Abstract
Peripheral neuropathic pain arises from trauma to sensory nerves. Other types of acute neurotrauma such as stroke and spinal cord injury are treated immediately, largely to prevent secondary damage. To pursue the possibility that neuropathic pain may also be amenable to early treatment, a rat model of neuropathic pain was induced using a 2-mm polyethylene cuff implanted around one sciatic nerve. Within 24 hours, hypersensitivity to von Frey hair stimulation appeared, as indicated by decreased paw withdrawal thresholds. When the cuff was removed 24 hours after implantation, readings returned to pre-implantation levels starting as early as day 18. When the cuff was removed after 4 days, there was a period of initial hypersensitivity, and then an increase toward baseline at two time points near the end of the study; therefore, only a partial recovery toward pre-implantation values occurred. Having established that a temporal reversal can occur, the next step examined possible pharmacological reversal. The tachykinin NK1 receptor antagonist, CP-96,345, produced a minor increase in withdrawal thresholds in animals with the cuff left permanently implanted. To determine the effect of early and repeated administration of CP-96,345, it was given daily on days 1–4. The cuff was removed on day 4. Six days later, readings showed reversal of tactile hypersensitivity. We suggest that persistent neuropathic pain occurs from processes that develop over several hours and days, and that some of these processes may be prevented by early medical intervention. Thus, nerve injury in the context of chronic neuropathic pain should be treated in a similar manner to nerve injury resulting from stroke, spinal cord injury, and other types of neurotrauma. We suggest that effective medical intervention within the first few hours after nerve injury may spare a patient from a chronic debilitating pain that may be refractory to later therapies.
Keywords: neurotrauma, neuroplasticity, nerve injury, neuropathy, chronic pain, tactile hypersensitivity
Introduction
Neuropathic pain is noteworthy for its permanence and its relative resistance to medical treatment. As the effects of neurotrauma in other conditions can be at least partially reversible, we set out to determine whether the tactile hypersensitivity that develops in a rat model of peripheral neuropathic pain could be reversed physiologically. Following stroke, most patients undergo an immediate period of treatment, beginning within the first few hours, with the aim of restoring function.1–3 As soon as the symptoms of a stroke are recognized, pharmacotherapy, consisting of thrombolytic and neuroprotective agents, is started in order to minimize damage to neurons, limiting the biochemical cascade that leads to irreversible injury and cell death.4,5 Spinal cord injury is also treated immediately.6 Evidence suggests that there are primary and secondary events that give rise to nerve cell death following spinal cord injury. Primary injury usually consists of spinal cord compression and the displacement and shock absorbed by the spinal cord.6 Secondary injury consists of the biochemical and molecular events that lead to cell death.6,7 Controlling the extent to which secondary injury occurs has a positive impact on patient outcome.6,7
The objective of the present study was to determine whether there might be a window of opportunity during which intervention might minimize or prevent the development of chronic tactile hypersensitivity in an animal model of neuropathic pain. Thus, the rationale for our study was to determine whether there may be a beneficial effect of removing the sciatic nerve cuff in the Mosconi and Kruger model,8 at various times after model induction. Ectopic activity in sensory fibers can be induced by irritation of the axons and it was rationalized, therefore, that by using the Mosconi and Kruger model of neuropathic pain, in which a polyethylene cuff is placed around the sciatic nerve, and removing the cuff at specific intervals after model induction, this would remove the peripheral irritation and possibly reverse or lessen the development of tactile hypersensitivity. When it became apparent that this reversal could take place, a study was done to investigate possible mechanisms leading to the irreversibility of the model, and the tachykinin NK1 receptor antagonist, CP-96,345, was given during the onset phase of the model.
Materials and methods
Animals
Male Sprague-Dawley rats (Charles River, QC) were housed in pairs and maintained on a 12-hour light cycle, with food and water available ad libitum. All procedures complied with the “Guidelines for the Care and Use of Experimental Animals”, Volumes I and II, of the Canadian Council on Animal Care. All procedures were also approved by the University Council on Animal Care, University of Western Ontario (London, ON) and the Animal Research Ethics Board, McMaster University (Hamilton, ON). Rats used for the permanent, 1-day and 4-day cuff models weighed 200–270 g on the day of cuff implantation. Rats used for the antagonist studies (in the permanent and 4-day cuff models) weighed 130–150 g on the day of cuff implantation.
Model induction
The method of inducing peripheral neuropathy was by implantation of a single cuff around the sciatic nerve, modified from the method described by Mosconi and Kruger,8 where two to four cuffs were used. Rats were anesthetized with a combination of ketamine (Ketalean; 5 mg/100 g; Bimeda-MTC Animal Health, Inc, Cambridge, ON), xylazine (Rompun; 0.5 mg/100 g; Bayer HealthCare, Toronto, ON), and acepromazine (Atravet; 0.1 mg/100 g; Ayerst Veterinary Laboratories, Guelph, ON) given intraperitoneally (ip). The left sciatic nerve was exposed after blunt dissection of overlying muscle and freed from surrounding tissue. A cuff made of a 2-mm segment of polyethylene tubing (Intramedic PE-90; Clay Adams, Division of Becton Dickinson, Parsippany, NJ) slit longitudinally was fitted around the nerve. Subsequently, the muscle was sutured, and the skin closed using suture clips. Antibiotic ointment (Furacin, nitrofurazone 0.2%; Vetoquinol N-A, Inc; Lavaltrie, QC) was applied over the wound, and 0.03 mL of antibiotic (Tribrissen 24%, trimethoprim-sulfadiazine; Schering Canada, Inc, Pointe Claire, QC) was injected subcutaneously. Animals were placed under a heating lamp until they recovered from the anesthetic, and then returned to their home cages.
Cuff removal
Animals were anesthetized and the sciatic nerve was exposed as described above. The cuff was located and then removed using fine forceps while care was taken not to damage the nerve. The muscle and skin were sutured as described above and the same postsurgical care procedures were followed. Cuff removal was done on either day 1 or day 4 after implantation.
Withdrawal threshold measurement
The testing chamber consisted of a 30 × 30 × 30 cm Plexiglas® box with a clear Plexiglas® floor. This floor contained 0.5-cm diameter holes that were spaced 1.5 cm apart, as described previously,9 and was positioned over an angled mirror that allowed an unobstructed view of the rat paws. Animals were placed in pairs in the testing chamber and allowed to acclimatize for 30 minutes prior to testing. Von Frey filaments (Stoelting Co, Wood Dale, IL) were applied to the soft tissue of the plantar surface of the hind paw to determine the withdrawal threshold. The first filament applied corresponded to a force of 2 g. If a negative response (no movement) was observed, a filament exerting greater force was then applied, and if a positive response (paw withdrawal) was observed, a filament of lesser force was applied next. Each filament was applied three times, at 3-second intervals. A 50% response threshold was calculated according to the response pattern observed described by Chaplan et al.10 The maximum score possible was 15 g, and the minimum was 0.25 g.
Drugs
All drugs were administered ip at a volume of 0.1 mL/100 g body weight. CP-96,345 (tachykinin NK1 receptor antagonist; a gift from Pfizer Central Research, Groton, CT) was given at a dose of 5 mg/kg, dissolved in physiological saline. SR 48969 (NK2 receptor antagonist; a gift from Sanofi-Synthelabo, Montpellier, France) was given at a dose of 5 mg/kg dissolved in distilled water. SR 142801 (NK3 receptor antagonist; a gift from Sanofi-Synthelabo) was given at a dose of 5 mg/kg and dissolved in 10% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St Louis, MO) in distilled water. Postdrug testing was done 30 minutes after injection of each antagonist.
Statistics
Data were analyzed using a repeated measures one-way analysis of variance (ANOVA) with the Bonferroni test for post hoc comparisons across days (Figures 1–3 and 5). Paired t-tests were used to compare pre- and postdrug values on each testing day (Figures 4 and 5). Analyses were performed using Prism, GraphPad Software, Inc (v 4.03; San Diego, CA).
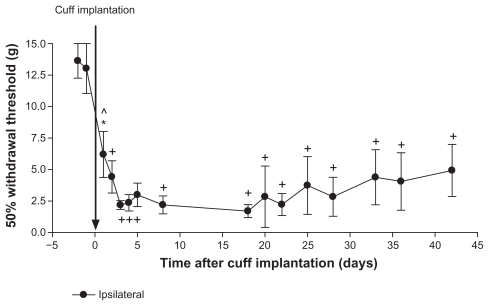
Figure 1.
Permanent cuff implantation. On day 0 rats (n = 6) were implanted with a 2-mm polyethylene cuff around the left sciatic nerve, which was kept in place for the duration of the study. Paw withdrawal thresholds were measured using von Frey filaments before and after model induction. There was a significant decrease in withdrawal thresholds after cuff implantation compared to pre-induction baseline values (days–2 and –1). No differences were observed between any of the testing days after cuff implantation.
Notes: Values are shown as means ± standard error of the mean of the ipsilateral paw. *P < 0.05; ^P < 0.01; +P < 0.001 compared to pre-implantation values.
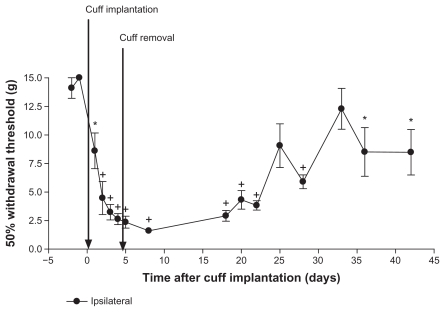
Figure 3.
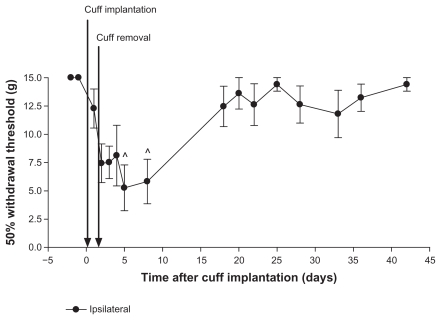
Four day cuff implantation. Rats (n = 7) were implanted with a cuff on day 0, and the cuff was removed on day 4. Paw withdrawal thresholds were measured using von Frey filament stimulation. There was a significant decrease in paw withdrawal thresholds after cuff implantation on most days compared to pre-induction baselines. Thresholds on days 25 and 28 were not significantly different from pre-induction baselines.
Notes: Values are shown as means ± standard error of the mean of the ipsilateral paw. *P < 0.05; +P < 0.001 compared to pre-implantation values.
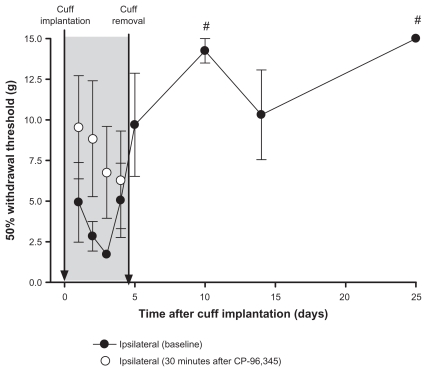
Figure 5.
Administration of a tachykinin NK1 receptor antagonist for 4 days in the 4-day cuff model. All rats (n = 4) were implanted with a cuff on day 0. Each day, for 4 days after cuff implantation, withdrawal thresholds were measured before (closed circles) and 30 minutes after (open circles) administration of CP-96,345, an NK1 receptor antagonist; shaded area. The cuff was removed on day 4, and treatment with CP-96,345 was stopped. There were no significant differences between predrug baselines and postdrug values on days 1 to 4. Baseline withdrawal thresholds were measured up to day 25 postcuff implantation. Withdrawal thresholds on days 10 and 25 were significantly higher than values on days 1, 2, 3, and 4 (#P at least <0.05).
Note: Values are shown as means ± standard error of the mean of the ipsilateral paw.
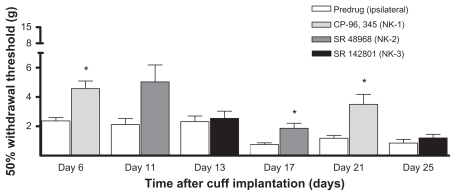
Figure 4.
Tachykinin receptor antagonists. Rats (n = 6) were implanted with a cuff on day 0 and readings of withdrawal thresholds were measured on selected days over the next 39 days. Baseline readings were stable on all testing days starting on day 1 to day 39 (there were no significant differences and all values were significantly lower than pre-implantation levels; data not shown). The effect of the antagonists to the tachykinin NK1, NK2 and NK3 receptors were tested: CP-96,345 on days 6 and 21; SR 48968 on days 11 and 17; and SR 142801 on days 13 and 25, respectively. Withdrawal thresholds were significantly higher than pre-drug values after the administration of CP-96,345 (on days 6 and 21; *P < 0.05) and SR 48968 (on day 17 only; *P < 0.05).
Note: Values are shown as means ± standard error of the mean of the ipsilateral paw.
Results
Effect of unilateral cuff implantation on withdrawal thresholds
Paw withdrawal thresholds were measured on days–2 and –1 before surgery on day 0 and for up to 42 days after cuff implantation (n = 6; Figure 1). There was a significant decrease in withdrawal thresholds on all days after cuff implantation compared to pre-induction baseline values (all P < 0.01 compared to days–2 and –1, with the exception of day–1 vs 1, P < 0.05). No significant differences were observed for all pair-wise comparisons between each of the testing days after cuff implantation. Therefore, all values after cuff implantation were lower than pre-induction values, yet not different from each other. Contralateral paw withdrawal thresholds were not different before or after cuff implantation (data not shown). Sham surgeries were not preformed as this experiment serves as the control group for the subsequent cuff removal studies (Figures 2, 3, and 5). In an experiment where the cuff was implanted near, but not around the sciatic nerve, no effects on withdrawal thresholds were observed (data not shown).
Figure 2.
One day cuff implantation. Rats (n = 6) were implanted with a cuff on day 0, and the cuff was removed on day 1. Paw withdrawal thresholds were measured using von Frey filament stimulation. There was a significant decrease in withdrawal thresholds on days 5 and 8 compared to pre-induction baseline values (^P < 0.01 compared to days–2 or –1). All subsequent values were not significantly different from pre-induction baselines.
Note: Values are shown as means ± standard error of the mean of the ipsilateral paw.
Effect of removing the cuff 24 hours after implantation
Paw withdrawal thresholds were measured on days–2 and –1 before surgery on day 0 and for up to 42 days after cuff implantation (n = 6; Figure 2). The cuff was removed on day 1. There was a significant decrease in withdrawal threshold on days 5 and 8 compared to pre-induction baseline values (days–2 and –1), all P < 0.01. Withdrawal thresholds on days 5 were significantly lower than those on days 20, 25, 36, and 42 (all P < 0.05); day 8 was lower than days 25 and 42 (P < 0.05). After this period of hypersensitivity, there was an increase in withdrawal thresholds, such that values observed starting at day 18 were not significantly different from pre-implantation values. Thus withdrawal thresholds transiently decreased on days 5 and 8, with a return to pre-induction values as early as day 18. Contralateral paw withdrawal thresholds after cuff implantation were not different from pre-implantation values (data not shown).
Effect of removing the cuff 4 days after implantation
Paw withdrawal thresholds were measured on days–2 and –1 before surgery on day 0 and for up to 42 days after cuff implantation (n = 7; Figure 3). The cuff was removed on day 4. There was a decrease in paw withdrawal threshold after cuff implantation compared to pre-induction baselines. With the exception of days 25 and 33, withdrawal thresholds were significantly lower on all days after cuff implantation compared to pre-induction baseline values (all P < 0.05 compared to days–2 and –1). Values near the end of the study, at days 25 and 33, were not significantly different from baseline values (days–2 and –1). Therefore, there was a partial recovery toward pre-induction values. Contralateral paw withdrawal thresholds were significantly lower than pre-implantation values only on day 8 (P < 0.05 compared to day–2 or –1; data not shown).
Effect of tachykinin receptor antagonists in permanently-implanted rats
Rats (n = 7) were implanted with a cuff on day 0 and withdrawal thresholds were measured across a 39-day observation period. One rat was removed from the study after day 13 (and all previous values were omitted from the analysis) because the ipsilateral paw did not show the typical signs of model induction (curled toes, redness, guarding, overgrown nails). As seen in Figure 1, there was a significant decrease in withdrawal thresholds after cuff implantation, which persisted for at least 39 days (day–1 versus each testing day, P < 0.001; n = 6; data not shown). Tachykinin receptor antagonists were given on the following days: CP-96,345 (NK1) on days 6 and 21; SR 48968 (NK2) on days 11 and 17; and SR 142801 (NK3) on days 13 and 25. Withdrawal thresholds were measured before and after drug administration (Figure 4). Withdrawal thresholds were significantly higher than predrug values after the administration of CP-96,345 (on both days tested, 6 and 21) and SR 48968 (on day 17 only); all P < 0.05. No effect of SR 48968 was observed on day 11, and there were no effects of SR 142801 on testing day, 13 or 25. The antagonists did not have any effect on the contralateral paw, or on naïve rats (data not shown). Administration of the antagonists did not have an effect on the progression of the permanent cuff model, as no significant differences were observed for all pair-wise comparisons between each of the testing days after cuff implantation (data not shown).
Effect of the NK1 receptor antagonist on development of hypersensitivity
Given that CP-96,345 had the most consistent effect (Figure 4), it was used to determine if early, repeated administration of this antagonist would have an effect on the development of hypersensitivity in this model of peripheral neuropathy. Figure 5 shows the withdrawal thresholds of the ipsilateral paw of rats (n = 4) that had a cuff implanted on day 0 and removed on day 4. Rats were treated daily on days 1 to 4 with CP-96,345. There were no significant differences between pre- and postdrug values on days 1 to 4. Baseline withdrawal thresholds were measured after cuff removal and drug administration was stopped, up to day 25 after cuff implantation. A repeated measures one-way ANOVA of baseline readings showed that withdrawal thresholds on day 10 were significantly higher than values on days 1 (P < 0.05), 2, 3 (P < 0.01), and 4 (P < 0.05). Values on day 25 were also significantly higher than values on days 1 (P < 0.05), 2 (P < 0.01), 3 (P < 0.001), and 4 (P < 0.05).
Discussion
These results demonstrate that the tactile hypersensitivity in an animal model of neuropathic pain can be reversed physiologically, by removing the source of the irritation to the sciatic nerve. It was found that while removal of the cuff at 24 hours after model induction led to full recovery to baseline values, if the cuff was removed after 4 days there was also a trend toward recovery, but it was not fully achieved.
The fact that removal of the cuff at 4 days resulted in only partial recovery suggests that the presence of the cuff set in place mechanisms that persist even after the source of the nerve irritation is no longer present. This suggests further that there is a difference between a short-term and a long-term irritation or injury to the nerve in terms of hypersensitivity to tactile stimulation to the peripheral receptive fields. Thus, there remains a “shadow” hypersensitivity when the cuff is removed after 4 days.
Full recovery occurred with removal of the cuff at 24 hours, in contrast to the partial recovery observed when the cuff was removed after 4 days. This suggests that the time of removal of the irritant condition is critical, and prompts the hypothesis that there may be an optimal window of time during which intervention may reduce and even prevent the development of a chronic tactile hypersensitivity, and that in humans there may be a window of time for effective therapeutic intervention to prevent the development of a persistent neuropathic pain. This may be a critical issue in view of the relative resistance of this type of pain in humans.
As it has been established that substance P is an important neuromodulator involved in the transmission of pain, the effects of antagonists to the receptors at which substance P acts were tested. Antagonists to the tachykinin NK1, NK2, and NK3 receptors were administered to rats with a permanent cuff. CP-96,345 (the NK1 receptor antagonist) significantly increased withdrawal thresholds on both days tested, ie, on days 6 and 21 after cuff implantation. The effect was modest, as withdrawal thresholds remained below 5 g. SR 48968, the NK2 receptor antagonist, also had a marginal effect, but only on day 17, not on day 11. These results indicate that substance P may be involved in the maintenance phase of neuropathic pain, similar to a report by Cahill and Coderre.11
In view of the observation that CP-96,345 had the most consistent effect, it was used to investigate possible mechanisms underlying the full versus partial reversibility of the model. The rationale for selecting this approach was that if it proved to be effective this might provide an avenue to explore a therapeutic approach to prevent or limit the development of neuropathic pain following peripheral nerve damage. Thus, in rats in which the cuff was to be removed 4 days after implantation, the antagonist was given starting 1 day after model induction and continuing daily until the cuff was removed at day 4. During this period there was a tendency for the readings 30 minutes after administration to be numerically higher (though not significant statistically) than those taken just before administration, possibly suggesting that a process is occurring in which NK1 receptors are involved in the induction of a state of hypersensitivity, particularly during the earliest days. Interestingly, the relative lack of an acute effect of CP-96,345 on day 4 suggests that other mechanisms may have begun to be recruited by this time. Intervention by early and repeated administration of the NK1 receptor antagonist facilitated recovery of the 4-day cuff model, within 6 days of the end of drug treatment. This supports the hypothesis that there may be a window of time during which the development of chronic pain may be prevented, and indicates that NK1 receptors may be intimately involved in establishing the permanence of hypersensitivity in this animal model of peripheral neuropathic pain. The data also support the concept that treatment for neuropathic pain should begin before the onset of symptoms whenever any damage to a nerve occurs or is suspected. Early, aggressive treatment with an appropriate drug may reduce the incidence or severity of the ensuing neuropathic pain.
Evidence from other types of trauma to the nervous system indicates that early treatment is of critical importance in determining outcome. Following a stroke, it is believed that the adequacy of treatment given in the first 24 to 48 hours is the most important factor for optimal outcome.4,12–15 Antithrombolytic medication to reestablish circulation to the affected areas of the central nervous system and thus to reduce secondary effects of the stroke, is effective only if given within 3 hours following the stroke.14,16 The onset of irreversible damage depends greatly on the duration and severity of ischemia.4 Neuroprotective agents are typically administered after a stroke to increase the resistance of the brain to ischemic damage.2,4,12
Following spinal cord injury, it is also standard procedure to attempt to reestablish function through early treatment.7 Evidence from basic science animal studies as well as from clinical studies suggests an overall positive clinical benefit of early surgical intervention.17 In the case of humans, surgical intervention often consists of stabilizing the spinal column to prevent further damage.6 However, acute spinal cord injury involves primary and secondary mechanisms of injury.18 While efforts are taken to reduce primary injury,19,20 steps are also taken to prevent the secondary injury resulting from changes in the tissues and the circulation.6,21
In the animal model of neuropathic pain used in this study, the presence of the sciatic nerve cuff appears to initiate processes that are fully reversible if the primary source of the injury is removed within 24 hours. However, these processes are only partially reversible if the primary injury is allowed to last for 4 days. In this case, the cuff leads to changes that persist even in its absence. As the onset of secondary mechanisms is a function of the duration of the injury, the establishment of neuropathic pain may be viewed as a degenerative process. The antagonist studies show that NK1 receptors may be involved in this degenerative process that gives rise to hypersensitivity to tactile stimulation, which is characteristic of this neuropathic pain model.
These results suggest that peripheral drive may be playing a governing role in neuropathic pain, as suggested recently.22 Spontaneous discharge in sensory fibers in models of peripheral neuropathy has been proposed in a number of models. Several studies have reported that systemic administration of lidocaine decreases ectopic activity recorded from injured peripheral sensory fibers.23–26 Furthermore, systematic reviews of human studies using systemic administration of local anesthetic drugs support their use for neuropathic pain.27,28 A governing role of afferent drive is also reflected in the data of Kim and Chung29 where nerve block transiently reversed the behavioral hypersensitivity to tactile stimulation. A predominant role of peripheral drive may also be reflected in a study by Sun and colleagues,30 who found that ectopic discharges in injured sensory nerves were highly correlated with hypersensitivity in the first 24 hours after spinal nerve ligation, and they suggest that ectopic discharge may be triggering neuropathic pain. The development of thermal and tactile hypersensitivity is permanently inhibited if spontaneous afferent activity is blocked immediately after nerve injury (for at least 3–5 days), again pointing to early spontaneous afferent activity as a trigger for pain behaviors.31 It is also interesting to note that previous studies have used the chronic constriction model of neuropathic pain, followed by decompression (removal of the ligatures) at postoperative week 4. At 8 weeks, they observed a reversal of thermal hyperalgesia and mechanical allodynia,32–34 an increase in the skin innervation index of substance P,33 an increase to normal levels of substance P and the δ-opioid receptor in the dorsal horn,34 and a decrease to normal levels of extracellular signal-regulated kinase (ERK) activation in the dorsal horn.32
In conclusion, these studies and our present data suggest that upon peripheral nerve injury a window of opportunity for effective medical intervention may be open, and, as time passes, the window closes progressively until, at some point, it is shut and the pain is entrenched as chronic neuropathic pain. In our model, full, spontaneous reversal with cuff removal after 24 hours but not after 4 days, and pharmacological reversal with cuff removal after 4 days, suggests that medical intervention may have a window of opportunity for effective treatment. We therefore suggest that the onset of chronic neuropathic pain is a process that occurs over several hours and days, and that effective intervention within the first few hours after nerve injury may spare a patient from a chronic debilitating neuropathic pain that may be refractory to later therapeutic interventions.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research and the Ontario Graduate Scholarship in Science and Technology. The generous gifts of CP-96,345 from Pfizer Central Research, and SR 48968 and SR 142801 from Sanofi-Synthelabo are gratefully acknowledged. The authors thank Suzanne MacDonald for assistance in setting up the protocol for this study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bassi P, Lattuada P, Tonietti S. Early phase combined therapeutic management of acute ischaemic stroke. Neurol Sci. 2005;26(Suppl 1):S29–S30. doi: 10.1007/s10072-005-0400-1. [DOI] [PubMed] [Google Scholar]
- 2.Nassisi D. Acute stroke: emergency management and future interventions. Mt Sinai J Med. 1997;64:241–248. [PubMed] [Google Scholar]
- 3.Sharma VK, Teoh HL, Wong LY, Su J, Ong BK, Chan BP. Recanalization therapies in acute ischemic stroke: pharmacological agents, devices, and combinations. Stroke Res Treat. 2010 doi: 10.4061/2010/672064.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albers GW. Rationale for early intervention in acute stroke. Am J Cardiol. 1997;80(4C):4D–10D. doi: 10.1016/s0002-9149(97)00579-1. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL. Target brain: neuroprotection and neurorestoration in ischemic stroke. Rev Neurol Dis. 2010;(7 Suppl 1):S14–21. [PubMed] [Google Scholar]
- 6.Carlson GD, Gordon C. Current developments in spinal cord injury research. Spine J. 2002;2:116–128. doi: 10.1016/s1529-9430(01)00029-8. [DOI] [PubMed] [Google Scholar]
- 7.Patel RV, DeLong W, Jr, Vresilovic EJ. Evaluation and treatment of spinal injuries in the patient with polytrauma. Clin Orthop Relat Res. 2004;422:43–54. doi: 10.1097/01.blo.0000130841.41657.d3. [DOI] [PubMed] [Google Scholar]
- 8.Mosconi T, Kruger L. Fixed-diameter polyethylene cuffs applied to the rat sciatic nerve induce a painful neuropathy: ultrastructural morpho-metric analysis of axonal alterations. Pain. 1996;64:37–57. doi: 10.1016/0304-3959(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 9.Pitcher GM, Ritchie J, Henry JL. Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. J Neurosci Meth. 1999;87:185–193. doi: 10.1016/s0165-0270(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 10.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 11.Cahill CM, Coderre TJ. Attenuation of hyperalgesia in a rat model of neuropathic pain after intrathecal pre- or post-treatment with a neurokinin-1 antagonist. Pain. 2002;95:277–285. doi: 10.1016/S0304-3959(01)00410-9. [DOI] [PubMed] [Google Scholar]
- 12.Camarata PJ, Heros RC, Latchaw RE. “Brain attack”: the rationale for treating stroke as a medical emergency. Neurosurgery. 1994;34:144–157. doi: 10.1097/00006123-199401000-00021. Discussion 157–148. [DOI] [PubMed] [Google Scholar]
- 13.ISTCG. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569–1581. [PubMed] [Google Scholar]
- 14.NINDS. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 15.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 16.Saver JL, Smith EE, Fonarow GC, et al. The “golden hour” and acute brain ischemia: presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41:1431–1439. doi: 10.1161/STROKEAHA.110.583815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albert TJ, Kim DH. Timing of surgical stabilization after cervical and thoracic trauma. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;3:182–190. doi: 10.3171/spi.2005.3.3.0182. [DOI] [PubMed] [Google Scholar]
- 18.Young W. Secondary injury mechanisms in acute spinal cord injury. J Emerg Med. 1993;11(Suppl 1):13–22. [PubMed] [Google Scholar]
- 19.La Rosa G, Conti A, Cardali S, Cacciola F, Tomasello F. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503–512. doi: 10.1038/sj.sc.3101627. [DOI] [PubMed] [Google Scholar]
- 20.Kishan S, Vives MJ, Reiter MF. Timing of surgery following spinal cord injury. J Spinal Cord Med. 2005;28:11–19. doi: 10.1080/10790268.2005.11753793. [DOI] [PubMed] [Google Scholar]
- 21.Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 22.Pitcher GM, Henry JL. Governing role of primary afferent drive in increased excitation of spinal nociceptive neurons in a model of sciatic neuropathy. Exp Neurol. 2008;214:219–228. doi: 10.1016/j.expneurol.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chabal C, Russell LC, Burchiel KJ. The effect of intravenous lidocaine, tocainide, and mexiletine on spontaneously active fibers originating in rat sciatic neuromas. Pain. 1989;38:333–338. doi: 10.1016/0304-3959(89)90220-0. [DOI] [PubMed] [Google Scholar]
- 24.Devor M, Wall PD, Catalan N. Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction. Pain. 1992;48:261–268. doi: 10.1016/0304-3959(92)90067-L. [DOI] [PubMed] [Google Scholar]
- 25.Omana-Zapata I, Khabbaz MA, Hunter JC, Bley KR. QX-314 inhibits ectopic nerve activity associated with neuropathic pain. Brain Res. 1997;771:228–237. doi: 10.1016/s0006-8993(97)00770-1. [DOI] [PubMed] [Google Scholar]
- 26.Sotgiu ML, Lacerenza M, Marchettini P. Effect of systemic lidocaine on dorsal horn neuron hyperactivity following chronic peripheral nerve injury in rats. Somatosens Mot Res. 1992;9:227–233. doi: 10.3109/08990229209144773. [DOI] [PubMed] [Google Scholar]
- 27.Abram SE. Neural blockade for neuropathic pain. Clin J Pain. 2000;16(2 Suppl):S56–S61. doi: 10.1097/00002508-200006001-00010. [DOI] [PubMed] [Google Scholar]
- 28.Kalso E, Tramer MR, McQuay HJ, Moore RA. Systemic local-anaesthetic- type drugs in chronic pain: a systematic review. Eur J Pain. 1998;2:3–14. doi: 10.1016/s1090-3801(98)90041-6. [DOI] [PubMed] [Google Scholar]
- 29.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 30.Sun Q, Tu H, Xing GG, Han JS, Wan Y. Ectopic discharges from injured nerve fibers are highly correlated with tactile allodynia only in early, but not late, stage in rats with spinal nerve ligation. Exp Neurol. 2005;191:128–136. doi: 10.1016/j.expneurol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Xie W, Strong JA, Meij JT, Zhang JM, Yu L. Neuropathic pain: early spontaneous afferent activity is the trigger. Pain. 2005;116:243–256. doi: 10.1016/j.pain.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng T, Hsieh Y, Hsieh S. Reversal of ERK activation in the dorsal horn after decompression in chronic constriction injury. Exp Neurol. 2007;206:17–23. doi: 10.1016/j.expneurol.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Tseng TJ, Chen CC, Hsieh YL, Hsieh ST. Effects of decompression on neuropathic pain behaviors and skin reinnervation in chronic constriction injury. Exp Neurol. 2007;204:574–582. doi: 10.1016/j.expneurol.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Tseng TJ, Chen CC, Hsieh YL, Hsieh ST. Influences of surgical decompression on the dorsal horn after chronic constriction injury: changes in peptidergic and delta-opioid receptor (+) nerve terminals. Neuroscience. 2008;156:758–768. doi: 10.1016/j.neuroscience.2008.08.010. [DOI] [PubMed] [Google Scholar]