Abstract
The dentate gyrus, an important anatomic structure of the hippocampal formation, is one of the major areas in which neurogenesis takes place in the adult mammalian brain. Neurogenesis in the dentate gyrus is thought to play an important role in hippocampus-dependent learning and memory. Neurogenesis has been reported to be increased in the dentate gyrus of patients with Alzheimer disease, but it is not known whether the newly generated neurons differentiate into mature neurons. In this study, the expression of the mature neuronal marker high molecular weight microtubule-associated protein (MAP) isoforms MAP2a and b was found to be dramatically decreased in Alzheimer disease dentate gyrus, as determined by immunohistochemistry and in situ hybridization. The total MAP2, including expression of the immature neuronal marker, the MAP2c isoform, was less affected. These findings suggest that newly generated neurons in Alzheimer disease dentate gyrus do not become mature neurons, although neuroproliferation is increased.
Keywords: Alzheimer disease, Hippocampus, Microtubule-associated protein 2, Neurogenesis
INTRODUCTION
Alzheimer disease (AD), the major cause of dementia in middle-aged to elderly individuals, is characterized clinically by progressive decline in cognitive abilities accompanied by behavioral abnormalities. The histopathologic hallmarks of AD are senile plaques containing amyloid-β peptide (1) and neurofibrillary tangles of paired helical or straight filaments of abnormally hyperphosphorylated tau (2, 3). High densities of plaques and neurofibrillary tangles are found predominantly in the hippocampus and the entorhinal cortex.
The dentate gyrus is the projecting target of the perforant pathway, which is the major cortical input from the entorhinal cortex to the hippocampus. The adult dentate gyrus has the unique property of persistent neurogenesis (4); in mice, this is detectable even at old ages, but at a slower rate (5). Neurogenesis is thought to play an important role in the maintenance of memory and associated learning (6–8). Thus, disruption of neurogenesis in the dentate gyrus can be involved in the age-associated decline of hippocampal learning and memory. Neurogenesis has 2 aspects: proliferation and differentiation. In AD brain, especially in the hippocampus, a marked imbalance of neurotrophic factors, including increased levels of fibroblast growth factor 2 (FGF-2) and decreased levels of the brain-derived neuro-trophic factor and neurotrophin 4, has been found (9, 10). Most likely, this changed environment adversely affects the maturation of multipotent neural progenitor cells into differentiated neurons. The expression of immature neuronal marker proteins that signal the birth of new neurons is increased in the hippocampus of AD patients (11), suggesting that neuroproliferation in the dentate gyrus is increased in this disease. However, it is not known whether these newly generated neurons can differentiate into mature neurons in the dentate gyrus in AD.
Microtubule-associated protein 2 (MAP2) is a family of heat-stable phosphoproteins expressed predominantly in the cell body and dendrites of neurons. Three major MAP2 isoforms, high molecular weight (HMW) MAP2a, b, and low molecular weight MAP2c, are differentially expressed during the development of the nervous system and have an important role in microtubule dynamics (12). Microtubule-associated protein 2c is present during early brain development and is absent from most adult brain areas. The switch in expression from MAP2c to MAP2a and b occurs during neuronal maturation and seems to be less complete in the dentate gyrus and the olfactory bulb, areas with persistent adult neurogenesis (13–16). This study examines the effect of the changed trophic environment on neural development in the hippocampus in AD by comparing alterations in the expression of the mature and the immature isoforms of MAP2 in the dentate gyrus from individuals with AD and age-matched controls.
MATERIALS AND METHODS
Tissue
Tissue sections of the hippocampus of 14 AD and 15 age-matched controls were obtained from the Brain Donation Program of the Sun Health Research Institute, Sun City, AZ (Table). All AD cases met the Consortium to Establish a Registry for Alzheimer’s Disease/National Institute on Aging–Reagan Institute criteria for definite AD (17, 18). All controls but 1, C7, were cognitively unimpaired according to their clinical records and also had no known neurologic diseases. Control Case C7 was found to have borderline dementia according to psychological assessment. The sections (40 μm) had been prepared from approximately 1-cm-thick blocks fixed in 4% paraformaldehyde for 24 to 48 hours. The sections were stored at −20°C in 30% ethylene glycol, 30% glycerol, 0.2 mol/L of sodium phosphate buffer, pH 7.4, until they were used.
TABLE.
Characteristics of Cases Used for this Study
| Case No. | Sex | Age (years) | Diagnosis | Postmortem Delay (hours) | ApoE Genotype | Braak* Score | NP CERAD |
|---|---|---|---|---|---|---|---|
| C1 | F | 81 | Control | 3.0 | 4,4 | II | A |
| C2 | F | 87 | Control | 4.0 | 3,3 | III | A |
| C3 | M | 83 | Control | 3.0 | 3,3 | III | A |
| C4 | F | 82 | Control | 2.0 | 3,3 | II | A |
| C5 | F | 70 | Control | 2.0 | 4,4 | I | A |
| C6 | M | 77 | Control | 2.5 | 3,3 | II | A |
| C7 | F | 88 | Control | 2.0 | 3,4 | III | A |
| C8 | M | 69 | Control | 2.2 | 3,4 | I | A |
| C9 | F | 86 | Control | 2.0 | 3,2 | II | O |
| C10 | F | 91 | Control | 2.5 | 2,2 | III | A |
| C11 | F | 94 | Control | 2.5 | 3,2 | III | B |
| C12 | M | 85 | Control | 3.2 | 3,3 | II | O |
| C13 | F | 91 | Control | 2.0 | 3,2 | II | O |
| C14 | M | 80 | Control | 3.3 | 3,3 | II | A |
| C15 | M | 90 | Control | 2.8 | 3,3 | III | A |
| Mean ± SD | 83.6 ± 7.4 | 2.6 ± 0.6 | |||||
| AD1 | M | 82 | AD | 2.3 | 3,4 | V | C |
| AD2 | M | 82 | AD | 1.8 | 3,4 | V | C |
| AD3 | M | 60 | AD | 3.3 | 3,33 | VI | C |
| AD4 | F | 73 | AD | 2.0 | 3,4 | V | C |
| AD5 | M | 94 | AD | 3.0 | 3,3 | IV | B |
| AD6 | M | 76 | AD | 2.3 | 3,3 | VI | C |
| AD7 | M | 72 | AD | 2.5 | 3,2 | VI | C |
| AD8 | F | 96 | AD | 2.8 | 3,3 | V | B |
| AD9 | F | 80 | AD | 2.3 | 3,3 | VI | C |
| AD10 | F | 85 | AD | 1.7 | 3,3 | V | C |
| AD11 | F | 89 | AD | 2.3 | 3,4 | V | C |
| AD12 | F | 60 | AD | 2.0 | 3,4 | VI | C |
| AD13 | F | 86 | AD | 1.8 | 3,4 | V | B |
| AD14 | M | 76 | AD | 4.0 | 4,4 | V | C |
| Mean ± SD | 79.4 ± 10.9 | 2.4 ± 0.6 |
CERAD plaque score denotes the frequency of neuritic plaques: 0, none; A, sparse; B, moderate; C, frequent.
Braak Score (32) denotes the progression of neurofibrillary changes with increasing severity from the transentorhinal stages I and II to the limbic stages III and IV, and, finally, to the cortical stages V and VI.
AD, Alzheimer disease; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; NP, neuritic plaque.
Western Blots and Immunohistochemistry
Brains (cerebra) were obtained from 5-month-old Wistar rats that had been euthanized by injection of 80 mg/kg (i.p.) of nembutal. Neural progenitor cells were prepared from the brains of a 3-month-old male Wister rat as previously described (19). Rats were killed according to the guidelines of the Institutional Animal Welfare Committee. For Western blots, brain homogenate and cell lysate were prepared in 50 mM TRIS-HCl, pH 6.8, containing 1% sodium dodecyl sulfate (SDS) and 1% β-mercaptoethanol and a cocktail of protease inhibitors (1 mmol/L of phenyl-methyl sulfonyl fluoride and 2 μg/mL each of aprotinin, leupeptin, and pepstatin A). Samples were electrophoresed on 7.5% SDS-polyacrylamide gels, transferred to immobilon, and developed with monoclonal antibodies SMI52 (1:1000; Sternberger Monoclonals, Baltimore, MD) or AP20 (1:2000; Chemicon International, Temecula, CA) to MAP2a,b; or HM2 to MAP2a,b,c (1:200; Sigma-Aldrich, St. Louis, MO). Bound antibodies were detected using peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagent (Pierce, Rockford, IL) as a substrate.
Immunohistochemistry was performed as previously described (20), except that floating sections were used. Sections were incubated overnight at 4°C with primary antibodies. Bound antibodies were detected using Vectastain Elite Avidin Biotin Kit (Vector, Burlingame, CA) with diaminobenzidine (Sigma-Aldrich) as substrate. All sections were treated identically using the same batches and concentrations of antibodies, incubation, and staining times. Only cases that showed clear immunoreactivity in the hippocampus with all 3 antibodies were used in the study.
In Situ Hybridization
Digoxigenin-labeled cRNA probes (anti-sense and sense probes) were prepared by in vitro transcription using the human MAP2 exon 8 and 1-265 cDNAs subcloned into pGEM-3Zf(+) vectors (Promega, Madison, WI) as templates in the presence of digoxigenin-labeled deoxyuridine triphosphate. MAP2 exon 8 is specific for MAP2a (21–23), and 1-265 is common for all the MAP2 isoforms, including MAP2a, b, and c (24). There is no unique DNA sequence for MAP2b, and that MAP2c is too short to be used as a probe. In situ hybridization was performed as described previously (25, 26). Sections (40 μm) were postfixed for 20 minutes in 4% formaldehyde, followed by a 5-minute wash in 0.1 mol/L of phosphate buffer, pH 7.2. Sections were treated with 0.001% proteinase-K (Promega) in 50 mM Tris-HCl, pH 7.2, 5 mM EDTA for 10 minutes, and subsequently for another 10 minutes with 0.1 mol/L of triethanolamine and 0.225% acetic acid anhydrous solution. After washing with 0.1 mol/L of phosphate buffer, sections were dehydrated through a series of increasing concentrations of ethanol and were air-dried. The sections were prehybridized for 30 minutes at 65°C in hybridization buffer (10% sodium dextran sulfate, 20 mmol/L of Tris-HCl, pH 8.0, 0.3 mol/L of NaCl, 0.2% sarcosine, 0.02% heat-denatured salmon sperm DNA, 1× Denhardt solution, 50% formamide) and then hybridized overnight at 65°C in hybridization solution with 500 ng/mL of cRNA probes. After rinsing in 5× standard saline citrate (0.75 mol/L of NaCl, 75 mmol/L of sodium citrate) at 60°C for 30 minutes, the sections were washed with 50% formamide/2× standard saline citrate (0.3 mol/L of NaCl, 30 mmol/L of sodium citrate) at 60°C for 30 minutes (high stringency wash). The sections were then subjected to 30 minutes RNase digestion at 37°C with 1 μg/mL of RNase A (Roche, Indianapolis, IN) in 10 mmol/L of Tris-HCl, pH 7.5, 1 mmol/L of ethylenediamine tetraacetic acid, 0.5 mol/L of NaCl, and then washed at high stringency. For detection of digoxigenin-labeled cRNA probes, the sections were treated with anti-digoxigenin conjugated to alkaline phosphatase (Roche) at a dilution of 1:500, and color was developed by incubation with 4-nitro blue tetrazolium chloride and 5-bromo-4 chloro-3 indolylphosphate solution (Roche).
Image Analysis
Levels of immunoreactivity and hybridization in AD and control dentate gyrus were assessed using a Zeiss Axiophot Microscope (Carl Zeiss, Thornwood, NY) with a 4× objective and 1.25 intermediate lens, yielding a spatial resolution of 5,525 pixels per millimeter. The National Institutes of Health Image J program, version 1.32j (http://rsb.info.nih.gov/ij/), was used to measure optical density (OD) of the images. All sections were examined under the same light intensity, brightness, and contrast settings. For each section, the OD was measured with reference to a gray scale from 0 to 255 pixels. In detail, each pixel of the image can take any gray scale value from completely black (0) to completely white (255). A fixed OD value was set as a positive threshold in each kind of staining. For each microscopic field examined, the threshold value was subtracted from the reading. The corrected gray values of all pixels belonging to the region of interest were used to calculate the mean OD value for that region (27). The OD values obtained were analyzed by 2-tailed t-test.
RESULTS
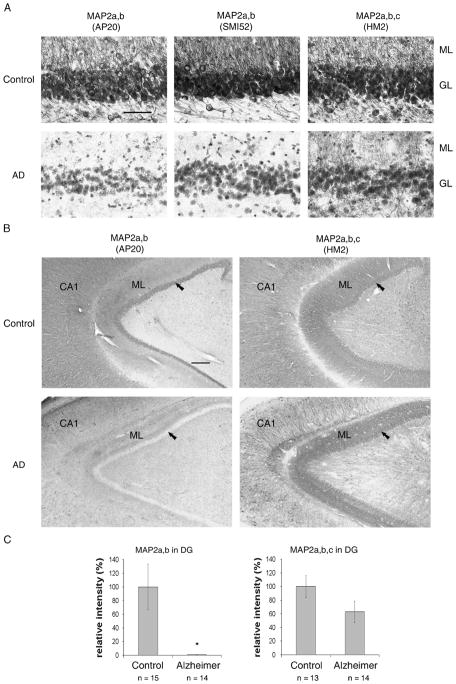
To investigate the effect of the changed trophic environment in AD hippocampus on neuronal development, we studied the expression of the mature HMW-MAP2 in the dentate gyrus of 14 AD and 15 control cases (Table). Using monoclonal antibody (mAb) AP20, which specifically labels MAP2a, MAP2b, but not the immature isoform MAP2c (Fig. 1), we found that this antibody only minimally stained the dentate gyrus in all 14 AD cases (Fig. 2A, B), whereas it robustly labeled the CA regions. In contrast, in 11 of 15 control cases, neurons in most hippocampal areas, including the dentate gyrus, were strongly labeled by this antibody. In 4 of 15 control cases (i.e. Cases C2, C8, C14, and C15), the staining pattern of the dentate gyrus was equal to that of the AD cases. These findings were confirmed with mAb SMI52, another mAb to MAP2a and MAP2b (Fig. 2A). In contrast to these 2 antibodies, HM2, which recognizes all 3 isoforms, MAP2a, MAP2b, and MAP2c (Fig. 1), stained most areas of the hippocampal formation, including the dentate gyrus, in both AD and controls, regardless of whether they were immunonegative or immunopositive with MAP2a and b antibodies (Fig. 2A, B). Quantitation of the immunostaining revealed that AD dentate gyrus had less than 1% of the MAP2a and b and approximately 60% of the MAP2a to c levels of the corresponding signals in age-matched control cases (Fig. 2C). These results suggest that MAP2c is predominantly expressed in the dentate gyrus in AD.
FIGURE 1.
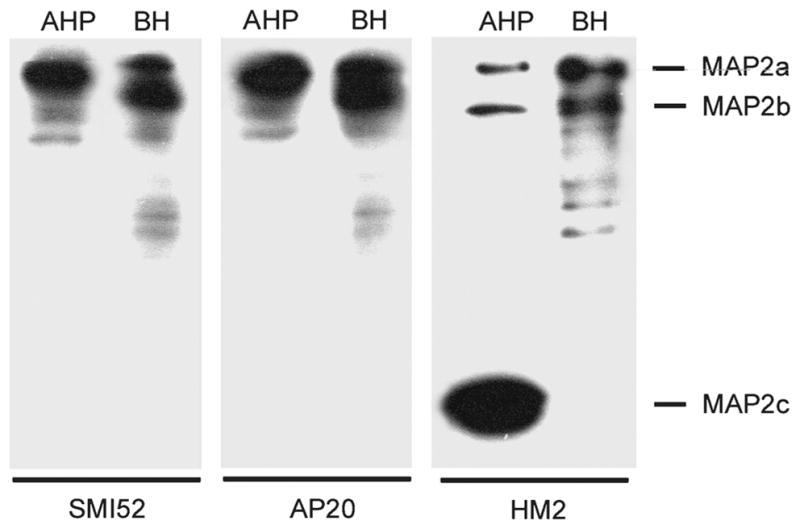
Western blots showing the specificity of the microtubule-associated protein (MAP)2 antibodies used. Cultured adult rat hippocampal progenitor (AHP) lysate (10 μg) and BH (2.5 μg) were electrophoresed on 7.5% sodium dodecyl sulfate–polyacrylamide gel under reducing conditions, and the Western blots were developed with mAb SMI52 and AP20 to MAP2a, b, and mAb HM2 to MAP2a to c. All 3 antibodies were of high specificity toward the MAP2 isoforms and did not cross-react with other brain proteins such as tau. In AHPs, the immature isoform MAP2c was the predominant MAP2, whereas in the adult brain, the high molecular weight (HMW)-MAPs were predominant, and MAP2c was not detectable. In both samples, MAP2a and MAP2b were expressed at similar ratios. BH, rat brain homogenate.
FIGURE 2.
Immunoreactivity of microtubule-associated protein (MAP)2a,b (mAb AP20, mAb SMI52) and MAP2a,b,c (mAb HM2) in dentate gyrus of control and Alzheimer disease (AD) brains. (A) All 3 antibodies strongly stained control dentate gyrus (upper 3 images), whereas the AD dentate gyrus was clearly labeled only by the antibody that also recognized MAP2c (lower right image) but not by the 2 antibodies specific to the mature MAP2 isoforms (lower left and middle images). Sections counterstained with hematoxylin. Scale bar = 50 μm. (B) Low-magnification images of an AD and a control case show the extent to which MAP2a and b in AD dentate gyrus is affected (lower left image); the granular layer (GL) (arrow) is immunonegative, and the molecular layer (ML) is weakly stained for MAP2a and b, whereas the CA1 area of the hippocampus is robustly stained. In contrast, in the control (upper left), the dentate gyrus is strongly immunopositive. The antibody to all MAP2 isoforms, including MAP2c, robustly stained both AD and control dentate gyri (lower and upper right images; scale bar = 300 μm). (C) Immunoreactivity of MAP2a, b (mAb AP20), and MAP2a to c (mAb HM2) in dentate gyrus (DG) of control and AD brains was quantitated by using ImageJ software. Mean ± SEM of quantitated intensity relative to control. Differences between AD and control cases were analyzed statistically by 2-tailed t-test (*, p < 0.01).
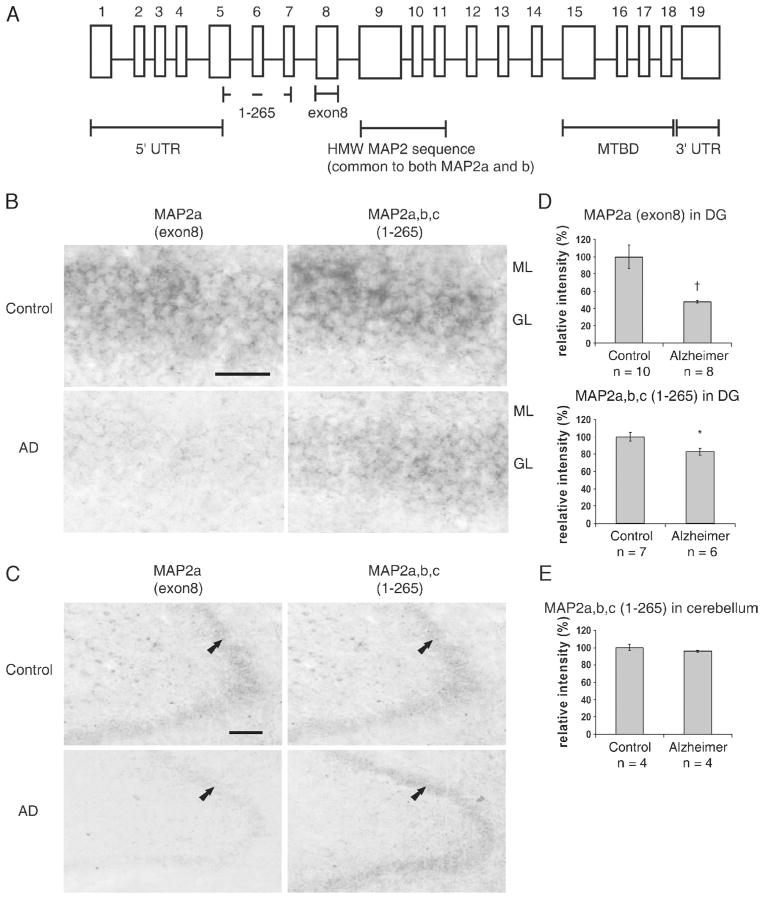
To assess the mRNA expression of the mature neuronal marker MAP2a in dentate gyrus, in situ hybridization histochemistry using cRNA probes of MAP2 exon 8 was performed on 10 control and 8 AD cases. The controls included C8, C14, and C15, which had been found to be immunohistochemically negative for HMW-MAP2 isoforms (C2 could not be studied because of unavailability of sections). cRNA probe of exon 8 was chosen because it is specific to MAP2a (21–23). In rat brain, MAP2a expression coincides with the time of synaptic formation and has been observed only in mature neurons (15). The first 265 bases (1–265) are common to all the MAP2 isoforms, including MAP2a to c (24), and the cRNA probe of MAP2 1 to 265 was used as a general MAP2 marker (Fig. 3A). For this analysis, we studied 7 controls (including C8, C14, and C15) and 6 AD cases. The level of MAP2a (exon 8) mRNA in the dentate gyrus was remarkably decreased in all AD cases compared with control cases (52%; p < 0.01), whereas the level of MAP2a to c (1–265) mRNA in the dentate gyrus was only approximately 17% (p < 0.05) decreased (Fig. 3B–D), indicating that it is the level of MAP2a mRNA that is mostly decreased, but that the level of MAP2c mRNA is minimally affected in AD dentate gyrus as compared with the controls. In 4 of 10 control cases studied, the expression of MAP2 exon 8 mRNA in dentate gyrus was as low as that of AD cases. Of these 4 cases, 3 (C8, C14, and C15) were also negative for MAP2a and b in immunohistochemistry, whereas C6 was immunohistochemically positive.
FIGURE 3.
(A) Schematic representation of the human microtubule-associated protein (MAP)2 gene. The exons are shown as boxes separated by the introns (solid lines). Under bars show the location of the probes and some of the important regions of the MAP2 gene. (B, C) Representative images of expressions of MAP2 exon 8 and MAP2 1 to 265 mRNAs in Alzheimer disease (AD) and control dentate gyri (AD4 and C11) as determined by digoxigenin-labeled in situ hybridization. (B) In the control, MAP2 exon 8 and MAP2 1 to 265 mRNAs (upper left and right images, respectively) were robustly expressed in the cytoplasm of the granular cell layer, whereas in AD, mostly exon 8 mRNA expression was reduced (lower left and right images). High magnification, scale bar = 50 μm. (C) Low magnification, scale bar = 500 μm. (D) Microtubule-associated protein 2 exon 8 and MAP2 1 to 265 mRNA signals from AD and control dentate gyrus (DG) were quantitated using ImageJ software. Mean ± SEM of quantitated intensity relative to control. Differences between AD and control cases were analyzed statistically by 2-tailed t-test (*, p < 0.05; †, p < 0.01). (E) Microtubule-associated protein 2 1 to 265 mRNA signals from AD and control cerebella. MTBD, microtubule binding domain; UTR, untranslated region; HMW, high molecular weight.
No difference was observed between AD and control cases in the expression of MAP2a to c (1–265) mRNA in cerebellum that served as an internal tissue control (Fig. 3E). This result suggests that the total MAP2 expression is not decreased in the nonaffected areas of the brain in AD. Furthermore, no hybridization signals were detected in the control experiments when sense RNA probes were used to indicate the specificity of the 2 anti-sense probes (data not shown).
DISCUSSION
Neurogenesis occurs throughout adult human life, especially in the dentate gyrus of the hippocampal formation (4). The hippocampus is one of the most affected areas in age-associated neurodegeneration and is critical for learning and memory (28, 29). The balance between neurogenesis and neurodegeneration in the hippocampus is critical for the maintenance of normal learning and memory (6). These cognitive abilities, however, require not only the birth of new neurons to replace the lost cells in the hippocampus but also the differentiation of these newborn cells into functional neurons. The present study demonstrates for the first time that this latter requirement, that is, differentiation of newly born cells into mature neurons, is compromised in AD.
Using immunohistochemistry, we found that the expression of the mature neuronal marker HMW-MAP2 is dramatically decreased in the dentate gyrus in AD. In addition, in situ hybridization demonstrated that MAP2a mRNA is only minimally expressed, whereas that of total MAP2, including the immature neuronal marker MAP2c, is decreased only slightly. Taken together, these findings suggest that the levels of the immature neuronal marker MAP2c are probably unchanged or increased in the dentate gyrus in AD. Thus, the newly generated neurons apparently do not mature into fully functional neurons. These findings are consistent with previous reports that have shown an increase in levels of the immature neuronal markers double-cortin, polysialylated nerve cell adhesion molecule, neurogenic differentiation factor, and TUC-4 in the hippocampus (11) and a decrease in the length and branching of dendrites and spine density of the dentate gyrus in AD (30).
Particularly in the hippocampus in AD brain, a marked imbalance of neurotrophic factors, including increased level of FGF-2, has been reported (9, 10). Previously, we have also shown that elevated levels of FGF-2 increase cell division and the level of nestin but decrease the levels of the neuronal lineage markers Tuj1 and MAP2a and b in adult hippocampal progenitor cells in culture (19, 20), indicating that an elevated level of FGF-2 drives the adult hippocampal progenitor cells toward an undifferentiated, actively dividing developmental stage. The marked decrease in the expression of MAP2a and b, but not MAP2c, in AD dentate gyrus found in this study is consistent with these reports.
The hippocampal formation, and especially the entorhinal cortex, CA1, and subiculum, are among the areas that are affected first and most severely in AD, whereas the granular layer of the dentate gyrus has generally been found to be spared (28, 31). The dramatic and specific decrease of the HMW-MAP2 isoforms in the AD dentate gyrus is, therefore, of great interest but difficult to explain. It is unlikely that the present findings are due to poor tissue viability because there was no loss of the MAP2c expression. Furthermore, the rest of the hippocampus was robustly stained by all antibodies, including those to the HMW-MAP2 isoforms. A review of the summaries of the clinical histories and neuropathology reports also did not reveal any condition besides plaques and tangles that might have been different between the AD and control groups. Although most AD cases had vascular or microvascular abnormalities that might have also affected the hippocampus, these findings were also seen in most of the age-matched controls. There was also no difference between AD and controls with respect to the occurrence of chronic congestive heart disease or liver failure. On the other hand, a connection to plaque and tangle pathology seems quite unlikely because 4 of the 15 control cases also exhibited an AD-like lack of the adult MAP2 isoforms. Thus, the decrease in HMW-MAP2 in the AD dentate gyrus is probably mainly attributable to an arrest in maturation of the developmentally immature granule cells in reaction to changes in the microenvironment and the AD-like control cases might represent presymptomatic AD.
In conclusion, using immunohistochemistry and in situ hybridization, we have determined that expression of the mature neuronal marker HMW-MAP2 is dramatically decreased in the dentate gyrus in AD, whereas the total MAP2, including expression of the immature neuronal marker MAP2c, is minimally decreased. These findings suggest that newly generated neurons in AD dentate gyrus do not fully mature, although neuroproliferation is increased. Promotion of the differentiation of immature neurons in the dentate gyrus into mature neurons might help improve cognition in AD patients.
Acknowledgments
We thank Drs. Tom Beach and Lucia Sue, Sun Health Research Institute, for the brain tissues; and Janet Murphy for secretarial assistance, including the preparation of the article.
This study was supported in part by the New York State Office of Mental Retardation and Developmental Disabilities, NIH grant AG019158, and the T.L.L. Temple Foundation Discovery Award for Alzheimer’s Disease Research from the Alzheimer’s Association.
References
- 1.Glenner GG, Wong CW. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–90. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 2.Grundke-Iqbal I, Iqbal K, Tung YC, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–17. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundke-Iqbal I, Iqbal K, Quinlan M, et al. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–89. [PubMed] [Google Scholar]
- 4.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–17. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 5.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–95. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 6.Gould E, Beylin A, Tanapat P, et al. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–65. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 7.Shors TJ, Miesegaes G, Beylin A, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–76. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 8.Snyder JS, Hong NS, McDonald RJ, et al. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–52. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Hock C, Heese K, Hulette C, et al. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57:846–51. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- 10.Stopa EG, Gonzalez AM, Chorsky R, et al. Basic fibroblast growth factor in Alzheimer’s disease. Biochem Biophys Res Commun. 1990;171:690–96. doi: 10.1016/0006-291x(90)91201-3. [DOI] [PubMed] [Google Scholar]
- 11.Jin K, Peel AL, Mao XO, et al. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:343–47. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shafit-Zagardo B, Kalcheva N. Making sense of the multiple MAP-2 transcripts and their role in the neuron. Mol Neurobiol. 1998;16:149–62. doi: 10.1007/BF02740642. [DOI] [PubMed] [Google Scholar]
- 13.Crandall JE, Fischer I. Developmental regulation of microtubule-associated protein 2 expression in regions of mouse brain. J Neurochem. 1989;53:1910–17. doi: 10.1111/j.1471-4159.1989.tb09261.x. [DOI] [PubMed] [Google Scholar]
- 14.Nunez J. Immature and mature variants of MAP2 and tau proteins and neuronal plasticity. Trends Neurosci. 1988;11:477–79. doi: 10.1016/0166-2236(88)90004-5. [DOI] [PubMed] [Google Scholar]
- 15.Riederer B, Matus A. Differential expression of distinct microtubule-associated proteins during brain development. Proc Natl Acad Sci U S A. 1985;82:6006–9. doi: 10.1073/pnas.82.17.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jalava NS, Lopez-Picon FR, Kukko-Lukjanov TK, et al. Changes in microtubule-associated protein–2 (MAP2) expression during development and after status epilepticus in the immature rat hippocampus. Int J Dev Neurosci. 2007;25:121–31. doi: 10.1016/j.ijdevneu.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). II Part, Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 18.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–97. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Tung YC, Li B, et al. Trophic factors counteract elevated FGF-2–induced inhibition of adult neurogenesis. Neurobiol Aging. 2007;28:1148–62. doi: 10.1016/j.neurobiolaging.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 20.Tatebayashi Y, Lee MH, Li L, et al. The dentate gyrus neurogenesis: A therapeutic target for Alzheimer’s disease. Acta Neuropathol (Berl) 2003;105:225–32. doi: 10.1007/s00401-002-0636-3. [DOI] [PubMed] [Google Scholar]
- 21.Chung WJ, Kindler S, Seidenbecher C, et al. MAP2a, an alternatively spliced variant of microtubule-associated protein 2. J Neurochem. 1996;66:1273–81. doi: 10.1046/j.1471-4159.1996.66031273.x. [DOI] [PubMed] [Google Scholar]
- 22.Kalcheva N, Weidenheim KM, Kress Y, et al. Expression of microtubule-associated protein–2a and other novel microtubule-associated protein–2 transcripts in human fetal spinal cord. J Neurochem. 1997;68:383–91. doi: 10.1046/j.1471-4159.1997.68010383.x. [DOI] [PubMed] [Google Scholar]
- 23.Shafit-Zagardo B, Kalcheva N, Dickson D, et al. Distribution and subcellular localization of high-molecular-weight microtubule-associated protein–2 expressing exon 8 in brain and spinal cord. J Neurochem. 1997;68:862–73. doi: 10.1046/j.1471-4159.1997.68020862.x. [DOI] [PubMed] [Google Scholar]
- 24.Kalcheva N, Albala J, O’Guin K, et al. Genomic structure of human microtubule-associated protein 2 (MAP-2) and characterization of additional MAP-2 isoforms. Proc Natl Acad Sci U S A. 1995;92:10894–98. doi: 10.1073/pnas.92.24.10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imaizumi K, Tsuda M, Wanaka A, et al. Differential expression of sgk mRNA, a member of the Ser/Thr protein kinase gene family, in rat brain after CNS injury. Brain Res Mol Brain Res. 1994;26:189–96. doi: 10.1016/0169-328x(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 26.Tanimukai H, Grundke-Iqbal I, Iqbal K. Inhibitors of protein phosphatase–2A: Topography and subcellular localization. Brain Res Mol Brain Res. 2004;126:146–56. doi: 10.1016/j.molbrainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Dolci C, Montaruli A, Roveda E, et al. Circadian variations in expression of the trkB receptor in adult rat hippocampus. Brain Res. 2003;994:67–72. doi: 10.1016/j.brainres.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 28.West MJ, Coleman PD, Flood DG, et al. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–72. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- 29.Morris RG, Moser EI, Riedel G, et al. Elements of a neurobiological theory of the hippocampus: The role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:773–86. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Einstein G, Buranosky R, Crain BJ. Dendritic pathology of granule cells in Alzheimer’s disease is unrelated to neuritic plaques. J Neurosci. 1994;14:5077–88. doi: 10.1523/JNEUROSCI.14-08-05077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobinski M, Wegiel J, Tarnawski M, et al. Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:414–20. doi: 10.1097/00005072-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]