Abstract
Objectives
The primary aim of the RELIEF study was to evaluate the efficacy and safety of two sequential intravenous (iv)/oral regimens: moxifloxacin iv/oral versus piperacillin/tazobactam (TZP) iv followed by oral amoxicillin/clavulanate (AMC).
Patients and methods
The study had a prospective, randomized, double-dummy, double-blind, multicentre design. Patients ≥18 years were prospectively stratified according to complicated skin and skin structure infection (cSSSI) subtype/diagnosis (major abscess, diabetic foot infection, wound infection or infected ischaemic ulcer), surgical intervention and severity of illness. Diagnoses and disease severity were based on predetermined criteria, documented by repeated photographs, and confirmed by an independent data review committee. Patients were randomized to receive either 400 mg of moxifloxacin iv once daily followed by 400 mg of moxifloxacin orally once daily or 4.0/0.5 g of TZP iv thrice daily followed by 875/125 mg of AMC orally twice daily for 7–21 days. The primary efficacy variable was clinical response at test of cure (TOC) for the per-protocol (PP) population. Clinical efficacy was assessed by the data review committee based on repeated photographs and case descriptions. Clinical trials registry number: NCT 00402727.
Results
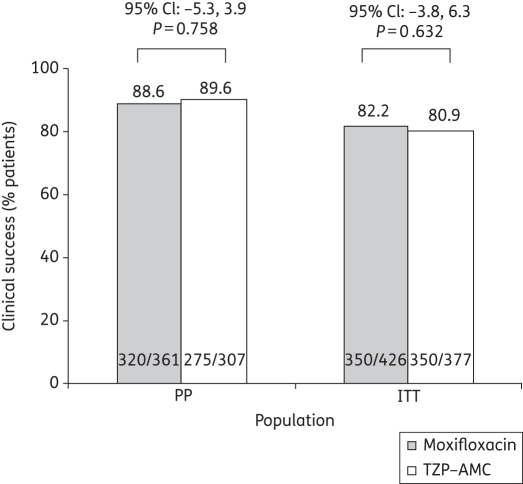
A total of 813 patients were randomized. Clinical success rates at TOC were similar for moxifloxacin and TZP–AMC in the PP [320/361 (88.6%) versus 275/307 (89.6%), respectively; P = 0.758] and intent-to-treat (ITT) [350/426 (82.2%) versus 305/377 (80.9%), respectively; P = 0.632] populations. Thus, moxifloxacin was non-inferior to TZP–AMC. Bacteriological success rates were high in both treatment arms [moxifloxacin: 432/497 (86.9%) versus TZP–AMC: 370/429 (86.2%), microbiologically valid (MBV) population]. Moxifloxacin was non-inferior to TZP–AMC at TOC in both the MBV and the ITT populations. Both treatments were well tolerated.
Conclusions
Once-daily iv/oral moxifloxacin monotherapy was clinically and bacteriologically non-inferior to iv TZP thrice daily followed by oral AMC twice daily in patients with cSSSIs.
Keywords: β-lactams, randomized controlled trials, soft tissue infections, fluoroquinolones, sequential therapy
Introduction
Skin and skin structure infections (SSSIs)1 may require hospitalization, especially among high-risk populations,2 and are a common indication for intravenous (iv) antibiotic use.3 SSSIs are considered complicated (cSSSIs) when they involve deeper soft tissue (fascia and/or muscle layers), require significant surgical intervention or occur in patients with a co-morbid condition that may compromise the response to treatment (e.g. diabetes).1,4 cSSSIs lead to significant morbidity and mortality, and impact considerably on healthcare resource utilization.5
cSSSIs are caused by Gram-positive and Gram-negative aerobic and anaerobic pathogens; the latter are more frequently associated with severe infections in the anogenital area or lower half of the body.3,6 Many different pathogens, alone or as part of a polymicrobial infection, are involved in cSSSIs, and will vary depending on the clinical situation, location of the infection and medical history of the patient.1 The management of cSSSIs normally involves both surgical debridement and empirical antibiotic therapy.
Moxifloxacin has an extended spectrum of activity against Gram-positive cocci, including methicillin-susceptible Staphylococcus aureus, and aerobic and anaerobic bacteria, and significant activity against many Gram-negative bacteria.7–11 It is active against non-growing Escherichia coli and Bacteroides fragilis in experimental abscesses,12 and its in vitro activity against S. aureus and E. coli is not reduced in an anaerobic milieu.13 It is not, however, recommended against methicillin-resistant S. aureus (MRSA). Moxifloxacin has good tissue penetration14,15 and iv to oral switch-down is simple, as the pharmacokinetic profiles for the two formulations are virtually interchangeable.16,17 Its once-daily formulation makes it a convenient antimicrobial option. These properties, combined with proven efficacy and tolerability versus β-lactam/β-lactamase inhibitors, make moxifloxacin a valuable treatment option, including in patients in whom β-lactams are contraindicated.3,18 While previous studies of iv/oral moxifloxacin in cSSSIs show it to be effective and well tolerated, they were limited by a lack of stratification with respect to infection severity and the requirement for baseline surgery. This common omission from cSSSI trials is addressed in the RELIEF study.
Patients and methods
Study design
This prospective, randomized, double-dummy, double-blind study (clinical trials registry number: NCT 00402727; http://clinicaltrials.gov/ct2/show/NCT00402727) was carried out from September 2006 to June 2008 in 61 centres worldwide (Belgium, Bulgaria, Germany, Greece, Hungary, Israel, Latvia, Lithuania, Poland, Romania, Russia, South Africa, Spain, Ukraine and the UK). Documented approval for the study was obtained from the Ethics Committee for all participating centres and written informed consent was sought from all participants. The study was carried out under Good Clinical Practice guidelines and in accordance with the Declaration of Helsinki. Dr Inge Gyssens had full access to the study data and is the guarantor of the data.
Definitions and diagnosis of cSSSI
A cSSSI was defined as a bacterial SSSI that required hospitalization, initial parenteral therapy for ≥48 h and which met at least one of the following criteria: deep soft tissue involvement; requirement for significant surgical intervention, including surgical drainage and/or debridement; and association with a complicating co-morbid condition. Prior to randomization, patients were stratified by the local investigator according to the type of infection [major abscess, diabetic foot infection (DFI), wound infection or infected ischaemic ulcer], requirement for surgical intervention and Wilson risk class.19 Major abscesses were defined as pus collection being associated with extensive cellulitis, and required antibiotic therapy in addition to surgical incision and drainage. DFIs were infections occurring below the ankle in patients with confirmed diabetes. DFIs were characterized, and their severity graded using the PEDIS (perfusion, extent/size, depth/tissue loss, infection and sensation), Wagner and University of Texas Wound classification systems.20–23 Wound infections included post-surgical (surgical incision), post-traumatic, human bite/clenched fist and animal bite wounds, and wounds associated with injection drug abuse. Infected ischaemic ulcers were defined as occurring in patients with peripheral vascular disease. The diagnosis of cSSSI was validated by a blinded independent data review committee (DRC) for all patients randomized in the study. Patients with an infection that had not been confirmed as a major abscess, a DFI, an infected ischaemic ulcer or a wound infection by the DRC were excluded from the primary efficacy analysis.
Patients
Men and women (≥18 years old) with a cSSSI of <21 days' duration and at least three of the following signs and symptoms were included: purulent drainage or discharge; erythema extending >1 cm from the wound edge; fluctuance, pain or tenderness to palpation; swelling or induration; fever; elevated total peripheral white blood cell (WBC) count >12 000/mm3 or >15% immature neutrophils regardless of total peripheral WBC; or C-reactive protein >20 mg/L. For all patients, a tissue or fluid specimen was obtained from the infected area for culture. Swabs were not accepted. Exclusion criteria included: necrotizing fasciitis; burn wound infections; secondary infections of a chronic skin disease; infection of a prosthesis; infections where a surgical procedure alone was definitive therapy, such as amputation through a clean site and after abscess drainage without extensive cellulitis or an uncomplicated SSSI (uSSSI); contraindications to any of the study drugs; a life expectancy <2 months; severe hepatic insufficiency (Child–Pugh C); or transaminases above five times the upper limit of normal or creatinine clearance <40 mL/min. Women who were lactating or pregnant were also excluded, as were patients with neutropenia, lymphocytopenia, an AIDS-defining event, antiretroviral therapy and chronic immunosuppressant therapy. Other exclusion criteria included antibacterial treatment for >24 h in the 7 days preceding study entry, unless the patient showed no response or had worsening of clinical signs and symptoms despite treatment for ≥3 days and a culture before enrolment showed persistence of a pathogen that was susceptible to the study drugs. Patients with an infection known to be due to MRSA, methicillin-resistant Staphylococcus epidermidis or vancomycin-resistant enterococci as the single pathogen were also excluded.
Surgical treatment
Patients had to receive a surgical intervention within 48 h of initiation of the study drug to qualify as having undergone baseline surgery.
Drug treatments
The randomization code was generated by the Department of Biometry at Bayer Healthcare AG. Eligible patients were randomly assigned in a 1 : 1 ratio to one of two treatment groups: moxifloxacin (Group 1) or piperacillin/tazobactam–amoxicillin/clavulanate (TZP–AMC; Group 2) using an interactive voice-response system. The sequence of the iv study drug administration was also randomized. Treatment groups were subdivided so that half of the patients received active treatment followed by placebo and half received the same treatment but in the opposite order (placebo followed by active treatment). Group 1 received 400 mg of moxifloxacin iv once daily and TZP placebo iv thrice daily, followed by 400 mg of oral moxifloxacin and oral AMC placebo twice daily. Group 2 received 4.0/0.5 g of piperacillin/tazobactam iv thrice daily and moxifloxacin placebo iv once daily, followed by 875/125 mg of oral amoxicillin/clavulanate twice daily and oral moxifloxacin placebo once daily. The decision to switch from iv to oral therapy was made by the investigator, but the patient had to have been afebrile for ≥24 h and received iv therapy for ≥3 days. The total treatment duration was 7–21 days. Blinding of the patients, investigators and contract research personnel was maintained throughout the study. Patients with a polymicrobial infection that included culture-confirmed MRSA, methicillin-resistant S. epidermidis or vancomycin-resistant enterococci could be treated with additional narrow-spectrum antibiotics.
Assessments
Clinical assessments were performed pre-treatment, during treatment (days 3–5), at end of treatment (EOT) (days 7–21) and at test of cure (TOC) (14–28 days post-therapy). At TOC, the clinical response was graded as cure, failure or indeterminate. Cure was the disappearance of acute signs and symptoms related to the infection or sufficient improvement, such that additional or alternative antimicrobial therapy was not required. Failure was insufficient lessening of the signs and symptoms of infection, such that additional or alternative antimicrobial therapy was required, or there was requirement for surgical intervention to treat a persistent infection or development of another wound infection at the original site of infection. Indeterminate was used for patients in whom a clinical assessment could not be determined. Clinical success was defined as cure at TOC for patients not classified as clinical failure at any evaluation visit earlier than TOC. The skin lesions of all patients were photographed panoramically, and from the front and side (to determine the precise location of the lesion) at entry (repeated prior to any surgery), on days 3–5, EOT, TOC and post-alternative therapy (if administered). Patients with a DFI had the affected extremities radiographed to assess for the presence of foreign materials, tissue gas or signs of osteomyelitis. Bacteriological assessment was carried out on pre-treatment samples (wound and blood) and samples collected during the study. Samples were collected via biopsy, curettage of the wound base after debridement, tissue or bone biopsy, aspiration of purulent secretions, or a leading-edge needle aspiration for patients with cellulitis. Samples were inoculated directly into preservative medium (BBL Port-A-Cul Transport vial or jar, Becton, Dickinson and Company, NJ, USA) and shipped within 72 h to the central laboratory [Eurofins medinet SAS (formerly Focus Bio-Inova Europe SAS), Plaisir cedex, France] for testing according to CLSI guidelines. MICs were determined using validated reference broth microdilution panels, and extended-spectrum β-lactamase activity was determined for Enterobacteriaceae species using a standardized method. Organisms were defined as ‘pathogenic’ based on blinded post-study evaluation. Common cSSSI pathogens, such as S. aureus and E. coli, were always considered pathogenic. Other bacteria (e.g. Aerococcus viridans) were never classified as a pathogen. Some species (e.g. Enterobacter cloacae or Enterococcus faecalis) were classified as a pathogen if certain semi-quantitative growth requirements or clinical signs were met. Bacteriological response was classified as: success (eradication or presumed eradication); failure (persistence, presumed persistence, recurrence, reinfection or superinfection); or indeterminate. Colonization was defined as the isolation of a new organism (colonizer) that did not require subsequent antimicrobial therapy. Safety assessments were based on physical examination, vital signs, pre-treatment and on-therapy electrocardiograms, adverse events (AEs), and standard laboratory tests. For all patients with an AE meeting the definition of diarrhoea, a stool sample was submitted to the central laboratory for Clostridium difficile toxin detection.
Study endpoints/variables
The primary efficacy endpoint was the clinical response determined by the DRC at TOC, i.e. 14–28 days after the last dose of the study drug. The DRC was blinded to the country and site, treatment allocation, and local investigator for the evaluation of efficacy. Members of the DRC received all patient data that were part of the study database for review. This included clinical signs and symptoms at each assessment visit, concomitant medications, microbiology results, laboratory tests, and photographs. Photographs were interpreted by a central photograph-reading contract research organization (Radpharm Inc., Princeton, NJ, USA). Patient data were reviewed independently by at least three out of five members of the DRC, where possible while the study was ongoing. Where there was no initial agreement on diagnosis and/or clinical response or data queries were raised, profiles and photographs were reviewed and discussed by the complete DRC until consensus was reached. A secondary efficacy variable was bacteriological response at days 3–5, EOT and TOC.
Populations for analysis
The patient populations for analysis were the safety/intent-to-treat (ITT) population (all randomized patients receiving at least one dose of study drug and having at least one observation), the per-protocol (PP) population (ITT patients with fully documented cSSSI diagnostic criteria, no protocol violations influencing outcome, no essential data missing and no other systemic/topical antimicrobial agent administered concomitantly during treatment unless the patient was a treatment failure) and the microbiologically valid (MBV) population (PP patients with baseline pathogens and a microbiological evaluation at TOC).
Statistical methods
Statistical analyses were adjusted for cSSSI subtype, severity of illness and surgical procedure. Fourteen strata (based on subtype, risk class and requirement for baseline surgery) were used for the analysis, but the study was not powered to look at differences in the clinical efficacy between subgroups. Mantel–Haenszel point estimates and 95% confidence intervals (CIs) for the differences in clinical success rates at TOC were used to compare the treatment groups (moxifloxacin group minus comparator group). The 95% CIs were calculated using Mantel–Haenszel weights based on the 14 strata defined. If the lower limit of this CI exceeded −10%, it was shown that moxifloxacin was clinically not less effective than the comparator regimen. If the lower limit of the CI exceeded 0, superiority of treatment with moxifloxacin was proven. The study was powered to demonstrate non-inferiority with respect to clinical cure, as assessed by the DRC for the PP population with supportive results in the ITT population.
AEs were classified according to the MedDRA code. Vital signs and laboratory data were analysed descriptively.
Sample size estimation was based on the primary efficacy variable (clinical response at TOC visit) and the following assumptions: a true failure rate of 20% in the control group; an equivalence (clinically relevant) δ = 10%; α = 5% (two sided); and β = 5%. This yielded a sample size estimate of 321 valid patients per treatment group, including an adjustment of 10% to account for the multicentre design of the study. Assuming a validity rate of 80%, 402 patients were needed in each treatment group.
Results
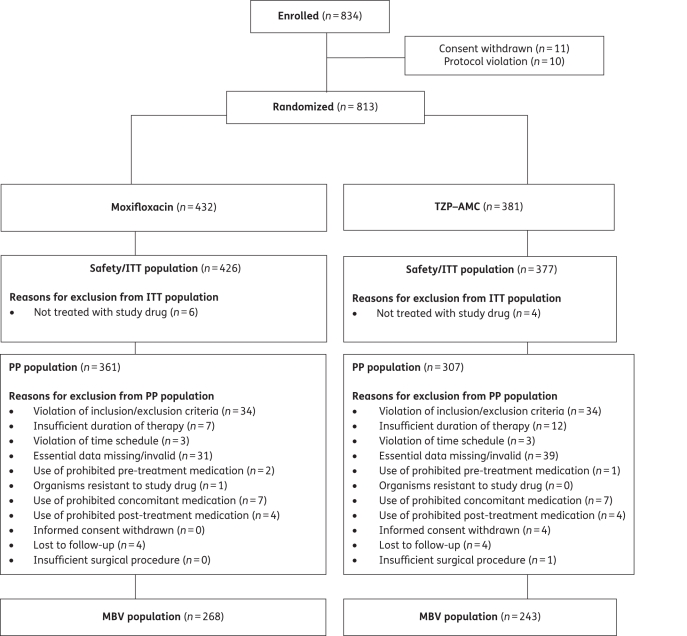
The first patient was enrolled in September 2006 and the last follow-up visit occurred in June 2008. The trial ended when the required patient sample size (n = 402) was reached. Patient flow through the study is shown in Figure 1. Patients were generally well matched in terms of demographics and other characteristics (Tables 1 and 2); although, in the PP population, WBC and glycosylated haemoglobin (HbA1c) values were higher in moxifloxacin-treated patients (P = 0.022 and 0.018, respectively). At baseline, cSSSI and disease-related characteristics were also well matched between groups (Table 2). A majority of patients with an abscess had systemic signs of infection (elevated WBC and/or C-reactive protein) and were febrile (mean temperature of 39.2°C). Abscesses frequently involved deep tissues or had complicated factors. Approximately 42% of valid PP patients with abscesses had involvement of the underlying fat tissue and a further 58% of patients had extension of the abscess to the underlying fascia or muscle. Lesions were either major abscesses, as evidenced by the mean size of the lesions (112 cm2), or carried a high risk of an anaerobic or Gram-negative involvement; the majority of such abscesses were in the genital or perianal regions. Approximately 80% of valid PP patients had surgical procedures performed within 48 h of initiation of study drug. Drainage, extensive or local debridement and amputations were the most frequent interventions. Most patients with an amputation were DFI patients, who had peripheral vascular disease, as evidenced by lower ankle-brachial indices (data not shown). In these patients, adequate debridement may require amputation at some level as a means of removing non-viable infected tissue, including bone.
Figure 1.
Patient flow through the study.
Table 1.
Patient demographics and baseline characteristics (ITT and PP populations)
| Characteristic | ITT |
PP |
||
|---|---|---|---|---|
| moxifloxacin (N = 426) | TZP–AMC (N = 377) | moxifloxacin (N = 361) | TZP–AMC (N = 307) | |
| Sex, male, n (%) | 267 (62.7) | 255 (67.6) | 229 (63.4) | 213 (69.4) |
| Mean (SD) age, years | 53.4 (16.2) | 52.8 (16.0) | 52.7 (16.4) | 51.8 (15.9) |
| Mean (SD) body mass index, kg/m2 | 28.2 (6.4) | 27.0 (5.0) | 27.8 (6.0) | 27.0 (4.9) |
| Mean (range) temperature, °C | 39.2 (36.4–41.5) | 39.2 (36.5–42.0) | 39.2 (36.7–41.5) | 39.2 (36.5–42.0) |
| Mean (SD) white blood cells, Giga/L | 10.8 (4.8) | 10.0 (3.9) | 10.9 (4.8) | 10.2 (3.9) |
| Mean (SD) HbA1c, % | 7.3 (2.5) | 7.0 (2.2) | 7.4 (2.6) | 7.0 (2.2) |
| Mean (SD) C-reactive protein, mg/L | 9.0 (9.1) | 8.7 (8.9) | 9.0 (8.9) | 8.9 (8.9) |
| Co-morbid condition, n (%) | ||||
| cardiac | 101 (23.7) | 81 (21.5) | 81 (22.4) | 60 (19.5) |
| malignancy | 7 (1.6) | 6 (1.6) | 5 (1.4) | 4 (1.3) |
| diabetes mellitus | 180 (42.3) | 136 (36.1) | 156 (43.2) | 113 (36.8) |
| hepatic | 7 (1.6) | 13 (3.4) | 6 (1.7) | 9 (2.9) |
| renal | 21 (4.9) | 21 (5.6) | 18 (5.0) | 15 (4.9) |
| respiratory | 21 (4.9) | 23 (6.1) | 17 (4.7) | 18 (5.9) |
| transplantation | 0 | 1 (0.3) | 0 | 1 (0.3) |
| vascular | 124 (29.1) | 119 (31.6) | 106 (29.4) | 89 (29.0) |
HbA1c, glycosylated haemoglobin.
Table 2.
cSSSI and disease-related characteristics at baseline (ITT and PP populations)
| ITT |
PP |
|||
|---|---|---|---|---|
| moxifloxacin (N = 426) | TZP–AMC (N = 377) | moxifloxacin (N = 361) | TZP–AMC (N = 307) | |
| cSSSI diagnosis, n (%) | ||||
| major abscess | 183 (43.0) | 169 (44.8) | 167 (46.3) | 153 (49.8) |
| diabetic foot infection | 123 (28.9) | 110 (29.2) | 110 (30.5) | 96 (31.3) |
| wound infection | 72 (16.9) | 55 (14.6) | 62 (17.2) | 47 (15.3) |
| infected ischaemic ulcer | 24 (5.6) | 18 (4.8) | 22 (6.1) | 11 (3.6) |
| other cSSSI | 4 (0.9) | 3 (0.8) | — | — |
| uSSSI or no infection | 20 (4.7) | 22 (5.8) | — | — |
| Source of infection | ||||
| community acquired, n (%) | 368 (86.4) | 345 (91.5) | 310 (85.9) | 280 (91.2) |
| hospital acquired, n (%) | 58 (13.6) | 32 (8.5) | 51 (14.1) | 27 (8.8) |
| Location of primary lesion, n (%) | ||||
| foot | 154 (36.2) | 132 (35.0) | 134 (37.1) | 110 (35.8) |
| legs | 82 (19.2) | 78 (20.7) | 57 (15.8) | 46 (15.0) |
| buttocks and genitals | 58 (13.6) | 61 (16.2) | 53 (14.7) | 55 (17.9) |
| arm | 37 (8.7) | 32 (8.5) | 35 (9.7) | 29 (9.4) |
| abdomen and groin | 39 (9.2) | 22 (5.8) | 32 (8.9) | 21 (6.8) |
| hand | 20 (4.7) | 20 (5.3) | 19 (5.3) | 19 (6.2) |
| head and neck | 22 (5.2) | 21 (5.6) | 19 (5.3) | 18 (5.9) |
| chest and back | 14 (3.3) | 11 (2.9) | 12 (3.3) | 9 (2.9) |
| Deepest plane involved, n (%) | ||||
| fascia | 153 (35.9) | 145 (38.5) | 125 (34.6) | 115 (37.5) |
| muscle | 117 (27.5) | 101 (26.8) | 97 (26.9) | 79 (25.7) |
| fat | 105 (24.6) | 78 (20.7) | 96 (26.6) | 72 (23.5) |
| bone | 20 (4.7) | 26 (6.9) | 18 (5.0) | 21 (6.8) |
| dermis | 17 (4.0) | 14 (3.7) | 14 (3.9) | 8 (2.6) |
| othera | 14 (3.3) | 13 (3.4) | 11 (3.0) | 12 (3.9) |
| Wilson score evaluationb | ||||
| mean (SD) score | 83.5 (31.3) | 81.4 (30.2) | 84.7 (32.4) | 83.3 (30.8) |
| risk class, n (%) | ||||
| I | 118 (27.7) | 116 (30.8) | 100 (27.7) | 99 (32.2) |
| II | 92 (21.6) | 65 (17.2) | 84 (23.2) | 54 (17.6) |
| III | 92 (21.6) | 87 (23.1) | 75 (20.8) | 73 (23.8) |
| IV | 124 (29.1) | 109 (28.9) | 102 (28.3) | 81 (26.4) |
| White blood cells ≥10 500 Giga/L, n (%) | 174 (40.8) | 111 (29.4) | 149 (41.3) | 97 (31.6) |
| C-reactive protein ≥0.5 mg/dL, n (%) | 378 (88.7) | 334 (88.6) | 328 (90.9) | 277 (90.2) |
| Temperature >38°C, n (%) | 385 (90.4) | 335 (88.9) | 329 (91.1) | 279 (90.9) |
| Initialc surgery, n (%) | 321 (75.4) | 298 (79.0) | 285 (78.9) | 256 (83.4) |
| Mean (SD) duration of anaesthesia (min) | 43.1 (48.9) | 38.8 (35.7) | 42.8 (49.1) | 37.6 (33.8) |
| Most common initial surgeriesc,d, n (%) | ||||
| local debridement | 99 (23.2) | 85 (22.5) | 87 (24.1) | 72 (23.5) |
| abscess drainage | 237 (55.6) | 222 (58.9) | 212 (58.7) | 199 (64.8) |
| extensive debridement | 113 (26.5) | 112 (29.7) | 97 (26.9) | 99 (32.2) |
| amputation | 58 (13.6) | 48 (12.7) | 53 (14.7) | 36 (11.7) |
Other cSSSIs in the moxifloxacin group were complicated erysipelas (n = 1), necrotizing fasciitis (n = 2) and other skin infection requiring surgery (n = 1), and in the comparator group were necrotizing fasciitis (n = 2) and other skin infection requiring surgery (n = 1).
aTendon, articulum/joint, aponeurosis and subcutaneous tissue.
bSee reference 19.
cBefore or within 48 h of initiation of study drug.
dPatients may have undergone more than one type of surgery.
Pathogens
No major difference was seen between treatment groups regarding the frequency or type of baseline pathogens in the MBV population (Table 3). A total of 497 pathogens were isolated from 268 moxifloxacin-treated patients and 429 pathogens from 243 comparator-treated patients. In the moxifloxacin arm, 129 (48.1%) patients had monomicrobial infections while 139 (51.9%) had polymicrobial infections. Corresponding numbers in the comparator arm were 132 (54.3%) and 111 (45.7%), respectively. Aerobic Gram-positive cocci were the most frequent pathogens, were isolated in 65% of patients and included mainly S. aureus (43%), β-haemolytic streptococci group A–G (20%) and E. faecalis (16%), followed by aerobic Gram-negative bacilli (mainly E. coli). MRSA was isolated from 23 MBV patients in the moxifloxacin arm and 15 MBV patients in the TZP–AMC arm; in 8/23 moxifloxacin patients and 6/15 TZP–AMC patients, MRSA was isolated as the sole pathogen at inclusion. Most MBV patients with an MRSA isolate were from Eastern Europe or the Russian Federation [n = 32/38 (84.2%)]. Overall, in the moxifloxacin arm, 1/5/18 MRSA isolates were susceptible/intermediate/resistant to moxifloxacin using European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints (≤0.5/1/>1 mg/L), respectively.
Table 3.
Commonly occurring baseline causative organisms (isolated in ≥1% of patients in either treatment group) from skin and skin structures, and bacteriological success (eradication and presumed eradication) rates by pathogen at TOC (MBV population)
| Causative organisms at baseline, n (%) |
Bacteriological success rate, n/N (%) |
|||
|---|---|---|---|---|
| moxifloxacin | TZP–AMC | moxifloxacin | TZP–AMC | |
| Total causative organisms | 497 | 429 | 432/497 (86.9) | 370/429 (86.2) |
| Gram-positive aerobic cocci | 320 (64.4) | 280 (65.3) | 276/320 (86.3) | 240/280 (85.7) |
| Staphylococcus aureus | ||||
| methicillin-susceptible | 152 (30.6) | 154 (35.9) | 134/152 (88.2) | 134/154 (87.0) |
| methicillin-resistant | 23 (4.6) | 17 (4.0) | 17/23 (73.9) | 15/17 (88.2) |
| Enterococcus faecalis | 61 (12.3) | 50 (11.7) | 48/61 (78.7) | 39/50 (78.0) |
| Streptococcus pyogenes | 36 (7.2) | 24 (5.6) | 34/36 (94.4) | 22/24 (91.7) |
| Streptococcus agalactiae | 31 (6.2) | 13 (3.0) | 26/31 (83.9) | 12/13 (92.3) |
| Streptococcus equisimilis | 13 (2.6) | 12 (2.8) | 12/13 (92.3) | 10/12 (83.3) |
| Gram-negative aerobic bacilli | 142 (28.6) | 119 (27.7) | 123/142 (86.6) | 103/119 (86.6) |
| Escherichia colia | 58 (11.7) | 55 (12.8) | 52/58 (89.7) | 49/55 (89.1) |
| Enterobacter cloacaea | 22 (4.4) | 19 (4.4) | 21/22 (95.5) | 19/19 (100.0) |
| Klebsiella pneumoniaea | 14 (2.8) | 9 (2.1) | 9/14 (64.3) | 5/9 (55.6) |
| Proteus mirabilisa | 8 (1.6) | 9 (2.1) | 7/8 (87.5) | 7/9 (77.8) |
| Acinetobacter baumannii | 11 (2.2) | 5 (1.2) | 10/11 (90.9) | 5/5 (100.0) |
| Klebsiella oxytocaa | 7 (1.4) | 6 (1.4) | 7/7 (100.0) | 6/6 (100.0) |
| Pseudomonas aeruginosa | 9 (1.8) | 4 (0.9) | 8/9 (88.9) | 2/4 (50.0) |
| Morganella morganiia | 6 (1.2) | 4 (0.9) | 3/6 (50.0) | 4/4 (100.0) |
| Proteus vulgaris | 3 (0.6) | 6 (1.4) | 3/3 (100.0) | 4/6 (66.7) |
| Anaerobes | 35 (7.0) | 30 (7.0) | 33/35 (94.3) | 27/30 (90.0) |
| Bacteroides fragilis | 26 (5.2) | 19 (4.4) | 24/26 (92.3) | 16/19 (84.2) |
n/N = number of bacteriological successes/number of organisms isolated.
aData shown are for extended-spectrum β-lactamase (ESBL) and non-ESBL producers. The following numbers of ESBL producers were isolated for each species (moxifloxacin and TZP–AMC, respectively): E. coli, 4 (<1%) and 1 (<1%); E. cloacae, 17 (3%) and 14 (3%); K. pneumoniae, 4 (<1%) and 4 (<1%); and P. mirabilis, 8 (2%) and 7 (2%).
Four patients (1%) had baseline blood pathogens: there was one case of methicillin-susceptible S. aureus in the moxifloxacin group and one case each of S. aureus (susceptibility not tested; isolate destroyed during transport to the central laboratory), Streptococcus parasanguis and Klebsiella oxytoca in the TZP–AMC group.
The susceptibility of the baseline pathogens to moxifloxacin, TZP and AMC is shown in Table S1 (available as Supplementary data at JAC Online).
Clinical efficacy
Overall, clinical success at TOC was similar for moxifloxacin and TZP–AMC in the PP and ITT populations (P = not significant) (Figure 2). Moxifloxacin was non-inferior to TZP–AMC in both populations as the lower limit of the 95% CI was above −10%. The highest cure rates by clinical category were seen in patients with abscesses and wound infections (Table 4). No statistically significant differences in clinical success rates were seen between treatments when patients were stratified for severity by Wilson risk class (Table S2, available as Supplementary data at JAC Online). Cure rates decreased with increasing severity (P < 0.001, Cochran–Mantel–Haenszel test stratified by treatment group). Overall, clinical cure rates were similar in patients with versus without initial surgery (90.6% versus 90.0%) and no differences were seen in the two treatment groups. Similar numbers of PP patients in each treatment arm required additional surgery (i.e. performed after 48 h of initiation of the study drug) during therapy [moxifloxacin: 60/361 (16.6%); TZP–AMC: 56/307 (18.2%)]. The most frequent surgical procedures included local debridement [moxifloxacin: 29/361 (8.0%); TZP–AMC: 24/307 (7.8%)], (primary) closure [moxifloxacin: 24/361 (6.6%); TZP–AMC: 20/307 (6.5%)] and amputation [moxifloxacin: 9/361 (2.5%); TZP–AMC: 17/307 (5.5%)]. Additional amputations were done in 8.2% (9/110) of moxifloxacin- and 16.7% (16/96) of TZP–AMC-treated patients with DFI, respectively. One PP TZP–AMC patient with ischaemic ulcer also had an additional amputation. A vast majority of patients with additional surgery were considered as clinical failures. No major differences were observed between treatment groups with regard to clinical cure rates of aerobic (86.6% overall) versus anaerobic (91.5% overall) bacterial infections. Clinical response rates were similar for patients with monomicrobial [moxifloxacin: 113/129 (87.6%); TZP–AMC: 120/131 (91.6%)] or polymicrobial [moxifloxacin: 120/139 (86.3%); TZP–AMC: 95/112 (84.8%)] infections.
Figure 2.
Clinical success at TOC (PP and ITT populations).
Table 4.
Clinical success at TOC by diagnosis (PP and ITT populations), as assessed by the DRC
| Clinical cure, n/N (%) |
||||||
|---|---|---|---|---|---|---|
| PP |
ITT |
|||||
| moxifloxacin (N = 361) | TZP–AMC (N = 307) | estimate: 95% CI | moxifloxacin (N = 426) | TZP–AMC (N = 377) | estimate: 95% CI | |
| All patients | 320/361 (88.6) | 275/307 (89.6) | −0.72: −5.3, 3.9 | 350/426 (82.2) | 305/377 (80.9) | 1.25: −3.8, 6.3 |
| Diagnosis | ||||||
| major abscess | 160/167 (95.8) | 147/153 (96.1) | 0.2: −4.2, 4.5 | 163/183 (89.1) | 151/169 (89.3) | 0.8: −5.6, 7.2 |
| diabetic foot infection | 84/110 (76.4) | 75/96 (78.1) | −2.8: −14.5, 9.0 | 86/123 (69.9) | 76/110 (69.1) | −0.1: −12.4, 12.1 |
| wound infection | 59/62 (95.2) | 45/47 (95.7) | −1.4: −9.7, 6.8 | 65/72 (90.3) | 48/55 (87.3) | 2.3: −8.6, 13.2 |
| infected ischaemic ulcer | 17/22 (77.3) | 8/11 (72.7) | 6.2: −27.2, 39.6 | 17/24 (70.8) | 9/18 (50.0) | 20.1: −9.0, 49.2 |
n/N, number of patients experiencing clinical cure/number of patients with a given diagnosis.
There were no major differences between the treatment groups regarding the types of patients assessed as clinical failures at TOC. Most were male, assigned to Wilson risk class IV, with a primary diagnosis of DFI and the deepest tissue involved was most frequently the muscle or fascia. Most had undergone surgical intervention, mainly abscess drainage, amputation, or local or extensive debridement. The most frequent baseline pathogen observed for these patients was methicillin-susceptible S. aureus, consistent with the main analysis populations. This pathogen was, however, eradicated from most clinical failures by the EOT or TOC visit.
Bacteriological efficacy
Overall, the bacteriological efficacy was high in both treatment arms. In the MBV population, moxifloxacin was non-inferior to TZP–AMC with respect to bacteriological efficacy at TOC (Table 3). These results are supported by those of the ITT population (data not shown). The bacteriological efficacy against the most commonly isolated organisms was similar between treatment arms (Table 3). Although MRSA infection was identified in some MBV patients (major abscess, n = 6; DFI, n = 22; wound infection, n = 5; and infected ischaemic ulcer, n = 5), the overall bacterial success rates for moxifloxacin and TZP–AMC against this pathogen were high [17/23 (73.9%) and 15/17 (88.2%), respectively]. No moxifloxacin-treated and one TZP–AMC-treated patient received concomitant anti-MRSA treatment.
Safety
Both treatment regimens were well tolerated (Table 5), with a total of 100/426 (23.5%) moxifloxacin- and 72/377 (19.1%) TZP–AMC-treated patients experiencing a treatment-emergent AE (P = 0.14). The incidence of drug-related AEs was similar in each group [moxifloxacin: 37/426 (8.7%) versus TZP–AMC: 28/377 (7.4%); P = 0.60], with the most commonly occurring drug-related AE in both arms being diarrhoea (Table 5). Tests for C. difficile in patients with confirmed diarrhoea indicated that three moxifloxacin- and one TZP–AMC-treated patient had C. difficile isolated or the C. difficile toxin detected. Withdrawal rates due to drug-related AEs were low in both arms [moxifloxacin: n = 8 (1.9%) versus TZP–AMC: n = 3 (0.8%); P = 0.23]. All events in each arm resolved. A serious AE was reported in 21 (4.9%) patients in the moxifloxacin group and 14 (3.7%) patients in the TZP–AMC group (P = 0.49). These events were considered to be drug related in six patients. Serious drug-related AEs in the moxifloxacin arm were prolonged QT interval (n = 2), increased blood alkaline phosphatase (n = 1) and wound infection (n = 1). In the TZP–AMC group, serious AEs were impaired healing (n = 1) and wound infection (n = 1). Four deaths occurred, none of which was considered to be drug related: deaths in the moxifloxacin group (n = 3) were due to cardiopulmonary failure, renal failure and shock, and pulmonary embolism; the death in the TZP–AMC group (n = 1) was due to pulmonary embolism.
Table 5.
Incidence of treatment-emergent AEs (ITT/safety population)
| Moxifloxacin (N = 426), n (%) | TZP–AMC (N = 377), n (%) | P value | |
|---|---|---|---|
| Any treatment-emergent AE | 100 (23.5) | 72 (19.1) | 0.14 |
| Any treatment-emergent drug-related AEsa | 37 (8.7) | 28 (7.4) | 0.60 |
| ventricular extrasystoles | 0 | 2 (0.5) | — |
| upper abdominal pain | 0 | 2 (0.5) | — |
| diarrhoea | 8 (1.9) | 4 (1.1) | — |
| flatulence | 0 | 2 (0.5) | — |
| nausea | 3 (0.7) | 2 (0.5) | — |
| vomiting | 1 (0.2) | 2 (0.5) | — |
| Withdrawal due to treatment-emergent drug-related AEb | 8 (1.9) | 3 (0.8) | 0.23 |
| Any treatment-emergent serious AE | 21 (4.9) | 14 (3.7) | 0.49 |
| Any treatment-emergent drug-related serious AE | 4 (0.9) | 2 (0.5) | 0.69 |
aIndividual events listed under treatment-emergent drug-related AEs occurring in ≥0.5% of patients in either treatment group.
bAEs leading to withdrawal, moxifloxacin: cytolytic hepatitis (n = 1); drug hypersensitivity (n = 1); QT prolongation (n = 2); increase in blood alkaline phosphatase (n = 1); gout (n = 1); dizziness (n = 1); and allergic dermatitis (n = 1). TZP–AMC: diarrhoea (n = 1); wound re-infection (n = 1); and allergic dermatitis (n = 1).
Discussion
In this comparative, randomized, double-blind, multicentre study, moxifloxacin iv/oral was non-inferior to TZP–AMC in the treatment of cSSSIs, stratified according to severity and the need for initial baseline surgery. Such stratification prior to randomization has not, to the authors' knowledge, been carried out in other clinical trials and its omission from cSSSI studies has been identified as a potential design flaw.24 The four infections that determined eligibility were clearly defined: major abscess; DFI; wound infection; and infected ischaemic ulcer. DFI was assessed using several severity scores (data not shown) to ensure that patients with moderate-to-severe infections were recruited. The characteristics of the subgroup of patients with abscesses reflected that of a population with major abscesses, based on the size and location of abscesses seen. Such patients have been identified as potentially benefiting from hospital admission and initial iv therapy in addition to surgical intervention, especially if diabetes is a co-morbidity.6 The strict methodology used provided an in-depth and accurate assessment of patients and disease characteristics. Assessment by a blinded DRC, prospective use of the Wilson risk class and standardized photographic assessment of the lesions all helped eliminate investigator bias. The use of repeated photographs as a diagnostic tool has been validated in other clinical specialties, e.g. dermatology,25 and was used in the current study to improve diagnostic consistency.24 Indeed, a recent meta-analysis of cSSSI trials24 concluded that the legitimacy of using antimicrobials could be confirmed by such an approach. Retrospective stratification by cSSSI subgroup is common in clinical trials, but prospective stratification by disease type and severity, and detailed assessments of patients' wound types and clinical responses have not, to our knowledge, been reported in other cSSSI studies. Therefore, the RELIEF study adds a unique dimension to similar studies in this area and overcomes many shortcomings of previous trials.24
Moxifloxacin was shown to be clinically non-inferior to TZP–AMC. Thus, the current data confirm those from previous studies of cSSSIs.3,18,26 As well as good overall efficacy, moxifloxacin had comparable efficacy to TZP–AMC in the different subgroups of cSSSI, with particularly good efficacy in patients with abscesses. Cure rates were lower in patients with compromised vascular perfusion (DFI and ischaemic ulcers); however, the results in patients with a DFI are similar to those seen in other trials of antimicrobial treatments in cSSSIs.26 The analysis was not powered to look at differences in clinical efficacy in subgroups.
The type and distribution of baseline pathogens was as expected from previous studies, with the most commonly occurring pathogens being Gram-positive aerobes (in ∼65% of PP patients), particularly methicillin-susceptible S. aureus, β-haemolytic streptococci and E. faecalis. The majority of the E. faecalis strains were isolated from DFIs (n = 59) and abscesses (n = 24). E. faecalis is known to be commonly isolated from patients with a DFI, though it is not clear whether this is contamination or colonization of the site. As in the present trial, previous studies have shown good clinical outcomes despite the use of agents that are not active against enterococci.27,28 Few anaerobic pathogens were isolated; it is possible these have a more important causative role to play in severe infections, such as necrotizing fasciitis.1 However, because this was a large multinational study, it is also possible that despite optimal transport containers and conditions, anaerobes did not survive the journey to the central laboratory as well as aerobes did. High bacteriological success rates were achieved in both arms, and success rates were generally similar between mono- and polymicrobial infections, as would be expected when using two broad-spectrum antibiotics. MRSA isolates were isolated relatively infrequently from patients with DFIs in this study (12.4%), although previous studies have isolated MRSA from 30%–50% of such infections.29–31 The reasons for this are unclear, but may be related to the exclusion criteria and/or differing epidemiology and geographical location of various study populations. Recent work has shown that across Europe, isolation rates of MRSA from cSSSIs can vary from 3% to 48%.32 Nonetheless, success rates against MRSA were similar and relatively good in both arms. As neither of the treatment regimens was specifically used for anti-MRSA activity, this clearance may be because the isolate was a colonizer rather than a pathogen or due to the role of extensive debridement; this is particularly likely in patients who received no anti-MRSA therapy but still experienced clinical cure. Given the limited activity of moxifloxacin and lack of activity of TZP–AMC against community-acquired MRSA (CA-MRSA), patients with known MRSA infections should not be treated with these regimens, despite the in vitro susceptibility of some isolates to moxifloxacin. In contrast to the USA, the prevalence of CA-MRSA remains relatively low (1%–3%) in most European countries.33
Safety data are comparable to those from previous clinical trials of moxifloxacin,34 which can be considered safe and well tolerated in the patient population enrolled to the study. Specifically, similar numbers and types of AEs were seen in each treatment arm and no cardiac events that could be surrogates of arrhythmia occurred. C. difficile-associated diarrhoea rates were notably low in the current study. The literature suggests that almost all classes of antibiotics may drive the selection of resistant strains of C. difficile that can cause C. difficile-associated diarrhoea outbreaks if infection control practices fail.35 Fluoroquinolones are probably not different in this respect from other classes of antibiotics.36 It is clear, however, that irrespective of the antibiotic being used, good antibiotic stewardship and scrupulous hygiene are key to limiting C. difficile-associated diarrhoea and should be exercised in all clinical situations.37
Possible weaknesses of the study include the limited analysis of microorganisms; this could lead to underestimation of the polymicrobial character of the cSSSIs. Citron et al.38 commented on the large number and variety of organisms that were isolated from specimens in patients with moderate-to-severe DFIs, and found that most patients had polymicrobial cultures. However, the contribution to the pathogenicity of many of the microorganisms isolated from open lesions is still unclear.
While surgical intervention and/or drainage are clinically important features of cSSSI management,1,4 it is unclear how they may affect outcomes in antibiotic trials. Although only patients with residual infection after surgery were included, the role of antibiotic therapy after surgery is more difficult to assess and this may work in favour of equivalence or non-inferiority. The current study did, however, stratify for surgical intervention in order to reduce any confounding effects.
Most patients in this study were recruited from Eastern Europe, despite concerted efforts to recruit from Western Europe, which raises the question of whether the data are applicable across geographical populations. Differences may exist between Western and Eastern Europe in terms of the pathogens isolated, the severity of infections treated or referred to hospital, patients' access to healthcare and possible variation in the accepted standards of surgical care. However, these differences are speculative and their impact is unknown. A review of the 2008 report of the European Antibiotic Resistance Surveillance System39 suggests that differences tend to be between Southern and Northern Europe (particularly in the incidence of MRSA) rather than between Western and Eastern Europe.
Conclusions
Once-daily iv/oral moxifloxacin monotherapy was clinically and bacteriologically non-inferior to a commonly used broad-spectrum antibiotic therapy (4.0/0.5 g of piperacillin/tazobactam iv thrice daily followed by 875/125 mg of oral amoxicillin/clavulanate twice daily) in patients with cSSSIs, across the range of infection severities included in this study. Moxifloxacin sequential monotherapy, together with surgery, is an appropriate option for patients with cSSSIs, including those with polymicrobial infections, when used in accordance with the prescribing guidelines.
Funding
This work was supported by a research grant from Bayer Healthcare AG. The editorial assistance was funded by Bayer Healthcare Pharmaceuticals.
Transparency declarations
I. C. G. has received research grants from MSD, Bayer, Boehringer Ingelheim and UCB Pharma, honoraria from Pfizer, consultancy fees from ECDC, MSD and Wyeth and royalties from Reed Business. M. D. has received research grants from Pfizer and Bayer and honoraria from Pfizer, Bayer, Novartis and Johnson and Johnson. P. K. has received honoraria (lecture fees) from Janssen-Cilag, Bayer, Novartis and Pfizer. D. N. has received research grants from Bayer; honoraria from Pfizer, Novartis, Wyeth and Johnson and Johnson and consultancy fees from Pfizer, Astellas and Wyeth. N. S. has received research grants from GlaxoSmithKline, NovoNordisk, Sanofi Aventis, Pfizer, LifeScan and Ferring and consultancy fees from DSM Pharmaceuticals and Pfizer. B. H., P. R. and P. A. are employees of Bayer Pharma AG. J. A. is an employee and stockholder of Bayer Healthcare.
Highfield Communication Consultancy provided editorial assistance. Bayer Healthcare Pharmaceuticals provided assistance with study design, data acquisition and statistical and other analyses.
Author contributions
I. C. G. was the main author of the paper and undertook the initial drafting of the paper. I. C. G., M. D., P. K., D. N. and N. S. formed the DRC committee and were responsible for the assessment of data. P. A. contributed significantly to the design of the study and the assessment of clinical data. J. A. assessed the microbiological data. B. H. was involved in the design and management of the study. P. R. undertook the statistical analyses. All the authors contributed to the development of this paper and approve this document. I. C. G. is the guarantor of the data.
Supplementary data
Tables S1 and S2 are available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Acknowledgements
Preliminary data from the RELIEF study were presented at the European Congress of Clinical Microbiology and Infectious Diseases, Helsinki, 2009 (Posters 1785 and 1786), the European Congress of Clinical Microbiology and Infectious Diseases, Vienna, 2010 (Poster 1550) and the European Association for the Study of Diabetes, Stockholm, Sweden, 2010 (Poster 1167).
We are grateful to all investigators and study centres who participated in the RELIEF study:
Belgium: Dr D. Ballaux, Antwerp; Prof. F. Jacobs, Brussels; Dr K. Spincemaille, Brussels; and Dr K. Van Acker, Bornem/Antwerp; Bulgaria: Assoc. Prof. M. Kadurina, Sofia; Dr G. Lefterov, Ruse; and Assoc. Prof. B. Ninov, Pleven; Germany: Prof. P. Altmeyer, Bochum; Prof. H. Gollnick, Magdeburg; Prof. C. Sunderkötter, Münster; and Prof. M. Winkler, Hanover; Greece: Prof. A. Skoutelis, Athens; Hungary: Prof. Á. Altorjay, Székesfehérvár; Dr L. Damjanovich, Debrecen; Dr F. Halmos, Kaposvár; Dr E. Kisida, Budapest; Prof. A. Nagy, Veszprém; and Prof. A. Oláh, Győr; Israel: Dr M. Chowers, Kfar Saba; Dr G. Rahav, Tel Hashomer; Prof. I. Potasman, Haifa; and Prof. R. Raz, Afula; Latvia: Dr M. Aizsilniece, Riga; D. Krievins, Riga; U. Kupcs, Valmiera; G. Pupelis, Riga; V. Rozitis, Liepāja; and Dr I. Satilovs, Daugavpils; Lithuania: S. Ausra, Šiauliai; R. Civilka, Ukmergė; A. Gradauskas, Vilnius; and E. Varanauskiene, Kaunas; Poland: Prof. W. Karnafel, Warsaw; Dr M. Melaniuk, Lublin; Dr A. Wolski, Lublin; and Dr J. Wronski, Lublin; Romania: Prof. R. M. Cosgarea, Cluj-Napoca; Prof. M. Graur, Iaşi; Prof. I. Popescu, Bucharest; Dr S. C. Tivadar, Bucharest; and Assoc. Prof. I. A. Veresiu, Cluj-Napoca; Russia: Prof. I. Gurieva, Moscow; Prof. N. Khachatryan, Moscow; Prof. A. Konychev, St Petersburg; Prof. A. Severtsev, Moscow; Prof. N. Vorochobina, St Petersburg; Prof. V. Yakusevich, Yaroslavl; and Prof. A. Zouzova, Smolensk; South Africa: Prof. J. H. R. Becker, Pretoria; Dr M. M. De Vries Basson, Bellville; Prof. J. E. J. Krige, Cape Town; Dr L. Van Zyl, Worcester; and Dr N. C. Wright, Johannesburg; Spain: Prof. J. M. Tellado, Madrid; Ukraine: O. Dziublyk, Kiev; I. Herych, Lviv; A. Lissov, Kiev; V. Mishalov, Kiev; O. Pyptiuk, Ivano-Frankivsk; and A. Zaychuk, Odessa; and UK: Prof. S. MacRury, Inverness.
References
- 1.DiNubile MJ, Lipsky BA. Complicated infections of skin and skin structures: when the infection is more than skin deep. J Antimicrob Chemother. 2004;53(Suppl S2):ii37–50. doi: 10.1093/jac/dkh202. doi:10.1093/jac/dkh202. [DOI] [PubMed] [Google Scholar]
- 2.CDC. Soft tissue infections among injection drug users – San Francisco, California, 1996–2000. MMWR. 2001;50:381–4. [PubMed] [Google Scholar]
- 3.Giordano P, Song J, Pertel P, et al. Sequential intravenous/oral moxifloxacin versus intravenous piperacillin–tazobactam followed by oral amoxicillin–clavulanate for the treatment of complicated skin and skin structure infection. Int J Antimicrob Agents. 2005;26:357–65. doi: 10.1016/j.ijantimicag.2005.07.017. doi:10.1016/j.ijantimicag.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Nichols RL. Optimal treatment of complicated skin and skin structure infections. J Antimicrob Chemother. 1999;44:19–23. doi: 10.1093/jac/44.suppl_1.19. doi:10.1093/jac/44.suppl_1.19. [DOI] [PubMed] [Google Scholar]
- 5.Lipsky BA, Weigelt JA, Gupta V, et al. Skin, soft tissue, bone, and joint infections in hospitalized patients: epidemiology and microbiological, clinical, and economic outcomes. J Antimicrob Chemother. 2007;60:370–6. doi: 10.1086/520743. doi:10.1093/jac/dkm130. [DOI] [PubMed] [Google Scholar]
- 6.Eron LJ, Lipsky BA, Low DE, et al. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(Suppl S1):i3–17. doi: 10.1093/jac/dkg466. doi:10.1093/jac/dkg466. [DOI] [PubMed] [Google Scholar]
- 7.Woodcock KM, Andrews JM, Boswell FJ, et al. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–6. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldridge KE, Ashcraft DS. Comparison of the in vitro activities of BAY 12-8039, a new quinolone, and other antimicrobials against clinically important anaerobes. Antimicrob Agents Chemother. 1997;41:709–11. doi: 10.1128/aac.41.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guay DRP. Moxifloxacin in the treatment of skin and skin structure infections. Ther Clin Risk Management. 2006;2:417–34. doi: 10.2147/tcrm.2006.2.4.417. doi:10.2147/tcrm.2006.2.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashour HM, el-Sharif A. Microbial spectrum and antibiotic susceptibility profile of Gram-positive aerobic bacteria isolated from cancer patients. J Clin Oncol. 2007;25:576–9. doi: 10.1200/JCO.2007.14.0947. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs E, Dalhoff A, Korfmann G. Susceptibility patterns of bacterial isolates from hospitalised patients with respiratory tract infections (MOXIAKTIV study) Int J Antimicrob Agents. 2009;33:52–7. doi: 10.1016/j.ijantimicag.2008.07.017. doi:10.1016/j.ijantimicag.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Stearne LE, Vonk AG, Kullberg BJ, et al. Effect of recombinant murine granulocyte colony-stimulating factor with or without fluoroquinolone therapy on mixed-infection abscesses in mice. Antimicrob Agents Chemother. 2005;49:3668–75. doi: 10.1128/AAC.49.9.3668-3675.2005. doi:10.1128/AAC.49.9.3668-3675.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubinstein E, Diamantstein L, Yoseph G, et al. The effects of albumin, globulin, pus and dead bacteria in aerobic and anaerobic conditions on the antibacterial activity of moxifloxacin, trovafloxacin and ciprofloxacin against Streptococcus pneumoniae, Staphylococcus aureus and Escherichia coli. Clin Microbiol Infect. 2000;6:678–81. doi: 10.1046/j.1469-0691.2000.00166.x. doi:10.1046/j.1469-0691.2000.00166.x. [DOI] [PubMed] [Google Scholar]
- 14.Müller M, Stass H, Brunner M, et al. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob Agents Chemother. 1999;43:2345–9. doi: 10.1128/aac.43.10.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkhardt O, Derendorf H, Jäger D, et al. Moxifloxacin distribution in the interstitial space of infected decubitus ulcer tissue of patients with spinal cord injury measured by in vivo microdialysis. Scand J Infect Dis. 2006;38:904–8. doi: 10.1080/00365540600664076. doi:10.1080/00365540600664076. [DOI] [PubMed] [Google Scholar]
- 16.Stass H, Kubitza D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother. 1999;43(Suppl B):83–90. doi: 10.1093/jac/43.suppl_2.83. doi:10.1093/jac/43.suppl_2.83. [DOI] [PubMed] [Google Scholar]
- 17.Wise R, Andrews JM, Marshall G, et al. Pharmacokinetics and inflammatory-fluid penetration of moxifloxacin following oral or intravenous administration. Antimicrob Agents Chemother. 1999;43:1508–10. doi: 10.1128/aac.43.6.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vick-Fragoso R, Hernández-Oliva G, Cruz-Alcázar J, et al. Efficacy and safety of sequential intravenous/oral moxifloxacin vs intravenous/oral amoxicillin/clavulanate for complicated skin and skin structure infections. Infection. 2009;37:407–17. doi: 10.1007/s15010-009-8468-x. doi:10.1007/s15010-009-8468-x. [DOI] [PubMed] [Google Scholar]
- 19.Wilson SE, Solomkin JS, Le V, et al. A severity score for complicated skin and soft tissue infections derived from Phase III studies of linezolid. Am J Surg. 2003;185:369–75. doi: 10.1016/s0002-9610(02)01411-3. doi:10.1016/S0002-9610(02)01411-3. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21:855–9. doi: 10.2337/diacare.21.5.855. doi:10.2337/diacare.21.5.855. [DOI] [PubMed] [Google Scholar]
- 21.Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev. 2004;20(Suppl 1):S90–5. doi: 10.1002/dmrr.464. doi:10.1002/dmrr.464. [DOI] [PubMed] [Google Scholar]
- 22.Wagner FW., Jr The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2:64–122. doi: 10.1177/107110078100200202. [DOI] [PubMed] [Google Scholar]
- 23.Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot wounds. J Foot Ankle Surg. 1996;35:528–31. doi: 10.1016/s1067-2516(96)80125-6. doi:10.1016/S1067-2516(96)80125-6. [DOI] [PubMed] [Google Scholar]
- 24.McClaine RJ, Husted TL, Hebbeler-Clark RS, et al. Meta-analysis of trials evaluating parenteral antimicrobial therapy for skin and soft tissue infections. Clin Infect Dis. 2010;50:1120–6. doi: 10.1086/651264. doi:10.1086/651264. [DOI] [PubMed] [Google Scholar]
- 25.Kvedar JC, Edwards RA, Menn ER, et al. The substitution of digital images for dermatologic physical examination. Arch Dermatol. 1997;133:161–7. doi:10.1001/archderm.133.2.161. [PubMed] [Google Scholar]
- 26.Lipsky BA, Giordano P, Choudhri S, et al. Treating diabetic foot infections with sequential intravenous to oral moxifloxacin compared with piperacillin–tazobactam/amoxicillin–clavulanate. J Antimicrob Chemother. 2007;60:370–6. doi: 10.1093/jac/dkm130. doi:10.1093/jac/dkm130. [DOI] [PubMed] [Google Scholar]
- 27.Lipsky BA, Itani K, Norden C, et al. Treating foot infections in diabetic patients: a randomized, multicenter, open-label trial of linezolid versus ampicillin–sulbactam/amoxicillin–clavulanate. Clin Infect Dis. 2004;38:17–24. doi: 10.1086/380449. doi:10.1086/380449. [DOI] [PubMed] [Google Scholar]
- 28.Lipsky BA, Armstrong DG, Citron DM, et al. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): prospective, randomised, controlled, double-blinded, multicentre trial. Lancet. 2005;366:1695–703. doi: 10.1016/S0140-6736(05)67694-5. doi:10.1016/S0140-6736(05)67694-5. [DOI] [PubMed] [Google Scholar]
- 29.Dang CN, Prasad YD, Boulton AJ, et al. Methicillin-resistant Staphylococcus aureus in the diabetic foot clinic: a worsening problem. Diabet Med. 2003;20:159–61. doi: 10.1046/j.1464-5491.2003.00860.x. doi:10.1046/j.1464-5491.2003.00860.x. [DOI] [PubMed] [Google Scholar]
- 30.Tentolouris N, Petrikkos G, Vallianou N, et al. Prevalence of methicillin-resistant Staphylococcus aureus in infected and uninfected diabetic foot ulcers. Clin Microbiol Infect. 2006;12:186–9. doi: 10.1111/j.1469-0691.2005.01279.x. doi:10.1111/j.1469-0691.2005.01279.x. [DOI] [PubMed] [Google Scholar]
- 31.Lecornet E, Robert J, Jacqueminet S, et al. Preemptive isolation to prevent meticillin-resistant Staphylococcus aureus cross-transmission in diabetic foot. Diabetes Care. 2007;30:2341–2. doi: 10.2337/dc07-0743. doi:10.2337/dc07-0743. [DOI] [PubMed] [Google Scholar]
- 32.Sader HS, Farrell DJ, Jones RN. Antimicrobial susceptibility of Gram-positive cocci isolated from skin and skin-structure infections in European medical centres. Int J Antimicrob Agents. 2010;36:28–32. doi: 10.1016/j.ijantimicag.2010.03.016. doi:10.1016/j.ijantimicag.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Ferry T, Etienne J. Community acquired MRSA in Europe. BMJ. 2007;335:947–8. doi: 10.1136/bmj.39373.465903.BE. doi:10.1136/bmj.39373.465903.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ball P, Stahlmann R, Kubin R, et al. Safety profile of oral and intravenous moxifloxacin: cumulative data from clinical trials and postmarketing studies. Clin Ther. 2004;26:940–50. doi: 10.1016/s0149-2918(04)90170-1. doi:10.1016/S0149-2918(04)90170-1. [DOI] [PubMed] [Google Scholar]
- 35.Vernaz N, Hill K, Leggeat S, et al. Temporal effects of antibiotic use and Clostridium difficile infections. J Antimicrob Chemother. 2009;63:1272–5. doi: 10.1093/jac/dkp128. doi:10.1093/jac/dkp128. [DOI] [PubMed] [Google Scholar]
- 36.Hensgens MP, Goorhuis A, van Kinschot CM, et al. Clostridium difficile infection in an endemic setting in the Netherlands. Eur J Clin Microbiol Infect Dis. 2011;30:587–93. doi: 10.1007/s10096-010-1127-4. doi:10.1007/s10096-010-1127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss K. Clostridium difficile and fluoroquinolones: is there a link? Int J Antimicrob Agents. 2009;33(Suppl 1):S29–32. doi: 10.1016/S0924-8579(09)70013-5. doi:10.1016/S0924-8579(09)70013-5. [DOI] [PubMed] [Google Scholar]
- 38.Citron DM, Goldstein EJC, Merriam CV, et al. Bacteriology of moderate–severe diabetic foot infections and in vitro activity of antimicrobial agents. J Clin Microbiol. 2007;45:2818–28. doi: 10.1128/JCM.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.EARS-NET 2009. Antimicrobial Resistance Surveillance in Europe 2009. http://www.ecdc.europa.eu/en/publications/Publications/1011_SUR_annual_EARS_Net_2009.pdf. (8 August 2011, date last accessed) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.