Abstract
BACKGROUND
Growth factors are proteins secreted by a number of cell types that are capable of modulating cellular growth, proliferation and cellular differentiation. It is well accepted that uterine cellular events such as proliferation and differentiation are regulated by sex steroids and their actions in target tissues are mediated by local production of growth factors acting through paracrine and/or autocrine mechanisms. Myometrial mass is ultimately modified in pregnancy as well as in tumour conditions such as leiomyoma and leiomyosarcoma. Leiomyomas, also known as fibroids, are benign tumours of the uterus, considered to be one of the most frequent causes of infertility in reproductive years in women.
METHODS
For this review, we searched the database MEDLINE and Google Scholar for articles with content related to growth factors acting on myometrium; the findings are hereby reviewed and discussed.
RESULTS
Different growth factors such as epidermal growth factor (EGF), transforming growth factor-α (TGF-α), heparin-binding EGF (HB-EGF), acidic fibroblast growth factor (aFGF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF) and TGF-β perform actions in myometrium and in leiomyomas. In addition to these growth factors, activin and myostatin have been recently identified in myometrium and leiomyoma.
CONCLUSIONS
Growth factors play an important role in the mechanisms involved in myometrial patho-physiology.
Keywords: myometrium, leiomyoma, growth factors, steroid hormones
Introduction
The myometrium is the muscular wall of the uterus that undergoes significant changes in size and cellular properties in specific physiological and pathological conditions. The myometrium mass undergoes moderate changes during each reproductive cycle (Burroughs et al., 2000) and dramatic changes throughout pregnancy (Johansson, 1984; Shynlova et al., 2006) and menopause (Wu et al., 2000). The uterine mass growth during pregnancy represents one of the most remarkable events in reproduction, with a massive increase in both size and number of myometrial smooth muscle cells, to allow the growing fetus to have the necessary support. During pregnancy, the myometrium mass undergoes changes in cellular phenotype, characterized by an early proliferative phase, an intermediate phase of cellular hypertrophy, and a final contractile/labour phase (Shynlova et al., 2009). Myometrial mass and cellular morphology are also modified in tumour conditions such as leiomyosarcoma and leiomyoma (Matsuo et al., 1999; Walker and Stewart, 2005). Leiomyosarcomas are rare aggressive malignant uterine smooth muscle tumours occurring mainly in post-menopausal women (Emoto et al., 1999). Uterine leiomyosarcoma usually presents features such as vaginal bleeding (77–95%), pelvic pain (33%), uterine enlargement or a palpable pelvic mass (20–50%; Iwamoto et al., 2005).
Uterine fibroids
Uterine leiomyomas (fibroids or myomas) are common benign tumours that arise from a single uterine smooth muscle cell (Wallach and Vlahos, 2004). Uterine leiomyomas are the most common cause of solid pelvic tumours, found in 20–40% of women during their reproductive years (Wallach and Vlahos, 2004). The common symptoms associated with uterine leiomyoma are irregular and excessive bleeding and anaemia, pelvic discomfort, bowel and bladder dysfunctions, pressure sensation in the lower abdomen and pain during intercourse (Marsh and Bulun, 2006). Moreover, leiomyomas have also been associated with infertility and recurrent abortion (Olive and Pritts, 2010). These tumours tend to grow rapidly during pregnancy and can therefore cause obstructed labour leading to fetal malpresentation and fetal anomalies that often require a Caesarean section, as well as post-partum haemorrhage secondary to uterine atony (Walker and Stewart, 2005). Pregnant myometrium and uterine fibroids are both characterized by an extraordinary myometrial growth rate, apposition of extracellular matrix and changes in physiological attributes, i.e. contraction. Leiomyomas share many characteristics with the parturient myometrium, including increased production of extra-cellular matrix components, the expression of receptors for peptide and steroid hormones and the expression of the gap junction protein connexin 43. The latter is required for cell–cell communication and the synchronous contractions at labour. However, unlike normal post-partum myometrium, leiomyomas fail to regress via apoptosis and to undergo normal dedifferentiation (Andersen et al., 1993; Andersen and Barbieri, 1995; Walker and Stewart, 2005). Leiomyomas have characteristics of the well-differentiated uterine smooth muscle cells of pregnancy as evidenced by the fact that these tumour cells resemble the myometrial cells of pregnancy more closely than they resemble typical myometrial cells of a non-gravid uterus (Andersen et al., 1993; Andersen and Barbieri, 1995; Cesen-Cummings et al., 2003). Leiomyoma and pregnant myometrium both present dysregulated patterns of cellular differentiation and gene expression. In general, the major differences when compared with normal non-pregnant myometrium are found for estrogen-regulated genes including those encoding structural proteins, i.e. collagens (Andersen and Barbieri, 1995; Shynlova et al., 2009).
Growth factors and myometrium
Growth factors are polypeptides or proteins that are secreted by a number of cell types, have a wide range of biological effects, and generally act over short distances either in an autocrine and/or paracrine manner. Growth factors exert most of their effects on target cells by interacting with specific cell-surface receptors, with subsequent signalling transmission via signal transduction systems in the cell (Pusztai et al., 1993). Importantly, growth factors are essential elements in controlling the cellular proliferation rate, and overexpression of either growth factors or their cognate receptors may contribute to tumourigenesis.
To date, factors known to act on myometrial cells are: epidermal growth factor (EGF) (Yeh et al., 1991), heparin-binding EGF (HB-EGF) (Mangrulkar et al., 1995), platelet-derived growth factor (PDGF) (Boehm et al., 1990), insulin-like growth factor (IGF) (Boehm et al., 1990; Gloudemans et al., 1990), transforming growth factor-α (TGF-α) (Dixon et al., 2000), vascular endothelial growth factor (VEGF) (Harrison-Woolrych et al., 1995; Dixon et al., 2000; Sanci et al., 2011), acidic fibroblast growth factor (aFGF) (Wolanska and Bankowski, 2006) and basic fibroblast growth factor (bFGF) (Mangrulkar et al., 1995). All the above cited growth factors are ligands of the well known and established receptor tyrosine kinases (RTK) that activate two critical signalling cascades such as the Ras-Erk/MAP kinase and phosphatidylinosite-3 kinase (P13K)-AKT-mTor pathways (Fig. 1; McKay and Morrison, 2007). In addition, members of the TGF-β superfamily, such as TGF-β (Chegini et al., 1994; Dou et al., 1996; Tang et al., 1997) as well as activin (Ciarmela et al., 2008, 2011) and myostatin (Ciarmela et al., 2009, 2011) are also important growth factors involved in myometrial cell biology, acting through activation of the serine/threonine kinase receptors/Smad pathway.
Figure 1.
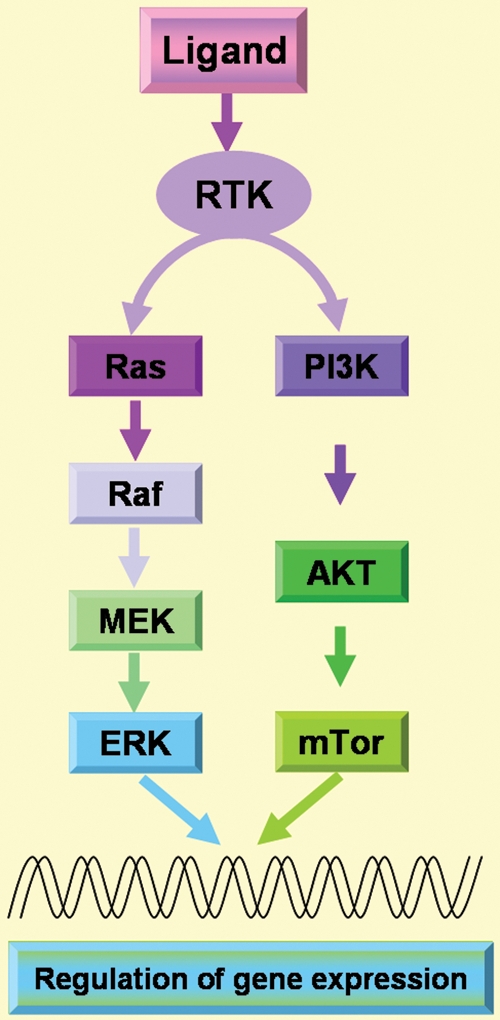
RTK pathways. Different growth factors (EGF, HB-EGF, PDGF, IGF, TGF-α, VEGF, aFGF, bFGF) can act as ligand of the RTK and therefore two different signalling cascades such as Ras-Erk/MAPK and P13K-AKT-mTor pathways can be activated. RTK, receptor tyrosine kinases; EGF, epidermal growth factor; HB-EGF, heparin-binding EGF-like growth factor; PDGF, platelet-derived growth factor; IGF, insulin-like growth factor; TGF-α, transforming growth factor-α; VEGF, vascular endothelial growth factor; aFGF, acidic fibroblast growth factor; bFGF, basic fibroblast growth factor; MAPK, mitogen-activated protein kinase; P13K, phosphatidylinosite-3 kinase.
Cripto, an EGF-CFC (Epidermal Growth Factor-Cripto, FRL-1, Cryptic) protein that was first isolated as a putative oncogene from a human teratocarcinoma cell line (Ciccodicola et al., 1989), also has growth factor-like activity and interacts with the functional signalling of other growth factors including TGF-β (Gray et al., 2006), activin (Gray et al., 2003), Nodal (Shen, 2007; Kelber et al., 2008) and myostatin (Ciarmela et al., 2011). Recently, Strizzi et al. (2007) reported Cripto-1 (CR-1) expression during leiomyosarcoma development of MMTV-CR-1 transgenic mice, with approximately one-fifth (19.7%) of the MMTV-CR-1 transgenic mice developing uterine leiomyosarcomas as opposed to 0% of the FVB/N normal control mice observed for the same time period. These authors further demonstrated that human leiomyosarcoma and leiomyoma had deranged Cripto mRNA expression and that normal uterine smooth muscle cells treated with exogenous Cripto exhibited increased proliferation and migration rates in comparison with non-treated cells (Strizzi et al., 2007).
Steroid hormones and growth factors
Uterine cellular proliferation and differentiation are regulated by steroid hormones. It is well established that ovarian steroids act in their target tissue, at least in part, through local modulation of a number of growth factors, cytokines and chemokines. This autocrine/paracrine signalling plays an important role in the events involved in myometrium cellular transformation and turnover that are ultimately involved in leiomyoma pathophysiology. In this scenario, many cytokines including interleukin (IL)-1, IL-6, IL-11, IL-13, IL-15, interferon (IFN)-γ, tumour necrosis factor (TNF)-α, granulocyte-macrophage colony-stimulating factor (GM-CSF) and erythropoietin have been documented in the myometriun and leiomyoma. These and other cytokines play a central role in regulating inflammation, angiogenesis and tissue remodelling and, importantly, cytokine and growth factor interactions have been associated with subepithelial airway fibrosis and leiomyoma pathophysiology. For a complete review on the implications of inflammatory-related mediators in the development of leiomyomas and their interaction with ovarian hormones and growth factors, the reader is referred to Chegini (2010). The mitogenic action of steroids in their target tissues is considered to be mediated by local production of growth factors acting through paracrine and/or autocrine mechanisms. The modulation of growth factor expression by steroids suggests growth factors represent the ultimate effectors of many steroid hormone actions. In addition to the genomic actions of steroid hormones on growth factor expression, non-genomic interactions of growth factors and hormone signalling pathways have also been demonstrated (Nierth-Simpson et al., 2009; Yu et al., 2010).
Recently, some reports have provided evidence for a non-genomic interface between estrogen (E2) and some cytoplasmic signalling pathways, such as TGF-β/Smad and MAP kinase (Hermon et al., 2008) pathways in leiomyoma cells via growth factor activation. In fact, the genomic effects of E2 can be enhanced or amplified by cytoplasmic signalling pathways triggered by growth factor receptors or by plasma membrane estrogen receptors (ERs). In addition, non-genomic E2 signalling might be coupled to feedback mechanisms leading to the regulation of growth factors through the activation of growth factor receptors (Yu et al., 2010).
This review aims to synthesize the knowledge currently available regarding the roles of different growth factors and their interactions with steroid hormones in the regulation of myometrial cell function.
Methods
To generate this review, multiple strategies were used to identify relevant studies between growth factors and myometrium. We conducted extensive Medline and Google Scholar search using combinations of the following search items: myometrium, leiomyoma, leiomyosarcoma, growth factors (EGF, TGF-α, HB-EGF, aFGF, bFGF, VEGF, IGF, PDGF, TGF-β, Activin and Myostatin), sex steroid hormones (estrogen, progesterone), cripto, extracellular matrix, myomectomy, hysterectomy, uterine artery embolization, magnetic resonance-guided focused ultrasonography, laparoscopic uterine artery occlusion, cryomyolysis, GnRHa, SPRMs, SERMs, RTK, MAPK/ERK/Smad pathway and P13/AKT/mTOR pathway. Bibliographies were cross-referenced to identify additional studies. All relevant articles and additional articles cited in primary references are included. We summarized the work related to growth factors and their regulation by sex steroids in myometrium and leiomyomas. This review highlights the role of growth factors in the pathogenesis and future therapy of uterine fibroids as well as the need for further research on the functions of growth factors and their regulation by steroids, as many studies have focused on expression only.
Epidermal growth factor
EGF, a 6045-kDa protein with 53 amino acid residues and three intramolecular disulfide bonds, signals via its transmembrane EGF-receptor (EGF-R also known as ErbB1 or HER1) to regulate key processes of cell biology such as proliferation, survival, and differentiation during development, tissue homeostasis and tumourigenesis (Carpenter and Cohen, 1990). The EGF-R is one of the most versatile signalling units in biology. EGF-induced activation of EGF-R results in phosphorylation of specific tyrosine residues within the cytoplasmic receptor tail. These phosphorylated tyrosine residues in turn serve as docking sites for proteins containing Src homology 2 (SH2) and phosphotyrosine-binding (PTB) domains that coordinate the activation of multiple downstream signalling pathways. In mammals, canonical EGF-R activation involves the binding of seven peptide growth factors including EGF, TGF-α and heparin-binding EGF (HB-EGF; Yarden and Sliwkowski, 2001).
Uterine effects
EGF and EGF-R mRNA have both been identified in myometrial and leiomyoma cells (Yeh et al., 1991) and immunolocalization of both proteins has also been described in the cytoplasm of smooth-muscle cells of leiomyomas and matched myometrium (Dixon et al., 2000). Interestingly, EGF-positive staining signals in leiomyoma tissue from the proliferative phase is significantly decreased compared with matched myometrium (Dixon et al., 2000). Myometrial and leiomyoma cells express an EGF-R isoform with a molecular mass of 133 kDa (Yeh et al., 1991; Shimomura et al., 1998). Moreover, leiomyoma and myometrium contain specific, high-affinity binding sites for EGF (Hofmann et al., 1984). EGF is mitogenic for both cultured myometrium and leiomyoma cells (Fayed et al., 1989; Rossi et al., 1992), and it has been demonstrated that EGF plays a crucial role as a local growth factor in regulating leiomyoma growth (Yeh et al., 1991; Rossi et al., 1992). A role for EGF in leiomyoma growth is also supported by the fact that the selective EGF-R blocker AG1478 is able to block leiomyoma cell proliferation (Shushan et al., 2004). Shushan et al. (2007) also demonstrated that leiomyoma cell growth is effectively blocked by TKS050, a new EGF-R inhibitor. TKS050 induced cell cycle arrest and apoptosis in a dose- and time-dependent manner. This newly developed inhibitor may be useful as a possible alternative therapy for leiomyomas in the years to come (Shushan et al., 2007). Recently, NADPH oxidase-derived ROS (reactive oxygen species) have been shown to be critical component of the MAP kinase mitogenic pathway of EGF signalling in leiomyoma smooth muscle cell proliferation. This ROS production is inhibited by the NADPH oxidase inhibitor (DPI), suggesting the presence of the NADPH oxidase system and its importance in mitogenic signalling pathways in leiomyoma smooth muscle cells. The development of new therapies for the treatment and/or prevention of uterine leiomyomas should take advantage of the discovery of the NADPH oxidase-derived ROS acting in EGF signalling pathways leading to cell proliferation (Mesquita et al., 2010; Fig. 2).
Figure 2.
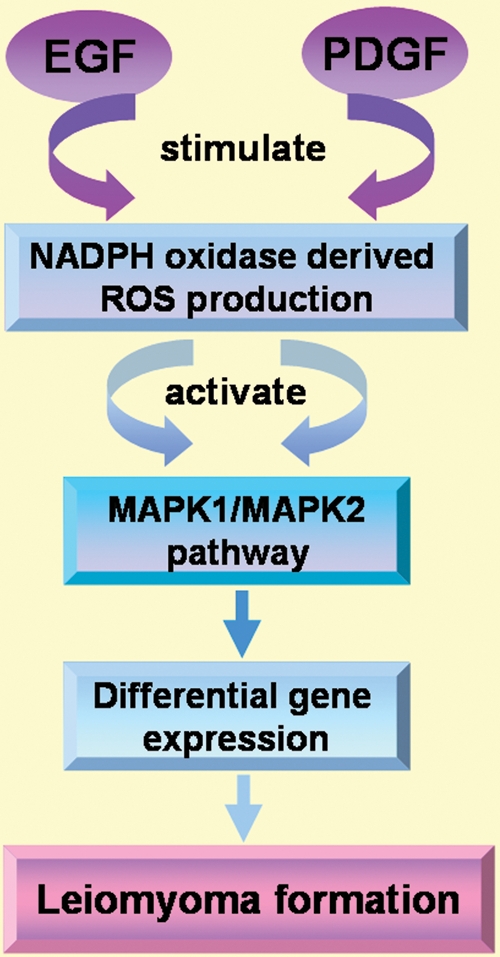
EGF and PDGF signalling in leiomyoma formation through MAPK pathway. NADPH oxidase-derived ROS have been shown to be a critical component of the MAPK pathway of EGF and PDGF signalling in leiomyoma smooth muscle cell proliferation. ROS, reactive oxygen species.
Steroid regulation
Steroid regulation of EGF has been long documented by several reports. Progesterone (P4) up-regulates EGF expression in leiomyoma cells (Shimomura et al., 1998; Matsuo et al., 1999; Maruo et al., 2003). Wang et al. (2006) recently found that asoprisnil (J867), a novel selective P4 receptor (PR) modulator down-regulated expression of EGF and its receptors in cultured uterine leiomyoma cells without altering EGF-induced proliferation and apoptosis rates in normal myometrial cells (Wang et al., 2006). E2 reduces the stimulatory effect of EGF and also inhibites 3H-thymidine incorporation by the smooth muscle cells (Rossi et al., 1992). It has also been found that E2 treatment reduces EGF expression but up-regulates the expression of EGF-R and proliferating cell nuclear antigen (PCNA), an endogenous marker of cell proliferation, in both myometrium and leiomyoma cells (Shimomura et al., 1998; Matsuo et al., 1999).
Transforming growth factor-α
TGF-α is single-chain, 50-amino acid polypeptide that is structurally similar to EGF and a potent member of the EGF family. Both TGF-α and EGF bind to the same receptor (EGF-R) triggering cell growth and proliferation in a broad range of cell types (Massague, 1983; Tam et al., 1984).
Uterine effects
TGF-α is reported to be minimally to not expressed in the cytoplasm of smooth-muscle cells of leiomyomas and matched myometrium in women in the proliferative phase of the menstrual cycle (Dixon et al., 2000). Moore et al. (2000) similarly showed that while TGF-α and its receptor, EGF-R, were intensely expressed in uterine leiomyosarcomas, their expression was absent in leiomyomas and also in control myometrium in B6C3F1 mice (an outbred strain originated from crossing C57BL/6 with C3H mice).
Steroid regulation
Newbold et al. (2002) have demonstrated that diethylstilbestrol (a synthetic estrogenic compound) induces uterine leiomyomas and, interestingly, these tumours showed focal areas of atypia expressing TGF-α. These findings are similar to previous reports performed in B6C3F1 mice (Moore et al., 2000). Altogether, available evidence suggests that TGF-α expression may be an early and necessary event for malignant transformation and also an important biomarker of malignancy in uterine smooth muscle tumours in mice (Newbold et al., 2002).
Heparin-binding EGF
HB-EGF is a 22 kDa EGF family member that was first identified in the macrophage-like cell-conditioned medium (Higashiyama et al., 1991). HB-EGF binds to its cognate receptors, human EGF receptor 1 (HER1) and HER4 (Elenius et al., 1997).
Uterine effects
HB-EGF is present in normal myometrium and in fibroid tumours (Nowak, 2000). Similar to EGF, HB-EGF shows a decreased expression in leiomyomas relative to normal myometrium (Mangrulkar et al., 1995). The receptor HER1 protein has been detected in leiomyoma and myometrial cells (Dixon et al., 2000) and HB-EGF stimulates the proliferation of both leiomyoma and myometrial cells and inhibits apoptosis through augmentation of HER1 expression. Treatment with HB-EGF results in a dose-dependent increase in PCNA expression in both cells compared with untreated control cultures. However, the proliferative potential of the myometrial cells responded better to HB-EGF than that of leiomyoma cells suggesting that HB-EGF may play a more vital role in normal myometrial growth than in leiomyoma growth (Wang et al., 2005).
Steroid regulation
While there is no evidence for sex steroid regulation of HB-EGF in myometrium, sex steroid regulation of HB-EGF has been documented in mouse (Wang et al., 1994) and rat (Zhang et al., 1994) uterus. In adult ovariectomized mouse uterus, E2 regulates HB-EGF expression in the luminal epithelium, whereas expression of HB-EGF in the stroma is regulated by both E2 and P4 (Wang et al., 1994). On the other hand, Zhang et al. (1994) demonstrated that P4 stimulated the expression of HB-EGF in isolated rat uterine stromal cells, but repressed HB-EGF expression in isolated uterine epithelial cells. Whereas E2 treatment strongly enhanced HB-EGF expression in epithelial cells, it had no effect on HB-EGF mRNA levels in stromal cells. P4 treatment followed by E2 injection still stimulated HB-EGF expression in isolated stromal cells and repressed expression in epithelial cells (Zhang et al., 1994).
Acidic fibroblast growth factor
Fibroblast growth factor-1 (FGF-1) or acidic FGF (aFGF) is a multifunctional peptide which is usually isolated as a form with a molecular mass of 16–18 kDa. It belongs to the large family of FGFs that signal through high-affinity tyrosine kinase-linked FGF receptors (FGFR1–4) to regulate cell growth, differentiation and other cell functions.
Uterine effects
aFGF mRNA expression was detected in uterine myometrial cells (Samathanam et al., 1998). Hague et al. (2000) observed aFGF expression also in leiomyomas (Hague et al., 2000). Later, aFGF mRNA was confirmed in both myometrium and uterine leiomyomas and an increased expression occurring during tumour growth was reported (Wolanska and Bankowski, 2006). aFGF mRNA was also overexpressed during mass myometrium conversion into leiomyoma (Wolanska et al., 2008).
Steroid regulation
Samathanam et al. (1998) found that FGF-1 immunoreactivity is robustly increased while its mRNA levels are not appreciably increased by E2, suggesting that the peptide abundance is regulated at the level of translation or protein stability (Samathanam et al., 1998).
Basic fibroblast growth factor
Basic fibroblast growth bFGF (or FGF-2) is a 18 kDa protein that belongs to the FGF family, which includes a number of mitogenic proteins characterized by their affinity for heparin and heparin-like molecules (Gospodarowicz et al., 1987). bFGF binds to two major receptors with high affinity; one is fibroblast growth factor type I receptor (FGFR-1) and other is the type II receptor (FGFR-2) (Fernig and Gallagher, 1994).
Uterine effects
Several researchers reported the expression of bFGF and its receptors FGFR-1 and FGFR-2 in both leiomyoma and myometrial cells (Pekonen et al., 1993; Mangrulkar et al., 1995; Dixon et al., 2000; Nowak, 2000; Wu et al., 2001; Flake et al., 2003; Wolanska and Bankowski, 2006), with more distinct expression of FGFR-1 in the tumours compared with myometrium (Wolanska and Bankowski, 2006). Although bFGF is mitogenic for both human uterine myometrial and leiomyoma cells, leiomyoma cells are less responsive (Rauk et al., 1995). bFGF also regulates angiogenesis (Anania et al., 1997; Hong et al., 2001). This factor can also bind to a component of the extracellular matrix of leiomyomas (Dixon et al., 2000).
Steroid regulation
E2 exhibits no synergism with the mitogenic effect of bFGF (Rauk et al., 1995). However, Rider et al. (1997) reported that E2 and P4 regulate bFGF mRNA expression in the rat uterus (Rider et al., 1997).
Vascular endothelial growth factor
VEGF is a secreted, heparin-binding homodimeric glycoprotein of ∼46 kDa that consists of six isoforms (VEGF121, VEGF145, VEGF165, VEGF183, VEGF189 and VEGF206) (Tischer et al., 1991; Poltorak et al., 1997; Lei et al., 1998). VEGF stimulates cellular responses by binding to tyrosine kinase receptors, VEGFR-1 (fms-like tyrosine kinase: flt-1) and VEGFR-2 (kinase domain-containing receptor: KDR/flk- 1).
Uterine effects
VEGF mRNA and protein expression have been identified in the smooth muscle cells of both normal myometrium and leiomyomas (Harrison-Woolrych et al., 1995; Dixon et al., 2000; Sanci et al., 2011). The VEGF receptors, VEGFR-1 and VEGFR-2 are also expressed in myometrium (Brown et al., 1997), leiomyomas and in cellular leiomyomas (Sanci et al., 2011). A stronger VEGF expression was found in leiomyomas than in adjacent myometrium, indicating that local angiogenesis may be important for the development and growth of these tumours (Gentry et al., 2001). Hong et al. (2001) reported that VEGF is significantly expressed in leiomyosarcoma compared with leiomyoma. VEGF stimulates angiogenic activity which is responsible for actively growing tumours and may enhance the growth of fibroids and disease progression in many carcinomas (Hong et al., 2001; Flake et al., 2003; Arita et al., 2005).
Steroid regulation
Both E2 end P4 induce VEGF expression in the rodent uterus (Hyder et al., 2000).
Insulin-like growth factor
IGFs are multifunctional peptides, structurally related to proinsulin (Duan, 2002) that consist of two ligands, IGF-I and IGF-II. IGFs bind to two cell-surface receptors (IGF-IR and IGF-IIR), and to a family of six high-affinity IGF-binding proteins (IGFBP 1–6) (Yu and Berkel, 1999).
Uterine effects
IGF-I and IGF-II mRNAs are present in leiomyoma and myometrium (Boehm et al., 1990; Gloudemans et al., 1990; Giudice et al., 1993; Van der Ven et al., 1997) as well as in leiomyosarcoma (Gloudemans et al., 1990; Van der Ven et al., 1997). Specifically, IGF-II mRNA is more highly expressed in leiomyosarcoma when compared with normal myometrium and leiomyoma (Gloudemans et al., 1990; Van der Ven et al., 1997). IGF-I content in leiomyomas is higher than in myometrium (Dixon et al., 2000; Wolanska and Bankowski, 2004; Yu et al., 2008; Zhao et al., 2008). IGF-I, but not IGF-II, is mitogenic in leiomyoma cell cultures (Strawn et al., 1995). In addition, IGF-I plays a crucial role in leiomyoma cell growth, not only by increasing PCNA expression, but also by up-regulating Bcl-2, an apoptosis-inhibiting gene product, in leiomyoma cells (Gao et al., 2001; Maruo et al., 2003). Peng et al. (2009) examined whether deregulation of IGFs and IGF signalling is a common event present in symptomatic leiomyomas and whether IGFs are associated with large fibroids. The results showed that IGF-I but not IGF-II levels are directly correlated with activation of p-AKT and p-S6K. Larger fibroids showed higher levels of IGF-1 and p-AKT activity when compared with small ones (Peng et al., 2009). Furthermore, IGF has also been demonstrated to promote increased cellular proliferation in uterine leiomyoma cells, through activation of the MAPK pathway, and such an effect was reversed by the use of a neutralizing antibody against the IGF-IRβ in vitro (Yu et al., 2008). In most situations, IGF-binding proteins inhibit the actions of IGFs by blocking their binding to the receptor; in certain circumstances, however, these binding proteins may be able to enhance IGF-I actions by binding to it and preventing its degradation, thereby increasing its bioavailability in target tissues (Yu and Berkel, 1999). The lower levels of IGF-binding protein-3 in leiomyomas than in myometrium could also be significant, as this would increase the bioavailability of free bioactive IGF in fibroids (Vollenhoven et al., 1993; Flake et al., 2003). It has been observed that IGF-I and IGF-binding protein (IGFBP) expression is associated with the proliferative phenotype during early pregnancy of rat. Specifically, high levels of IGF-I and its binding proteins (IGFBP-I and IGFBP-III) were observed during Days 6–14 of gestation (Shynlova et al., 2007b, 2009).
Steroid regulation
IGF-I, similarly to progesterone receptor (PR), transcripts in both leiomyoma and myometrium in the proliferative phase were significantly higher than those after GnRH-a treatment, indicating that the leiomyoma fibroid shrinkage after steroid deprivation is associated with alterations in IGF-I and PR expression (Wu et al., 2002). Significantly, decreased IGF-I mRNA and protein expression were observed in cultured leiomyoma cells with the treatment of P4 either alone or in combination with E2, whereas E2 treatment alone was unable to cause any significant effect, demonstrating a role for P4 in modulating IGF-I expression in leiomyoma (Yamada et al., 2004). Specifically, Ying and Weiyuan (2009) examined whether P4 affects IGF-I mRNA expression through P4 receptor B (PRB) mRNA in leiomyoma. Their finding revealed that P4 down-regulated IGF-I expression through PRB, and PRA appeared to inhibit this function of P4 by inhibiting the transcription of PRB; thus, the action of P4 on leiomyoma growth may depend on different ratios of PRA and PRB (Ying and Weiyuan, 2009). In cultured leiomyoma cells, P4 treatment inhibited the expression of IGF-I as well as the cytokine TNF-α and, conversely, augmented the expression of Bcl-2 (Maruo et al., 2003). The PR modulator, asoprisnil (J867), also inhibited the expression of IGF-I and their receptor (IGF-IRα) in cultured uterine leiomyoma cells but not in normal myometrial cells (Wang et al., 2006). Asoprisnil (J867) also decreased Bcl-2 expression in leiomyoma cells but not in normal myometrial cells (Ohara et al., 2007). These two different studies provide evidence that asoprisnil inhibits proliferation by down-regulating the expression of specific growth factors and their receptors and by allowing apoptosis in leiomyoma cells without affecting proliferation and apoptosis in normal myometrial cells.
IGF-I is classically considered an estrogen-regulated gene. Interestingly, Swartz et al. (2005) performed microarray studies comparing estrogen-treated uterine leiomyoma cells and normal myometrial cells to untreated cells and found several genes that were differentially expressed, including IGF-I. It has also been demonstrated that low concentrations of genistein, a soy-derived phytoestrogen, have a stimulatory effect in human leiomyoma cells but not in smooth muscle cells (Moore et al., 2007) where it induces ER-alpha (ER-α) and IGF-I receptor interactions and cell proliferation (Di et al., 2008). It is likely that the binding of estrogens to ER-α in the cytoplasm induces interactions between ER-α and IGF-IR leading to the activation of MAPK. This may then in turn lead to activation of ER resulting in enhanced ER transcriptional activity and up-regulation of IGF expression thus creating a positive feedback regulatory mechanism and/or autocrine mechanism that stimulates leiomyoma cell proliferation (Di et al., 2008).
Platelet-derived growth factor
PDGF is a 125-amino acid dimeric glycoprotein with both homodimeric (AA and BB) and heterodimeric (AB) forms linked by disulphide bonds (Dalla-Favera et al., 1982; Betsholtz et al., 1986). PDGF exists in five different isoforms (PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC and PDGF-DD) that activate cellular responses through two different receptors, PDGF-Rα and PDGF-Rβ. Specifically, PDGF-Rα, binds the A-, B- and C-chains with high affinity while PDGF-Rβ, binds only the B- and D-chains (Heldin et al., 2002).
Uterine effects
PDGF and PDGF-R expression have been documented in both normal myometrial and leiomyoma tissues (Boehm et al., 1990; Mangrulkar et al., 1995; Nowak, 2000) and cells (Palman et al., 1992; Rossi et al., 1992) but discordant data have been reported regarding their levels of expression. More than 20 years ago it was reported that more PDGF-R sites are present in cultured leiomyoma cells than in myometrial cells, but that their receptor affinity is lower in leiomyoma than in myometrium (Fayed et al., 1989). Liang et al. (2006) reported higher expression of PDGF-AA, PDGF-BB and receptors in leiomyoma than in myometrium (Liang et al., 2006), while later Hwu et al. (2008) reported that there are no significant differences in the above factors and also of PDGF-DD in leiomyoma compared with adjacent myometrial tissues (Hwu et al., 2008). PDGF-CC has been reported to be more highly expressed in leiomyoma tissues compared with adjacent myometrial tissues (Hwu et al., 2008) as well as in fibroid-derived smooth muscle cells compared with myometrial-derived smooth muscle cells (Suo et al., 2009). PDGF stimulates DNA synthesis as well as protein synthesis (Fayed et al., 1989), increases collagen alpha1 expression (Liang et al., 2006) and modulates the rate of cell proliferation in myometrium and leiomyoma cells (Arici and Sozen, 2003; Liang et al., 2006). Taniguchi et al. (2001) examined whether or not PDGF would be able to stimulate the expression of VEGF in cultured human myometrial smooth muscle cells. They reported that PDGF treatment enhanced VEGF immunoreactivity and stimulated cell proliferation (Taniguchi et al., 2001). Mesquita et al. (2010) demonstrated that NADPH oxidase-derived ROS is a necessary component of the MAP kinase mitogenic pathway activated by PDGF in leiomyoma smooth muscle cells. Their experimental results showed that stimulation of these cells with PDGF caused a marked increase in intracellular ROS production, while a NADPH oxidase inhibitor (DPI) blocked ROS production in a dose-dependent fashion (Fig. 2). Moreover, PDGF treatment caused an increase in 3H-thymidine incorporation by leiomyoma smooth muscle cells (Mesquita et al., 2010). PDGF also interacts with other growth factors such as TGFβ and EGF to enhance proliferation (Fayed et al., 1989; Wolanska and Bankowski, 2007).
Steroid regulation
Steroid hormones regulate PDGF expression. An E2 role in activating PDGF expression has been reported by the use of an antiestrogenic compound (ICI 182780) in cultured leiomyoma cells. This compound was able to prevent E2-induced PDGF expression, thus revealing that PDGF is in fact, one of the growth factors involved in the proliferative response of leiomyoma smooth muscle cells to estrogen stimulation in vitro (Barbarisi et al., 2001). Nonetheless, E2 inhibited 3H-thymidine incorporation by the smooth muscle cells and at the same time reduced the stimulatory effect of PDGF in human myometrial tissue (Rossi et al., 1992). The reduced levels of PDGF production observed after GnRH-a treatment and the relationship between decreased PDGF expression and greater shrinkage in uterine volume suggest a mitogenic action of PDGF in leiomyomas (Di Lieto et al., 2002).
Transforming growth factor-β
TGF-β is a dimeric protein composed of two identical 112 amino acid subunits. In humans, there are three TGF-β isoforms described so far (TGF-β1, TGF-β2 and TGF-β3), which are encoded by separate genes (Sozen and Arici, 2002). All TGF-β isoforms accomplish their biological functions through three types of transmembrane receptors: type I (ALK-5), type II and type III (betaglycan) (Massague et al., 1994).
Uterine effects
TGF-β and its receptors are expressed in human myometrium (Chegini et al., 1994; Tang et al., 1997) and leiomyomas (Dou et al., 1996; Chegini et al., 2003; Xu et al., 2003; Ding et al., 2004). Their putative role in leiomyoma pathogenesis has been hypothesized because TGF-βs, their receptors and downstream signalling mediators, like Smad 2/3 complexes, are overexpressed in leiomyoma compared with normal myometrium (Dou et al., 1996; Arici and Sozen, 2000; Lee and Nowak, 2001; De Falco et al., 2006; Norian et al., 2009). Moreover, evidence from a genetically susceptible animal model indicates that TGF-β signalling may be critical for the maintenance of the disease. Moreover, in mutant rats that develop uterine leiomyomas spontaneously, treatment with a synthetic TGF-βR1 kinase inhibitor, SB-525334, decreased the incidence, number and size of these tumours (Laping et al., 2007).
Many reports have investigated the TGF-β effects on human uterine smooth muscle cells in vitro, demonstrating that it is the prototype of a bimodal regulator of cell growth, which can either inhibit or stimulate the proliferation of smooth muscle cells, depending of the growth factor concentration range. At low concentrations, all TGF-β isoforms induce the proliferation of human uterine smooth muscle cells in a dose-dependent manner (Tang et al., 1997). Equally, two independent studies have shown that TGF-β3 at low concentrations stimulates the proliferation of leiomyoma cells (Arici and Sozen, 2000; Lee and Nowak, 2001). In a similar fashion, TGF-β at low concentrations enhances DNA replication in human vascular smooth muscle cells, whereas this effect disappears at higher concentrations (Battegay et al., 1990).
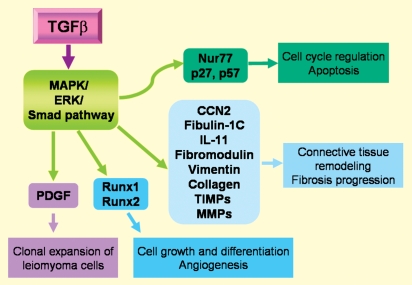
Figure 3 summarizes how TGF-β affects other paracrine and autocrine regulators of cell proliferation and tumour expansion. TGF-β3 induced a molecular phenotype in myometrial and leiomyoma cells with elevated production of ECM-related genes and decreased production of ECM degradation-related genes (Joseph et al., 2010). Arici and Sozen (2000) also reported that TGF-β3 induces fibronectin (a high-molecular weight extracellular matrix glycoprotein) expression in leiomyoma cells, suggesting that TGF-β3 may mediate the growth-promoting effects on leiomyomas by playing a role in the fibrogenic process that characterizes these tumours (Arici and Sozen, 2000). Wolanska and Bankowski (2007) reported that TGF-β induces proliferation of cells at low concentrations by stimulating autocrine PDGF secretion. In contrast, higher concentrations of TGF-β1 evoke the opposite effect via down-regulation of the PDGF receptor and by direct growth inhibition (Wolanska and Bankowski, 2007). TGF-β is also able to modulate the production of paracrine and autocrine mediators of the inflammatory response, cell growth, apoptosis and tissue remodelling (Luo et al., 2005, 2006; Yen-Ping Ho et al., 2009; Fig. 3). TGF-β acts through the activation of MAPK/ERK/Smad pathways and regulates the expression of different types of genes whose products may, at least in part, influence the outcome of leiomyoma growth and regression (Ding et al., 2004; Levens et al., 2005; Luo et al., 2006; Fig. 3).
Figure 3.
Local regulatory proteins that mediate some of the actions of TGF-β in human myometrium and leiomyoma. TGF-β acts through MAPK/ERK/Smad pathway, regulates the expression of different genes and thereby modulates different actions (in the squares) that may contribute to the leiomyoma formation.
Steroid regulation
Regarding the physiological and pharmacological changes due to sex steroid hormones, the highest level of TGF-β3 mRNA is observed in leiomyoma from the mid-secretory phase of menstrual cycle, suggesting that P4, possibly in combination with E2 (which up-regulates progesterone receptor), stimulates TGF-β3 expression in myometrium and leiomyoma (Arici and Sozen, 2000). In line with this hypothesis, the selective PR modulator asoprisnil inhibits the expression of TGF-β3 and TGF-βRII in cultured uterine leiomyoma cells (Wang et al., 2006).
Interestingly, the expression of TGF-β3 (De Falco et al., 2006) and TGF-βR (Chegini et al., 2003; Xu et al., 2003) is down-regulated in uterine leiomyomas from women undergoing GnRH-a therapy. This effect is a consequence of the antiproliferative state induced by the pharmacological suppression of ovarian steroids, a classical mechanism of GnRH-a action. In leiomyoma cells, the DNA synthesis induced by TGF-β1 can be inhibited by the addition of GnRH-a in the culture medium (Chegini et al., 2002), suggesting that the anti-proliferative effect of GnRH-a on these tumours is partly mediated by a direct effect on their cells.
The steroid modulation of TGF-β expression is demonstrated also by the study on pregnant rat myometrium. TGF-β1 gene expression increases during the second half of gestation, whereas TGF-β3 gene expression and protein levels display a bimodal pattern, with a first peak at mid-gestation and a second peak at term (Shynlova et al., 2007a). The expression of TGF-β3 can be artificially modulated in this animal model, being stimulated by the PR antagonist mifepristone and inhibited by P4 at late gestation (Shynlova et al., 2007a).
Activin
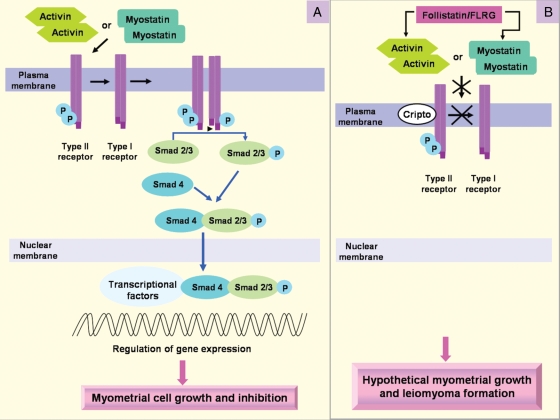
Activin-A is a dimer of two activin βA subunits belonging to the TGF-β superfamily. Activin binds to cell-surface receptor complexes displaying two distinct classes of receptor serine kinases called type I and type II. Activin first binds to type II activin receptors, either ActRIIA or ActRIIB, and only binds a type I receptor, activin receptor-like kinase 4 (ALK4), once bound to the type II receptors. Within the receptor complex, type II receptors phosphorylate, and thereby activate, ALK4, which in turn phosphorylates intracellular substrates such as the Smad proteins. Activin induces ALK4 dependent phosphorylation of Smad 2 and Smad 3, which mediate transcriptional regulation of activin target genes. Activin signalling is also regulated by several membrane (Cripto) and extracellular factors, including the receptor antagonist, inhibin, and the activin-binding proteins, follistatin and follistatin-related gene (FLRG) (Vale et al., 2004; Fig. 4).
Figure 4.
Activin and myostatin transduction signalling (A) and binding proteins (B) in myometrial system. Activin or myostatin first binds to type II receptors, and only binds type I receptors once bound to type II receptors. Within the receptor complex, type II receptors phosphorylate, and thereby activate, type I receptors, which in turn phosphorylates intracellular substrates such as the Smad proteins which mediate transcriptional regulation of activin target genes. Activin signalling is also regulated by several membrane (Cripto) and extracellular factors, including the activin-binding proteins, follistatin and FLRG. The higher expression of the binding proteins in leiomyoma may produce reduced sensitivity to the anti-proliferative effects of activin and myostatin on myometrial cells and therefore contribute to leiomyoma formation.
Uterine effects
The role of activin in myometrium has been controversial. Binding of iodinated activin-A was reported in rats (Draper et al., 1997), and expression of activin receptors was detected in one study (Hayashi et al., 2003) but not detected in another (Schneider-Kolsky et al., 2001). Importantly, it was recently demonstrated that activin-A can regulate myometrial cell functions (Ciarmela et al., 2008). Uterine tissue and two well-characterized myometrial cell lines, PHM1 (pregnant human myometrial 1) (Monga et al., 1996) and hTERT HM (telomerase reverse transcriptase-infected human myometrial) (Condon et al., 2002) respond to activin-A as measured by phosphorylation of Smad-2. Those cell lines express a full complement of activin receptors, as well as activin βA subunit and follistatin. Activin inhibited proliferation of PHM1 and hTERT HM cells in a time and dose-dependent manner, with more extensive growth inhibition observed in PHM1. In PHM1, activin-A decreased oxytocin receptor and HoxA-10 mRNA expression but did not alter total PR, cyclooxygenase-2 (Cox-2), and connexin 43 mRNA expression levels. Furthermore, treatment of PHM1 myometrial cells with activin-A attenuated oxytocin and thromboxaneA2 induced intracellular Ca2+ accumulation. Compared with untreated controls, PHM1 cells cultured with activin-A appeared larger, and morphometric analysis confirmed that both their area and volume were increased. The change in the microscopical appearance and size of activin-treated PHM1 cells suggested that activin-A could have altered their levels of matrix proteins (Ciarmela et al., 2008).
Steroid regulation
In vivo experiments demonstrated that activin mRNA expression is regulated in rodent uterus during the estrous cycle (Jones et al., 2006) and in response to steroid deprivation and replacement (Ciarmela et al., 2009). E2 treatment of human myometrial and leiomyoma tissue explants reduced activin-A mRNA levels while no changes were observed after P4 treatment. Steroidal regulation was also confirmed by the finding that activin mRNA levels were higher in menopausal than in fertile myometrial specimens (Ciarmela et al., 2011).
Myostatin
Myostatin is a highly conserved TGF-β family member that functions as an endogenous inhibitor of muscle growth in diverse species (Grobet et al., 1997; Kambadur et al., 1997; McPherron et al., 1997; McPherron and Lee, 1997; Schuelke et al., 2004; Mosher et al., 2007). Targeted disruption of the myostatin gene in mice leads to muscle hypertrophy and hyperplasia with an approximate doubling of muscle mass (McPherron et al., 1997).
Myostatin and activins share some receptors and signalling transduction molecules. Myostatin stimulates target cells by assembling a cell-surface receptor complex containing type I and II receptors. Myostatin binds the type II Ser/Thr kinase receptor, ActRIIB, and then partners with a type I receptor, either ALK4 (or ActRIB) or ALK5 (TGF-βRI). These complexes induce phosphorylation of Smad-2 and activate an activin/ TGF-β-like signalling pathway (Rebbapragada et al., 2003; Fig. 4).
Uterine effects
The expression of myostatin and the specific type I receptor, ALK5, has been demonstrated in PHM1 cells by RT–PCR. The rat uterus expresses myostatin, and the levels of myostatin mRNA correspond to higher myometrial cell contents. It has also been shown that myostatin induces Smad signalling in PHM1 cells as well as in rat uterine explants, suggesting that myostatin is able to initiate a signalling cascade in myometrium (Ciarmela et al., 2009).
Myostatin treatment decreased PHM1 cell proliferation at different time points analysed. Using a range of myostatin doses, it was revealed that the inhibition of growth of PHM1 cells was dose-dependent, with an EC50 of ∼1.1 nM, comparable with other systems reported myostatin responses (Thomas et al., 2000; Ciarmela et al., 2009).
Steroid regulation
In contrast to skeletal muscle, myostatin expression in the uterus is regulated by steroid hormones. It has been shown that ovariectomized rats have higher myostatin expression than normal cycling rats and that estrogen treatment abrogates myostatin expression. Subsequently, it was reported that myostatin is not expressed in uterus in the presence of high estrogen levels, either pharmacologically induced or during physiological circumstances, such as the proestrus–early estrous stage. Instead, myostatin expression is elevated in the presence of low steroid levels such as during diestrus, and this expression is even higher in the absence of steroids after ovariectomy. In addition, myostatin expression correlates negatively with the growing phases of the uterus: its levels are low during the swelling, whereas its expression is high when the uterus shrinks (Ciarmela et al., 2009).
Recently, steroid regulation of myostatin has been documented also in human myometrium. Specifically, it has been observed that myostatin mRNA levels in tissue explants were reduced after E2 treatment but were unchanged after P4 treatment. In addition, the expression levels were higher in menopausal than in fertile myometrial specimens (Ciarmela et al., 2011).
Activin and myostatin signalling in uterine fibroid
Activin-A and myostatin transmit their signals from plasma membrane to nucleus through the intracellular Smad pathway by simultaneously binding to two types of transmembrane serine/threonine kinase receptors, type II (ActRIIA or ActRIIB) and type I (ALK4/ActRIB or ALK5/TGF-βRI). During their signalling, receptor-regulated Smads (Smad2, Smad3) and common mediator Smad (Smad4) play an important role to facilitate the function of activin-A and myostatin (Fig. 4), whereas Smad7 inhibits signal transduction by blocking type I receptor phosphorylation of Smad2 and Smad3 (Nakao et al., 1997; Lebrun et al., 1999). Furthermore, Smad7 expression is induced by both activin-A (Bilezikjian et al., 2001) and myostatin (Zhu et al., 2004) providing a negative feedback loop. Activin-A and myostatin signalling is also regulated by extracellular activin-binding proteins like follistation (FST) and FLRG (Tsuchida et al., 2000; Hill et al., 2002). Cripto is a GPI-anchored cell-surface protein that can also regulate the function of activin (Kelber et al., 2008) and myostatin (Ciarmela et al., 2011).
Experimental data have shown that activin-A and myostatin expression levels are higher in leiomyoma compared with adjacent myometrium but that their signalling is disrupted in leiomyoma. It has been demonstrated that the expression levels of all the receptors (ALK4, ALK5 and ActRII, ActRIIb) and Smad7 were not different in leiomyoma compared with normal myometrium suggesting higher activin-A and myostatin expression in leiomyoma does not correspond to the expression of receptors and Smad7. Activin-A and myostatin were able to increase Smad7 expression levels in myometrium but not in leiomyoma indicating that leiomyomas are less responsive to these ligands.
It has been observed that FLRG expression is higher in leiomyoma compared with matched healthy myometrium whereas follistatin expression was unchanged. Activin and myostatin evaluated as a ratio with follistatin were still higher in leiomyoma compared with matched healthy myometrium. On the other hand, activin and myostatin evaluated as ratio with FLRG were not significantly higher in leiomyoma compared with matched healthy myometrium. Moreover it has been documented that Cripto mRNA is expressed in almost all the fibrotic specimens examined but was not detected in healthy myometrium (Ciarmela et al., 2011). The current hypothesis is that alterations in the activin- and myostatin-related protein systems may produce loss of sensitivity to the antiproliferative effects of activin and myostatin and that increased expression of FLRG and Cripto may contribute to the growth of these tumours (Ciarmela et al., 2011).
Extracellular matrix and growth factors
As we stated in the introduction section, in the pathogenesis of uterine fibroids the role of growth factors and extracellular matrix proteins is essential (Joseph et al., 2010; Malik et al., 2010). In a recent study, the interactions between human uterine leiomyoma cells and uterine leiomyoma-derived fibroblasts, and their importance in cell growth and extracellular matrix protein production has been investigated using a co-culture system. The researchers found enhanced cell proliferation, and elevated levels of extracellular matrix, collagen type I and IGFBP-3 after co-culturing. There was also increased secretion of VEGF, EGF, bFGF and PDGFA and B in the media of leiomyma cells co-cultured with fibroblasts. Protein arrays revealed increased phosphorylated RTKs of the above growth factor ligands, and immunoblots showed elevated levels of the RTK downstream effector, MAPK, in co-cultured leiomyoma cells. There was also increased secretion of TGF-β 1 and 3. The TGF-β, activin and myostatin downstream effectors phospho-Smad 2 and 3 levels were also increased in co-cultured cells. However, none of the above effects was seen in normal myometrial cells co-cultured with fibroblasts. The soluble factors released by tumour-derived fibroblasts and/or leiomyoma cells, and activation of the growth factor receptors and their pathways stimulated the proliferation of leiomyoma cells and enhanced the production of extracellular matrix proteins (Moore et al., 2010).
Therapeutic directions for uterine fibroid management
Despite the high prevalence and symptomatic nature of leiomyomas, relatively little is understood about their growth and development. Consequently, perfect therapeutic options are still limited. Hysterectomy is the definitive treatment for women with symptomatic uterine fibroids (Farquhar and Steiner, 2002). However, this surgical option is unsuitable for patients wishing to remain fertile. Myomectomy is another surgical option which is suitable for women wishing to remain fertile and to retain their uterus (Frishman and Jurema, 2005). Unfortunately, myomectomy is associated with significant morbidity including haemorrhage, adhesion formation, leiomyoma recurrence, blood transfusion, bowel injury and rarely hysterectomy (Olufowobi et al., 2004). Many new technologies have been added for the treatment of leiomyoma including uterine artery embolization, magnetic resonance-guided focused ultrasonography, laparoscopic uterine artery occlusion and cryomyolysis. But these are not satisfactory due to their significant complication rate although there is sound evidence for initial symptom reduction, shorter hospital stays and quicker returns to work (Sharp, 2006). To date, GnRHa is widely used as short-term effective therapy for leiomyoma management (Takeuchi et al., 2000; Stewart, 2001; Sankaran and Manyonda, 2008). However, this treatment also has drawbacks including profound estrogen deficiency and a decrease in bone mineral density (Dodin et al., 1991). Therefore, there is an obvious need for alternative therapy/pharmacologic agents that would be useful to inhibit the leiomyoma growth without the unwanted side effects. Discovery of progesterone antagonists, selective progesterone receptor modulators (SPRMs), selective estrogen receptor modulators (SERMs), leiomyoma-related growth factors, growth factor inhibitors and growth factor signalling modulators could aid in discovering new alternative therapeutic strategies.
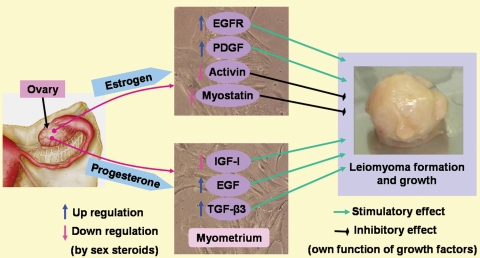
A great deal of evidence supports the concepts that sex steroids (E2 and P4) exert their effects on myometrium and leiomyoma biology by up-regulating and down-regulating various growth factors (summarized in Table I and Fig. 5). Estrogen may promote the growth of uterine leiomyomas through up-regulation of EGFR and PDGF and down-regulating activin and myostatin expression. Similarly, P4 may influence leiomyoma growth by up-regulating EGF and TGF-β3 expression. In contrast, P4 also shows inhibitory actions on leiomyoma growth by down-regulating IGF-I expression. The actual role of sex steroids, how they interact with growth factors and how they influence or regulate leiomyoma growth are not well understood. However, using the concept of a growth-influencing ability of sex steroids, several progesterone antagonists/antiprogestins, SPRMs and SERMs have been proposed as having therapeutic potential for leiomyoma management.
Table I.
Growth factors and related proteins in human myometrium and leiomyoma: presence, action and steroid regulation.
| Growth factors | Receptors binding proteins | Presence in myometrium and or in leiomyoma | Action on myometrial cells | Regulated by sex steroids (estrogen/progesterone) |
|---|---|---|---|---|
| EGF | EGF-R (HER1) | Expression in myometrium and reduced expression in leiomyomas (Yeh et al., 1991; Dixon et al., 2000) | Mitogenic for myometrium and leiomyomas (Fayed et al., 1989; Rossi et al., 1992). Regulates leiomyoma growth (Yeh et al., 1991; Rossi et al., 1992) | P4 up-regulates EGF expression in leiomyoma (Shimomura et al., 1998; Matsuo et al., 1999; Maruo et al., 2003). E2 reduces EGF but up-regulates EGF-R expression in both leiomyoma and myometrium (Shimomura et al., 1998; Matsuo et al., 1999) |
| TGF-α | EGF-R (HER1) | Minimal expression in leiomyomas and myometrium (Dixon et al., 2000). Expressed in leiomyosarcomas (Moore et al., 2000) | ||
| HB-EGF | HER1 (EGFR1), HER4 | Expression in myometrium and reduced expression in leiomyoma (Mangrulkar et al., 1995; Dixon et al., 2000) | Stimulates leiomyoma and myometrial cell proliferation. Inhibits apoptosis (Wang et al., 2005) | P4 and E2 regulate HB-EGF expression in mouse uterus (Wang et al., 1994). P4 stimulates HB-EGF expression in rat uterine stromal cells (Zhang et al., 1994). E2 enhances HB-EGF expression in rat epithelial cells (Zhang et al., 1994) |
| PDGF | PDGF-Rα, PDGF-Rβ | Expression in leiomyomas and myometrium (Boehm et al., 1990; Mangrulkar et al., 1995). Higher expression of PDGF-CC in leiomyoma than in myometrium (Hwu et al., 2008) | Modulates proliferation of leiomyoma and myometrial cells (Arici and Sozen, 2003). Stimulates DNA synthesis (Fayed et al., 1989). Stimulates VEGF production (Taniguchi et al., 2001) | E2 induces PDGF expression in leiomyoma (Barbarisi et al., 2001) |
| IGF- | IGF-IR, IGF-IIR; IGFBP 1–6 | Expression in leiomyoma and myometrium (Boehm et al., 1990; Gloudemans et al., 1990; Giudice et al., 1993; Van der Ven et al., 1997) as well as leiomyosarcoma (Gloudemans et al., 1990; Van der Ven et al., 1997). Higher IGF-I expression in leiomyomas than in myometrium (Dixon et al., 2000; Wolanska and Bankowski, 2004; Yu et al., 2008; Zhao et al., 2008) | IGF-I stimulates proliferation of leiomyoma cells (Strawn et al., 1995). IGF-I up-regulates leiomyoma cell growth (Gao et al., 2001; Maruo et al., 2003). | P4 modulates IGF-I expression in leiomyoma (Yamada et al., 2004) P4 inhibits the IGF-I expression in cultured leiomyoma cells (Maruo et al., 2003). E2 stimulates IGF-I expression in leiomyoma cells (Di et al., 2008) |
| TGF-β | TGF-β R type I, II, and III | Expression in myometrium (Chegini et al., 1994; Tang et al., 1997) and increased expression in leiomyoma (Dou et al., 1996) | Stimulates leiomyoma cell proliferation (Arici and Sozen, 2000; Lee and Nowak, 2001). Low concentrations stimulate autocrine PDGF secretion (Wolanska and Bankowski, 2007) | P4 appears to stimulate TGF-β3 expression in myometrium and leiomyoma (Arici and Sozen, 2000) |
| VEGF | flt (VEGFR-1), KDR (VEGFR-2) | Expression in myometrium and leiomyomas (Harrison-Woolrych et al., 1995; Dixon et al., 2000; Sanci et al., 2011). Increased expression in leiomyomas (Gentry et al., 2001) | Stimulates angiogenic activity (Flake et al., 2003; Arita et al., 2005; Hong et al., 2001) | E2 and P4 induce VEGF expression in rodent uterus (Hyder et al., 2000) |
| aFGF | FGFR1–4 | Expression in myometrium and increased expression in leiomyomas (Wolanska and Bankowski, 2006) | ||
| bFGF | FGFR-1, FGFR-2 | Expression in leiomyomas and myometrium (Pekonen et al., 1993; Mangrulkar et al., 1995; Dixon et al., 2000; Nowak, 2000; Wu et al., 2001; Wolanska and Bankowski, 2006). Increased expression of FGFR-1 in leiomyomas (Wolanska and Bankowski, 2006) | Regulates angiogenesis (Anania et al., 1997; Hong et al., 2001). Mitogenic for both human uterine myometrial and leiomyoma cells (Rauk et al., 1995) | E2 and P4 regulates bFGF mRNA expression in rat uterus (Rider et al., 1997) |
| Activin | Alk4, ActRIIA, ActRIIB; Follistatin, FLRG | Expression in PHM1 (pregnant human myometrium) cells (Ciarmela et al., 2008) and human myometrium and leiomyoma specimens (Ciarmela et al., 2011) | Inhibits myometrial cell growth. Decreases oxytocin receptor and HoxA-10 mRNA expression. Attenuates of oxytocin and thromboxaneA2 induced intracellular Ca2+ accumulation. Modifies cell morphology (Ciarmela et al., 2008) | E2 modulates activin-A mRNA levels in ovariectomized and steroid-replaced rat (Ciarmela et al., 2009). E2 reduces activin-A mRNA levels in human myometrium (Ciarmela et al., 2011) |
| Myostatin | Alk4, Alk5, ActRIIB; Follistatin, FLRG | Expression in PHM1 cells (Ciarmela et al., 2009) and human myometrium and leiomyoma specimens (Ciarmela et al., 2011). | Inhibits myometrial cell growth (Ciarmela et al., 2009). | E2 modulates the myostatin mRNA levels in ovariectomized and steroid-replaced rat (Ciarmela et al., 2009). E2 reduces myostatin mRNA levels in human myometrium (Ciarmela et al., 2011) |
Figure 5.
Steroid hormone regulation of growth factors and effects of the growth factors on leiomyoma formation. Estrogen and progesterone, produced by the ovary, regulate the expression levels of different growth factors in the myometrium. The growth factors are considered the ultimate effectors of the steroid hormone actions because they have stimulatory or inhibitory effects on cell proliferation and probably leiomyoma formation.
RU486 (Mifepristone) is a well-studied antiprogestin that is relatively effective for presurgical management of leiomyoma-related uterobleeding along and has a capability of reducing leiomyoma volume (Engman et al., 2009). However, recently another study has reported that improvements in health-related quality of life after treatment with mifepristone are partly explained by improvements in pain and bleeding, but not uterine size (Feng et al., 2010). Therefore, more studies are needed to understand the real role of mifepristone and other antiprogestins on leiomyoma growth.
Well-studied SPRMs such as asoprisnil (J867) (Wang et al., 2006; Williams et al., 2007), CDB-4124 (Luo et al., 2010), CDB-2914 (Levens et al., 2008; Yoshida et al., 2010), CP8863 and CP8947 (Catherino et al., 2010) have shown promising therapeutic potential and are still under investigation (Ohara, 2008; Spitz, 2009). Some are currently undergoing clinical trials for the treatment of leiomyomas. SERMs, such as tamoxifen and raloxifene (Stewart, 2001), have also been clinically evaluated for the treatment of leiomyoma. Recently it has been demonstrated in primates that ERα and PR expression in the myometrium is indeed influenced by treatment with hormonally active agents (Hill et al., 2011).
Currently, scientists have given topmost priority to the exploration of new therapeutic strategies for leiomyoma treatment by understanding leiomyoma-related growth factors, growth factor inhibitors and their signalling in leiomyoma biology. The most studied growth factors EGF, PDGF, TGF-β, IGFs (since 1990) and recently added activin and myostatin have shown encouraging results that could open new therapeutic strategies for leiomyoma management. To date, selective EGF-R blocker (AG1478) (Shushan et al., 2004), EGF-R inhibitor (TKS050) (Shushan et al., 2007) and TGF-βR kinase inhibitor (SB-525334) (Laping et al., 2007) have shown therapeutic possibilities. However, more studies are needed to explore their therapeutic role on leiomyoma growth. NADPH oxidase-derived ROS have been shown to be a critical component for mitogenic signalling of EGF and PDGF in leiomyoma smooth muscle cells proliferation. More importantly, NADPH oxidase inhibitor (DPI) blocks the ROS production providing a novel therapeutic target for the management of uterine leiomyomas through the targeting of intracellular ROS and specifically, NADPH oxidase (Mesquita et al., 2010).
Because RTK (Zwick et al., 2001), as well as TGF-β (Nagaraj and Datta, 2010), signalling pathways are considered as candidate targets for cancer intervention strategies, they could also be considered for uterine fibroid therapy. Many therapeutic strategies designed to block RTK signalling networks have been recently developed and different RTK-based drugs are currently being investigated in clinical trials (reviewed in Yarden and Sliwkowski, 2001; Zwick et al., 2001; Faivre et al., 2006). Strategies towards the prevention and interception of RTK signalling include: (i) monoclonal antibodies (e.g. Herceptin) that are directed against the extracellular domain of RTK; (ii) small-molecule tyrosine kinase inhibitors (e.g. tyrphostins) that selectively interfere with intrinsic tyrosine kinase activity thereby blocking receptor autophosphorylation and activation of downstream signal transducers; and (iii) antisense oligonucleotides that are designed to interact with the mRNA to block the transcription and thus the expression of specific target proteins (Zwick et al., 2001). RTK can signal through the P13K/AKT/mTOR pathway. Rapamycin and rapamycin derivatives that specifically block mTOR have been developed during the past 10 years as potential anticancer agents (Faivre et al., 2006). The use of rapamycin to target mTOR in myometrium both during pregnancy (Jaffer et al., 2009) and in uterine leiomyoma (Crabtree et al., 2009) has been recently investigated. Interestingly, in vivo and in vitro studies have confirmed the activation of the mTOR signalling pathway in leiomyomata, and treatment of Eker rats with the rapamycin analogue WAY-129327 has inhibited mTOR signalling and decreased tumour incidence, multiplicity and size. These data show the important role of mTOR signalling in leiomyoma etiology and the potential pharmacotherapeutic opportunities for targeting this pathway in the treatment of this disease (Crabtree et al., 2009). Targeting mTOR could also be a way to interfere at the same time with RTK and TGF-β superfamily pathways. In fact, a functional cross-talk between the mTOR and Smad signalling devices has been identified in several human cancer cells, and also in non-transformed cells (Law et al., 2002; van der Poel, 2004; Langenfeld et al., 2005; Amirouche et al., 2009; Osman et al., 2009).
The potential importance of activin, myostatin and TGF-β as novel drug targets for cancer as well as immune, endocrine and metabolic disorders is being currently reported (Tsuchida, 2004; Vale et al., 2004; Harrison et al., 2005). Example of molecules proposed are: an antagonist of the activin receptor [activin Met108Ala (Harrison et al., 2004)], activin-A/C chimeras (Muenster et al., 2005), activin A/bone morphogenetic protein (BMP) chimeras (Korupolu et al., 2008), small-molecule inhibitors of ALK4 (SB-431542 and SB-505124), neutralizing antibodies and the soluble ActRII ECD (Harrison et al., 2005), as well as chemically developed TGF-β inhibitors such as LY364947, A-83-01 and Ki26894 (Tsuchida et al., 2008). Finally, further possibilities could involve the use of receptor antisense oligonucleotides targeting all the growth factors or the use of the known binding proteins (e.g. IGF BP).
Conclusions
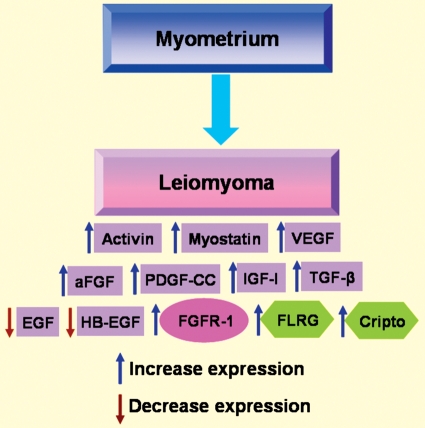
This review discloses that there are many growth factors that have been identified in myometrium (pregnant and non-pregnant) and in leiomyoma. However, for many of these there is only partial information available. Only a few factors have been thoroughly investigated with regard to their expression, regulation by steroids and, most importantly, their mode of signalling and regulation. For example, activin and myostatin are emerging growth factors that have been studied both in vivo and in vitro. Interestingly, estrogen down-regulates both activin and myostatin expression in human myometrium and, although expression of activin and myostatin is increased in leiomyoma, their signalling is disrupted in this condition, apparently due to increased FLRG and/or Cripto expression (Ciarmela et al., 2011). This finding may encourage us to explore the mechanisms of action of activin and myostatin and their regulators with the aim of establishing new strategies for the treatment of leiomyomas. In summary, we believe it is necessary to continue to perform extended and in depth research on the growth factors that control normal and pathological myometrial cellular biology. Figure 6 shows the expression level changes of growth factors, their receptors and related proteins in fibroid myometrium compared with normal non-pregnant myometrium. We have summarized the knowledge available so far regarding the roles of various growth factors in controlling the behaviour of myometrial cells. The main take-home message is that multiple molecular targets for potential myometrial dysfunctions and therapeutic innovation have been elucidated in the last years. However, it is necessary to improve present knowledge mainly in the regulatory and functional aspects, and much room is available for future research.
Figure 6.
Expression level changes of growth factors (violet), their receptors (pink) and related proteins (green) in leiomyoma compared with normal myometrium.
Authors' roles
Conceived and designed the study: P.C., F.P., W.V., M.C.; Performed the search: P.C., M.S.I., P.C.G.; Analysed the data: P.C., M.S.I., F.M.R., E.B., P.C.G.; Wrote the paper: P.C., M.S.I., F.M.R., P.C.G., E.B., F.P., W.V., M.C.
Funding
This work was supported in part by the Grants4Targets initiative from Bayer Schering Pharma (to P.C.). This work was also supported in part by the Department of Defense W81XWH-10-1-0891 (to P.G.) and by the Clayton Medical Research Foundation and Grant Number P30CA014195 from the National Cancer Institute (to P.G. and W.V.). W.V. is a Senior CMRF Investigator.
Conflict of interest
W.V. is a co-founder, consultant, equity holder, member of the Board of Directors and Scientific Advisory Board of Acceleron Pharma Inc. In accordance with Salk Institute policy, W.V. derives patent and licensing income in the activin field. All the other authors have nothing to declare.
References
- Amirouche A, Durieux AC, Banzet S, Koulmann N, Bonnefoy R, Mouret C, Bigard X, Peinnequin A, Freyssenet D. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology. 2009;150:286–294. doi: 10.1210/en.2008-0959. [DOI] [PubMed] [Google Scholar]
- Anania CA, Stewart EA, Quade BJ, Hill JA, Nowak RA. Expression of the fibroblast growth factor receptor in women with leiomyomas and abnormal uterine bleeding. Mol Hum Reprod. 1997;3:685–691. doi: 10.1093/molehr/3.8.685. [DOI] [PubMed] [Google Scholar]
- Andersen J, Barbieri RL. Abnormal gene expression in uterine leiomyomas. J Soc Gynecol Investig. 1995;2:663–672. doi: 10.1016/1071-5576(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Andersen J, Grine E, Eng CL, Zhao K, Barbieri RL, Chumas JC, Brink PR. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266–1276. doi: 10.1016/0002-9378(93)90293-r. [DOI] [PubMed] [Google Scholar]
- Arici A, Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73:1006–1011. doi: 10.1016/s0015-0282(00)00418-0. [DOI] [PubMed] [Google Scholar]
- Arici A, Sozen I. Expression, menstrual cycle-dependent activation, and bimodal mitogenic effect of transforming growth factor-beta1 in human myometrium and leiomyoma. Am J Obstet Gynecol. 2003;188:76–83. doi: 10.1067/mob.2003.118. [DOI] [PubMed] [Google Scholar]
- Arita S, Kikkawa F, Kajiyama H, Shibata K, Kawai M, Mizuno K, Nagasaka T, Ino K, Nomura S. Prognostic importance of vascular endothelial growth factor and its receptors in the uterine sarcoma. Int J Gynecol Cancer. 2005;15:329–336. doi: 10.1111/j.1525-1438.2005.15225.x. [DOI] [PubMed] [Google Scholar]
- Barbarisi A, Petillo O, Di Lieto A, Melone MA, Margarucci S, Cannas M, Peluso G. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. J Cell Physiol. 2001;186:414–424. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1040>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990;63:515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- Betsholtz C, Johnsson A, Heldin CH, Westermark B, Lind P, Urdea MS, Eddy R, Shows TB, Philpott K, Mellor AL, et al. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986;320:695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Corrigan AZ, Blount AL, Chen Y, Vale WW. Regulation and actions of Smad7 in the modulation of activin, inhibin, and transforming growth factor-beta signaling in anterior pituitary cells. Endocrinology. 2001;142:1065–1072. doi: 10.1210/endo.142.3.8028. [DOI] [PubMed] [Google Scholar]
- Boehm KD, Daimon M, Gorodeski IG, Sheean LA, Utian WH, Ilan J. Expression of the insulin-like and platelet-derived growth factor genes in human uterine tissues. Mol Reprod Dev. 1990;27:93–101. doi: 10.1002/mrd.1080270203. [DOI] [PubMed] [Google Scholar]
- Brown LF, Detmar M, Tognazzi K, Abu-Jawdeh G, Iruela-Arispe ML. Uterine smooth muscle cells express functional receptors (flt-1 and KDR) for vascular permeability factor/vascular endothelial growth factor. Lab Invest. 1997;76:245–255. [PubMed] [Google Scholar]
- Burroughs KD, Fuchs-Young R, Davis B, Walker CL. Altered hormonal responsiveness of proliferation and apoptosis during myometrial maturation and the development of uterine leiomyomas in the rat. Biol Reprod. 2000;63:1322–1330. doi: 10.1095/biolreprod63.5.1322. [DOI] [PubMed] [Google Scholar]
- Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- Catherino WH, Malik M, Driggers P, Chappel S, Segars J, Davis J. Novel, orally active selective progesterone receptor modulator CP8947 inhibits leiomyoma cell proliferation without adversely affecting endometrium or myometrium. J Steroid Biochem Mol Biol. 2010;122:279–286. doi: 10.1016/j.jsbmb.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesen-Cummings K, Houston KD, Copland JA, Moorman VJ, Walker CL, Davis BJ. Uterine leiomyomas express myometrial contractile-associated proteins involved in pregnancy-related hormone signaling. J Soc Gynecol Investig. 2003;10:11–20. [PubMed] [Google Scholar]
- Chegini N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med. 2010;28:180–203. doi: 10.1055/s-0030-1251476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chegini N, Zhao Y, Williams RS, Flanders KC. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-beta 1 (TGF beta 1), TGF beta 2, TGF beta 3, and TGF beta type II receptor messenger ribonucleic acid and protein and contains [125I]TGF beta 1-binding sites. Endocrinology. 1994;135:439–449. doi: 10.1210/endo.135.1.8013382. [DOI] [PubMed] [Google Scholar]
- Chegini N, Ma C, Tang XM, Williams RS. Effects of GnRH analogues, ‘add-back’ steroid therapy, antiestrogen and antiprogestins on leiomyoma and myometrial smooth muscle cell growth and transforming growth factor-beta expression. Mol Hum Reprod. 2002;8:1071–1078. doi: 10.1093/molehr/8.12.1071. [DOI] [PubMed] [Google Scholar]
- Chegini N, Luo X, Ding L, Ripley D. The expression of Smads and transforming growth factor beta receptors in leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Mol Cell Endocrinol. 2003;209:9–16. doi: 10.1016/j.mce.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Ciarmela P, Wiater E, Vale W. Activin-A in myometrium: characterization of the actions on myometrial cells. Endocrinology. 2008;149:2506–2516. doi: 10.1210/en.2007-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarmela P, Wiater E, Smith SM, Vale W. Presence, actions, and regulation of myostatin in rat uterus and myometrial cells. Endocrinology. 2009;150:906–914. doi: 10.1210/en.2008-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarmela P, Bloise E, Gray PC, Carrarelli P, Islam MS, De Pascalis F, Severi FM, Vale W, Castellucci M, Petraglia F. Activin-A and myostatin response and steroid regulation in human myometrium: disruption of Their signalling in uterine fibroid. J Clin Endocrinol Metab. 2011;96:755–765. doi: 10.1210/jc.2010-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccodicola A, Dono R, Obici S, Simeone A, Zollo M, Persico MG. Molecular characterization of a gene of the ‘EGF family’ expressed in undifferentiated human NTERA2 teratocarcinoma cells. Embo J. 1989;8:1987–1991. doi: 10.1002/j.1460-2075.1989.tb03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW, Rainey WE. Telomerase immortalization of human myometrial cells. Biol Reprod. 2002;67:506–514. doi: 10.1095/biolreprod67.2.506. [DOI] [PubMed] [Google Scholar]
- Crabtree JS, Jelinsky SA, Harris HA, Choe SE, Cotreau MM, Kimberland ML, Wilson E, Saraf KA, Liu W, McCampbell AS, et al. Comparison of human and rat uterine leiomyomata: identification of a dysregulated mammalian target of rapamycin pathway. Cancer Res. 2009;69:6171–6178. doi: 10.1158/0008-5472.CAN-08-4471. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R, Gallo RC, Giallongo A, Croce CM. Chromosomal localization of the human homolog (c-sis) of the simian sarcoma virus onc gene. Science. 1982;218:686–688. doi: 10.1126/science.6291150. [DOI] [PubMed] [Google Scholar]
- De Falco M, Staibano S, D'Armiento FP, Mascolo M, Salvatore G, Busiello A, Carbone IF, Pollio F, Di Lieto A. Preoperative treatment of uterine leiomyomas: clinical findings and expression of transforming growth factor-beta3 and connective tissue growth factor. J Soc Gynecol Investig. 2006;13:297–303. doi: 10.1016/j.jsgi.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Di X, Yu L, Moore AB, Castro L, Zheng X, Hermon T, Dixon D. A low concentration of genistein induces estrogen receptor-alpha and insulin-like growth factor-I receptor interactions and proliferation in uterine leiomyoma cells. Hum Reprod. 2008;23:1873–1883. doi: 10.1093/humrep/den087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lieto A, De Rosa G, De Falco M, Iannotti F, Staibano S, Pollio F, Scaramellino M, Salvatore G. Relationship between platelet-derived growth factor expression in leiomyomas and uterine volume changes after gonadotropin-releasing hormone agonist treatment. Hum Pathol. 2002;33:220–224. doi: 10.1053/hupa.2002.31298. [DOI] [PubMed] [Google Scholar]
- Ding L, Xu J, Luo X, Chegini N. Gonadotropin releasing hormone and transforming growth factor beta activate mitogen-activated protein kinase/extracellularly regulated kinase and differentially regulate fibronectin, type I collagen, and plasminogen activator inhibitor-1 expression in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab. 2004;89:5549–5557. doi: 10.1210/jc.2004-0161. [DOI] [PubMed] [Google Scholar]
- Dixon D, He H, Haseman JK. Immunohistochemical localization of growth factors and their receptors in uterine leiomyomas and matched myometrium. Environ Health Perspect. 2000;108(Suppl 5):795–802. doi: 10.1289/ehp.00108s5795. [DOI] [PubMed] [Google Scholar]
- Dodin S, Lemay A, Maheux R, Dumont M, Turcot-Lemay L. Bone mass in endometriosis patients treated with GnRH agonist implant or danazol. Obstet Gynecol. 1991;77:410–415. [PubMed] [Google Scholar]
- Dou Q, Zhao Y, Tarnuzzer RW, Rong H, Williams RS, Schultz GS, Chegini N. Suppression of transforming growth factor-beta (TGF beta) and TGF beta receptor messenger ribonucleic acid and protein expression in leiomyomata in women receiving gonadotropin-releasing hormone agonist therapy. J Clin Endocrinol Metab. 1996;81:3222–3230. doi: 10.1210/jcem.81.9.8784073. [DOI] [PubMed] [Google Scholar]
- Draper LB, Chong H, Wang E, Woodruff TK. The uterine myometrium is a target for increased levels of activin A during pregnancy. Endocrinology. 1997;138:3042–3046. doi: 10.1210/endo.138.7.5231. [DOI] [PubMed] [Google Scholar]
- Duan C. Specifying the cellular responses to IGF signals: roles of IGF-binding proteins. J Endocrinol. 2002;175:41–54. doi: 10.1677/joe.0.1750041. [DOI] [PubMed] [Google Scholar]
- Elenius K, Paul S, Allison G, Sun J, Klagsbrun M. Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. Embo J. 1997;16:1268–1278. doi: 10.1093/emboj/16.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto M, Iwasaki H, Ishiguro M, Kikuchi M, Horiuchi S, Saito T, Tsukamoto N, Kawarabayashi T. Angiogenesis in carcinosarcomas of the uterus: differences in the microvessel density and expression of vascular endothelial growth factor between the epithelial and mesenchymal elements. Hum Pathol. 1999;30:1232–1241. doi: 10.1016/s0046-8177(99)90043-6. [DOI] [PubMed] [Google Scholar]
- Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PG, Gemzell-Danielsson K. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod. 2009;24:1870–1879. doi: 10.1093/humrep/dep100. [DOI] [PubMed] [Google Scholar]
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229–234. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- Fayed YM, Tsibris JC, Langenberg PW, Robertson AL., Jr. Human uterine leiomyoma cells: binding and growth responses to epidermal growth factor, platelet-derived growth factor, and insulin. Lab Invest. 1989;60:30–37. [PubMed] [Google Scholar]
- Feng C, Meldrum S, Fiscella K. Improved quality of life is partly explained by fewer symptoms after treatment of fibroids with mifepristone. Int J Gynaecol Obstet. 2010;109:121–124. doi: 10.1016/j.ijgo.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernig DG, Gallagher JT. Fibroblast growth factors and their receptors: an information network controlling tissue growth, morphogenesis and repair. Prog Growth Factor Res. 1994;5:353–377. doi: 10.1016/0955-2235(94)00007-8. [DOI] [PubMed] [Google Scholar]
- Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111:1037–1054. doi: 10.1289/ehp.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishman GN, Jurema MW. Myomas and myomectomy. J Minim Invasive Gynecol. 2005;12:443–456. doi: 10.1016/j.jmig.2005.05.023. quiz 457–448. [DOI] [PubMed] [Google Scholar]
- Gao Z, Matsuo H, Wang Y, Nakago S, Maruo T. Up-regulation by IGF-I of proliferating cell nuclear antigen and Bcl-2 protein expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2001;86:5593–5599. doi: 10.1210/jcem.86.11.8008. [DOI] [PubMed] [Google Scholar]
- Gentry CC, Okolo SO, Fong LF, Crow JC, Maclean AB, Perrett CW. Quantification of vascular endothelial growth factor-A in leiomyomas and adjacent myometrium. Clin Sci (Lond) 2001;101:691–695. [PubMed] [Google Scholar]
- Giudice LC, Irwin JC, Dsupin BA, Pannier EM, Jin IH, Vu TH, Hoffman AR. Insulin-like growth factor (IGF), IGF binding protein (IGFBP), and IGF receptor gene expression and IGFBP synthesis in human uterine leiomyomata. Hum Reprod. 1993;8:1796–1806. doi: 10.1093/oxfordjournals.humrep.a137937. [DOI] [PubMed] [Google Scholar]
- Gloudemans T, Prinsen I, Van Unnik JA, Lips CJ, Den Otter W, Sussenbach JS. Insulin-like growth factor gene expression in human smooth muscle tumors. Cancer Res. 1990;50:6689–6695. [PubMed] [Google Scholar]
- Gospodarowicz D, Ferrara N, Schweigerer L, Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987;8:95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Gray PC, Harrison CA, Vale W. Cripto forms a complex with activin and type II activin receptors and can block activin signaling. Proc Natl Acad Sci USA. 2003;100:5193–5198. doi: 10.1073/pnas.0531290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PC, Shani G, Aung K, Kelber J, Vale W. Cripto binds transforming growth factor beta (TGF-beta) and inhibits TGF-beta signaling. Mol Cell Biol. 2006;26:9268–9278. doi: 10.1128/MCB.01168-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17:71–74. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- Hague S, Zhang L, Oehler MK, Manek S, MacKenzie IZ, Bicknell R, Rees MC. Expression of the hypoxically regulated angiogenic factor adrenomedullin correlates with uterine leiomyoma vascular density. Clin Cancer Res. 2000;6:2808–2814. [PubMed] [Google Scholar]
- Harrison CA, Gray PC, Fischer WH, Donaldson C, Choe S, Vale W. An activin mutant with disrupted ALK4 binding blocks signaling via type II receptors. J Biol Chem. 2004;279:28036–28044. doi: 10.1074/jbc.M402782200. [DOI] [PubMed] [Google Scholar]
- Harrison CA, Gray PC, Vale WW, Robertson DM. Antagonists of activin signaling: mechanisms and potential biological applications. Trends Endocrinol Metab. 2005;16:73–78. doi: 10.1016/j.tem.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Harrison-Woolrych ML, Sharkey AM, Charnock-Jones DS, Smith SK. Localization and quantification of vascular endothelial growth factor messenger ribonucleic acid in human myometrium and leiomyomata. J Clin Endocrinol Metab. 1995;80:1853–1858. doi: 10.1210/jcem.80.6.7775632. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Carpenter KD, Gray CA, Spencer TE. The activin-follistatin system in the neonatal ovine uterus. Biol Reprod. 2003;69:843–850. doi: 10.1095/biolreprod.103.016287. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Eriksson U, Ostman A. New members of the platelet-derived growth factor family of mitogens. Arch Biochem Biophys. 2002;398:284–290. doi: 10.1006/abbi.2001.2707. [DOI] [PubMed] [Google Scholar]
- Hermon TL, Moore AB, Yu L, Kissling GE, Castora FJ, Dixon D. Estrogen receptor alpha (ERalpha) phospho-serine-118 is highly expressed in human uterine leiomyomas compared to matched myometrium. Virchows Arch. 2008;453:557–569. doi: 10.1007/s00428-008-0679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem. 2002;277:40735–40741. doi: 10.1074/jbc.M206379200. [DOI] [PubMed] [Google Scholar]
- Hill GD, Moore AB, Kissling GE, Flagler ND, Ney E, Cline JM, Dixon D. Effects of hormonally active agents on steroid hormone receptor expression and cell proliferation in the myometrium of ovariectomized macaques. Toxicol Pathol. 2011;39:508–515. doi: 10.1177/0192623311401045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann GE, Rao CV, Barrows GH, Schultz GS, Sanfilippo JS. Binding sites for epidermal growth factor in human uterine tissues and leiomyomas. J Clin Endocrinol Metab. 1984;58:880–884. doi: 10.1210/jcem-58-5-880. [DOI] [PubMed] [Google Scholar]
- Hong T, Shimada Y, Uchida S, Itami A, Li Z, Ding Y, Kaganoi J, Komoto I, Sakurai T, Imamura M. Expression of angiogenic factors and apoptotic factors in leiomyosarcoma and leiomyoma. Int J Mol Med. 2001;8:141–148. doi: 10.3892/ijmm.8.2.141. [DOI] [PubMed] [Google Scholar]
- Hwu YM, Li SH, Lee RK, Tsai YH, Yeh TS, Lin SY. Increased expression of platelet-derived growth factor C messenger ribonucleic acid in uterine leiomyomata. Fertil Steril. 2008;89:468–471. doi: 10.1016/j.fertnstert.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Huang JC, Nawaz Z, Boettger-Tong H, Makela S, Chiappetta C, Stancel GM. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect. 2000;108(Suppl 5):785–790. doi: 10.1289/ehp.00108s5785. [DOI] [PubMed] [Google Scholar]
- Iwamoto I, Fujino T, Higashi Y, Tsuji T, Nakamura N, Komokata T, Douchi T. Metastasis of uterine leiomyosarcoma to the pancreas. J Obstet Gynaecol Res. 2005;31:531–534. doi: 10.1111/j.1447-0756.2005.00344.x. [DOI] [PubMed] [Google Scholar]
- Jaffer S, Shynlova O, Lye S. Mammalian target of rapamycin is activated in association with myometrial proliferation during pregnancy. Endocrinology. 2009;150:4672–4680. doi: 10.1210/en.2009-0419. [DOI] [PubMed] [Google Scholar]
- Johansson B. Different types of smooth muscle hypertrophy. Hypertension. 1984;6:III64–III68. doi: 10.1161/01.hyp.6.6_pt_2.iii64. [DOI] [PubMed] [Google Scholar]
- Jones RL, Kaitu'u-Lino TJ, Nie G, Sanchez-Partida LG, Findlay JK, Salamonsen LA. Complex expression patterns support potential roles for maternally derived activins in the establishment of pregnancy in mouse. Reproduction. 2006;132:799–810. doi: 10.1530/REP-06-0034. [DOI] [PubMed] [Google Scholar]
- Joseph DS, Malik M, Nurudeen S, Catherino WH. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril. 2010;93:1500–1508. doi: 10.1016/j.fertnstert.2009.01.081. [DOI] [PubMed] [Google Scholar]
- Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7:910–916. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- Kelber JA, Shani G, Booker EC, Vale WW, Gray PC. Cripto is a noncompetitive activin antagonist that forms analogous signaling complexes with activin and nodal. J Biol Chem. 2008;283:4490–4500. doi: 10.1074/jbc.M704960200. [DOI] [PubMed] [Google Scholar]
- Korupolu RV, Muenster U, Read JD, Vale W, Fischer WH. Activin A/bone morphogenetic protein (BMP) chimeras exhibit BMP-like activity and antagonize activin and myostatin. J Biol Chem. 2008;283:3782–3790. doi: 10.1074/jbc.M704530200. [DOI] [PubMed] [Google Scholar]
- Langenfeld EM, Kong Y, Langenfeld J. Bone morphogenetic protein-2-induced transformation involves the activation of mammalian target of rapamycin. Mol Cancer Res. 2005;3:679–684. doi: 10.1158/1541-7786.MCR-05-0124. [DOI] [PubMed] [Google Scholar]
- Laping NJ, Everitt JI, Frazier KS, Burgert M, Portis MJ, Cadacio C, Gold LI, Walker CL. Tumor-specific efficacy of transforming growth factor-beta RI inhibition in Eker rats. Clin Cancer Res. 2007;13:3087–3099. doi: 10.1158/1078-0432.CCR-06-1811. [DOI] [PubMed] [Google Scholar]
- Law BK, Chytil A, Dumont N, Hamilton EG, Waltner-Law ME, Aakre ME, Covington C, Moses HL. Rapamycin potentiates transforming growth factor beta-induced growth arrest in nontransformed, oncogene-transformed, and human cancer cells. Mol Cell Biol. 2002;22:8184–8198. doi: 10.1128/MCB.22.23.8184-8198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun JJ, Takabe K, Chen Y, Vale W. Roles of pathway-specific and inhibitory Smads in activin receptor signaling. Mol Endocrinol. 1999;13:15–23. doi: 10.1210/mend.13.1.0218. [DOI] [PubMed] [Google Scholar]
- Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab. 2001;86:913–920. doi: 10.1210/jcem.86.2.7237. [DOI] [PubMed] [Google Scholar]
- Lei J, Jiang A, Pei D. Identification and characterization of a new splicing variant of vascular endothelial growth factor: VEGF183. Biochim Biophys Acta. 1998;1443:400–406. doi: 10.1016/s0167-4781(98)00240-1. [DOI] [PubMed] [Google Scholar]
- Levens E, Luo X, Ding L, Williams RS, Chegini N. Fibromodulin is expressed in leiomyoma and myometrium and regulated by gonadotropin-releasing hormone analogue therapy and TGF-beta through Smad and MAPK-mediated signalling. Mol Hum Reprod. 2005;11:489–494. doi: 10.1093/molehr/gah187. [DOI] [PubMed] [Google Scholar]
- Levens ED, Potlog-Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL, Blocker W, Nieman LK. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol. 2008;111:1129–1136. doi: 10.1097/AOG.0b013e3181705d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Wang H, Zhang Y, Lu S, Wang Z. Expression and functional analysis of platelet-derived growth factor in uterine leiomyomata. Cancer Biol Ther. 2006;5:28–33. doi: 10.4161/cbt.5.1.2234. [DOI] [PubMed] [Google Scholar]
- Luo X, Ding L, Xu J, Chegini N. Gene expression profiling of leiomyoma and myometrial smooth muscle cells in response to transforming growth factor-beta. Endocrinology. 2005;146:1097–1118. doi: 10.1210/en.2004-1377. [DOI] [PubMed] [Google Scholar]
- Luo X, Ding L, Chegini N. CCNs, fibulin-1C and S100A4 expression in leiomyoma and myometrium: inverse association with TGF-beta and regulation by TGF-beta in leiomyoma and myometrial smooth muscle cells. Mol Hum Reprod. 2006;12:245–256. doi: 10.1093/molehr/gal015. [DOI] [PubMed] [Google Scholar]
- Luo X, Yin P, Coon VJ, Cheng YH, Wiehle RD, Bulun SE. The selective progesterone receptor modulator CDB4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertil Steril. 2010;93:2668–2673. doi: 10.1016/j.fertnstert.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, Norian J, McCarthy-Keith D, Britten J, Catherino WH. Why leiomyomas are called fibroids: the central role of extracellular matrix in symptomatic women. Semin Reprod Med. 2010;28:169–179. doi: 10.1055/s-0030-1251475. [DOI] [PubMed] [Google Scholar]
- Mangrulkar RS, Ono M, Ishikawa M, Takashima S, Klagsbrun M, Nowak RA. Isolation and characterization of heparin-binding growth factors in human leiomyomas and normal myometrium. Biol Reprod. 1995;53:636–646. doi: 10.1095/biolreprod53.3.636. [DOI] [PubMed] [Google Scholar]
- Marsh EE, Bulun SE. Steroid hormones and leiomyomas. Obstet Gynecol Clin North Am. 2006;33:59–67. doi: 10.1016/j.ogc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Maruo T, Matsuo H, Shimomura Y, Kurachi O, Gao Z, Nakago S, Yamada T, Chen W, Wang J. Effects of progesterone on growth factor expression in human uterine leiomyoma. Steroids. 2003;68:817–824. doi: 10.1016/j.steroids.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Massague J. Epidermal growth factor-like transforming growth factor. I. Isolation, chemical characterization, and potentiation by other transforming factors from feline sarcoma virus-transformed rat cells. J Biol Chem. 1983;258:13606–13613. [PubMed] [Google Scholar]
- Massague J, Attisano L, Wrana JL. The TGF-beta family and its composite receptors. Trends Cell Biol. 1994;4:172–178. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Kurachi O, Shimomura Y, Samoto T, Maruo T. Molecular bases for the actions of ovarian sex steroids in the regulation of proliferation and apoptosis of human uterine leiomyoma. Oncology. 1999;57(Suppl 2):49–58. doi: 10.1159/000055275. [DOI] [PubMed] [Google Scholar]
- McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Mesquita FS, Dyer SN, Heinrich DA, Bulun SE, Marsh EE, Nowak RA. Reactive oxygen species mediate mitogenic growth factor signaling pathways in human leiomyoma smooth muscle cells. Biol Reprod. 2010;82:341–351. doi: 10.1095/biolreprod.108.075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga M, Ku CY, Dodge K, Sanborn BM. Oxytocin-stimulated responses in a pregnant human immortalized myometrial cell line. Biol Reprod. 1996;55:427–432. doi: 10.1095/biolreprod55.2.427. [DOI] [PubMed] [Google Scholar]
- Moore AB, He H, Yoshida A, Rico PJ, Haseman JK, Dixon D. Transforming growth factor-alpha, epidermal growth factor receptor, and PCNA immunoexpression in uterine leiomyosarcomas and leiomyomas in B6C3F1 mice. Exp Toxicol Pathol. 2000;52:195–200. doi: 10.1016/S0940-2993(00)80028-7. [DOI] [PubMed] [Google Scholar]
- Moore AB, Castro L, Yu L, Zheng X, Di X, Sifre MI, Kissling GE, Newbold RR, Bortner CD, Dixon D. Stimulatory and inhibitory effects of genistein on human uterine leiomyoma cell proliferation are influenced by the concentration. Hum Reprod. 2007;22:2623–2631. doi: 10.1093/humrep/dem185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AB, Yu L, Swartz CD, Zheng X, Wang L, Castro L, Kissling GE, Walmer DK, Robboy SJ, Dixon D. Human uterine leiomyoma-derived fibroblasts stimulate uterine leiomyoma cell proliferation and collagen type I production, and activate RTKs and TGF beta receptor signaling in coculture. Cell Commun Signal. 2010;8:10. doi: 10.1186/1478-811X-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007;3:e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenster U, Harrison CA, Donaldson C, Vale W, Fischer WH. An activin-A/C chimera exhibits activin and myostatin antagonistic properties. J Biol Chem. 2005;280:36626–36632. doi: 10.1074/jbc.M507236200. [DOI] [PubMed] [Google Scholar]
- Nagaraj NS, Datta PK. Targeting the transforming growth factor-beta signaling pathway in human cancer. Expert Opin Investig Drugs. 2010;19:77–91. doi: 10.1517/13543780903382609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Moore AB, Dixon D. Characterization of uterine leiomyomas in CD-1 mice following developmental exposure to diethylstilbestrol (DES) Toxicol Pathol. 2002;30:611–616. doi: 10.1080/01926230290105839. [DOI] [PubMed] [Google Scholar]
- Nierth-Simpson EN, Martin MM, Chiang TC, Melnik LI, Rhodes LV, Muir SE, Burow ME, McLachlan JA. Human uterine smooth muscle and leiomyoma cells differ in their rapid 17beta-estradiol signaling: implications for proliferation. Endocrinology. 2009;150:2436–2445. doi: 10.1210/en.2008-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norian JM, Malik M, Parker CY, Joseph D, Leppert PC, Segars JH, Catherino WH. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci. 2009;16:1153–1164. doi: 10.1177/1933719109343310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak RA. Novel therapeutic strategies for leiomyomas: targeting growth factors and their receptors. Environ Health Perspect. 2000;108(Suppl 5):849–853. doi: 10.1289/ehp.00108s5849. [DOI] [PubMed] [Google Scholar]
- Ohara N. Action of progesterone receptor modulators on uterine leiomyomas. Clin Exp Obstet Gynecol. 2008;35:165–166. [PubMed] [Google Scholar]
- Ohara N, Morikawa A, Chen W, Wang J, DeManno DA, Chwalisz K, Maruo T. Comparative effects of SPRM asoprisnil (J867) on proliferation, apoptosis, and the expression of growth factors in cultured uterine leiomyoma cells and normal myometrial cells. Reprod Sci. 2007;14:20–27. doi: 10.1177/1933719107311464. [DOI] [PubMed] [Google Scholar]
- Olive DL, Pritts EA. Fibroids and reproduction. Semin Reprod Med. 2010;28:218–227. doi: 10.1055/s-0030-1251478. [DOI] [PubMed] [Google Scholar]
- Olufowobi O, Sharif K, Papaionnou S, Neelakantan D, Mohammed H, Afnan M. Are the anticipated benefits of myomectomy achieved in women of reproductive age? A 5-year review of the results at a UK tertiary hospital. J Obstet Gynaecol. 2004;24:434–440. doi: 10.1080/01443610410001685600. [DOI] [PubMed] [Google Scholar]
- Osman B, Doller A, Akool el S, Holdener M, Hintermann E, Pfeilschifter J, Eberhardt W. Rapamycin induces the TGFbeta1/Smad signaling cascade in renal mesangial cells upstream of mTOR. Cell Signal. 2009;21:1806–1817. doi: 10.1016/j.cellsig.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Palman C, Bowen-Pope DF, Brooks JJ. Platelet-derived growth factor receptor (beta-subunit) immunoreactivity in soft tissue tumors. Lab Invest. 1992;66:108–115. [PubMed] [Google Scholar]
- Pekonen F, Nyman T, Rutanen EM. Differential expression of keratinocyte growth factor and its receptor in the human uterus. Mol Cell Endocrinol. 1993;95:43–49. doi: 10.1016/0303-7207(93)90027-h. [DOI] [PubMed] [Google Scholar]
- Peng L, Wen Y, Han Y, Wei A, Shi G, Mizuguchi M, Lee P, Hernando E, Mittal K, Wei JJ. Expression of insulin-like growth factors (IGFs) and IGF signaling: molecular complexity in uterine leiomyomas. Fertil Steril. 2009;91:2664–2675. doi: 10.1016/j.fertnstert.2007.10.083. [DOI] [PubMed] [Google Scholar]
- Poltorak Z, Cohen T, Sivan R, Kandelis Y, Spira G, Vlodavsky I, Keshet E, Neufeld G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J Biol Chem. 1997;272:7151–7158. doi: 10.1074/jbc.272.11.7151. [DOI] [PubMed] [Google Scholar]
- Pusztai L, Lewis CE, Lorenzen J, McGee JO. Growth factors: regulation of normal and neoplastic growth. J Pathol. 1993;169:191–201. doi: 10.1002/path.1711690204. [DOI] [PubMed] [Google Scholar]
- Rauk PN, Surti U, Roberts JM, Michalopoulos G. Mitogenic effect of basic fibroblast growth factor and estradiol on cultured human myometrial and leiomyoma cells. Am J Obstet Gynecol. 1995;173:571–577. doi: 10.1016/0002-9378(95)90284-8. [DOI] [PubMed] [Google Scholar]
- Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230–7242. doi: 10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider V, Carlone DL, Foster RT. Oestrogen and progesterone control basic fibroblast growth factor mRNA in the rat uterus. J Endocrinol. 1997;154:75–84. doi: 10.1677/joe.0.1540075. [DOI] [PubMed] [Google Scholar]
- Rossi MJ, Chegini N, Masterson BJ. Presence of epidermal growth factor, platelet-derived growth factor, and their receptors in human myometrial tissue and smooth muscle cells: their action in smooth muscle cells in vitro. Endocrinology. 1992;130:1716–1727. doi: 10.1210/endo.130.3.1311246. [DOI] [PubMed] [Google Scholar]
- Samathanam CA, Adesanya OO, Zhou J, Wang J, Bondy CA. Fibroblast growth factors 1 and 2 in the primate uterus. Biol Reprod. 1998;59:491–496. doi: 10.1095/biolreprod59.3.491. [DOI] [PubMed] [Google Scholar]
- Sanci M, Dikis C, Inan S, Turkoz E, Dicle N, Ispahi C. Immunolocalization of VEGF, VEGF receptors, EGF-R and Ki-67 in leiomyoma, cellular leiomyoma and leiomyosarcoma. Acta Histochem. 2011;113:317–325. doi: 10.1016/j.acthis.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Sankaran S, Manyonda IT. Medical management of fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:655–676. doi: 10.1016/j.bpobgyn.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Schneider-Kolsky M, Manuelpillai U, Gargett C, Wallace EM. Activin betaA-subunit and activin receptors in human myometrium at term and during labour. BJOG. 2001;108:869–874. doi: 10.1111/j.1471-0528.2001.00202.x. [DOI] [PubMed] [Google Scholar]
- Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- Sharp HT. Assessment of new technology in the treatment of idiopathic menorrhagia and uterine leiomyomata. Obstet Gynecol. 2006;108:990–1003. doi: 10.1097/01.AOG.0000232618.26261.75. [DOI] [PubMed] [Google Scholar]
- Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Matsuo H, Samoto T, Maruo T. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab. 1998;83:2192–2198. doi: 10.1210/jcem.83.6.4879. [DOI] [PubMed] [Google Scholar]
- Shushan A, Rojansky N, Laufer N, Klein BY, Shlomai Z, Levitzki R, Hartzstark Z, Ben-Bassat H. The AG1478 tyrosine kinase inhibitor is an effective suppressor of leiomyoma cell growth. Hum Reprod. 2004;19:1957–1967. doi: 10.1093/humrep/deh355. [DOI] [PubMed] [Google Scholar]
- Shushan A, Ben-Bassat H, Mishani E, Laufer N, Klein BY, Rojansky N. Inhibition of leiomyoma cell proliferation in vitro by genistein and the protein tyrosine kinase inhibitor TKS050. Fertil Steril. 2007;87:127–135. doi: 10.1016/j.fertnstert.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Oldenhof A, Dorogin A, Xu Q, Mu J, Nashman N, Lye SJ. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod. 2006;74:839–849. doi: 10.1095/biolreprod.105.048124. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Tsui P, Dorogin A, Langille BL, Lye SJ. The expression of transforming growth factor beta in pregnant rat myometrium is hormone and stretch dependent. Reproduction. 2007a;134:503–511. doi: 10.1530/REP-07-0004. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Tsui P, Dorogin A, Langille BL, Lye SJ. Insulin-like growth factors and their binding proteins define specific phases of myometrial differentiation during pregnancy in the rat. Biol Reprod. 2007b;76:571–578. doi: 10.1095/biolreprod.106.056929. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S2–10. doi: 10.1016/j.ejogrb.2009.02.044. [DOI] [PubMed] [Google Scholar]
- Sozen I, Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril. 2002;78:1–12. doi: 10.1016/s0015-0282(02)03154-0. [DOI] [PubMed] [Google Scholar]
- Spitz IM. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol. 2009;21:318–324. doi: 10.1097/GCO.0b013e32832e07e8. [DOI] [PubMed] [Google Scholar]
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- Strawn EY, Jr, Novy MJ, Burry KA, Bethea CL. Insulin-like growth factor I promotes leiomyoma cell growth in vitro. Am J Obstet Gynecol. 1995;172:1837–1843. doi: 10.1016/0002-9378(95)91420-x. discussion 1843–1834. [DOI] [PubMed] [Google Scholar]
- Strizzi L, Bianco C, Hirota M, Watanabe K, Mancino M, Hamada S, Raafat A, Lawson S, Ebert AD, D'Antonio A, et al. Development of leiomyosarcoma of the uterus in MMTV-CR-1 transgenic mice. J Pathol. 2007;211:36–44. doi: 10.1002/path.2083. [DOI] [PubMed] [Google Scholar]
- Suo G, Jiang Y, Cowan B, Wang JY. Platelet-derived growth factor C is upregulated in human uterine fibroids and regulates uterine smooth muscle cell growth. Biol Reprod. 2009;81:749–758. doi: 10.1095/biolreprod.109.076869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz CD, Afshari CA, Yu L, Hall KE, Dixon D. Estrogen-induced changes in IGF-I, Myb family and MAP kinase pathway genes in human uterine leiomyoma and normal uterine smooth muscle cell lines. Mol Hum Reprod. 2005;11:441–450. doi: 10.1093/molehr/gah174. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Kobori H, Kikuchi I, Sato Y, Mitsuhashi N. A prospective randomized study comparing endocrinological and clinical effects of two types of GnRH agonists in cases of uterine leiomyomas or endometriosis. J Obstet Gynaecol Res. 2000;26:325–331. doi: 10.1111/j.1447-0756.2000.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Tam JP, Marquardt H, Rosberger DF, Wong TW, Todaro GJ. Synthesis of biologically active rat transforming growth factor I. Nature. 1984;309:376–378. doi: 10.1038/309376a0. [DOI] [PubMed] [Google Scholar]
- Tang XM, Dou Q, Zhao Y, McLean F, Davis J, Chegini N. The expression of transforming growth factor-beta s and TGF-beta receptor mRNA and protein and the effect of TGF-beta s on human myometrial smooth muscle cells in vitro. Mol Hum Reprod. 1997;3:233–240. doi: 10.1093/molehr/3.3.233. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Morita I, Kubota T, Murota S, Aso T. Human uterine myometrial smooth muscle cell proliferation and vascular endothelial growth-factor production in response to platelet-derived growth factor. J Endocrinol. 2001;169:79–86. doi: 10.1677/joe.0.1690079. [DOI] [PubMed] [Google Scholar]
- Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- Tsuchida K. Activins, myostatin and related TGF-beta family members as novel therapeutic targets for endocrine, metabolic and immune disorders. Curr Drug Targets Immune Endocr Metabol Disord. 2004;4:157–166. doi: 10.2174/1568008043339901. [DOI] [PubMed] [Google Scholar]
- Tsuchida K, Arai KY, Kuramoto Y, Yamakawa N, Hasegawa Y, Sugino H. Identification and characterization of a novel follistatin-like protein as a binding protein for the TGF-beta family. J Biol Chem. 2000;275:40788–40796. doi: 10.1074/jbc.M006114200. [DOI] [PubMed] [Google Scholar]
- Tsuchida K, Nakatani M, Uezumi A, Murakami T, Cui X. Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer. Endocr J. 2008;55:11–21. doi: 10.1507/endocrj.kr-110. [DOI] [PubMed] [Google Scholar]
- Vale W, Wiater E, Gray P, Harrison C, Bilezikjian L, Choe S. Activins and inhibins and their signaling. Ann N Y Acad Sci. 2004;1038:142–147. doi: 10.1196/annals.1315.023. [DOI] [PubMed] [Google Scholar]
- van der Poel HG. Mammalian target of rapamycin and 3-phosphatidylinositol 3-kinase pathway inhibition enhances growth inhibition of transforming growth factor-beta1 in prostate cancer cells. J Urol. 2004;172:1333–1337. doi: 10.1097/01.ju.0000138829.97838.19. [DOI] [PubMed] [Google Scholar]
- Van der Ven LT, Roholl PJ, Gloudemans T, Van Buul-Offers SC, Welters MJ, Bladergroen BA, Faber JA, Sussenbach JS, Den Otter W. Expression of insulin-like growth factors (IGFs), their receptors and IGF binding protein-3 in normal, benign and malignant smooth muscle tissues. Br J Cancer. 1997;75:1631–1640. doi: 10.1038/bjc.1997.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenhoven BJ, Herington AC, Healy DL. Messenger ribonucleic acid expression of the insulin-like growth factors and their binding proteins in uterine fibroids and myometrium. J Clin Endocrinol Metab. 1993;76:1106–1110. doi: 10.1210/jcem.76.5.7684390. [DOI] [PubMed] [Google Scholar]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104:393–406. doi: 10.1097/01.AOG.0000136079.62513.39. [DOI] [PubMed] [Google Scholar]
- Wang XN, Das SK, Damm D, Klagsbrun M, Abraham JA, Dey SK. Differential regulation of heparin-binding epidermal growth factor-like growth factor in the adult ovariectomized mouse uterus by progesterone and estrogen. Endocrinology. 1994;135:1264–1271. doi: 10.1210/endo.135.3.8070372. [DOI] [PubMed] [Google Scholar]
- Wang J, Ohara N, Takekida S, Xu Q, Maruo T. Comparative effects of heparin-binding epidermal growth factor-like growth factor on the growth of cultured human uterine leiomyoma cells and myometrial cells. Hum Reprod. 2005;20:1456–1465. doi: 10.1093/humrep/deh842. [DOI] [PubMed] [Google Scholar]
- Wang J, Ohara N, Wang Z, Chen W, Morikawa A, Sasaki H, DeManno DA, Chwalisz K, Maruo T. A novel selective progesterone receptor modulator asoprisnil (J867) down-regulates the expression of EGF, IGF-I, TGFbeta3 and their receptors in cultured uterine leiomyoma cells. Hum Reprod. 2006;21:1869–1877. doi: 10.1093/humrep/del035. [DOI] [PubMed] [Google Scholar]
- Williams AR, Critchley HO, Osei J, Ingamells S, Cameron IT, Han C, Chwalisz K. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum Reprod. 2007;22:1696–1704. doi: 10.1093/humrep/dem026. [DOI] [PubMed] [Google Scholar]
- Wolanska M, Bankowski E. An accumulation of insulin-like growth factor I (IGF-I) in human myometrium and uterine leiomyomas in various stages of tumour growth. Eur Cytokine Netw. 2004;15:359–363. [PubMed] [Google Scholar]
- Wolanska M, Bankowski E. Fibroblast growth factors (FGF) in human myometrium and uterine leiomyomas in various stages of tumour growth. Biochimie. 2006;88:141–146. doi: 10.1016/j.biochi.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Wolanska M, Bankowski E. Transforming growth factor beta and platelet-derived growth factor in human myometrium and in uterine leiomyomas at various stages of tumour growth. Eur J Obstet Gynecol Reprod Biol. 2007;130:238–244. doi: 10.1016/j.ejogrb.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Wolanska M, Bankowska-Guszczyn E, Jaworski S. [Fibroblast growth factor gene expression in uterine leiomyomas] Ginekol Pol. 2008;79:555–559. [PubMed] [Google Scholar]
- Wu X, Blanck A, Olovsson M, Moller B, Favini R, Lindblom B. Apoptosis, cellular proliferation and expression of p53 in human uterine leiomyomas and myometrium during the menstrual cycle and after menopause. Acta Obstet Gynecol Scand. 2000;79:397–404. [PubMed] [Google Scholar]
- Wu X, Blanck A, Olovsson M, Moller B, Lindblom B. Expression of basic fibroblast growth factor (bFGF), FGF receptor 1 and FGF receptor 2 in uterine leiomyomas and myometrium during the menstrual cycle, after menopause and GnRHa treatment. Acta Obstet Gynecol Scand. 2001;80:497–504. [PubMed] [Google Scholar]
- Wu X, Wang H, Englund K, Blanck A, Lindblom B, Sahlin L. Expression of progesterone receptors A and B and insulin-like growth factor-I in human myometrium and fibroids after treatment with a gonadotropin-releasing hormone analogue. Fertil Steril. 2002;78:985–993. doi: 10.1016/s0015-0282(02)03378-2. [DOI] [PubMed] [Google Scholar]
- Xu J, Luo X, Chegini N. Differential expression, regulation, and induction of Smads, transforming growth factor-beta signal transduction pathway in leiomyoma, and myometrial smooth muscle cells and alteration by gonadotropin-releasing hormone analog. J Clin Endocrinol Metab. 2003;88:1350–1361. doi: 10.1210/jc.2002-021325. [DOI] [PubMed] [Google Scholar]
- Yamada T, Nakago S, Kurachi O, Wang J, Takekida S, Matsuo H, Maruo T. Progesterone down-regulates insulin-like growth factor-I expression in cultured human uterine leiomyoma cells. Hum Reprod. 2004;19:815–821. doi: 10.1093/humrep/deh146. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Yeh J, Rein M, Nowak R. Presence of messenger ribonucleic acid for epidermal growth factor (EGF) and EGF receptor demonstrable in monolayer cell cultures of myometria and leiomyomata. Fertil Steril. 1991;56:997–1000. doi: 10.1016/s0015-0282(16)54681-0. [DOI] [PubMed] [Google Scholar]
- Yen-Ping Ho J, Man WC, Wen Y, Polan ML, Shih-Chu Ho E, Chen B. Transforming growth interacting factor expression in leiomyoma compared with myometrium. Fertil Steril. 2010;94:1078–1083. doi: 10.1016/j.fertnstert.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Weiyuan Z. Dual actions of progesterone on uterine leiomyoma correlate with the ratio of progesterone receptor A:B. Gynecol Endocrinol. 2009;25:520–523. doi: 10.1080/09513590902972117. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ohara N, Xu Q, Chen W, Wang J, Nakabayashi K, Sasaki H, Morikawa A, Maruo T. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Semin Reprod Med. 2010;28:260–273. doi: 10.1055/s-0030-1251483. [DOI] [PubMed] [Google Scholar]
- Yu H, Berkel H. Insulin-like growth factors and cancer. J La State Med Soc. 1999;151:218–223. [PubMed] [Google Scholar]
- Yu L, Saile K, Swartz CD, He H, Zheng X, Kissling GE, Di X, Lucas S, Robboy SJ, Dixon D. Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas. Mol Med. 2008;14:264–275. doi: 10.2119/2007-00101.Yu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Moore AB, Dixon D. Receptor tyrosine kinases and their hormonal regulation in uterine leiomyoma. Semin Reprod Med. 2010;28:250–259. doi: 10.1055/s-0030-1251482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Funk C, Roy D, Glasser S, Mulholland J. Heparin-binding epidermal growth factor-like growth factor is differentially regulated by progesterone and estradiol in rat uterine epithelial and stromal cells. Endocrinology. 1994;134:1089–1094. doi: 10.1210/endo.134.3.8119147. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang W, Wang S. The expression of estrogen receptor isoforms alpha, beta and insulin-like growth factor-I in uterine leiomyoma. Gynecol Endocrinol. 2008;24:549–554. doi: 10.1080/09513590802340522. [DOI] [PubMed] [Google Scholar]
- Zhu X, Topouzis S, Liang LF, Stotish RL. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine. 2004;26:262–272. doi: 10.1016/j.cyto.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Zwick E, Bange J, Ullrich A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr Relat Cancer. 2001;8:161–173. doi: 10.1677/erc.0.0080161. [DOI] [PubMed] [Google Scholar]