Abstract
Background
Concern has been raised over the practice of unnecessary double anaerobic coverage therapy (DACT) in the hospital setting. However, the incidence of and risk factors for unnecessary DACT are not well studied. On 8 September 2008, the antimicrobial stewardship programme (ASP) at our institution was modified such that several antibiotics, including ampicillin/sulbactam and metronidazole, no longer required pre-approval. We anticipated that this change would increase both unnecessary DACT and target antibiotic consumption.
Methods
A nested case–control study was conducted to determine the cumulative incidence of and risk factors for unnecessary DACT. Cases were subjects who received unnecessary DACT while controls were subjects who did not receive DACT or who received necessary DACT. Segmented regression analysis was subsequently performed to evaluate the impact of ASP changes on unnecessary DACT and consumption of target antibiotics.
Results
From October 2007 to September 2009, the cumulative incidence of unnecessary DACT was 2.3% [95% confidence interval (CI) 1.7–3.1]. Independent risk factors for unnecessary DACT [adjusted odds ratio (95% CI); P value] included hospitalization on a surgical ward [3.51 (1.03–12.02); P = 0.002], hospitalization on an obstetrics and gynaecology ward [9.07 (2.54–32.40); P = 0.002] and underlying metastatic malignancy [3.18 (1.38–7.09); P = 0.006]. The ASP change was associated with an increase in ampicillin/sulbactam and metronidazole consumption. However, there was no significant impact on unnecessary DACT prescribing.
Conclusions
Although uncommon, unnecessary DACT is more prevalent in specific services. Future qualitative studies focusing on these specific subgroups would be useful in elucidating this problem more clearly. The ASP changes were not associated with increases in unnecessary DACT.
Keywords: ampicillin/sulbactam, metronidazole, antibiotic stewardship programme
Introduction
Double anaerobic coverage therapy (DACT; i.e. two agents with overlapping coverage of anaerobic organisms) has been identified as a potentially important component of inappropriate prescribing. Using metronidazole as an adjunctive therapy to other anti-anaerobic agents has never been shown to improve clinical outcomes.1 On the other hand, metronidazole therapy is associated with many negative consequences, including increasing antibiotic cost, increasing the risk of acquisition of drug-resistant pathogens and increasing the risk of adverse drug reactions.2,3
While much work has investigated the inappropriate use of agents with broad Gram-positive or Gram-negative activity, little is known about usage patterns of agents with activity primarily against anaerobic bacteria. To our knowledge, the incidence of and risk factors for DACT have not been studied. The goal of this study was to determine the cumulative incidence of and risk factors for unnecessary DACT. In particular, we sought to determine the impact of recent changes in our institution's antimicrobial stewardship programme (ASP) on unnecessary DACT.
Methods
The study was conducted at the Hospital of the University of Pennsylvania (HUP), a 725 bed academic tertiary and quaternary medical centre. The University of Pennsylvania Institutional Review Board reviewed and approved this study. The cumulative incidence of unnecessary DACT was obtained from the entire study population while risk factors for unnecessary DACT were investigated through the nested case–control study. We focused on ampicillin/sulbactam, which is the most commonly used β-lactam/β-lactamase inhibitor in patients hospitalized at our institution. Data on consumption of target antibiotics was obtained directly from the HUP pharmacy database.
Under the ASP at HUP, a programme described in detail previously,4 prescriptions of ampicillin/sulbactam if administered for >3 days and parenteral metronidazole (if administered at any frequency other than every 12 h) required pre-approval. On 28 September 2008, these ASP policies were revised such that approval from the ASP was no longer required for any ampicillin/sulbactam and metronidazole orders. Therefore, we anticipated that partial discontinuation of the ASP may increase unnecessary DACT and consumption of ampicillin/sulbactam and metronidazole.
From 1 October 2007 to 30 September 2009, all hospitalized patients who received ampicillin/sulbactam for at least 2 days were eligible for the study. Inpatient medical records of all eligible subjects who were simultaneously prescribed metronidazole for at least one dose were reviewed to validate the co-administration of ampicillin/sulbactam and metronidazole as well as the indication for metronidazole. Cases were defined as those patients who received unnecessary DACT. Controls were those who did not receive DACT or those who received necessary DACT. All eligible controls from the study population were included. For each eligible admission, we included only the first course of ampicillin/sulbactam for a given patient.
Because the only rational indication for adding metronidazole to ampicillin/sulbactam is for treatment of Clostridium difficile-associated disease (CDAD),5 all DACT prescriptions were considered unnecessary unless at least one of the following criteria was true: (i) metronidazole was administered at the standard dosage for CDAD (500 mg every 8 h), indicating a clinical suspicion of CDAD; (ii) suspicion or diagnosis of CDAD was noted in the medical record within 24 h before/after the initiation of metronidazole; and (iii) a test for C. difficile toxin was requested within 24 h before/after the initiation of metronidazole regardless of the result.
Data, including demographics, hospital course, inpatient antimicrobial therapy and immunosuppressive therapy, presence of C. difficile toxin test and the result, and discharge status, were obtained directly from the Pennsylvania Integrated Clinical and Administrative Research Database (PICARD).6,7 Charlson co-morbidities were evaluated by applying the Enhanced ICD-9-CM coding algorithms8 to primary and secondary ICD-9 codes obtained from the PICARD.
Categorical variables were expressed as percentages while continuous variables were expressed in terms of mean or median when appropriate. The cumulative incidence of unnecessary DACT was calculated by dividing the total number of admissions with unnecessary DACT by the total number of admissions with ampicillin/sulbactam therapy. Data on ampicillin/sulbactam and metronidazole consumption were reported in terms of defined daily dose9 per hospital admission in a given period (DDD/admission).
To assess unadjusted associations between potential risk factors and unnecessary DACT, the χ2 test or Fisher's exact test was used for categorical data and Student's t-test or the Mann–Whitney U-test was used for continuous data depending on the sample distributions. We then performed multiple logistic regression analysis including all variables that were associated with unnecessary DACT (P ≤ 0.20) in univariate analyses. In the multivariable model, we included the duration of the ampicillin/sulbactam course as an estimate of time at risk.
We performed segmented logistic regression analysis to compare the cumulative incidence of unnecessary DACT during the restriction period (1 October 2007 to 30 September 2008) and the non-restriction period (1 October 2008 to 30 September 2009). Additionally, we performed segmented linear regression analysis to evaluate the DDD/admission of ampicillin/sulbactam and metronidazole during the restriction and non-restriction periods. Thus, the analysis was performed by month; there were 12 timepoints before and 12 timepoints after the ASP change.
A two-tailed P value of <0.05 was considered significant. All statistical calculations were performed by using STATA version 10.0 (STATA Corp, College Station, TX, USA).
Results
During the study period, 1783 admissions of 1686 unique patients were eligible for the study. DACT was dispensed in 83 admissions, 41 (49.4%) of which were unnecessary. The cumulative incidence of unnecessary DACT was 2.3% [95% confidence interval (CI) 1.7–3.1].
Baseline characteristics, co-morbidities and recent drug exposure of cases and controls were compared (Table S1, available as Supplementary data at JAC Online). Independent risk factors for unnecessary DACT from the multivariable analysis [adjusted odds ratio (95% CI); P value] included hospitalization on a surgical ward [3.51 (1.03–12.02); P = 0.002], hospitalization on an obstetrics and gynaecology ward [9.07 (2.54–32.40); P = 0.002] and underlying metastatic malignancy [3.18 (1.38–7.09); P = 0.006].
Data from chart review revealed that unnecessary DACT was prescribed to treat intra-abdominal infections in 78.9% of surgical patients (15/19). Of all obstetrics and gynaecology patients who received unnecessary DACT, 38.5% (5/13) were treated for intra-abdominal infections and 38.5% (5/13) for genital infections, including ovarian abscesses, endometritis and cervicitis. Moreover, we found that just over one-third (3/8) of patients with metastatic cancer were prescribed unnecessary DACT to treat head and neck infections and another one-third to treat post-surgical intra-abdominal infections.
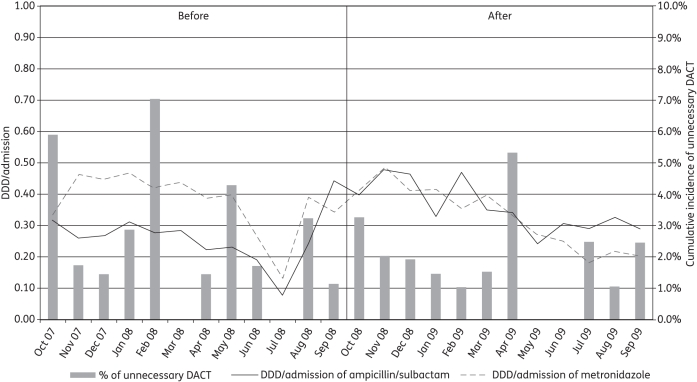
The cumulative incidence of unnecessary DACT before and after the ASP changes was 2.8% (22/781) and 1.9% (19/1002), respectively. The DDD/admission of ampicillin/sulbactam was higher in the non-restriction period (0.260 ± 0.086 versus 0.357 ± 0.079), whereas the DDD/admission of metronidazole was slightly lower in the non-restriction period (0.373 ± 0.097 versus 0.327 ± 0.100). Figure 1 shows the cumulative incidence of unnecessary DACT (represented as a bar graph) and the DDD/admission of ampicillin/sulbactam and metronidazole for each calendar month.
Figure 1.
Cumulative incidence of unnecessary DACT for each calendar month (represented as a bar graph) and DDD/admission of ampicillin/sulbactam and DDD/admission of metronidazole for each calendar month (represented as a line graph).
For unnecessary DACT, neither the immediate effect nor the change in slope resulting from the ASP change was statistically significant (Table 1). However, the DDD/admission of ampicillin/sulbactam significantly increased after the change in ASP [coefficient of level change = 0.219 (0.09–0.342); P = 0.002]. Although the DDD/admission of metronidazole at baseline decreased slightly over time [coefficient of baseline trend = −0.139 (−0.025–0.002); P = 0.02], it increased significantly after the change in ASP [coefficient of level change = 0.201 (0.087–0.314); P = 0.001].
Table 1.
Parameter estimates, CIs and P values from the segmented models of cumulative incidence of unnecessary DACT, DDD/admission of ampicillin/sulbactam and DDD/admission of metronidazole
| Parameters | Cumulative incidence of unnecessary DACT |
DDD/admission of ampicillin/sulbactam |
DDD/admission of metronidazole |
|||
|---|---|---|---|---|---|---|
| coefficient (95% CI) | P value | coefficient (95% CI) | P value | coefficient (95% CI) | P value | |
| Intercept | −4.734 (−6.428 to −3.039) | <0.001 | 0.280 (0.185–0.376) | <0.001 | 0.463 (0.378–0.549) | <0.001 |
| Baseline trend | −0.088 (−0.212 to 0.036) | 0.16 | −0.003 (−0.016 to 0.010) | 0.62 | −0.014 (−0.025 to −0.002) | 0.02 |
| Level change after partial discontinuation of ASP | 0.227 (−1.059 to 1.514) | 0.73 | 0.219 (0.092–0.346) | 0.002 | 0.201 (0.087–0.314) | 0.001 |
| Trend change after partial discontinuation of ASP | 0.065 (−0.115 to 0.245) | 0.48 | −0.013 (−0.031 to 0.005) | 0.15 | −0.012 (−0.029 to 0.004) | 0.13 |
Discussion
The reasons for the higher rates of unnecessary DACT on surgery and obstetrics and gynaecology wards are not clear. Perhaps physicians working in these areas were uncomfortable with ampicillin/sulbactam's coverage of anaerobic organisms or were unaware of its activity. A qualitative study of knowledge and attitudes among these physicians to better identify the reasons for higher rates of unnecessary DACT would be useful. Having metastatic cancer was also found to be a risk factor for unnecessary DACT. We hypothesize that it may be a proxy for a greater level of illness.
Although antibiotic pre-approval programmes have been shown to be effective in reducing antibiotic consumption,4,10 little is known about the impact of discontinuation of such interventions. As expected, we found increasing consumption of ampicillin/sulbactam and metronidazole after the change in ASP. However, we did not detect any change in unnecessary DACT prescribing.
Our study had several potential limitations. Because of the low cumulative incidence of unnecessary DACT, small associations could possibly have been missed. Second, data from the HUP pharmacy database and PICARD may not truly represent drug administration. To minimize misclassification of necessary DACT as unnecessary DACT, we validated our study population with individual chart reviews. Inpatient medical records of all subjects who were simultaneously prescribed metronidazole for at least one dose were reviewed to confirm the duration of DACT and the indication for metronidazole therapy. Third, the lack of data on antibiotic exposure prior to hospitalization may result in information bias, although it is unlikely this would result in differential bias. Finally, this study was conducted at a tertiary care hospital that has a comprehensive ASP and focused only on ampicillin/sulbactam and metronidazole; therefore the generalizability of the results may be an issue.
In summary, the problem of unnecessary DACT should be brought to the attention of clinicians, emphasizing education in services where the practice appears more common. This study highlights the need to provide customized ASPs for different prescribers and/or patient groups. Furthermore, our study confirms that the partial discontinuation of an ASP has a negative impact on target antibiotic consumption.
Funding
This study was supported by National Institutes of Health (NIH) grant K24-AI080942 (E. L.).
Transparency declarations
E. L. has received research support from Merck, Ortho-McNeil, Cubist and AstraZeneca. All other authors: none to declare.
Supplementary data
Table S1 is available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
References
- 1.Brook I. Treatment of anaerobic infection. Expert Rev Anti Infect Ther. 2007;5:991–1006. doi: 10.1586/14787210.5.6.991. doi:10.1586/14787210.5.6.991. [DOI] [PubMed] [Google Scholar]
- 2.Lucas GM, Lechtzin N, Puryear DW, et al. Vancomycin-resistant and vancomycin-susceptible enterococcal bacteremia: comparison of clinical features and outcomes. Clin Infect Dis. 1998;26:1127–33. doi: 10.1086/520311. doi:10.1086/520311. [DOI] [PubMed] [Google Scholar]
- 3.Kazmier FJ. A significant interaction between metronidazole and warfarin. Mayo Clin Proc. 1976;51:782–4. [PubMed] [Google Scholar]
- 4.Gross R, Morgan AS, Kinky DE, et al. Impact of a hospital-based antimicrobial management program on clinical and economic outcomes. Clin Infect Dis. 2001;33:289–95. doi: 10.1086/321880. doi:10.1086/321880. [DOI] [PubMed] [Google Scholar]
- 5.Nelson R. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database Syst Rev. 2007;(issue 3):CD004610. doi: 10.1002/14651858.CD004610.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Lautenbach E, Synnestvedt M, Weiner MG, et al. Epidemiology and impact of imipenem resistance in Acinetobacter baumannii. Infect Control Hosp Epidemiol. 2009;30:1186–92. doi: 10.1086/648450. doi:10.1086/648450. [DOI] [PubMed] [Google Scholar]
- 7.Lautenbach E, Synnestvedt M, Weiner MG, et al. Imipenem resistance in Pseudomonas aeruginosa: emergence, epidemiology, and impact on clinical and economic outcomes. Infect Control Hosp Epidemiol. 2010;31:47–53. doi: 10.1086/649021. doi:10.1086/649021. [DOI] [PubMed] [Google Scholar]
- 8.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. doi:10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 9.Merlo J, Wessling A, Melander A. Comparison of dose standard units for drug utilisation studies. Eur J Clin Pharmacol. 1996;50:27–30. doi: 10.1007/s002280050064. doi:10.1007/s002280050064. [DOI] [PubMed] [Google Scholar]
- 10.Rattanaumpawan P, Sutha P, Thamlikitkul V. Effectiveness of drug use evaluation and antibiotic authorization on patients' clinical outcomes, antibiotic consumption, and antibiotic expenditures. Am J Infect Control. 2010;38:38–43. doi: 10.1016/j.ajic.2009.04.288. doi:10.1016/j.ajic.2009.04.288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.