Abstract
Background
Completion axillary lymph node dissection (CALND) is recommended in the setting of positive sentinel lymph node biopsy (SLNB) but is associated with a higher rate of postoperative complications. In this study, the characteristics and outcomes of patients who did and did not have CALND are compared.
Methods
We identified all patients with breast cancer with positive sentinel lymph nodes (SLNs) who did not have concurrent CALND from 2003 to 2006 using a prospectively collected database (British Columbia Cancer Breast Outcomes database) and retrospective chart review. Patient and tumour characteristics were compared between those who received CALND and those who did not.
Results
Among 185 patients with positive SLNs identified by SLNB, 90 had a CALND and 95 had no further surgical therapy. Patients who did not receive CALND had more sentinel nodes removed (p < 0.001), a lower percentage of positive SLNs (p < 0.001) and lower pathologic N stage (p = 0.044) than those who did receive CALND. The size of the breast lesion, size of the largest SLN deposit, estrogen receptor status, grade, lymphovascular invasion, histology and multifocality were not significantly different between groups. Sixty-two percent of women who did not have CALND received radiation to the axilla. Postoperative complication rates (including lymphedema) were higher in the CALND group (21%) compared with the SLNB group (7%). The rates of locoregional recurrence (1% in both groups) and systemic metastases (6% in the CALND group v. 8% in the SLNB group) were similar at 36 months’ follow-up.
Conclusion
Compared with women who had CALND, women who did not receive CALND had on average a lower N stage with 3 or more SLNs removed and less than 50% node positivity. Most of these women received radiation therapy to the axilla and had comparable recurrence rates to those who had CALND.
Abstract
Contexte
L’ablation des ganglions lymphatiques axillaires (AGLA) est recommandée lorsque la biopsie des ganglions lymphatiques sentinelles donne des résultats positifs, mais cette intervention est associée à un taux plus élevé de complications postopératoires que la seule biopsie. Cette étude a comparé les caractéristiques et les résultats chez les patientes qui ont subi une AGLA et chez celles qui n’ont pas subi cette intervention.
Méthodes
Nous avons repéré toutes les patientes atteintes de cancer du sein chez lesquelles la biopsie des ganglions lymphatiques sentinelles a donné un résultat positif et qui n’avaient pas subi une AGLA simultanée, de 2003 à 2006, à partir d’une base de données recueillies de façon prospective (base de données sur l’issue du cancer du sein en Colombie-Britannique) et nous avons effectué une étude rétrospective des dossiers. Nous avons comparé les caractéristiques des patientes et celles de la tumeur chez les patientes qui ont subi une AGLA et chez celles qui n’en ont pas subi.
Résultats
Sur les 185 patientes dont les ganglions lymphatiques sentinelles avaient été jugé positif à la suite d’une biopsie, 90 avaient subi une AGLA et 95 n’avaient pas subi d’autre traitement chirurgical. Les patientes qui n’ont pas subi une AGLA avaient subi l’ablation d’un plus grand nombre de ganglions sentinelles (p < 0,001), présentaient un pourcentage moins élevé de ganglions sentinelles positifs (p < 0,001) et se trouvaient à un stade pathologique N moins élevé (p = 0,044) que celles qui ont subi une AGLA. Il n’y avait pas de différences significatives entre les groupes quand à la grosseur de la lésion du sein, à la taille de la concentration la plus importante dans les ganglions lymphatiques sentinelles, à l’état des récepteurs de l’œstrogène, au grade, à l’envahissement lymphovasculaire, à l’histologie et à la multifocalité. Soixante-deux pour cent des femmes qui n’ont pas subi d’AGLA ont reçu une irradiation de l’aisselle. Les taux de complications postopératoires (y compris de lymphœdème) ont été plus élevés chez les patientes qui ont subi une AGLA (21 %) que chez celles qui ont subi une biopsie des ganglions lymphatiques sentinelles (7 %). Les taux de récidive locorégionale (1 % dans les 2 groupes) et de métastases généralisées (6 % chez les patientes qui ont subi une AGLA c. 8 % chez celles qui ont eu une biopsie des ganglions lymphatiques sentinelles) étaient semblables au suivi à 36 mois.
Conclusion
Chez les femmes qui n’ont pas subi d’AGLA, par rapport aux femmes qui ont subi cette intervention, le stade N était en moyenne moins élevé, elles ont subi l’ablation de 3 ganglions lymphatiques sentinelles ou plus et moins de 50 % des ganglions étaient positifs. La plupart de ces femmes ont reçu une radiothérapie à l’aisselle et leurs taux de récidive étaient comparables à ceux que l’on a constatés chez celles qui avaient subi une AGLA.
Sentinel lymph node biopsy (SLNB) is the preferred method of staging the axilla in patients with early-stage breast cancer. The American Society of Clinical Oncology (ASCO) guidelines recognize SLNB as an appropriate alternative to axillary lymph node dissection (ALND) in patients with T1- or T2-stage breast cancers that are clinically node negative.1 For the 25% of patients who will have a positive sentinel lymph node (SLN) identified by SLNB,2 ASCO recommends a completion axillary lymph node dissection (CALND).1 However, less than half (48%) of these patients will actually have additional nodal disease identified on CALND.3 There has been much debate recently about whether a CALND is necessary for all patients with positive SLNs.4 The American College of Surgeons Oncology Group Z0011 trial, which compared outcomes for patients with positive SLNs with and without ALND, has not shown any difference in recurrence or survival between the 2 groups.5 Although this trial closed prematurely owing to low accrual, it is the largest trial of its kind and is unlikely to be duplicated. Retrospective outcomes research can provide additional information on current practices in treating women with positive SLNs and thus help guide clinical decisions.
Compared with ALND, SLNB is associated with less morbidity, including better arm mobility, less pain, decreased lymphedema and seroma formation.6,7 The rate of overall complications associated with CALND has been reported to be as high as 70% compared with 25% for SLNB.8 Low locoregional recurrence rates have been reported in selected patients with positive SLNs who do not undergo CALND.9 In particular, in the setting of microscopic SLN metastases, there appears to be no advantage to performing CALND.2,10 Given the advantages for SLNB, Van Zee and colleagues11 developed a nomogram to predict the likelihood of non-SLN metastases in patients with positive SLNs, which has been validated.12 Although this nomogram will produce a percent likelihood for having additional nodal metastases, it does not provide any instruction on when this risk is low enough to safely avoid CALND. The role of radiation to the axilla as a potential alternative to CALND is currently being tested in the EORTC trial13 and has been previously postulated to result in similar long-term survival for early breast cancer.14
In British Columbia, as in other jurisdictions, CALND is recommended after positive SLNs are identified by SLNB. However, there are a considerable number of patients who have positive SLNs but do not undergo CALND. The goal of the current study was to look at patient-, tumour- and lymph node–related factors associated with CALND after a positive SLN is identified by SLNB. Relapse data are also reported.
Methods
We used prospectively collected data from the British Columbia Breast Cancer Outcomes Unit database to obtain details on patients with breast cancer with positive SLNs who did or did not receive CALND from January 2003 to August 2006. The database collects data from all referred patients and represents 85% of all incident breast cancer diagnosis in the province. We excluded patients from the study if they died within 6 months of diagnosis, had preoperative systemic chemotherapy or hormone treatment, did not have any adjuvant systemic chemotherapy or hormone therapy, or had CALND on the same day as SLNB.
Staging classifications were based on the American Joint Committee on Cancer Manual, 6th edition.15 We evaluated the timing of node dissections, radiation therapy and chemotherapy. The total number of biopsied nodes, the number of metastatic deposits and the size of positive nodes were reviewed for both groups. Lymph nodes were designated as having microscopic metastases if the size of the largest deposit was greater than 0.2 mm but less than or equal to 2.0 mm.15 Patients with isolated tumour cells (< 0.2 mm) in SLNs were considered to be node-negative and thus we did not include them in this study. We performed a retrospective chart review to determine the rate of complications, follow-up and rate of recurrence. The nomogram scores predicting likelihood of non-SLN involvement were calculated using the Memorial Sloan-Kettering Cancer Center nomogram (www.mskcc.org/nomograms).10 The BC Cancer Agency Research Ethics Board approved our study protocol.
Statistical analysis
We compared patient and tumour characteristics for known values between patients receiving CALND and those receiving SLNBs only. We tested continuous variables with the Wilcoxon rank-sum test and categorical variables with the χ2 test. For the TNM staging, the N stage was tested using the Fisher exact test. We compared the rate of CALND for each group with risk scores above and below 10% using the χ2 test. Crude complication and recurrence rates were calculated by dividing the number of complications or recurrences in each group by the number of patients in each group. We compared complication rates and total recurrence rates using a χ2 test, and local recurrence was compared using the Fisher exact test. All analyses were univariate.
Results
Among a total of 185 eligible patients, we identified 95 who had SLNBs only and 90 who had a subsequent CALND. Tumour characteristics were similar between the 2 groups (Table 1). Patients were more likely to have CALND if they had fewer SLNs removed during their first operation (3 in SLNB v. 2 in CALND groups, p < 0.001), if a higher percentage of these SLNs were positive (38% in the SLNB v. 63% in the CALND groups, p < 0.001) and if more than 3 nodes were positive (3% in the SLNB v. 11% in the CALND groups, p = 0.044). The median age in the SLNB group was slightly less than that in the CALND group, but this difference was not statistically significant (56 v. 59, p = 0.06).
Table 1.
Tumour characteristics of patients with breast cancer who had sentinel lymph node biopsy alone (SLNB) or SLNB followed by completion axillary lymph node dissection (CALND)
| Characteristic | Group; no (%)* | p value | |
|---|---|---|---|
| SLNB, n = 95 | CALND, n = 90 | ||
| Median age at diagnosis | 59 (47–73) | 56 (48–64) | 0.06 |
| Tumour characteristics | |||
| Tumour size, median (range) mm | 19 (13–25) | 18 (12–25) | 0.80 |
| ER status† | |||
| Positive | 91 (95.8) | 81 (90.0) | 0.19 |
| Negative | 4 (4.2) | 8 (8.9) | |
| Lymphovascular invasion† | |||
| Positive | 33 (34.7) | 22 (24.4) | 0.14 |
| Negative | 61 (64.2) | 66 (73.3) | |
| Histology | |||
| Ductal | 83 (87.4) | 83 (92.2) | 0.28 |
| Lobular | 12 (12.6) | 7 (7.8) | |
| Grade† | |||
| Grade 1 | 24 (25.3) | 25 (27.8) | 0.72 |
| Grade 2 | 46 (48.4) | 45 (50.0) | |
| Grade 3 | 25 (26.3) | 19 (21.1) | |
| Multifocal | |||
| Yes | 9 (9.5) | 10 (11.1) | 0.71 |
| No | 86 (90.5) | 80 (88.9) | |
| T Stage | |||
| T1 | 52 (54.7) | 48 (53.3) | 0.85 |
| T2 | 39 (41.1) | 38 (42.2) | |
| T3 | 3 (3.2) | 3 (3.3) | |
| T4 | 1 (1.1) | 1 (1.1) | |
ER = estrogen receptor.
Unless otherwise indicated.
Data not available for all patients.
Since Her2 testing was not routinely performed before 2004, there were incomplete data available to analyze this variable. There were more total nodes removed in the CALND group than the SLNB group (11 v. 3), and 48% percent of women in the SLNB group had only microscopic deposits in the lymph nodes compared with 34% in the CALND group (p = 0.05; Table 2).
Table 2.
Characteristics of nodes in the sentinel lymph node biopsy and completion axillary lymph node dissection groups
| Characteristic | SLNB, n = 95 | CALND, n = 90 | p value |
|---|---|---|---|
| Nodes removed, total no. (range) | 3 (2–5) | 11 (8–14) | NA |
| SLNs, total no. (range) | 3] (2–5) | 2 (2–4) | < 0.001 |
| Positive SLN, no. (range) | 1 (1–2) | 1 (1–2) | 0.13 |
| Percent positive SLN, % (range) | 37.5 (25–50) | 63.4 (40–100) | < 0.001 |
| Largest sentinel node deposit, mean (range) mm | 2.5 (1–6) | 3 (1.5–5) | 0.72 |
| Microscopic SLN deposits, no. (%) | 46 (48.4) | 31 (34.4) | 0.05 |
| N Stage, no. (%) | |||
| N1 | 92/95 (96.8) | 80/90 (88.9) | 0.044 |
| N2 | 3/95 (3.2) | 10/90 (11.1) | |
CALND = completion axillary lymph node dissection; NA = not applicable; SLN = sentinel lymph nodes; SLNB = sentinel lymph node biopsy.
Although a comparable percentage of women received radiation to the axilla in the CALND (62%) and SLNB (54%) groups, a higher percentage in the CALND group had radiation to the breast and chest wall alone compared with the SLNB group (30% v. 18%; Table 3). Similar percentages of women received adjuvant hormonal therapy in the CALND (88%) and SLNB (88%) groups (p = 0.72), but significantly more patients in the CALND group had systemic chemotherapy compared with the SLNB group (76% v. 46%, p < 0.001; Table 3). When a CALND was performed, 29% of women had additional positive non-SLNs.
Table 3.
Rates of adjuvant treatment in the sentinel lymph node biopsy and completion axillary lymph node dissection groups
| Adjuvant treatment | Group; no. (%) | p value | |
|---|---|---|---|
| SLNB, n = 95 | CALND, n = 90 | ||
| Radiation | 0.15 | ||
| None | 19 (20.0) | 14 (15.6) | |
| Breast/chest wall | 17 (17.9) | 27 (30.0) | |
| Breast/chest wall and axilla | 59 (62.1) | 49 (54.4) | |
| Systemic chemotherapy | 44 (46.3) | 68 (75.6) | < 0.001 |
| Intervening chemotherapy between SLNB and CALND | 9 (10) | ||
| Hormonal therapy | 85 (89.5) | 79 (87.8) | 0.72 |
CALND = axillary lymph node dissection; NA = not applicable; SLNB = sentinel lymph node biopsy.
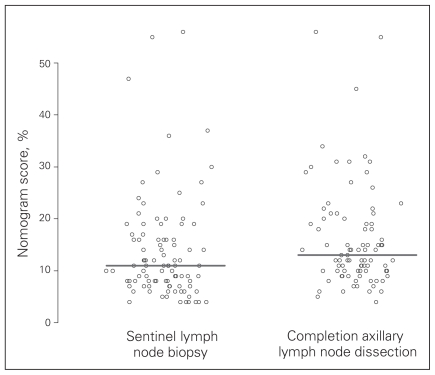
Nomogram scores were assigned to all patients based on criteria specified in the Memorial Sloan-Kettering Cancer Center nomogram. Lower scores predict a lower risk of further positive nodes on CALND, and there was a tendency for lower scores among the SLNB compared with the CALND group (Fig. 1). Those who had a less than 10% chance of having additional non-SLN involvement based on their nomogram scores were statistically more likely to have SLNBs only compared to those with a greater than 10% chance (32% v. 55%, p = 0.004; Table 4). There was a higher percentage of patients on the lower end of the nomogram score spectrum; there were no patients with nomogram risk scores higher than 56%, and most patients were concentrated in the 5%–25% range.
Fig. 1.
Memorial Sloan-Kettering Cancer Center nomogram score distribution for patients in the sentinel lymph node biopsy versus the completion axillary lymph node dissection groups. The line represents the median score for each group.
Table 4.
Patients with Memorial Sloan-Kettering Cancer Center nomogram scores below and above 10% and the corresponding percentage of patients who underwent completion axillary lymph node dissection
| Nomogram score | No. patients | No. (%) patients who underwent CALND | p value |
|---|---|---|---|
| < 10% | 64 | 19 (32.2) | 0.004 |
| ≥ 10% | 130 | 67 (54.9) |
CALND = completion axillary lymph node dissection.
The median follow-up for both groups was 1.9 years. On chart review, postoperative complications were more common in the CALND group than the SLNB group (21% v. 7%, p = 0.003), including a higher rate of lymphedema (3% v. 8%, p = 0.12; Table 5). There was a wider range of complications related directly to the axillary portion of the operation in the CALND group than the SLNB group (Table 6). Within the limited follow-up period of this study, recurrence rates, including rates of axillary recurrence, were similar between the 2 groups (Table 5).
Table 5.
Comparison of complication and recurrence rates for sentinel lymph node biopsy and completion axillary lymph node dissection groups
| Event | Group; no. (%) | p value | |
|---|---|---|---|
| SLNB, n = 107 | CALND, n = 91 | ||
| Complications | |||
| All | 7 (7.4) | 19 (21.1) | 0.007 |
| Lymphedema | 3 (3.2) | 7 (7.8) | 0.16 |
| Other complications | 5 (5.3) | 17 (18.9) | 0.004 |
| Recurrences | |||
| All (metastatic, axillary, local) | 7 (7.4) | 8 (8.9) | 0.70 |
| Axillary | 1 (1.1) | 1 (1.1) | 1.00 |
CALND = completion axillary lymph node dissection; SLNB = sentinel lymph node biopsy.
Table 6.
Number of patients with complications other than lymphedema in the sentinel lymph node biopsy and completion axillary lymph node dissection groups
| Complication | SLNB | CALND |
|---|---|---|
| Delayed wound healing | 0 | 2 |
| Arm tenderness | 0 | 1 |
| Wound infection | 0 | 1 |
| Reduced shoulder range of motion | 0 | 5 |
| Carpal tunnel syndrome | 0 | 1 |
| Pain | 1 | 3 |
| Tethering or stiffness in the axilla | 1 | 3 |
| Numbness | 0 | 1 |
| Radiation pneumonitis | 1 | 0 |
CALND = completion axillary lymph node dissection; SLNB = sentinel lymph node biopsy.
Discussion
In this population-based study, a high proportion of patients with positive SLNs identified by SLNB did not proceed to CALND. A higher sentinel node count, lower percent nodal positivity and lower Memorial Sloan-Kettering Cancer Center nomogram score were factors associated with patients not receiving CALND.
There was a tendency to avoid CALND when many nodes were harvested during the initial SLNB, likely relating to the uncertainty regarding further yield on the number of nodes during a subsequent CALND. Since the total number of nodes removed on CALND was expected to be higher than that in SLNB, we did not perform a statistical comparison. However, a higher percentage of positive SLNs was strongly predictive of further CALND, a course of action supported by the literature, which shows a higher percentage of positive SLNs to be predictive of higher like-lihood of axillary lymph node (ALN) involvement16 as well as decreased survival.17 Interestingly, the size of metastasis in the largest node was not a predictor of further CALND, but having microscopic disease made it less likely for a patient to undergo further CALND. This is consistent with other studies that have found the presence of micrometastases to be the only important size factor in predicting further ALN involvement.9
The median age of the CALND group was higher than that in the SLNB group, but this did not reach statistical significance. Although age alone is not a deterrent from performing further surgery, CALND has been shown to have little effect on survival in women older than 60 years.18 Older age can also be a surrogate for other comorbidities, which may discourage patients and surgeons from proceeding to CALND. However, with only a 3-year age difference between the 2 groups in this study, comorbidity may not be the major driving factor in avoiding CALND.
Tumour characteristics did not appear to influence the decision to perform CALND. Breast lesions exceeding 2 cm in size have been reported to be predictors of a high number of lymph node metastases.16 In this study, however, the size of breast lesions was similar between the 2 groups at a median of less than 2 cm. Since more than 50% of tumours were T1 stage, the size of the tumour did not appear to influence the type of surgery. Lymphovascular invasion, estrogen receptor status, grade, histology and multifocality, all of which are factors deemed to be important in determining further ALN involvement,11 were not significantly associated with a patient receiving CALND. Whether or not having a Her2-negative cancer would change this pattern could not be answered by this study. The lack of difference in characteristics between groups is likely owing to the surgical decision being based mainly on the status of the axilla itself, as indicated by the SLN status, since CALND is thought to be most beneficial in controlling locoregional recurrence in the axilla.14
In our study, the decision not to perform CALND was generally a combination of physician advice and patient preference. Physician advice usually involves an estimate of the likelihood of having additional positive nodes should a CALND be performed. At our centre, the Van Zee nomogram often has a role in this discussion. It is not surprising that patients who are quoted a less than 10% risk of further ALN involvement would choose not to proceed with CALND given its associated morbidity. The 10% risk level used in our calculation is based on previous findings of it being a meaningful cut-off.19 The trend toward lower nomogram scores is likely the explanation for the difference in the rates of systemic therapy between the 2 groups, since patients at higher risk of recurrence are more likely to have systemic chemotherapy. The nomogram score distribution of patients in this study suggests an overall lower risk population compared with other studies.12
Complications evaluated in this study are based on chart review. The 20% complication rate is much lower than the 70% rate reported in other studies.8 Owing to the retrospective nature of the study, not all complications could be captured. However, clinically relevant complications that cause restrictions in daily life, such as severe lymphedema, would be more likely to be noted in follow-up encounters and, as such, detected in our review.
Radiation has been proposed as an alternative to CALND14 in select patients, and this is currently the subject of the EORTC trial.13 In our study, an equal percentage of patients in both groups received radiation to the axilla, indicating that radiation was not routinely used as a substitute for CALND.
The follow-up period in this study was somewhat limited, ending at just under 2 years, which is insufficient time to definitively determine whether there were differences in recurrence between the 2 groups. Previous research at our institution has shown that for breast cancer most loco-regional recurrence will occur within 2 years of treatment.20 Therefore, further follow-up is unlikely to reveal significant differences in recurrence rates.
Conclusion
Positive lymph nodes were identified during CALND for less than one-third of patients. If one could identify this high-risk group after SLNB, the other two-thirds of patients could avoid undergoing a second operation. Our results are in keeping with those recently reported in the Z0011 trial,5 which did not demonstrate an advantage for ALND in patients with early breast cancer who had 1–2 positive SLNs. In our study, patients who do not receive CALND have 3 or more nodes removed on SLNB, a lower pathologic N stage and less than 50% node positivity. Just over half of these women received radiation to the axilla, with similar rates of recurrence. The higher complication rate associated with CALND, in addition to prediction models like the Van Zee nomogram, may explain why many women with positive SLNs identified by SLNB do not have CALND despite this being the current standard of care.
Footnotes
Presented at the Canadian Surgery Forum, Sept. 10–13, 2009, in Victoria, BC
Competing interests: None declared.
Contributors: Drs. Davis, Kennecke and Swanson designed the study. Drs. Aslani and Kennecke acquired the data, which Drs. Kennecke and Mr. Woods analyzed. Drs. Aslani, Davis and Kennecke wrote the article, which Drs. Davis, Kennecke and Swanson and Mr. Woods reviewed. All authors approved the article’s publication.
References
- 1.Lyman GH, Giuliano AE, Somerfield MR, et al. American society of clinical oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946–53. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 3.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a meta-analysis. Cancer. 2006;106:4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 4.Katz A, Niemierko A, Gage I, et al. Can axillary dissection be avoided in patients with sentinel lymph node metastasis? J Surg Oncol. 2006;93:550–8. doi: 10.1002/jso.20514. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano AE, McCall LM, Beitsch PD, et al. ACOSOG Z0011: a randomized trial of axillary node dissection in women with clinical T1-2 N0 M0 breast cancer who have a positive sentinel node [presentation]. J Clin Oncol; ASCO Annual Meeting 2010; 2010. 2010. p. CRA506. [Google Scholar]
- 6.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–53. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard DK, Donohue JH, Reynolds C, et al. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg. 2003;138:482–7. doi: 10.1001/archsurg.138.5.482. [DOI] [PubMed] [Google Scholar]
- 8.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–63. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 9.Liang WC, Sickle-Santanello BJ, Nims TA. Is a completion axillary dissection indicated for micrometastases in the sentinel lymph node? Am J Surg. 2001;182:365–8. doi: 10.1016/s0002-9610(01)00738-3. [DOI] [PubMed] [Google Scholar]
- 10.Hwang RF, Gonzalez-Angulo AM, Yi M, et al. Low locoregional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer. 2007;110:723–30. doi: 10.1002/cncr.22847. [DOI] [PubMed] [Google Scholar]
- 11.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–51. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Park J, Fey JV, Naik AM, et al. A declining rate of completion axillary dissection in sentinel lymph node-positive breast cancer patients is associated with the use of a multivariate nomogram. Ann Surg. 2007;245:462–8. doi: 10.1097/01.sla.0000250439.86020.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meijnen P, Rutgers EJT, van de Velde CJH, et al. EORTC 10981-22023 trial. AMAROS: After mapping of the axilla: Radiotherapy or surgery? Trial update. Eur J Cancer. 2004;2(Suppl 3):A-79. [Google Scholar]
- 14.Louis-Sylvestre C, Clough K, Asselain B, et al. Axillary treatment in conservative management of operable breast cancer: Dissection or radiotherapy? Results of a randomized study with 15 years of follow-up. J Clin Oncol. 2004;22:97–101. doi: 10.1200/JCO.2004.12.108. [DOI] [PubMed] [Google Scholar]
- 15.Greene FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual. 6th ed. Chicago (IL): Springer; 2002. [Google Scholar]
- 16.Rivers AK, Griffith KA, Hunt KK, et al. Clinicopathologic features associated with having four or more metastatic axillary nodes in breast cancer patients with a positive sentinel lymph node. Ann Surg Oncol. 2006;13:36–44. doi: 10.1245/ASO.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 17.Lale Atahan I, Yildiz F, Ozyigit G, et al. Percent positive axillary lymph node metastasis predicts survival in patients with non-metastatic breast cancer. Acta Oncol. 2008;47:232–8. doi: 10.1080/02841860701678761. [DOI] [PubMed] [Google Scholar]
- 18.International Breast Cancer Study. Rudenstam CM, Zahrieh D, et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group Trial 10–93. J Clin Oncol. 2006;24:337–44. doi: 10.1200/JCO.2005.01.5784. [DOI] [PubMed] [Google Scholar]
- 19.Poirier E, Sideris L, Dube P, et al. Analysis of clinical applicability of the breast cancer nomogram for positive sentinel lymph node: the Canadian experience. Ann Surg Oncol. 2008;15:2562–7. doi: 10.1245/s10434-008-0033-9. [DOI] [PubMed] [Google Scholar]
- 20.Konkin DE, Tyldesley S, Kennecke H, et al. Management and outcomes of isolated axillary node recurrence in breast cancer. Arch Surg. 2006;141:867–72. doi: 10.1001/archsurg.141.9.867. [DOI] [PubMed] [Google Scholar]