Abstract
Background
Deep wound infection after spinal surgery is a severe complication that often requires prolonged medical and surgical management. It can compromise the outcome of the deformity correction, especially in patients requiring surgical intervention with subsequent removal of implants. Ascertaining the incidence and risk factors leading to infection may help to prevent this problem.
Methods
We reviewed the hospital charts of all patients who underwent spinal deformity correction from 1996 to 2005.
Results
In all, 227 patients were identified (139 idiopathic, 57 neuromuscular, 8 syndromic, 6 congenital, 17 other); 191 patients were treated with posterior instrumentation and fusion, 11 with anterior-only procedures and 24 with combined anterior and posterior procedures. Final follow-up ranged from 1 to 9.5 years. Infection developed in 14 patients. The overall incidence of infection was 6.2%. Drainage and back pain were the most common presenting symptoms. The incidence of infection was higher among patients with nonidiopathic diagnoses (risk ratio [RR] 8.65, p < 0.001). Use of allograft bone was associated with a higher rate of infection (RR 9.66, p < 0.001) even when stratified by diagnosis (nonidiopathic diagnoses, RR 7.6, p = 0.012). Higher volume of instrumentation was also a risk factor for infection (p = 0.022). Coagulase-negative Staphyloccocus was the most commonly identified organism, followed by Propionibacterium acnes and Pseudomonas.
Conclusion
Development of infection following scoliosis surgery was found to be associated with several risk factors, including a nonidiopathic diagnosis, the use of allograft and a higher volume of instrumentation. Preventative measures addressing these factors may decrease the rate of infection.
Abstract
Contexte
L’infection profonde de la plaie après une intervention chirurgicale à la colonne est une complication grave qui nécessite souvent un traitement médical et chirurgical prolongé. L’infection peut compromettre le résultat de la correction d’une malformation, surtout chez les patients qui doivent subir une autre intervention chirurgicale pour retirer des implants. La détermination de l’incidence et des facteurs de risque d’infection pourrait aider à éviter le problème.
Méthodes
Nous avons examiné les dossiers d’hôpital de tous les patients qui ont subi, de 1996 à 2005, une intervention chirurgicale à la colonne visant à corriger une malformation.
Résultats
Au total, nous avons trouvé 227 patients (problème idiopathique dans 139 cas, neuromusculaire dans 57, syndromique dans 8, congénital dans 6, et autre dans 17 cas); 191 patients ont été traités au moyen d’une exploration instrumentale et d’une fusion postérieure, 11 ont subi des interventions antérieures seulement et 24, des interventions antérieures et postérieures combinées. Le suivi final a varié de 1 à 9,5 ans. Quatorze patients ont eu une infection. L’incidence globale de l’infection s’est établie à 6,2 %. L’écoulement et les douleurs dorsales ont constitué les symptômes les plus fréquents. L’incidence de l’infection a été plus élevée chez les patients qui avaient reçu un diagnostic de problème non idiopathique (risque relatif [RR] de 8,65, p < 0,001). On a établi un lien entre une allogreffe osseuse et un taux d’infection plus élevé (RR de 9,66, p < 0,001), même après stratification selon le diagnostic (diagnostic non idiopathique, RR de 7,6, p = 0,012). Le nombre plus élevé d’instruments a aussi constitué un facteur de risque d’infection (p = 0,022). Le micro-organisme identifié le plus souvent a été le Staphylocoque négatif (quant à la coagulase), suivi de Propionibacterium acnes et de Pseudomonas.
Conclusion
On a établi un lien entre l’apparition d’une infection à la suite d’une intervention chirurgicale visant à corriger une scoliose et plusieurs facteurs de risque, y compris un diagnostic non idiopathique, l’usage d’une allogreffe et le nombre des instruments. Des mesures de prévention pour corriger ces facteurs pourraient réduire le taux d’infection.
Deep wound infection after surgery for scoliosis correction is a severe complication that requires prolonged medical and surgical management.1 It is defined as a surgical site infection in which there is a direct communication between the associated infected material and the spinal instrumentation and bone graft/fusion mass.1–4 Infection can occur early or late, with signs of late infection appearing at an average of 3.1 (range 1.2–8.5) years postoperatively.5,6
The incidence of postoperative wound infection in patients with idiopathic scoliosis ranges from 1% to 5%, with the reported incidence being highly variable.1,5,7–9 By contrast, in patients with neuromuscular causes of scoliosis the rate is much higher, ranging from 4%–14% in some studies.1,4,10–13 Impaired immune status, poor personal hygiene and soiling of the wound have been suggested to contribute to the higher incidence of infection in patients with neuromuscular disorders.4 Other suggested risk factors include a higher degree of cognitive impairment,1 malnourishment,1,14 prolonged duration of surgery, large volumes of instrumentation and the presence of another remote infectious lesion.15 The use of allograft has also been suggested to increase the risk of infection, but this conclusion remains controversial.4,16,17
The diagnosis of infection is difficult to establish but is suggested by pain,18 the presence of a sinus tract, persistent or late postoperative wound drainage, erythema, wound dehiscence, positive culture from a wound aspirate, instrumentation failure/pseudoarthrosis and pathologic confirmation of the presence of inflammatory cells and/or the offending organism(s).1,3,5,6,19 Staphylococcus aureus1,12,15 and S. epidermidis2,5,20 have been reported to be the most common causative organisms. A high erythrocyte sedimentation rate (ESR) has also been reported to be associated with late infection.20
There is a substantial patient cost associated with the development of deep wound infection following scoliosis surgery. It can compromise the outcome of the deformity correction; especially in patients who require subsequent removal of the implants for effective eradication of the infectious agents.1 Other associated complications, including the development of sepsis, vertebral osteomyelitis, neurologic compromise and clinically important soft tissue defects, are additional sources of patient morbidity that in some cases may be life-threatening.1 As such, the identification of risk factors associated with the development of deep wound infection is paramount to develop measures to help prevent this devastating complication. To this end, the aim of our study was 2-fold: first, we sought to determine the incidence of deep wound infection following pediatric scoliosis surgery, and second, we sought to identify significant risk factors predisposing these patients to infection.
Methods
We performed a retrospective chart review of all patients who had undergone scoliosis correction surgery at the local children’s hospital over a 10-year period from 1996 to 2005. The patients were identified from our institution database (Clinibase; Logibec) using International Classification of Diseases (ICD)-9 and ICD-10 diagnostic codes for the years identified for study. We considered all diagnoses, and a minimum postoperative follow-up of 1 year was a prerequisite for inclusion in our study. Patients who had undergone spinal surgery for any reason other than scoliosis correction or who had undergone revision surgery for reasons other than the treatment of infection were excluded from this study. This study was approved by the university’s Conjoint Health Research Ethics Board.
The primary outcome variable of interest was to determine the incidence of deep wound infection following pediatric scoliosis surgery. Deep wound infection for the purpose of this study, and as described in the literature,1,2,4,20 is defined as a surgical site infection in which there is communication between the associated infected material and the spinal instrumentation and bone graft/fusion mass, as proven by surgical exploration.
Secondary outcome variables of interest included primary diagnosis, age at surgical intervention, type of surgery (anterior approach only, posterior approach only, combined anterior and posterior approaches), duration of surgery, perioperative prophylactic antibiotic administration, intraoperative blood loss, presence of concurrent remote infection, perioperative administration of medications to reduce blood loss (e.g., tranexamic acid), the need for perioperative blood products (e.g., blood transfusion, clotting factors), type of bone graft used (allograft, autogenous graft, or synthetic bone substitutes), number of fusion levels, volume of instrumentation (number of implants), development of pseudoarthrosis and/or hardware failure, total lymphocyte count (an indicator of preoperative nutritional status)1,21 and cultured organism(s).
Statistical analysis
We analyzed the collected data using both descriptive and inferential statistics. As this was a retrospective chart review without any sample size calculation, inferential statistics were performed on an exploratory basis only. We used unpaired Student t tests to compare infected and noninfected groups with respect to secondary variables of interest. Owing to small numbers, multivariate analysis was not feasible. As such, stratification of the data analysis was carried through different stages to determine the presence of any confounders or effect modifiers. We used the χ2 and Fisher exact tests to determine difference in proportions. Risk ratios and risk differences with associated confidence intervals (CIs) were also determined. We considered results to be significant at p < 0.05.
Results
In all, 227 patients were eligible for inclusion in our study, and we reviewed their hospital charts. Of these patients, 167 were girls with mean age 14.4 years, and 60 were boys with mean age of 14.7 years. All patients were preoperatively screened for pre-existing concurrent remote infection with a urinalysis and ESR. If either of these screening tests suggested or confirmed the presence of an infection, the surgery was postponed until the infection was treated and cleared. The patients were grouped by diagnoses and stratified into adolescent idiopathic and nonadolescent idiopathic groups (Table 1). The mean preoperative Cobb angle was 58° (range 35°–87°) in the adolescent idiopathic group and 75° (range 30°–127°) in the nonadolescent idiopathic group. Posterior instrumentation and fusion surgery was performed in 192 patients, anterior-only surgery was performed in 11 patients, and 24 patients underwent both anterior and posterior surgery. Allograft was retrieved from the institution’s regional bone bank and was used as the primary source of bone graft in 87 patients (38%). In the remaining 140 patients, autogenous graft harvested from the iliac crest or local spinous processes was used. A synthetic bone graft extender (Osteoset; Wright Medical Technology, Inc.) was used to augment the use of autogenous bone graft in 8 of these patients. The average follow-up was 4.4 (range 1.0–9.5) years.
Table 1.
Clinical characteristics of 227 patients undergoing pediatric scoliosis surgery from 1996 to 2005
| Diagnosis | Girls, no. | Boys, no. | Age, mean yr | Cobb angle, mean ° | Total no. |
|---|---|---|---|---|---|
| Adolescent idiopathic | 114 | 20 | 15.2 | 58 | 134 |
| Nonadolescent idiopathic | |||||
| Juvenile idiopathic | 4 | 3 | 12.6 | 85 | 7 |
| Neuromuscular | 28 | 28 | 14.3 | 75 | 56 |
| Syndromic | 4 | 4 | 12.6 | 70 | 8 |
| Thoracic insufficiency syndrome | 4 | 3 | 5.4 | 52 | 7 |
| Congenital | 5 | 1 | 12.6 | 55 | 6 |
| Kyphosis | 4 | 1 | 16.8 | 74 | 5 |
| Other | 4 | 0 | 12.9 | 59 | 4 |
| Total | 167 | 60 | 14.5 | 63 | 227 |
The 10-year cumulative incidence of infection was 6.2% (14 patients). The cumulative incidence for patients who underwent posterior-only and anterior–posterior surgery was 5.2% (10 of 192 patients) and 16.7% (4 of 24 patients), respectively. All infections were identified in the posterior wound only. There were no infections in the anterior-only group. When analyzed by diagnosis, patients with neuromuscular etiologies had the highest cumulative incidence (14.3%, 8 of 56 patients). Patients with adolescent idiopathic scoliosis had the lowest incidence of infection (1.5%, 2 of 134 patients). The remaining 4 patients with infection were distributed evenly among the congenital, syndromic and other diagnostic groups.
There was a significant difference in the risk of infection between the adolescent idiopathic and nonadolescent idiopathic groups. The nonadolescent idiopathic group had a significantly higher risk of infection with an estimated risk ratio (RR) of 8.65 (p < 0.001, 95% CI 1.98–37.7). In comparing the adolescent idiopathic scoliosis group to the neuromuscular subgroup, the latter had a significantly higher risk of infection with an estimated RR of 9.5 (p < 0.001, 95% CI 2.1–43.7).
An early deep wound infection (within 3 wk) developed in 6 patients (42.8%), and infection developed at a later stage postoperatively (mean 37.3, range 5–72 wk) in the remaining 8 patients (57.1%). Drainage and back pain were the 2 most common presenting symptoms; 4 of the 8 patients presenting with late infection had pain as their initial concern. All patients with infections underwent surgical exploration, copious irrigation with normal saline and débridement of devitalized tissue. A cultured organism was identified in 12 of 14 patients (85.7%). Staphlococcus epidermidis was the most commonly isolated organism (6 patients) followed by Propionibacterium acnes (3 patients) and Pseudomonas (2 patients). Two or more organisms were isolated from 3 patients, including Enterococcus, methicillin-resistant S. aureus and Streptococcus (in addition to the organisms previously listed). Interestingly, S. aureus was isolated in only 1 patient. More virulent enteric and gram-negative organisms were more commonly isolated from early-onset infections (e.g., Pseudomonas, Enterococcus) whereas low-virulence cutaneous organisms were more commonly cultured from later-onset infections (e.g., P. acnes, S. epidermidis). The clinical characteristics of the 14 patients who experienced an infection are presented in Table 2.
Table 2.
Clinical characteristics of patients with diagnosed deep wound infections following pediatric scoliosis surgery
| No. | Diagnosis | Age, yr | Sex | Surgical approach | Bone graft | Levels fused | Onset, wk | Initial symptoms | Cultured organism | Pseudoarthrosis | Wound closure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AIS | 19 | F | Posterior | Allograft | T6–L2 | 8 | Drainage | Negative culture | No | VAC |
| 2 | AIS | 14 | F | Posterior | Autogenous | T5–L2 | 72 | Pain | P. acnes | Yes | VAC |
| 3 | Congenital scoliosis | 16 | F | Anterior–posterior | Allograft | T4–L3 | 1 | Drainage | Enterococcus, MRSA | No | VAC |
| 4 | Juvenile idiopathic scoliosis | 11 | F | Anterior–posterior | Allograft | T2–L4 | 19 | Drainage | P. acnes, S. epidermidis | No | VAC |
| 5 | Juvenile idiopathic scoliosis | 13 | M | Posterior | Autogenous | T4–L2 | 3 | Drainage | S. aureus | No | Primary |
| 6 | Duchenne muscular dystrophy | 14 | M | Posterior | Allograft | T2–pelvis | 5 | Drainage | S. epidermidis | No | VAC |
| 7 | Cerebral palsy | 13 | F | Anterior–posterior | Allograft | T2–pelvis | 1 | Drainage | Streptococcus | No | Primary |
| 8 | Rett syndrome | 17 | F | Posterior | Allograft | T2–L5 | 1 | Drainage | S. epidermidis, Pseudomonas, C. albicans | No | VAC |
| 9 | Myelomeningocele | 18 | M | Posterior | Allograft | T2–pelvis | 42 | Pain | S. epidermidis | Yes | VAC |
| 10 | Cerebral palsy | 13 | F | Anterior–posterior | Allograft | T2–pelvis | 72 | Drainage | S. epidermidis | No | VAC |
| 11 | Cerebral palsy | 14 | M | Anterior–posterior | Allograft | T2–pelvis | 33 | Pain | S. epidermidis | No | VAC |
| 12 | Cerebral palsy | 13 | M | Posterior | Allograft | T2–pelvis | 46 | Pain | P. acnes | Yes | Secondary |
| 13 | Duchenne muscular dystrophy | 17 | M | Posterior | Allograft | T2–pelvis | 3 | Drainage | Pseudomonas | No | Primary |
| 14 | Osteogenesis imperfecta | 12 | M | Posterior | Allograft | T4–L2 | 21 | Drainage | Negative culture | No | Primary |
AIS = adolescent idiopathic scoliosis; C. = Candida; F = female; M = male; MRSA = methicillin-resistant Staphylococcus aureus; P. = Propionibacterium; S. = Staphylococcus; VAC = vacuum-assisted closure.
Allograft bone was used in 87 (38%) patients, with 55 of these patients being in the nonadolescent idiopathic group and the remainder in the adolescent idiopathic group. The incidence of infection for patients who had been treated with and without allograft was 16% (12 of 75 patients) and 1.4% (2 of 138 patients), respectively. The use of allograft was associated with a higher risk of infection with an estimated RR of 9.66 (p < 0.001, 95% CI 2.21–42.11). Further stratification of the analysis by diagnosis demonstrated that the use of allograft remained a significant risk factor in the nonadolescent idiopathic group (estimated RR 7.6, p = 0.014, 95% CI 1.02–56.4). All patients with a neuromuscular diagnosis who experienced an infection were treated with allograft (8 of 44, 18.2%), whereas none of this subgroup experienced an infection when autogenous bone graft alone was used (0 of 12 patients).
Considering the entire cohort, we found that using larger volumes of instrumentation was a significant risk factor, with the mean number of implants being 14 in the group without infection and 22 in the group with infection (p < 0.001). Owing to the increased number of levels typically instrumented for patients in the neuromuscular subgroup (often, sublaminar wires from the upper thoracic spine to the pelvis) compared with the remaining patients in the cohort, the data were analyzed accordingly. Though many of the cases of patients with neuromuscular disorders involved instrumentation and fusion from the upper thoracic spine to the pelvis, several patients treated earlier in the cohort had more selective, shorter fusions. Stratifying the analysis by diagnosis, the volume of instrumentation as a risk factor remained significant when analyzed within the neuromuscular subgroup alone (p = 0.022).
Blood loss, the use of perioperative medications to reduce blood loss, duration of surgery, preoperative Cobb angle and number of levels fused were not found to be significant risk factors for infection after stratification by diagnosis. We investigated preoperative nutritional status indirectly by assessing the patients’ total lymphocyte counts, but we did not find this to be a significant risk factor.
About 88% of the patients in this cohort (201 of 227) received a first-generation cephalosporin (cefazolin) at the beginning of the surgery for infection prophylaxis. It was standard practice at our institution that the timing of the administration of antibiotics (and all other medications) be documented on the intraoperative medical record sheet by the treating anesthesiologist for every surgery. Neither the timing of dosing nor the type of antibiotic used was found to be a significant risk factor for the development of infection.
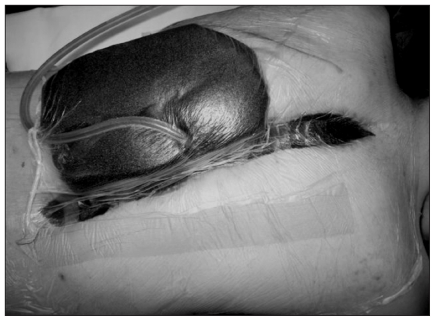
Following surgical irrigation and débridement, 5 (35.7%) patients with infections were closed primarily, 8 (57.1%) were treated with serial débridements and vacuum-assisted closure (VAC) dressing-delayed wound closure (continuous suction, 125 mm Hg; KCI Medical Inc.), and 1 (7.1%) was left to heal by secondary intention (Fig. 1). Patients treated by VAC underwent an average of 10 vacuum dressing changes (range 3–17) before delayed wound closure. Several of the patients treated with VAC dressings required simple fascio-cutaneous advancement flaps to facilitate wound closure; we found induration and adherence of subcutaneous tissue to the underlying fascial plane to be common impediments to subsequent skin closure. One patient required a musculo-cutaneous rotation flap for soft-tissue coverage owing to clinically important paraspinal muscle loss after repeated débridements. The presence of a pseudoarthrosis was identified in 3 of 14 (21.4%) patients with infections at an average of 2.9 years of follow-up, but none required revision as they were asymptomatic and there was no evidence of deformity progression. There were no recurrences of infection after the completion of a single course of surgical management, and all patients retained their spinal implants. All patients with diagnosed infections were treated concurrently with a prolonged course of intravenous antibiotics under the direction of a pediatric infectious diseases consultant.
Fig. 1.
Vacuum-assisted closure after anterior–posterior surgery in a 16-year-old girl. Note that the large sponge is for pressure relief only and is not applied to the wound.
Discussion
The development of deep wound infection following pediatric scoliosis surgery is a devastating complication that typically requires prolonged courses of surgical and medical management to achieve successful eradication of the offending organism(s). Identification of significant risk factors for the development of infection — with subsequent execution of a factor-based targeted prophylaxis protocol — may help to decrease the incidence of this complication in patients undergoing pediatric scoliosis surgery.22
The 10-year cumulative incidence of infection in this study was 6.2%, with the lowest risk being in patients with adolescent idiopathic scoliosis (1.5%). This is consistent with the lower end of the range of incidence reported in previous studies.1,5,8 The neuromuscular subgroup had the highest incidence of infection (14.3%) in our study. In this subgroup, spastic quadriplegia (cerebral palsy) was the most commonly encountered diagnosis. The increased risk of infection found in our study is consistent with that reported in other studies and is commonly linked to poor hygiene, fecal contamination of the wound and a level of immune compromise.4 Impaired nutritional status has also been implicated as a risk factor in these patients.1,14 Unfortunately, in the current study, serum albumin and other superior measures of nutritional status were not consistently measured and, as such, we used the total lymphocyte count as the only available proxy. It is known that the total lymphocyte count has relatively low sensitivity and specificity as a determinant of nutritional status and, as such, conclusions based on its results should be interpreted with caution.21 Nonetheless, we did not find total lymphocyte count to be a significant risk factor for the development of infection. In a multicentre study by Sponseller and colleagues,4 the only independent risk factors identified for this diagnostic subgroup were the degree of cognitive impairment and the use of allograft bone.
The use of allograft in posterior fusion for scoliosis has been addressed in several previous studies and, on the whole, has been reported to be safe and effective for spinal fusion.16,17,23 Despite this, our data support the study by Sponseller and colleagues,4 identifying the use of allograft as a significant risk factor within the entire cohort and when adjusted for effect modifiers (e.g., nonidiopathic or neuromuscular diagnoses). The reasons for this finding are not clear, but may include the overwhelming of host defenses by the presence of a large amount of devitalized bone in patients who were already relatively immunocompromised. Barriga and colleagues24 had found positive bacterial cultures, including Pseudomonas, S. epidermidis and others, in 46% of allograft swabbed before implantation for posterior scoliosis surgery. They postulated that contamination likely occurred during allograft preparation intra-operatively (i.e., during thawing and morsellization) as the rates of contamination were markedly higher than the initial cultures taken at the bone bank. In a recent study of 220 children with cerebral palsy treated with unit rod fixation, the addition of gentamicin to freeze-dried cortico-cancellous allograft significantly reduced the incidence of infection (3.9%) compared with allograft without antibiotic impregnation (15.2%).25 The summation of these findings suggests that, contrary to previously held beliefs, the use of allograft is likely a significant risk factor for infection, especially in children with nonidiopathic diagnoses. Standardization of allograft preparation at the bone bank, with or without antibiotic impregnation, may help to avoid this route of allograft contamination and potentially reduce the incidence of infection following scoliosis surgery.
In our study, offending organisms were identified in 85.7% of patients, with S. epidermidis being the most commonly isolated, followed by P. acnes and Pseudomonas. These results were similar to those reported in several previous studies.4,22,26 Preoperative antibiotic prophylaxis with a first generation cephalosporin has been the accepted practice in orthopedic surgery owing to the identification of S. aureus as the most common cause of infections associated with internal fixation devices.27 The shift in predominant pathogens from S. aureus to low-virulence cutaneous and gram-negative organisms has been suggested to result from this common practice.22 Accordingly, particularly for patients with nonidiopathic diagnoses, an argument could be made supporting the use of more broad-spectrum antibiotic prophylaxis to account for this shift. Adding gentamicin to complement the use of a first-generation cephalosporin might prove more efficacious given that the aminoglycoside has been shown to be beneficial in treating biofilm-producing P. acnes28 and in decreasing the incidence of postoperative infection when added to allograft.25 Further trials are necessary to investigate the efficacy of this change in antibiotic prophylaxis.
Volume of instrumentation has been previously reported to be a significant risk factor for the development of infection, and our results support these findings.5 Theoretically, the increased volume of metal provides more surface area for bacterial adherence and thus precipitates the development of substantial numbers of colony-forming units resistant to both the host immune system and to systemic antibiotic treatment. Two recent studies investigating the role of implant materials in the formation of biofilms for low-virulence cutaneous organisms — S. epidermidis and P. acnes — demonstrated that these organisms are extremely resistant to treatment once a biofilm has formed.28,29 Likely, this is the reason why many previous studies have suggested that the only way to completely eradicate these infections is by implant removal.30
The removal of spinal implants at an early postoperative stage can compromise fusion and curve correction. As such, retention of implants should be one of the goals when treating infection. Even after fusion has been achieved, bending of the fusion mass has been described following implant removal, and thus this practice should be avoided if possible.31 In our study, most patients with diagnosed deep wound infections following posterior surgery underwent irrigation with copious amounts of normal saline solution, débridement of devitalized tissue and the use of VAC. With VAC dressings, the controlled application of continuous subatmospheric pressure (–125 mm Hg) induces the formation of granulation tissue, promotes angiogenesis, evacuates edematous fluid and provides a sterile barrier dressing during treatment.32 At the time of most recent follow-up, none of the patients in our study required implant removal. Two recent studies investigating the use of vacuum dressings in the treatment of infection following scoliosis surgery support these results, with none of the 20 patients in both studies requiring implant removal.32,33 Though onerous in the number of dressing changes typically required in the treatment of infection, the ability to retain the patient’s spinal instrumentation has substantial benefits, and its use should be encouraged.
Limitations
The limitations of this study are inherent to its retrospective design and include the availability and accuracy of the medical record. Despite this, most of the data for the desired outcome variables were easily retrieved from the operative reports and perioperative and intraoperative records without a substantial amount of missing information. Eliminating confounders, particularly in the neuromuscular group, was another challenge as the smaller numbers in this cohort precluded a multivariate analysis. As such, the elimination of confounders and effect modifiers were addressed through classical data stratification. Despite the demonstration of statistical significance for several determined risk factors, wide confidence intervals were demonstrated. This was an unavoidable issue related to the small number of patients with diagnosed infections and was another inherent limitation for this study.
Conclusion
By synthesizing the results of this and other studies, our institution has adopted a standardized prophylaxis protocol for subsequent patients undergoing scoliosis surgery (Box 1). This protocol includes the use of a preclosure dilute betadine irrigation solution reported in a recent randomized controlled trial to reduce the incidence of postoperative infection in adult spine patients.34 Future prospective studies are required to determine if these measures serve to reduce the reported incidence of infection following pediatric scoliosis surgery.
Box 1. Proposed prophylaxis protocol for patients undergoing scoliosis surgery.
Screening for remote infection with preoperative erythrocyte sedimentation rate and urine cultures
Closed-room policy to reduce air-flow contaminants
Chlorhexidine scrub followed by skin preparation with chlorhexidine
Antibiotic administration (cefazolin) within 30 minutes before skin incision
Addition of gentamicin to cefazolin for prophalaxis in high-risk groups (e.g., neuromuscular disorders)
Limiting the use of allograft where possible; using Bone Bank prepackaged/morcellized allograft when needed
Thorough débridement of devitalized tissue before closure
Use of 3.5% dilute betadine irrigation before application of bone graft as per Cheng and colleagues34
Surgical-site reinforcement of sterile dressings to prevent fecal contamination
No dressing changes for first 72 hours postoperatively to allow for initial wound healing
Footnotes
Competing interests: None declared.
Contributors: Drs. Aleissa, Parsons and Howard designed the study, analyzed the data and wrote the article. Drs. Aleissa, Parsons, Grant, Harder and Howard acquired the data. Drs. Parsons, Grant, Harder and Howard reviewed the article. All authors approved its publication.
References
- 1.Szöke G, Lipton G, Miller F, et al. Wound infection after spinal fusion in children with cerebral palsy. J Pediatr Orthop. 1998;18:727–33. [PubMed] [Google Scholar]
- 2.Cook S, Asher M, Lai SM, et al. Reoperation after primary posterior instrumentation and fusion for idiopathic scoliosis. Toward defining late operative site pain of unknown cause. Spine. 2000;25:463–8. doi: 10.1097/00007632-200002150-00012. [DOI] [PubMed] [Google Scholar]
- 3.Lonstein J, Winter R, Moe J, et al. Wound infection with Harrington instrumentation and spine fusion for scoliosis. Clin Orthop Relat Res. 1973;96:222–33. [PubMed] [Google Scholar]
- 4.Sponseller PD, LaPorte DM, Hungerford MW, et al. Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine. 2000;25:2461–6. doi: 10.1097/00007632-200010010-00007. [DOI] [PubMed] [Google Scholar]
- 5.Clark CE, Shufflebarger HL. Late-developing infection in instrumented idiopathic scoliosis. Spine. 1999;24:1909–12. doi: 10.1097/00007632-199909150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Soultanis K, Mantelos G, Pagiatakis A, et al. Late infection in patients with scoliosis treated with spinal instrumentation. Clin Orthop Relat Res. 2003;411:116–23. doi: 10.1097/01.blo.0000068357.47147.10. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey R, Rapp RP, Young B, et al. Prophylactic parenteral antibiotics in clean neurosurgical procedures: a review. J Neurosurg. 1988;69:52–7. doi: 10.3171/jns.1988.69.1.0052. [DOI] [PubMed] [Google Scholar]
- 8.Lonstein JE. Scoliosis: surgical versus nonsurgical treatment. Clin Orthop Relat Res. 2006;443:248–59. doi: 10.1097/01.blo.0000198725.54891.73. [DOI] [PubMed] [Google Scholar]
- 9.Petty W, Spanier S, Shuster JJ, et al. The influence of skeletal implants on incidence of infection. Experiments in a canine model. J Bone Joint Surg Am. 1985;67:1236–44. [PubMed] [Google Scholar]
- 10.Broom MJ, Banta JV, Renshaw TS. Spinal fusion augmented by luque-rod segmental instrumentation for neuromuscular scoliosis. J Bone Joint Surg Am. 1989;71:32–44. [PubMed] [Google Scholar]
- 11.Gersoff WK, Renshaw TS. The treatment of scoliosis in cerebral palsy by posterior spinal fusion with Luque-rod segmental instrumentation. J Bone Joint Surg Am. 1988;70:41–4. [PubMed] [Google Scholar]
- 12.Stevens DB, Beard C. Segmental spinal instrumentation for neuromuscular spinal deformity. Clin Orthop Relat Res. 1989;242:164–8. [PubMed] [Google Scholar]
- 13.Sullivan JA, Conner SB. Comparison of Harrington instrumentation and segmental spinal instrumentation in the management of neuromuscular spinal deformity. Spine. 1982;7:299–304. doi: 10.1097/00007632-198205000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Jevsevar DS, Karlin LI. The relationship between preoperative nutritional status and complications after an operation for scoliosis in patients who have cerebral palsy. J Bone Joint Surg Am. 1993;75:880–4. doi: 10.2106/00004623-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Grosman R, Vlach O, Leznar M. [Infections in surgery of idiopathic scoliosis] [Article in Czech] Acta Chir Orthop Traumatol Cech. 2002;69:175–8. [PubMed] [Google Scholar]
- 16.Knapp DR, Jr, Jones ET, Blanco JS, et al. Allograft bone in spinal fusion for adolescent idiopathic scoliosis. J Spinal Disord Tech. 2005;18(Suppl):S73–6. doi: 10.1097/01.bsd.0000128694.21405.80. [DOI] [PubMed] [Google Scholar]
- 17.Yazici M, Asher MA. Freeze-dried allograft for posterior spinal fusion in patients with neuromuscular spinal deformities. Spine. 1997;22:1467–71. doi: 10.1097/00007632-199707010-00008. [DOI] [PubMed] [Google Scholar]
- 18.Gaine WJ, Andrew SM, Chadwick P, et al. Late operative site pain with isola posterior instrumentation requiring implant removal: Infection or metal reaction? Spine. 2001;26:583–7. doi: 10.1097/00007632-200103010-00027. [DOI] [PubMed] [Google Scholar]
- 19.Jones KB, Erkula G, Sponseller PD, et al. Spine deformity correction in Marfan syndrome. Spine. 2002;27:2003–12. doi: 10.1097/00007632-200209150-00008. [DOI] [PubMed] [Google Scholar]
- 20.Viola RW, King HA, Adler SM, et al. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine. 1997;22:2444–50. doi: 10.1097/00007632-199710150-00023. discussion 2450–1. [DOI] [PubMed] [Google Scholar]
- 21.Jensen JE, Jensen TG, Smith TK, et al. Nutrition in orthopaedic surgery. J Bone Joint Surg Am. 1982;64:1263–72. [PubMed] [Google Scholar]
- 22.Labbé AC, Demers AM, Rodrigues R, et al. Surgical-site infection following spinal fusion: a case–control study in a children’s hospital. Infect Control Hosp Epidemiol. 2003;24:591–5. doi: 10.1086/502259. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy RE, Peek RD, Morrissy RT, et al. Allograft bone in spinal fusion for paralytic scoliosis. J Bone Joint Surg Am. 1986;68:370–5. [PubMed] [Google Scholar]
- 24.Barriga A, Diaz-de-Rada P, Barroso JL, et al. Frozen cancellous bone allografts: positive cultures of implanted grafts in posterior fusions of the spine. Eur Spine J. 2004;13:152–6. doi: 10.1007/s00586-003-0633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borkhuu B, Borowski A, Shah SA, et al. Antibiotic-loaded allograft decreases the rate of acute deep wound infection after spinal fusion in cerebral palsy. Spine. 2008;33:2300–4. doi: 10.1097/BRS.0b013e31818786ff. [DOI] [PubMed] [Google Scholar]
- 26.Hahn F, Zbinden R, Min K. Late implant infections caused by Propionibacterium acnes in scoliosis surgery. Eur Spine J. 2005;14:783–8. doi: 10.1007/s00586-004-0854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trampuz A, Zimmerli W. Antimicrobial agents in orthopaedic surgery: prophylaxis and treatment. Drugs. 2006;66:1089–105. doi: 10.2165/00003495-200666080-00005. [DOI] [PubMed] [Google Scholar]
- 28.Ramage G, Tunney MM, Patrick S, et al. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials. 2003;24:3221–7. doi: 10.1016/s0142-9612(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 29.Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine. 2005;30:38–43. doi: 10.1097/01.brs.0000147801.63304.8a. [DOI] [PubMed] [Google Scholar]
- 30.Ho C, Skaggs DL, Weiss JM, et al. Management of infection after instrumented posterior spine fusion in pediatric scoliosis. Spine. 2007;32:2739–44. doi: 10.1097/BRS.0b013e31815a5a86. [DOI] [PubMed] [Google Scholar]
- 31.Potter BK, Kirk KL, Shah SA, et al. Loss of coronal correction following instrumentation removal in adolescent idiopathic scoliosis. Spine. 2006;31:67–72. doi: 10.1097/01.brs.0000192721.51511.fe. [DOI] [PubMed] [Google Scholar]
- 32.Canavese F, Gupta S, Krajbich JI, et al. Vacuum-assisted closure for deep infection after spinal instrumentation for scoliosis. J Bone Joint Surg Br. 2008;90:377–81. doi: 10.1302/0301-620X.90B3.19890. [DOI] [PubMed] [Google Scholar]
- 33.van Rhee MA, de Klerk LW, Verhaar JA. Vacuum-assisted wound closure of deep infections after instrumented spinal fusion in six children with neuromuscular scoliosis. Spine J. 2007;7:596–600. doi: 10.1016/j.spinee.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Cheng MT, Chang MC, Wang ST, et al. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine. 2005;30:1689–93. doi: 10.1097/01.brs.0000171907.60775.85. [DOI] [PubMed] [Google Scholar]