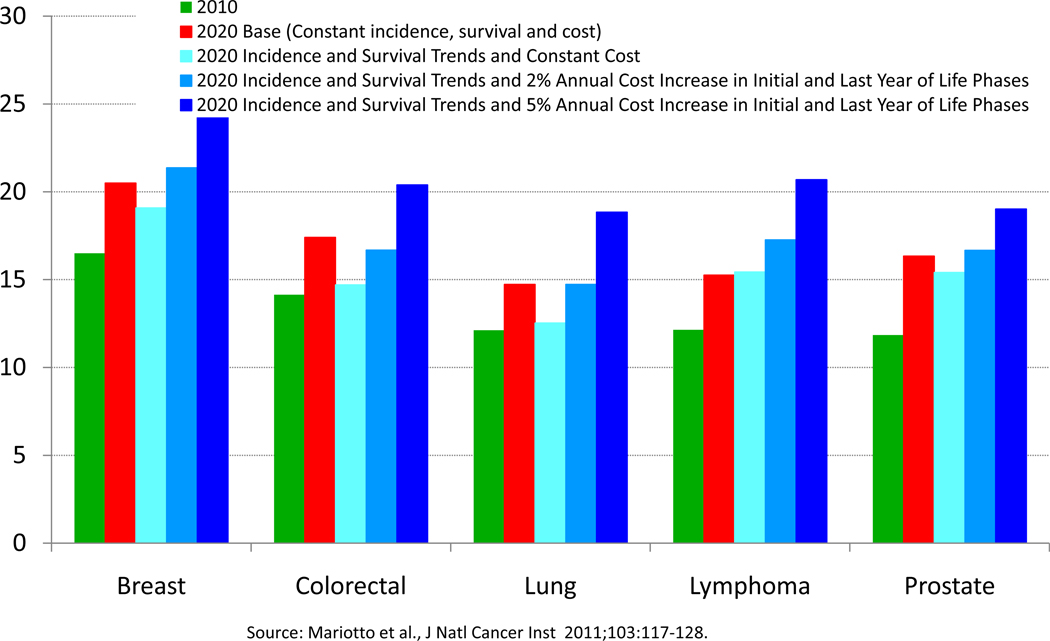
If cancer incidence, survival, and costs of care remained at constant levels, by the year 2020, the costs of cancer care in the US were projected to increase to $157.8 billion dollars from $124.5 billion dollars in 2010 (2). This 27% increase in projected costs reflects only US population changes. If recent trends of declining incidence, improving survival, and increasing costs continue, the estimated cost of cancer care would increase to $172.8 billion dollars in 2020, a 39% increase from 2010. As shown in Figure 4, assumptions about trends in incidence, survival and costs of care have different impacts on the costs for different cancer sites.
Figure 4.
Estimates of National Expenditures for Selected Cancers in 2010 and 2020: Impact of Assumptions About Trends in Incidence, Survival and Costs of Care
The majority of estimates of the direct medical costs of cancer have focused on the elderly population aged 65 and older, the population with the greatest cancer prevalence. Less work has been conducted in the population of cancer survivors under the age of 65, including survivors of childhood and adult cancers, and those few studies have predominately reported prevalence costs (22,23,23–25). Improving national estimates and projections of the direct medical costs of care for cancer survivors under the age of 65, particularly for incidence costs, will be a critical area for additional research, because this group has been reported to receive more aggressive care than older cancer patients (26–28) and may also experience the consequences of any lasting effects of disease and its treatment for more years than their elderly counterparts.
Indirect costs
Indirect costs of cancer are the monetary losses associated with time spent receiving medical care, time lost from work or other usual activities (morbidity costs), and lost productivity due to premature death (mortality costs). These costs are incurred by patients as well as their caregivers and families. Because these lost opportunities are not typically reflected in monetary transactions, the value of lost time must be approximated. The main approaches for valuing time are the human capital and the willingness-to-pay (WTP) methods. In the human capital approach, gender- and age- specific average earnings are combined with time lost from work or years of working life lost due to premature death to estimate unrealized earnings. This approach explicitly values the time of individuals or populations with greater earnings as greater than the time of individuals or populations with less earnings. WTP approaches, in contrast, incorporate both lost productivity and the intrinsic value of life, by estimating the amount an average individual or populations of individuals would be willing to pay for an additional year of life. Because cancer incidence and mortality rates are highest in the elderly, a population less likely to be in the workforce than their younger counterparts, these valid, but conceptually different approaches yield very different estimates of the indirect costs of cancer.
Patient time and morbidity costs
Patient and caregiver time data, including travel to and from care, waiting for and receiving care, are not routinely collected. In the few studies of time costs that have been conducted, time estimates have been based on patterns of medical care use with service specific estimates of time (12) or retrospective surveys with questions about time spent receiving care or providing assistance (29–32), and then combined with human capital or WTP estimates of the value of that time. These time costs vary by the type of cancer (12,29,33), phase of care (12,33), and by stage of disease at diagnosis (29). Morbidity due to disease or its treatment can be conceptualized as lost productivity (i.e., days lost from work) or more broadly as the loss of ability to participate in usual activities, including leisure. From the perspective of employers, costs associated with lost productivity due to employee disability and absenteeism among cancer survivors are likely to be substantial (21,34). Comprehensive national estimates of the incidence or prevalence costs associated with patient and caregiver time or morbidity are not currently available in the US.
Mortality costs
Few studies have assessed the mortality costs associated with premature death from cancer. Mortality costs are the combination of estimates of the future person years of life lost among individuals who die in a specific year with a monetary value of time, yielding an estimate that reflects lost productivity in the future. Two companion studies of mortality costs in the year 2000 based on the same population estimates and mortality rates, reported the value of life lost due to early death from cancer to be $960.6 billion (35) using the WTP method, compared with $115.8 billion (36) using the employment-based human capital method. As expected, the biggest differences in these estimates were in the elderly, due to differences in how years of life lost for the elderly were valued. Similar to national estimates of the direct medical costs of cancer, mortality costs were highest for lung, breast, and colorectal cancers with both approaches, although the magnitude of estimates varied. Others have estimated the indirect costs for specific cancers using similar data sources and assumptions (37,38). Regardless of the approach, in the few studies that estimate both mortality costs and direct costs of medical care, mortality cost estimates are generally at least the equivalent of the direct costs of medical care (39,40).
Projections of the indirect and productivity costs associated with cancer are also affected by expected changes in the US population. If cancer mortality rates remain constant at 2000 levels, lost productivity valued with the human capital approach is estimated to increase from $115.8 billion in 2000 to $147.6 billion in 2020, a 27.5% increase due only to population growth and aging (36). Studies of the indirect burden of cancer for individuals of all ages, particularly those that provide comprehensive national estimates of patient and caregiver time costs, lost productivity, and mortality costs will be important for future research. Inclusion of these estimates along with direct medical costs will give a more complete picture of the burden of cancer in the US. Additionally, patient and caregiver time costs are recommended for inclusion in cost-effectiveness analyses (41); their exclusion may lead to a bias towards underestimating the costs of interventions that place a higher burden on the cancer patient and informal caregivers (42).
Improving understanding of the economic burden of cancer in the US
In this section, we discuss common data sources for estimating cancer burden and identify areas for improving data sources and methods. We also highlight key areas for future research, including detailed projections of the burden of cancer, evaluation of targeted therapies, and investigation of financial burden to cancer survivors and their families.
Data sources for estimating the burden of cancer
Most data sources in the US were not developed for research in or developing estimates of the economic burden of cancer, and as a result have limitations associated with their use for estimating direct or indirect costs (43). Selected data sources for estimating the economic burden of cancer are detailed in Table 1. Individual level longitudinal data across the trajectory of illness are only available within the context of covered populations in discrete health insurance programs (e.g., linked SEER – Medicare data) and are not nationally representative. By definition, information about care received for patients who are uninsured is not available. Importantly, information about care received outside of an insurance program, and in some cases, in cancer centers or as part of clinical trials are not be available. Because a high proportion of children with cancer receive care at cancer centers or in clinical trials (i.e., more than 50% under age 14 enter an NCI-sponsored clinical trial (44)), existing health insurance program data are limited for estimating economic burden in this population.
Table 1.
Characteristics of Selected Data Sources/Research Resources in the US for Estimating Burden of Cancer
| SEER-Medicare | MEPS | H-CUP | MCBS | CRN | |
|---|---|---|---|---|---|
| Description | SEER tumor registries linked to Medicare claims |
Nationally representative in-person survey with provider data collection |
Inpatient discharge data from sampled hospitals |
National survey linked to Medicare claims |
Research centers in managed care organizations |
| Data characteristics | |||||
| National or nationally representative | geographically defined | √ | √ | √ | geographically defined |
| Individual –level longitudinal data | √ | 5 panels over 2 years | √ | √ | |
| Approximate number of cancer survivors in 2007 | >1,000,000 | <2,000 | >1,000,000 | <2,000 | >400,000 |
| Duration of information | Medicare eligibility through death | 2 years | Hospital admission through discharge | Enrollment through disenrollment or death | |
| Health insurance type | Medicare fee-for-service only | All payors | All payors | All payors | Managed care only |
| Patients information | |||||
| Age distribution | Aged 65+ or disabled (any age) | Aged 18+ | All ages | Aged 65+ or disabled (any age) | Aged 18+ |
| Information about patients without cancer | In cancer registry regions | √ | √ | √ | √ |
| Cancer information | |||||
| Cancer diagnosis | Registry, procedure or diagnosis codes | Self-report, procedure or diagnosis codes | Procedure or diagnosis codes | Self-report, procedure or diagnosis codes | Registry, procedure or diagnosis codes |
| Stage at diagnosis | √ | √ | |||
| Treatment | √ | √ | Inpatient hospital only | √ | √ |
| Type of cost estimate | |||||
| Incidence | √ | √ | |||
| Prevalence in a specific year | √ | √ | √ | √ | √ |
| Direct medical cost components | |||||
| Hospital | √ | √ | √ | √ | √ |
| Physician and other outpatient services | √ | √ | √ | √ | |
| Outpatient pharmacy | √* | √ | √* | √ | |
| Out of pocket | √ | √ | |||
| Indirect cost components | |||||
| Productivity loss (e.g., days lost from work) | √ | ||||
| Patient time | √ | ||||
| Caregiver time |
Data on Medicare Part D prescription drug services are available starting in 2006. Before 2006, drugs administered parenterally and their administration was covered by Medicare Part B, as were Prodrugs, the oral drug equivalent of drugs administered parenterally.
SEER-Medicare = Surveillance Epidemiology and End Results –Medicare, MEPS = Medical Expenditure Panel Survey, H-CUP = Healthcare Costs and Utilization Program, MCBS = Medicare Current Beneficiary Survey, CRN = Cancer Research Network
Nationally representative surveys (e.g., Medical Expenditure Panel Survey (MEPS)) and discharge databases (e.g., Healthcare Cost and Utilization Project (HCUP)) include information from all payers as well as for patients who are uninsured, but are typically cross-sectional or of limited duration, and may not include key clinical details about cancer patients such as stage of disease at diagnosis or the full course of treatment. Hospital discharge data are limited to a specific type of service – inpatient hospitalizations – and can be used for estimating direct medical costs of hospitalization or patient time associated with hospitalization. Employer-sponsored commercially insured databases have also been used in estimating cancer costs (21,34,45), but these data typically do not provide details about employers, covered individuals and their families, or stage and date of diagnosis. Few data sources include information about patient and family out-of-pocket costs.
Until recently, the MEPS had limited utility for estimating cancer burden because only the subset of individuals receiving treatment for cancer during a specific period were identified, rather than all patients with a history of a cancer diagnosis (“treated prevalence”). Recent changes in the identification of cancer survivors combined with efforts to add households with cancer survivors to the MEPS will allow for more complete estimates of the burden of cancer care for adult cancer survivors (46). This effort will also field a questionnaire about patient experiences with cancer, including employment and productivity and other measures of indirect costs that can be evaluated with medical costs of care.
Increasingly data linkages, between cancer registry and insurance claims, surveys and discharge databases, and between registry and multiple health insurance data sources within states will help to bridge some data gaps (46–48). Ongoing efforts to improve research resources through the linkage and enhancement of existing data sources, including information from clinical trials and cancer centers, is an important area for additional work in estimating direct medical and indirect costs of cancer care.
Methods for estimating burden of cancer
Use of different data sources which cover different patient populations, payors, and types of services or other measures can yield very different estimates of the costs of care associated with cancer (22,49). Further, even within the same data sources, the methods for identifying cancer survivors or caregivers can yield different estimates of cost (17,23,49). For example, in studies using the same data source, years of data, observation periods, and exclusion criteria, use of Medicare claims data to identify colorectal cancer survivors overestimated annual prevalence costs compared with using cancer registry data to identify survivors (mean net per-person annual costs $8736 vs. $5457, respectively (49)). Identifying a “treated prevalence” sample using claims for cancer care excludes longer-term cancer survivors who may be receiving care for late effects of cancer treatment, but not receiving cancer-related surgery, chemotherapy, or radiation therapy. Identifying a “treated prevalence” sample for estimating patient morbidity or time costs will also overestimate costs compared with a representative sample of individuals with a cancer history. Currently, global estimates of the burden of cancer including direct and indirect costs are developed using different data sources, patient populations and method for identifying patients, with little consideration of how best to combine these estimates in a meaningful way. Head-to-head comparisons of different data sources and methods using the same standard study criteria, patient samples and definitions will inform the combination of estimates across data sources and populations. Comparisons using standard definitions and methods across health care systems within and between countries (50) will also enhance understanding of the role of systems of health care delivery on the burden of cancer.
Projections of the economic burden of cancer
The projections of direct and indirect medical costs associated with cancer presented earlier are based in broad assumptions that current or recent incidence, survival or mortality rates remain the same, trends from prior years continue, or trends continue at a hypothetical level (e.g., 2% increase annually) (2,35,36). To assess the impact of specific interventions, such as efforts to increase the use of effective chemotherapy on the costs of care, more detailed microsimulation models of the natural history of disease that include information about risk factor prevalence, screening uptake and performance, and treatment uptake and effectiveness, such as those included in the NCI-sponsored Cancer Intervention Surveillance Modeling Network (CISNET) (51), are needed. Such simulation efforts can be particularly useful for policy makers faced with having to choose how best to allocate constrained resources to reduce the burden of cancer in the future.
Targeted therapies
The average cost of newly developed cancer treatments, primarily targeted therapies (e.g., monoclonal antibodies and small molecule drugs), has risen dramatically over the past decade (5,6). For example, one month of bevacizumab for the treatment of colorectal cancer and one month of ofatumumab for chronic lymphocytic leukemia may cost up to $9,000 and $16,000, respectively. Pralatrexate for the treatment of peripheral T-cell lymphoma can amount to $30,000 per month while the cost of one week of clofarabine treatment for pediatric leukemia may reach as high as $34,000 (52). Currently, nine monoclonal antibodies are approved by the US Food and Drug Administration for the treatment of cancer (53) and many others are in development.
Advances in identification of genetic mutations associated with treatment response allow the tailoring of treatment to individual patients. Limiting the use of expensive therapies to patients with genetic profiles associated with treatment response may reduce the program costs of these treatments. For example, colorectal cancer patients carrying KRAS mutations do not respond to treatment with cetuximab or panitumumab (54), and by restricting the use of cetuximab to patients without the KRAS mutations, the incremental cost effectiveness ratio is more favorable (55). These estimates excluded other treatment-related costs, including hourly infusion and oncology visit costs and patient waiting and travel time (56), which can be significant. Further, because the goal is to identify patients most likely to respond to targeted therapies, all potentially eligible patients must be tested, including those for whom treatment will not ultimately be indicated. Finally, the increase in median survival attributed to treatment with many of these targeted therapies, even among those most likely to respond, is typically only a few additional months (6). Currently, little is known about the utilization and effectiveness of targeted therapies in community practice. Estimating and projecting their uptake and cost, appropriate use of genetic tests to personalize therapy, and impact on patient morbidity and survival are important areas for future research.
Financial burden of cancer
The emerging use of targeted therapies highlights a critical data and research gap – out-of-pocket costs and financial burden to patients and their families. Because most health insurance plans require some form of cost-sharing for drug therapy (20% copayment is typical), patients and their families with health insurance may face bills of tens of thousands of dollars for a full course of treatment. Patients may delay treatment or fail to seek care because of high patient cost-sharing. High costs pose an even greater problem for the uninsured. A recent national study estimated that more than 2 million cancer survivors in the US did not receive needed medical services because of cost (57). Medical care expenses were recently reported to be the primary reason for many bankruptcies, with cancer as a leading cause (58).
Because health insurance in the US is predominantly employment-based in the working age population, its relationship with the economic burden of cancer survivorship is complex. A cancer diagnosis may limit employment opportunities, which in turn may lead to a loss of health insurance. Informal caregiving may also influence employment for caregivers, potentially limiting a caregiver’s ability to hold a full-time position or resulting in higher rates of absenteeism, particularly when the patient travels long distances for specialized care and/or when the patient is a child (59). Alternatively, maintaining health insurance coverage may lead cancer survivors or their employed family caregivers to work longer hours (60,61) or continue working (62) and delay retirement. Evaluation of financial burden and employment and health insurance trajectories in cancer survivors and their families will be an important area for additional research, particularly in relation to changes in health insurance from the Affordable Care Act (ACA).
Economic burden of long term survivorship
To date, little work has focused on the economic burden of long term cancer survivorship, outside of the initial period following diagnosis or the end of life (63), including late or lasting effects of treatment or recurrences. Late effects of treatment and recurrences are not routinely collected by most cancer registries, nor are they clearly identifiable from claims data. In the few studies that have been conducted, survivors of adult and childhood cancers have poorer health outcomes, increased functional limitations, and elevated health-related unemployment and underemployment levels compared to similar individuals without cancer, even many years after diagnosis (20,60,64,65). Few studies have measured trajectories of medical costs, health outcomes, or employment over a lifetime. Such studies are particularly relevant for survivors of childhood cancer. Even though they are a small proportion of cancer survivors (< 5% (1)), they have many years to experience any late or lasting effects of treatment, including risk of subsequent cancers. Additional work estimating and projecting the economic burden for longer term cancer survivors, including direct medical costs, indirect costs, and impacts on employment, productivity loss and financial burden will be important for future research.
In summary, the economic burden of cancer in the US is substantial and is expected to increase significantly due to population changes alone. Key areas for future efforts include: developing and enhancing research resources; improving estimates and projections of burden, particularly indirect costs; evaluating use and effectiveness of targeted therapies; and financial burden of cancer for patients and their families. This work will inform efforts by health care policy makers, healthcare systems, and employers to improve the cancer survivorship experience in the US.
ACKNOWKLEDGEMENT
The authors wish to acknowledge Martin Brown for thoughtful comments on an earlier version of the paper.
References
- 1.Howlander N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States.: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinan MA, Curtis LH, Hammill BG, Patz EF, Jr, Abernethy AP, Shea AM, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999–2006. JAMA. 2010;303:1625–1631. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]
- 5.Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009;360:626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 6.Schrag D. The price tag on progress - chemotherapy for colorectal cancer. N Engl J Med. 2004;351:317–319. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 7.Tangka FK, Trogdon JG, Richardson LC, Howard D, Sabatino SA, Finkelstein EA. Cancer treatment cost in the United States. Cancer. 2010;116:3477–3484. doi: 10.1002/cncr.25150. [DOI] [PubMed] [Google Scholar]
- 8.Woodward RM, Brown ML, Stewart ST, Cronin KA, Cutler DM. The value of medical interventions for lung cancer in the elderly: results from SEER-CMHSF. Cancer. 2007;110:2511–2518. doi: 10.1002/cncr.23058. [DOI] [PubMed] [Google Scholar]
- 9.Howard DH, Kauh J, Lipscomb J. The value of new chemotherapeutic agents for metastatic colorectal cancer. Arch Intern Med. 2010;170:537–542. doi: 10.1001/archinternmed.2010.36. [DOI] [PubMed] [Google Scholar]
- 10.Wong Y-N, Meropol NJ, Speier W, Sargent D, Goldberg RM, Beck JR. Cost implication of new treatments for advanced colorectal cancer. Cancer. 2009;115:2081–2091. doi: 10.1002/cncr.24246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkin EB, Bach PB. Cancer's next frontier: addressing high and increasing costs. JAMA. 2010;303:1086–1087. doi: 10.1001/jama.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yabroff KR, Davis WW, Lamont EB, Fahey A, Topor M, Brown ML, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14–23. doi: 10.1093/jnci/djk001. [DOI] [PubMed] [Google Scholar]
- 13.Riley GF, Potosky AL, Lubitz JD, Kessler LG. Medicare payments from diagnosis to death for elderly cancer patients by stage and diagnosis. Med Care. 1995;33:828–841. doi: 10.1097/00005650-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Taplin SH, Barlow W, Urban N, Mandelson MT, Timlin DJ, Ichikawa L, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87:417–426. doi: 10.1093/jnci/87.6.417. [DOI] [PubMed] [Google Scholar]
- 15.Fireman BH, Quesenberry CP, Somkin CP, Jacobson AS, Baer D, West D, et al. Cost of care for cancer in a health maintenance organization. Health Care Financ Rev. 1997;18:51–76. [PMC free article] [PubMed] [Google Scholar]
- 16.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 17.Yabroff KR, Warren JL, Schrag D, Meekins A, Topor M, Brown ML. Comparison of approaches for estimating incidence costs of care for colorectal cancer patients. Med Care. 2009;47:S56–S63. doi: 10.1097/MLR.0b013e3181a4f482. [DOI] [PubMed] [Google Scholar]
- 18.Brown ML, Riley GF, Schussler N, Etzioni RD. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40(8 Suppl):104–117. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 19.Barlow W. Overview of methods to estimate the medical costs of cancer. Med Care. 2009;47:S33–S36. doi: 10.1097/MLR.0b013e3181a2d847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 21.Barnett A, Birnbaum H, Cremieux PY, Fendrick AM, Slavin M. The costs of cancer to a major employer in the United States: a case-control analysis. Am J Manag Care. 2000;6:1243–1251. [PubMed] [Google Scholar]
- 22.Howard DH, Molinari N-A, Thorpe KE. National estimates of medical costs incurred by nonelderly cancer patients. Cancer. 2004;100:883–891. doi: 10.1002/cncr.20063. [DOI] [PubMed] [Google Scholar]
- 23.Short PF, Moran JR, Rajeshwari P. Medical expenditures of adult cancer survivors aged <65 years in the United States. Cancer. 2011;117:2791–2800. doi: 10.1002/cncr.25835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finkelstein EA, Tangka FK, Trogdon JG, Sabatino SA, Richardson LC. The personal financial burden of cancer for the working age population. Am J Manag Care. 2011;15:801–806. [PubMed] [Google Scholar]
- 25.Thorpe KE, Howard D. Health insurance and spending among cancer patients. Health Affairs - Web Exclusive. 2003:W3-189–W3-198. doi: 10.1377/hlthaff.w3.189. [DOI] [PubMed] [Google Scholar]
- 26.Smith TJ, Penberthy L, Desch CE, et al. Differences in initial treatment patterns and outcomes of lung cancer in the elderly. Lung Cancer. 1995;13:235–252. doi: 10.1016/0169-5002(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 27.Farrow DC, Hunt WC, Samet JM. Geographic variation in the treatment of localized breast cancer. N Engl J Med. 1992;326:1097–1101. doi: 10.1056/NEJM199204233261701. [DOI] [PubMed] [Google Scholar]
- 28.Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazil A, Key CR. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst. 2000;92:269–271. doi: 10.1093/jnci/92.3.269. [DOI] [PubMed] [Google Scholar]
- 29.Yabroff KR, Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115:4362–4373. doi: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]
- 30.Jonas DE, Russell LB, Sandler RS, Chou J, Pignone M. Value of patient time invested in the colonoscopy screening process: time requirements for colonoscopy study. Med Decis Making. 2008;28:56–65. doi: 10.1177/0272989X07309643. [DOI] [PubMed] [Google Scholar]
- 31.Hayman JA, Langa KM, Kabeto MU, Katz SH, DeMonner SM, Chernew ME, et al. Estimating the cost of informal caregiving for elderly patients with cancer. J Clin Oncol. 2001;19:3219–3225. doi: 10.1200/JCO.2001.19.13.3219. [DOI] [PubMed] [Google Scholar]
- 32.Stommel M, Given CW, Given BA. The cost of cancer home care to families. Cancer. 1993;71:1867–1874. doi: 10.1002/1097-0142(19930301)71:5<1867::aid-cncr2820710525>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Van Houtven CH, Ramsey SD, Hornbrook MC, Atienza AA, van Ryn M. Economic burden for informal caregivers of lung and colorectal cancer patients. Oncologist. 2010;15:883–893. doi: 10.1634/theoncologist.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang S, Long SR, Kutikova L, Bowman L, Finley D, Crown WH, et al. Estimating the cost of cancer: results on the basis of claims data analyses for cancer patients diagnosed with seven types of cancer during 1999 to 2000. J Clin Oncol. 2004;22:3524–3530. doi: 10.1200/JCO.2004.10.170. [DOI] [PubMed] [Google Scholar]
- 35.Yabroff KR, Bradley CJ, Mariotto AB, Brown ML, Feuer EJ. Estimates and projections of value of life lost from cancer deaths in the United States. J Natl Cancer Inst. 2008;100:1755–1762. doi: 10.1093/jnci/djn383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradley CJ, Yabroff KR, Dahman B, Feuer EJ, Mariotto A, Brown ML. Productivity costs of cancer mortality in the United States: 2000–2020. J Natl Cancer Inst. 2008;100:1763–1770. doi: 10.1093/jnci/djn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ekwueme DU, Chesson HW, Zhang KB, Balamurugan A. Years of potential life lost and productivity costs because of cancer mortality and for specific cancer sites where human papillomavirus may be a risk factor for carcinogenisis - United States, 2003. Cancer. 2008;113:2936–2945. doi: 10.1002/cncr.23761. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Ekwueme DU, Rim SH, Tangka FK. Years of potential life lost and productivity losses from male urogential cancer deaths - United States, 2004. Urology. 2010;76:528–535. doi: 10.1016/j.urology.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 39.Max W, Rice DP, Sung HY, Michel M, Breuer W, Zhang X. The economic burden of prostate cancer, California, 1998. Cancer. 2002;94:2906–2913. doi: 10.1002/cncr.10532. [DOI] [PubMed] [Google Scholar]
- 40.Max W, Rice DP, Sung HY, Michel M, Breuer W, Zhang X. The economic burden of gynecologic cancers in California, 1998. Gynecologic Oncology. 2003;88:96–103. doi: 10.1016/s0090-8258(02)00101-4. [DOI] [PubMed] [Google Scholar]
- 41.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 42.Russell LB. Completing costs: patients' time. Medical Care. 2009;47:S89–S93. doi: 10.1097/MLR.0b013e31819bc077. [DOI] [PubMed] [Google Scholar]
- 43.Lund JL, Yabroff KR, Ibuka Y, Russell LB, Barnett PG, Lipscomb J, et al. Inventory of data sources for estimating health care costs in the United States. Med Care. 2009;47:S127–S142. doi: 10.1097/MLR.0b013e3181a55c3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Care for Children and Adolescents With Cancer. [Accessed August 15, 2011];2011 http://www cancer gov/cancertopics/factsheet/NCI/children-adolescents.
- 45.Shih Y-CT, Xu Y, Cormier JN, Giordano S, Ridner SH, Buchholz TA, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009;27:2007–2014. doi: 10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 46.Medical Expenditure Panel Survey (MEPS): Cancer Survivorship Supplement. [Accessed August 8, 2011];2011 http://healthservices cancer gov/surveys/meps/
- 47.Bradley CJ, Given CW, Luo Z, Roberts C, Copeland G, Virnig BA. Medicaid, Medicare, and the Michigan Tumor Registry: a linkage strategy. Med Decis Making. 2007;27:352–363. doi: 10.1177/0272989X07302129. [DOI] [PubMed] [Google Scholar]
- 48.Schrag D, Virnig BA, Warren JL. Linking tumor registry and Medicaid claims to evaluate cancer care delivery. Health Care Financ Rev. 2009;30:61–73. [PMC free article] [PubMed] [Google Scholar]
- 49.Yabroff KR, Warren JL, Banthin J, Schrag D, Mariotto AB, Lawrence W, et al. Comparison of approaches for estimating prevalence costs of care for cancer patients: what is the impact of data source? Med Care. 2009;47:S64–S69. doi: 10.1097/MLR.0b013e3181a23e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warren JL, Barbera L, Bremner KE, Yabroff KR, Hoch JS, Barrett MJ, et al. End-of-life care for lung cancer patients in the United States and Ontario. J Natl Cancer Inst. 2011 doi: 10.1093/jnci/djr145. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Cancer Institute. Cancer Intervention and Surveillance Modeling Network (CISNET) [accessed August 15, 2011];2007 http://cisnet cancer gov/
- 52.Kolata G. Costly cancer drug offers hope, but also a dilemma. The New York Times. 2011 [Google Scholar]
- 53.American Cancer Society. Monoclonal antibodies. [Accessed March 2. 2011];2011
- 54.Amado RG, Wolf M, Peeters M, van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 55.Mittmann N, Au H-J, Tu D, O'Callaghan CJ, Isogai PK, Karapetis CS, et al. Prospective cost-effectiveness analysis of cetuximab in metastatic colorectal cancer: economic evaluation of National Cancer Insitute of Canada Clinical Trials Group CO.17 trial. J Natl Cancer Inst. 2009;101:1182–1192. doi: 10.1093/jnci/djp232. [DOI] [PubMed] [Google Scholar]
- 56.Elkin EB, Weinstein MC, Winer EP, Kuntz KM, Schnitt SJ, Weeks JC. HER-2 testing and trastuzumab therapy for metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol. 2004;22:863. doi: 10.1200/JCO.2004.04.158. [DOI] [PubMed] [Google Scholar]
- 57.Weaver KE, Rowland JH, Bellizzi KM, Aziz NM. Forgoing medical care because of cost: assessing disparities in healthcare access among cancer survivors living in the United States. Cancer. 2010;116:3493–3504. doi: 10.1002/cncr.25209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Himmelstein DU, Thorne D, Warren E, Woolhandler S. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med. 2009;122:741–746. doi: 10.1016/j.amjmed.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 59.Bloom BS, Knorr RS, Evans AE. The epidemiology of disease expenses: the costs of caring for children with cancer. JAMA. 1985;253:2393–2397. [PubMed] [Google Scholar]
- 60.Bradley CJ, Bednarek HL, Neumark D. Breast cancer and women's labor supply. Health Serv Res. 2002;37:1309–1328. doi: 10.1111/1475-6773.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hollenbeak CS, Short PF, Moran J. The implications of cancer survivorship for spousal employment. J Cancer Surviv. 2011 doi: 10.1007/s11764-011-0175-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradley CJ, Neumark D, Luo Z, Bednarek HL. Employment-contingent health insurance, illness, and labor supply of women: evidence from married women with breast cancer. Health Econ. 2007;16:19–37. doi: 10.1002/hec.1191. [DOI] [PubMed] [Google Scholar]
- 63.Yabroff KR, Warren JL, Brown ML. Costs of cancer care in the USA: a descriptive review. Nat Clin Pract Oncol. 2007;4:643–656. doi: 10.1038/ncponc0978. [DOI] [PubMed] [Google Scholar]
- 64.Dowling E, Yabroff KR, Mariotto AB, McNeel T, Zeruto C, Buckman D. Burden of illness in adult survivors of childhood cancers: findings from a population-based national sample. Cancer. 2010;116:3712–3721. doi: 10.1002/cncr.25141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moran JR, Short PF, Hollenbeak CS. Long-term employment effects of surviving cancer. J Health Economics. 2011;30:505–514. doi: 10.1016/j.jhealeco.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]