Abstract
Purpose
To investigate the effects of prolonged vaginal distension and beta-aminopropionitrile (BAPN) on urinary patterns and urethral structure in female virgin rats.
Materials and Methods
Female virgin rats were randomly divided into 3 groups of 7 rats each. The control group received no intervention; the VD group was treated with prolonged vaginal distension (VD) via balloon inflation; the VD+BAPN group was treated with VD plus i.p. injection of 150 mg/kg of BAPN every 3 days. Three weeks later all rats were subjected to conscious cystometric analysis and then sacrificed for histological analysis of the urethra.
Results
Conscious cystometry identified 0, 3, and 5 rats in the control, VD, and VD+BAPN groups, respectively, as having abnormal voiding pattern. Urethral collagen content was significantly lower in the VD and VD+BAPN rats when compared to control rats. Urethral elastic fibers were disorganized and shorter in the VD and VD+BAPN rats, and were fragmented, lacking the inter-muscle connections in the VD+BAPN rats. Urethral striated muscle fibers were shorter and more widely spaced in VD and VD+BAPN rats than in control rats. Additionally, those in the VD+BAPN group exhibited an abnormal wavy shape suggestive of lacking architectural support.
Conclusions
Prolonged vaginal distension caused urodynamic changes and histological abnormalities in the urethra including reduced collagen content, fragmented elastic fibers, as well as sparsely arranged and shortened striated muscle fibers. BAPN appears to interfere with the restoration of collagen and elastic fibers.
Keywords: stress urinary incontinence, birth trauma, beta-aminopropionitrile, elastin, collagen, striated muscle, smooth muscle
INTRODUCTION
Urinary incontinence (UI) afflicts more than 200 million people worldwide 1 and costs nearly 20 billion dollars in the United States alone in 2000 2. In a recent survey of 4,229 women older than 20 years, 49.6% responded as having UI symptoms and among them 84.1% reported as having the stress type of UI (SUI) 3. However, despite the recognition of SUI’s importance in healthcare and a wealth of knowledge about its risk factors, our understanding of SUI’s causal mechanism remains limited. Historically, the hypermobility theory, which considers that SUI is predominantly associated with a lack of urethral support, has dominated the field 4. However, it is well known that many women with urethral hypermobility remain continent 5 and surgical improvement of urethral support still fails a significant number of women 6. Furthermore, a recent case-control study concluded that “maximal urethral closure pressure and not urethral support is the factor most strongly associated with SUI,” and its authors suggested that “improving urethral function may have therapeutic promise.” 7
Therapy through improving urethral function requires an understanding of SUI-associated changes in the urethra. However, patients’ urethral tissues are usually unavailable for research, and thus animal models mimicking human SUI are valuable tools for both urodynamic and pathological studies. In the past decade such an animal model has permitted the identification of several SUI-related changes at the molecular and cellular levels 8-11. It has also allowed the recognition of the involvement of both the cellular and the extracelluar compartments in the pathogenesis of SUI. In the latter instance we first noted that SUI was associated with a decrease of type I collagen and fragmentation of elastic fibers 12. We also found that the elastic fiber abnormality was exacerbated by estrogen supplementation 13. However, while we consider that these structural changes are likely the consequence of birth trauma, interpretation as such is hampered by the fact that the SUI model was generated by a combination of pregnancy, delivery, prolonged vaginal distension, and ovariectomy. Therefore, in the present study we conducted our study with virgin female rats that were treated with prolonged vaginal distension without ovariectomy. We also performed experiments with an additional group of rats that were treated with beta-aminopropionitrile (BAPN) as well - in the hope that this additional treatment would allow us to gain a better understanding of the extracellular matrix’s role in SUI.
BAPN has been used extensively for studying the contribution of extracellular matrix to normal tissue function and disease processes. By specific inhibition of lysyl oxidase, it interferes with the cross-linking step of collagen and elastic fiber biosynthesis 14. In animals treated with BAPN collagens become abnormally soluble and elastic fibers weakened in tensile strength 15. Thus, BAPN has proven useful in the creation of animal models such as aortic aneurysm that have weakened collagen and elastic fibers 16. In the present study we show that BAPN exacerbated the impact of prolonged vaginal distension on urinary continence and urethral structure.
MATERIALS AND METHODS
Animals
Twenty-one 2-month-old female virgin Sprague-Dawley rats, weighing approximately 200 g, were obtained from Charles River Laboratories (Wilmington, MA). They were equally randomized into a Control group, a Vaginal Distension (VD) group, and a VD plus BAPN (VD+BAPN) group. The Control group received no treatment while the VD group was treated with prolonged vaginal distension: Under anesthesia with ketamine (90 mg/kg, i.p.) and xylazine (10 mg/ kg, i.p.), the rats were placed on a heating pad to maintain isothermia at 37 °C. The balloon of a transurethral catheter (18 F; Bard, Covington, GA, USA) was placed intravaginally and was filled with 3 mL of water. A 130-g weight was placed on the suspended end of the catheter providing a constant pull to direct the force to the pelvic floor and this catheter was left in situ for 4 h. The VD+BAPN group was treated the same way plus i.p. injection of 150 mg/kg of BAPN every three days. Three weeks later, all animals underwent assessment of urinary function by conscious cystometry. All experimental protocols were approved by the Institutional Animal Care and Use Committee at University of California San Francisco.
Conscious cystometry
Under isoflurane anesthesia, a transvesical catheter was inserted 24 hr prior to urodynamic testing. The tip of a polyethylene-90 (PE-90) tubing (Clay-Adams, Parsippany, NJ) was heated to create a collar and the catheter tip was implanted at the bladder dome. A second probe was placed in the intraabdominal space to measure the abdominal component of the vesical pressure. A piece of PE-90 tubing was fitted with a small balloon and tied in place with a suture. The tubing was passed through the abdominal wall muscle and tunneled subcutaneously to emerge at the dorsum of the neck. For conscious cystometry, the animal was restrained in a tunnel attached to a metabolic cage grid (Braintree Scientific, Braintree, MA). The bladder was filled at a rate of 0.1 ml/min using an infusion pump (KD Scientific, Holliston, MA). Intravesical pressure changes were recorded by a computer with LabView 6.0 software (National Instruments, Austin, TX) at a rate of 10 samples/sec. The voided urine was recorded by an electronic scale connected to the LabView software. After stabilization of the micturition cycle for 10 min, the bladder was emptied by aspiration and micturition cycles were recorded for 40 min. Multiple cystometric variables were recorded by obtaining mean values of 4 voiding cycles. Baseline pressure (BP) was defined as the lowest bladder pressure between voids. Voiding threshold pressure was the pressure where urine was first detected at the urethral meatus. The voiding function of each rat was classified as “abnormal” if bladder filling was accompanied by frequent, low volume bladder contractions with urethral leakage. Residual urine was determined by withdrawing urine from the bladder at the termination of cystometry. Upon completion of cystometry the animals were euthanized and their urethras harvested for histology.
Urethral tissue preparation
The urethra was fixed in cold 2% formaldehyde and 0.002% saturated picric acid in 0.1 M phosphate buffer, pH 8.0, for 4 h followed by overnight immersion in buffer containing 30% sucrose. The specimens were then embedded in OCT Compound (Sakura Finetic USA, Torrance, CA) and stored at −70 °C until use. Fixed frozen tissue specimens were cut at 10 microns, mounted onto SuperFrost-Plus charged slides (Fisher Scientific, Pittsburgh, PA) and air dried for 5 min.
Phalloidin stain for muscle fibers
Tissue sections were incubated with Alexa-488-conjugated phalloidin (Invitrogen, Carlsbad, CA), which was diluted 1:100 in 1% bovine serum albumin, for 20 min at room temperature. After rinse with PBS, the tissues were stained with 4′,6-diamidino-2-phenylindole (DAPI, for nuclear staining, 1 μg/ml, Sigma-Aldrich, St. Louis, MO). The stained tissues were examined with Nikon Eclipse E600 fluorescence microscope.
Masson’s trichrome stain
Urethral tissue sections were prepared as above, immersed in warm (58°C) Bouin solution for 15 min, rinsed, stained with Weigert Hematoxylin for 10 min, and then rinsed until only nuclei remained stained. The sections were then stained with Biebrich Scarlet-Acid Fuchsin for 3 min, rinsed, and immersed in phosphomolybdic acid for 45 min. Next, the sections was stained with Aniline Blue for 3 min, rinsed in distilled water for 2 min, immersed in 1% acetic acid for 2 min, and rinsed in distilled water for 2 min twice. Finally, the sections were dehydrated through increasing concentrations of ethanol, left to air dry, and mounted. To prevent variations in staining, all samples were stained simultaneously using this procedure.
Image analysis and quantification
Five randomly selected fields per tissue section at 400× for elastic fibers or at 200× for muscle fibers and collagen were photographed and recorded using a Retiga Q Image digital still camera and ACT-1 software (Nikon Instruments Inc., Melville, NY). The images were then quantified for length of elastic and muscle fibers or for pixel number of collagen stain using Image-Pro Plus image software (Media Cybernetics, Silver Spring, MD).
Statistical analysis
Data were analyzed with Prism 4 (GraphPad Software, San Diego, CA). Analysis of variance (ANOVA) followed by paired T test was used to determine the difference between different treatment groups. Difference was considered significant when p<0.05. All data are shown as mean ± standard deviation (SD).
RESULTS
Effects of VD and BAPN on voiding patterns
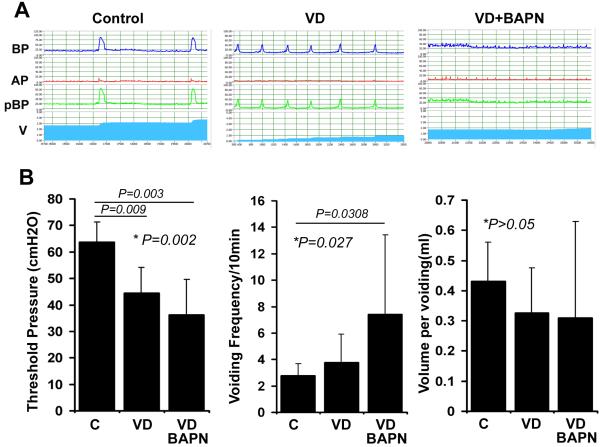
Conscious cystometry has defined abnormal voiding patterns as continuous or intermittent urine leakage at low bladder pressure during the filling phase 17. Based on this definition, cystometric analyses in the present study have identified 0/7, 3/7, and 5/7 of rats in the control, VD, and VD+BAPN groups, respectively, as having abnormal voiding patterns (Fig. 1). On average, rats in the VD group had significantly lower voiding threshold pressure than control rats, and rats in the VD+BAPN group had significantly lower voiding threshold pressure when compared to VD rats. Likewise, rats in the VD group had significantly higher voiding frequency than control rats, and rats in the VD+BAPN group had significantly higher voiding frequency when compared to VD rats. On the other hand, no significant difference in voiding volume was found between groups.
Figure 1.
Changes in urinary patterns. A. Representative cystometric charts of 10 min recording. BP: Bladder Pressure; AP: Abdominal Pressure; pBP: Bladder Pressure without abdominal pressure; V: Voiding; C: Control rats; VD: VD-treated rats; VD+BAPN: VD+BAPN-treated rats. B. Compilation of cystometric analysis results. Each bar represents the average of 7 rats.
Effects of VD and BAPN on urethral collagen
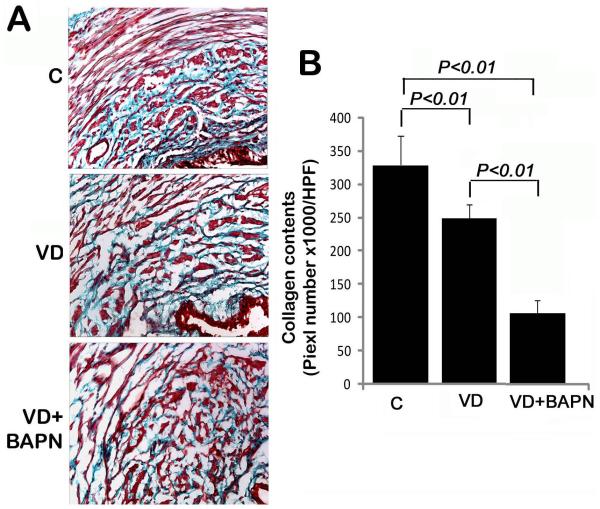
Collagen, the major extracellular component of female urethra 18, was mainly found in the inner circle (smooth muscle layer) of the urethra in normal rats (Fig. 2A). Its amount was significantly reduced in VD rats, and was further reduced in VD+BAPN rats (Fig. 2A&B).
Figure 2.
Changes in urethral collagen. A. Representative images of trichrome-stained urethral tissues of control (C), VD-treated (VD), and VD+BAPN-treated (VD+BAPN) rats. Urethral lumen is located at the lower right corner of each image. Original magnification is 200×. B. Compilation of quantification data. Each bar represents the average of 7 rats.
Effects of VD and BAPN on urethral elastic fibers
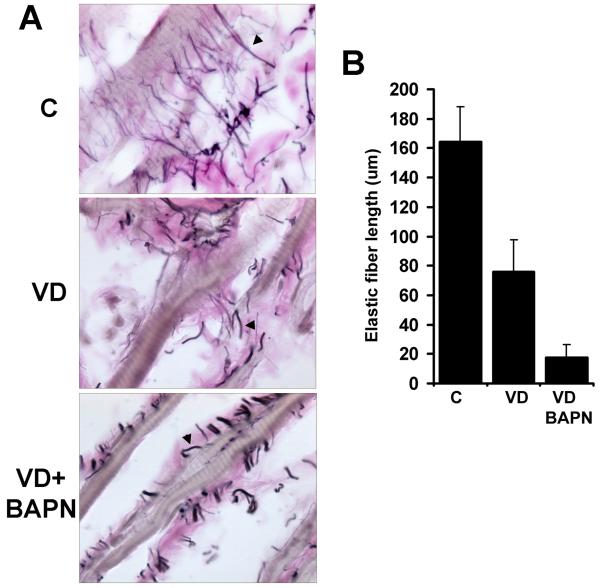
In the urethra of control rats, elastic fibers appeared to connect between striated muscle fibers (Fig. 3). In VD rats, the elastic fibers were shorter and not as well organized (Fig. 3). In VD+BAPN rats, the elastic fibers were fragmented and lost the inter-muscle connections (Fig. 3).
Figure 3.
Changes in urethral elastic fibers. A. Representative images of urethral tissues stained for elastic fibers (arrowhead) in the striated muscle layer of control (C), VD-treated (VD), and VD+BAPN-treated (VD+BAPN) rats. Original magnification is 1000x. B. Compilation of quantification data. Each bar represents the average of 7 rats.
Effects of VD and BAPN on urethral muscle
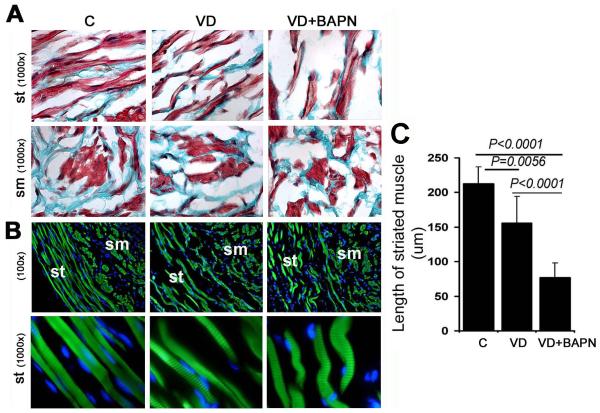
Muscular abnormalities were visible in trichrome-stained urethras of VD and VD+BAPN rats (Fig. 2A & 4A). Specifically, the striated muscle fibers were more widely spaced and shorter in VD and VD+BAPN rats than in control rats. Phalloidin stain, which specifically stains muscle fibers, further demonstrated these differences in muscle morphology between control and treated groups (Fig. 4B). In particular, the striated muscle fibers in the VD+BAPN group exhibited a “worm-like” shape (Fig. 4B). For quantitative analysis, the average lengths of the striated muscle fibers were compared between control and treated groups (Fig. 4C).
Figure 4.
Changes in urethral muscle. A. Representative images of trichrome-stained urethral tissues of control (C), VD-treated (VD), and VD+BAPN-treated (VD+BAPN) rats. sm: smooth muscle; st: striated muscle (both stained red). B. Representative images of phalloidin-stained urethral tissues. Both smooth (sm) and striated (st) muscles stained green while cell nuclei stained blue with DAPI. Note the striation in the striated muscle fibers in the 1000× magnification. C. Compilation of quantification data. Each bar represents the average of 7 rats.
DISCUSSION
Historically the hypermobility theory has dominated the field of research that aimed at identifying SUI’s causes. The acceptance of this theory and tissue availability issues may explain why most histological studies in SUI research have focused on periurethral rather than urethral tissues. However, the study by DeLancey et al 7, which concludes that changes in the urethra play the most significant role in SUI development, point to the need to refocus our attention on the urethra. For the undertaking of this challenge, urethral tissues from SUI animal models are practical alternatives to human tissues.
The rat’s urethra is anatomically similar to humans’ and therefore suitable for studying SUI-related changes 19. Since the majority of SUI patients are parous and menopausal, we established a rat SUI model by treating postpartum rats with VD and ovariectomy. While this rat model has been useful for assessing SUI-related functional and histological changes, interpretation of the experimental data has been complicated by the multifactorial nature of the intervention procedure. Thus, in the present study we focused on testing VD, which is singularly the most critical factor for inducing SUI symptoms 11, in combination with BAPN, which is well known for its effects on the extracellular matrix but has not been investigated in a urological setting. The results show that, at 3 weeks post-treatment, the majority of VD rats exhibited normal urinary patterns while the majority of VD+BAPN rats abnormal. This was paralleled by changes in the urethral collagen, elastic fiber, and striated muscle as VD alone had milder effects when compared to VD+BAPN.
By examining urethral needle biopsy specimens of continent and SUI patients under electron microscopy, FitzGerald et al 20 observed SUI-related alterations in collagen fibril morphologic characteristics. While it is not known whether urethral collagen is quantitatively affected in SUI patients, we have previously shown that urethral collagen content was decreased in rats with SUI symptoms 12, and others have reported decreased periurethral collagen content in SUI patients 21-23. In addition, increased collagenolysis in the vaginal mucosa of patients with prolapse disorder has also been observed 24. Thus, it appears that that a decrease in collagen content is associated with SUI-related urethral, periurethral, and vaginal abnormalities.
In the periurethral tissue of SUI women with a hypotonic urethra, irregular fragmented elastic fibers have been observed 25. In lysyl oxidase like-1 knockout (LOXL1-KO) mice, which are unable to assemble elastic fibers properly and exhibit SUI symptoms, a disorganized elastic fiber system in the urethra has also been demonstrated 26. In our SUI rat model we also found that the urethral elastic fibers were disorganized and fragmented 12,13. In the present study we found that urethral elastic fibers were slightly affected by VD but became fragmented in VD+BAPN rats. Thus, it appears that birth trauma, as represented by VD, disturb the elastic fiber system, which further becomes fragmented due to BAPN’s blockade of elastic fiber biosynthesis.
Magnetic resonance imaging has found that the urethral striated muscle layer was significantly thinner in women with SUI than in continent women 27. In the present study we found that the urethral striated muscle fibers became sparse and shorter in VD and VD+BAPN rats. In particular, those in the VD+BAPN group also exhibited a crooked “worm-like” shape suggestive of lacking architectural support. Since it has been shown that normal muscular architecture requires an intact elastic fiber system 28, it is possible that the fragmentation of elastic fibers and their loss of inter-muscle fiber connections in VD+BAPN rats are responsible for the abnormal striated muscle morphology.
Most SUI patients incurred parturition-induced urethral injuries years or decades earlier. However, current SUI animal models simulate the acute but not the clinically relevant chronic phase of disease progression. Specifically, we and others have observed that VD-induced incontinent rats spontaneously regained normal urinary function 11,29. Thus, in order to better simulate clinical SUI, it is necessary to inhibit this spontaneous recovery of urinary function; and, to this end, our present study points to the feasibility of adding BAPN to the SUI-induction regimen. By chronically suppressing collagen and elastic fiber biosynthesis, BAPN is expected to delay or prevent the spontaneous recovery of urinary function in VD-treated rats and thus make them more clinically relevant.
As mentioned in Introduction, BAPN has been used to create animal models of aortic aneurysm 16. In regard to treatments for aortic aneurysm, one of the most promising strategies is the administration of doxycycline (DOX), an inhibitor of matrix metalloproteinases 30. In our ongoing research, we are also testing the effects of DOX on SUI. Thus, while the present study reports the utilization of BAPN for the creation of a long-term SUI animal model, we have also obtained valuable data on the effects of DOX on SUI (manuscript in preparation). We believe that this combination of exploring BAPN as a negative effector and DOX as a positive effector of urinary continence is the best approach in the search of effective treatments for SUI.
CONCLUSIONS
Prolonged vaginal distension, which simulates prolonged labor, caused birth trauma-like urodynamic changes and histological abnormalities in the urethra including reduced collagen content, fragmented elastic fibers, as well as sparsely arranged and shortened striated muscle fibers. BAPN may interfere with the biosynthesis or restoration of the collagen and elastic fibers.
ACKNOWLEDGEMENTS
This work was supported by grants from the Rock Foundation, and the National Institutes of Health (DK64538 and DK069655).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Norton P, Brubaker L. Urinary incontinence in women. Lancet. 2006;367:57–67. doi: 10.1016/S0140-6736(06)67925-7. [DOI] [PubMed] [Google Scholar]
- 2.Hu TW, Wagner TH, Bentkover JD, et al. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63:461–465. doi: 10.1016/j.urology.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 3.Dooley Y, Kenton K, Cao G, et al. Urinary Incontinence Prevalence: Results From the National Health and Nutrition Examination Survey. J Urol. 2007 doi: 10.1016/j.juro.2007.09.081. [DOI] [PubMed] [Google Scholar]
- 4.Delancey JO. Why do women have stress urinary incontinence? Neurourol Urodyn. 2010;29(Suppl 1):S13–17. doi: 10.1002/nau.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang JM. Factors affecting urethrocystographic parameters in urinary continent women. J Clin Ultrasound. 1996;24:249–255. doi: 10.1002/(SICI)1097-0096(199606)24:5<249::AID-JCU4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Ward KL, Hilton P. A prospective multicenter randomized trial of tension-free vaginal tape and colposuspension for primary urodynamic stress incontinence: two-year follow-up. Am J Obstet Gynecol. 2004;190:324–331. doi: 10.1016/j.ajog.2003.07.029. [DOI] [PubMed] [Google Scholar]
- 7.DeLancey JO, Trowbridge ER, Miller JM, et al. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. 2008;179:2286–2290. doi: 10.1016/j.juro.2008.01.098. discussion 2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banie L, Lin G, Ning H, et al. Effects of estrogen, raloxifene and levormeloxifene on alpha1A-adrenergic receptor expression. J Urol. 2008;180:2241–2246. doi: 10.1016/j.juro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Lin G, Shindel AW, Banie L, et al. Molecular mechanisms related to parturition-induced stress urinary incontinence. Eur Urol. 2009;55:1213–1222. doi: 10.1016/j.eururo.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Resplande J, Gholami SS, Graziottin TM, et al. Long-term effect of ovariectomy and simulated birth trauma on the lower urinary tract of female rats. J Urol. 2002;168:323–330. [PubMed] [Google Scholar]
- 11.Sievert KD, Bakircioglu M Emre, Tsai T, et al. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change. J Urol. 2001;166:311–317. [PubMed] [Google Scholar]
- 12.Breyer BN, Wang G, Lin G, et al. The effect of long-term hormonal treatment on voiding patterns during filling cystometry and on urethral histology in a postpartum, ovariectomized female rat. BJU Int. 2010 doi: 10.1111/j.1464-410X.2010.09268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin G, Ning H, Wang G, et al. Effects of Birth Trauma and Estrogen on Urethral Elastic Fibers and Elastin Expression. Urology. 2010 doi: 10.1016/j.urology.2010.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page RC, Benditt EP. Molecular diseases of connective and vascular tissues. II. Amine oxidase inhibition by the lathyrogen, beta-aminopropionitrile. Biochemistry. 1967;6:1142–1148. doi: 10.1021/bi00856a025. [DOI] [PubMed] [Google Scholar]
- 15.Pasquali-Ronchetti I, Fornieri C, Castellani I, et al. Alterations of the connective tissue components induced by beta-aminopropionitrile. Exp Mol Pathol. 1981;35:42–56. doi: 10.1016/0014-4800(81)90006-x. [DOI] [PubMed] [Google Scholar]
- 16.Kanematsu Y, Kanematsu M, Kurihara C, et al. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension. 2010;55:1267–1274. doi: 10.1161/HYPERTENSIONAHA.109.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tantiwongse K, Fandel TM, Wang G, et al. The potential of hormones and selective oestrogen receptor modulators in preventing voiding dysfunction in rats. BJU Int. 2008;102:242–246. doi: 10.1111/j.1464-410X.2008.07582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickey DS, Phillips JI, Hukins DW. Arrangements of collagen fibrils and muscle fibres in the female urethra and their implications for the control of micturition. Br J Urol. 1982;54:556–561. doi: 10.1111/j.1464-410x.1982.tb13590.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim RJ, Kerns JM, Liu S, et al. Striated muscle and nerve fascicle distribution in the female rat urethral sphincter. Anat Rec (Hoboken) 2007;290:145–154. doi: 10.1002/ar.20420. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald MP, Mollenhauer J, Hale DS, et al. Urethral collagen morphologic characteristics among women with genuine stress incontinence. Am J Obstet Gynecol. 2000;182:1565–1574. doi: 10.1067/mob.2000.107327. [DOI] [PubMed] [Google Scholar]
- 21.Keane DP, Sims TJ, Abrams P, et al. Analysis of collagen status in premenopausal nulliparous women with genuine stress incontinence. Br J Obstet Gynaecol. 1997;104:994–998. doi: 10.1111/j.1471-0528.1997.tb12055.x. [DOI] [PubMed] [Google Scholar]
- 22.Goepel C, Hefler L, Methfessel HD, et al. Periurethral connective tissue status of postmenopausal women with genital prolapse with and without stress incontinence. Acta Obstet Gynecol Scand. 2003;82:659–664. doi: 10.1034/j.1600-0412.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 23.Moalli PA, Shand SH, Zyczynski HM, et al. Remodeling of vaginal connective tissue in patients with prolapse. Obstet Gynecol. 2005;106:953–963. doi: 10.1097/01.AOG.0000182584.15087.dd. [DOI] [PubMed] [Google Scholar]
- 24.Borges LF, Gutierrez PS, Marana HR, et al. Picrosirius-polarization staining method as an efficient histopathological tool for collagenolysis detection in vesical prolapse lesions. Micron. 2007;38:580–583. doi: 10.1016/j.micron.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Goepel C, Thomssen C. Changes in the extracellular matrix in periurethral tissue of women with stress urinary incontinence. Acta Histochem. 2006;108:441–445. doi: 10.1016/j.acthis.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Lee UJ, Gustilo-Ashby AM, Daneshgari F, et al. Lower urogenital tract anatomical and functional phenotype in lysyl oxidase like-1 knockout mice resembles female pelvic floor dysfunction in humans. Am J Physiol Renal Physiol. 2008;295:F545–555. doi: 10.1152/ajprenal.00063.2008. [DOI] [PubMed] [Google Scholar]
- 27.Kim JK, Kim YJ, Choo MS, et al. The urethra and its supporting structures in women with stress urinary incontinence: MR imaging using an endovaginal coil. AJR Am J Roentgenol. 2003;180:1037–1044. doi: 10.2214/ajr.180.4.1801037. [DOI] [PubMed] [Google Scholar]
- 28.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 29.Pan HQ, Kerns JM, Lin DL, et al. Increased duration of simulated childbirth injuries results in increased time to recovery. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1738–1744. doi: 10.1152/ajpregu.00784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindeman JH, Abdul-Hussien H, van Bockel JH, et al. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation. 2009;119:2209–2216. doi: 10.1161/CIRCULATIONAHA.108.806505. [DOI] [PubMed] [Google Scholar]