Abstract
Objectives
The presence of herpes simplex virus-2 (HSV-2) shedding episodes correlates with transmission to sexual partners and neonates, and some episodes correlate with disease manifestations. HSV-2-targeted guanosine analogues are effective when given on a prophylactic basis, but do not completely eliminate recurrences, asymptomatic shedding or transmission. We sought to describe the impact of twice-daily aciclovir and famciclovir on shedding episodes.
Methods
We used pooled results from crossover clinical trials to construct frequency histograms for viral shedding episode duration, peak copy number, expansion kinetics and decay kinetics.
Results
Suppressive aciclovir and famciclovir decreased the frequency of episodes of >24 h duration by 50%, lowered the mean early episode expansion rate (from 8.2 to 7.2 HSV DNA logs/day, P = 0.004), decreased the mean peak values for shedding episodes (from 4.9 to 3.9 log10 HSV DNA copies/mL, P < 0.001) and lowered the mean episode duration (from 4.8 to 2.1 days, P < 0.001) primarily by decreasing the probability of viral re-expansion during episodes. The mean rate of late viral decay was similar for persons on and off antiviral medications (−6.0 versus −5.8 HSV DNA logs/day, P = 0.61).
Conclusions
HSV-2-targeted antiviral therapy limits episode severity by decreasing the rate of early viral expansion and the likelihood of episode re-expansion. Late clearance of episodes in the immunocompetent host is not affected by antiviral therapy, suggesting that local immune response is critical for clearance of episodes both on and off treatment.
Keywords: viral dynamics, aciclovir, famciclovir
Introduction
The most commonly used antiviral medications for herpes simplex virus (HSV) infection are aciclovir, its pro-drug valaciclovir and famciclovir, a pro-drug of penciclovir. Both aciclovir and penciclovir enter HSV-infected cells and are converted into aciclovir-triphosphate and penciclovir-triphosphate by viral and cellular thymidine kinases. Aciclovir-triphosphate and penciclovir-triphosphate are guanosine analogues, which incorporate into viral DNA and thereby serve as chain terminators and inhibitors of viral DNA polymerase.1–3 The specificity of these agents for HSV-infected cells explains their excellent safety profile and therapeutic efficacy.4–11 Indications for treatment include genital and oral recurrences of HSV infection, as well as prophylaxis against recurrences, and reduction of transmission risk to sexual partners when given on a daily basis.
Various studies have shown that aciclovir, valaciclovir and famciclovir, while clinically effective, are imperfect for several therapeutic and preventative indications. Prophylactic administration of the DNA nucleoside inhibitors does not eliminate all mucosal viral shedding or transmission from persons with HSV-2 infection.12,13 When given to HSV-2-seropositive partners within serodiscordant relationships on a daily basis, valaciclovir prevented only 48% of transmissions over an 8 month period.14 Subclinical reactivation of HSV-2 in genital skin and mucosa occurs in aciclovir and valaciclovir therapy. This is associated with infiltration of HSV-specific CCR5+, CD4+ lymphocytes into the genital skin,15 a factor that appears to increase the risk of HIV-1 acquisition to16,17 and transmission from18 HSV-2-infected hosts. In two clinical trials, daily aciclovir taken twice daily did not decrease the HIV-1 acquisition rate in high-risk hosts.19,20 Similarly, in another large clinical trial, administration of aciclovir did not prevent transmission of HIV-1 from HIV-1/HSV-2 co-infected persons to their HIV-1-negative partners.21 It is not known why HSV-directed agents do not completely eliminate viral shedding and HSV-directed inflammation in the genital tract.
Frequency histograms are useful tools for describing quantitative aspects of viral shedding.22,23 Here we use frequency histograms to describe shedding episodes documented in persons on and off HSV-directed therapy.12,13,24 We describe episodes according to their frequency, duration, viral production and kinetics of expansion and decay. We identify decreased episode expansion rates and frequency of episodes of more than 1 day as key results of antiviral therapy, while episodes decay at the same rate whether or not antiviral agents are present.
Materials and methods
Ethics statement
The University of Washington institutional review board approved all included studies. Study participants provided written informed consent.
Study subjects
We studied previously published data from immunocompetent HSV-2-infected subjects who performed daily genital swabbing for detection of HSV-2 by PCR while enrolled in placebo-controlled trials that measured the frequency and severity of HSV-2 reactivation. During these trials, subjects received the study drug (aciclovir or famciclovir) dosed twice daily or placebo for at least 30 days, followed by a 1week washout period. They then crossed over to either placebo or study drug and received at least 30 days of therapy.12,13,24 Samples for shedding of HSV were collected on a daily basis. We included all HSV-2-seropositive, HIV-uninfected persons who contributed at least 30 consecutive days of genital lesion swabbing and diaries. Given reasonably similar results in comparative studies of aciclovir, valaciclovir and famciclovir, we pooled the results of these studies.12,24
Shedding outcomes
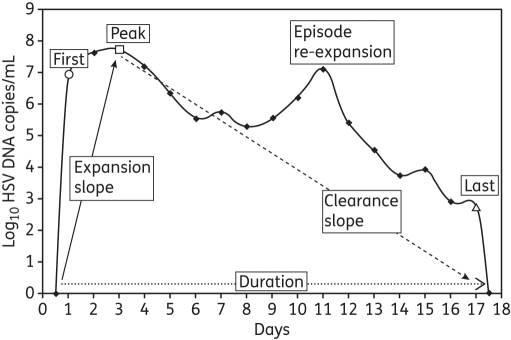
Shedding rate was defined as the number of swabs with HSV DNA detected by PCR out of the swabs collected. Lesion rate was the number of days with reported lesions out of the days with swabs collected. Shedding episodes were defined by a series of consecutive swabs containing HSV DNA ≥150 copies/mL (which has been defined as the optimal cut-off point for shedding in our studies),25 and not including more than one consecutive timepoint with a negative or missed swab. Episodes of certain duration were those that started and ended with two negative swabs. To ensure that results were not subject to undue bias based on overrepresentation of study subjects who contributed more data, we described the first 10 episodes observed from each subject. We described each shedding episode according to its duration, peak copy number, first positive copy number, last positive copy number, initiation to peak expansion slope and peak to termination clearance slope (Figure 1), and arranged all outcomes in frequency histograms to define ranges for these outcomes.
Figure 1.
HSV-2 shedding episode of 17 day duration derived from daily sampling. Each episode on and off therapy was analysed for duration (dotted arrow), initiation to peak regression slope (continuous arrow), peak to termination regression slope (dashed arrow), first positive HSV DNA copy number (open circle), peak HSV DNA copy number (open square), last HSV DNA copy number (open triangle) and presence of re-expansion (second peak, defined as a decrease of ≥0.5 log HSV DNA copies/mL followed by an expansion of ≥0.5 log HSV DNA copies/mL).
Shedding episode frequency
To calculate relevant population-level estimates of annualized episode rate, we enumerated the number of shedding episodes initiated during the observation period, divided this number by the total number of daily swabs performed and multiplied by 365. We calculated episode frequency with all available swabs.
Shedding episode duration
As swabs were taken every 24 h, episodes may have initiated within 0–24 h of the first positive swab of the episode and terminated within 0–24 h of the last positive swab. We assumed that the midpoint of this interval (12 h) would provide an unbiased estimate of length,23,26 and that missed swabs either preceding or following positive swabs contained HSV DNA when calculating episode duration (to account for the fact that the longest episodes were most likely to have missing intervening swabs).
Shedding episode peak, expansion and decay
For measures of episode peak, expansion and decay, we only included episodes of known duration (those episodes preceded and followed by at least two negative swabs). We defined episode peak according to the swab during an episode that contained the greatest amount of HSV DNA/mL.
Because of rapid kinetics of viral expansion, values for the first positive swab reflected in part the duration of the episode at the time of the swab, which was in turn likely to be randomly distributed between 0 and 24 h. Therefore, for calculation of the median expansion rate, we assumed that the median duration of an episode was approximately 12 h at the time of the first positive swab. We calculated the expansion rate during the first 12 h of an episode by dividing the median value for the first positive swab by 0.5 days. Similarly, we calculated an exponential slope of decay during the final 12 h of an episode by dividing the median value for the last positive swab by 0.5 days.
We calculated the rate of viral increase from initiation to the peak of each episode by computing the slope of a linear regression line over the copy numbers up to and including the maximum copy; for this calculation we set the time of the most proximal negative swab at 0.5 days prior to the first positive swab. We calculated the rate of decrease from peak to termination of each episode similarly by setting the time of termination to 0.5 days after the last positive swab (Figure 1). We calculated a median for each of these two slope measures based on results from all included episodes.
We performed separate analyses of episode characteristics for episodes with and without re-expansion (defined as an increase in HSV copy number by at least 0.5 logs after a prior decline within the episode of at least 0.5 logs; Figure 1) and for episodes with and without associated genital lesions.
Statistical methods for comparisons
Rate outcomes like shedding and lesion rates were compared by arm within persons using generalized linear mixed models with a log link, testing for within-person effects of the drug as rate ratios. Continuous outcomes like maximum copy obtained per episode were similarly compared using linear mixed models.
Laboratory methods
HSV serological testing was performed by western blot.27 Swabs were placed into vials containing 1 mL of PCR transport medium and refrigerated until laboratory processing.28 HSV DNA was detected using a quantitative PCR assay, and the HSV DNA level was expressed as copies/mL of transport medium with a cut-off of 150 copies/mL for defining positive samples.25 The initial PCR assay used type-common primers to the HSV gene encoding glycoprotein B. Positive samples were subsequently analysed using type-specific primers to examine whether the DNA detected was HSV-1, HSV-2 or both.29 An internal control was included to ensure that HSV-negative swabs were not due to inhibition. Laboratory personnel were blinded to clinical data.
Results
Study participants
A total of 158 persons who completed four different studies were eligible for inclusion. Two persons took valaciclovir (500 mg twice daily), 92 subjects took aciclovir (400 mg twice daily) and 64 took famciclovir (250 twice daily) while in the drug arm of the crossover trials. Since only two persons took valaciclovir, we limited our analysis to the 156 persons who took either aciclovir or famciclovir. Fifty-four (35%) participants were men; the median age was 35 years (interquartile range 28–45; range 21–68). Fifty-five subjects (35%) were dually infected with HSV-1 and 132 (85%) reported a history of recognized lesions. The median time since initial acquisition of genital herpes was 2 years (interquartile range 1–10; range 0–37).
Quantitative shedding frequency on and off twice-daily nucleoside analogues
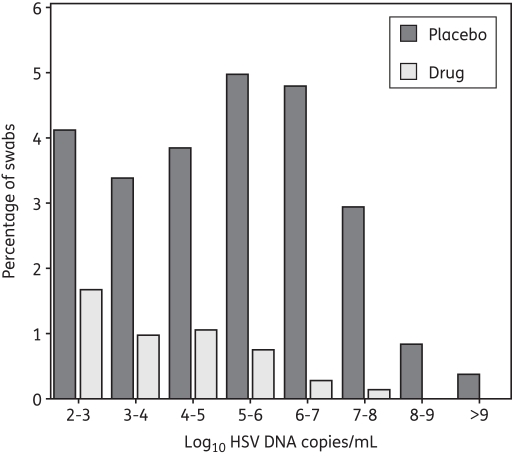
Of 8142 swabs performed in persons who were not receiving aciclovir or famciclovir, 2054 (25%) samples contained HSV DNA ≥150 copies/mL. Of 7509 swabs performed in persons receiving an antiviral drug, 368 (4.9%) contained HSV DNA ≥150 copies/mL (P < 0.001). The presence of study drug also resulted in a lower proportion of high-copy shedding: during placebo administration, a relatively similar proportion of swabs (3%–5%) contained 102–103, 103–104, 104–105, 105–106 or 106–107 HSV DNA copies/mL, while only 1.0% of swabs contained ≥108 HSV DNA copies; during antiviral drug administration, the proportion of swabs within each increasing log of virion copies decreased. Within each logarithmic strata ≥103 HSV DNA copies/mL, the proportion of swabs was approximately ≤1% (Figure 2).
Figure 2.
Frequency histogram of shedding frequency. Results are from 8142 genital swabs performed in 156 study subjects not receiving the study drug and 7509 swabs performed in the same subjects who received either 400 mg of aciclovir orally twice daily (92 subjects) or 250 mg of famciclovir orally twice daily (64 subjects).
Shedding episode frequency
In persons not receiving a study drug, we observed 401 episode onsets during 8529 sampled days and calculated an annualized episode rate of 17.2 (95% CI 15.3–19.3). In persons on antiviral medication we observed 178 episode onsets during 7925 sampled days and calculated an annualized episode rate of 8.0 (95% CI 6.9–9.9). Thus episode frequency was decreased by 53.5% (P < 0.001).
Shedding episode duration
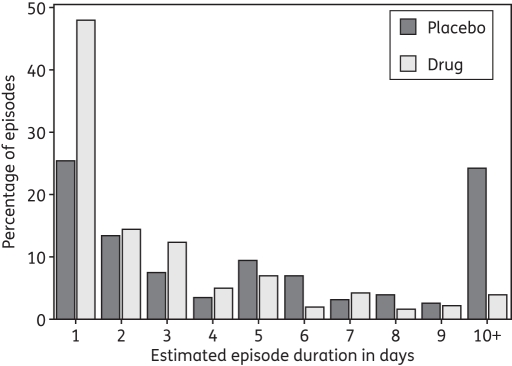
Of the 401 and 178 total accrued episodes in persons off and on a study drug, 383 (95.5%) and 175 (98.3%), respectively, terminated within the swabbing protocol and were therefore appropriate for analysis of duration. A total of 262 (65%) and 115 (65%) episodes were of certain duration (preceded and followed by two negative PCR swabs) and were within the first 10 episodes observed per subject; these episodes were analysed for first, last and peak positive HSV DNA copy number, as well as slope from initiation to peak and peak to termination.
Shedding episodes were 56% shorter (P < 0.001) in persons on antiviral therapy (mean = 2.1 days, median = 1.0 day) than in persons off of the drug (mean = 4.8 days, median = 3.0 days (Figure 3).
Figure 3.
Frequency histogram of shedding episode duration. Results are from 383 shedding episodes detected in 156 study subjects not receiving the study drug and 175 episodes detected in the same subjects while receiving the study drug.
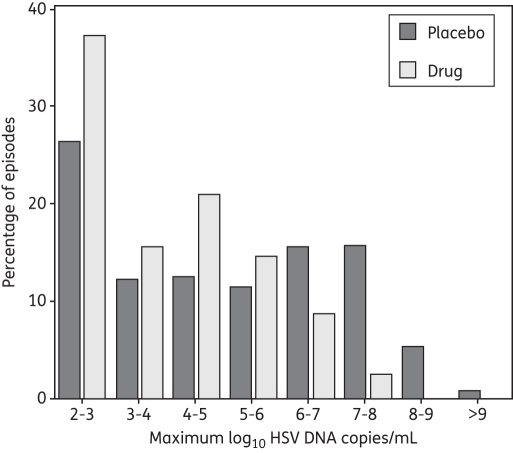
Peak viral production per shedding episode
Antiviral therapy reduced peak HSV DNA copy numbers (mean = 3.9 log10 HSV DNA copies/mL, median = 3.5 log10 HSV DNA copies/mL, versus mean = 4.9 log10 HSV DNA copies/mL, median = 4.9 log10 HSV DNA copies/mL on placebo, P < 0.001). For persons off and on the study drug, respectively, 26% and 37% of episodes peaked at less than 103 HSV DNA copies/mL. For persons off of the drug, there was a relatively constant proportion of peak copy numbers of 11%–16% within each of the five successive quantitative strata between 103 and 108 HSV DNA copies/mL. However, in persons receiving aciclovir or famciclovir, the proportion of episodes within each successively higher strata of HSV DNA copy number ≥105 HSV DNA copies/mL decreased. Fewer than 5% of episodes peaked at ≥107 HSV DNA copies/mL. Overall, a larger proportion of episodes that occurred while off of the study drug contained higher peak copy numbers of HSV DNA (Figure 4).
Figure 4.
Frequency histogram of shedding episode peak. Results are from 262 shedding episodes detected in 156 study subjects not receiving the study drug and 115 episodes detected in the same subjects while receiving the study drug.
Rate of shedding episode expansion
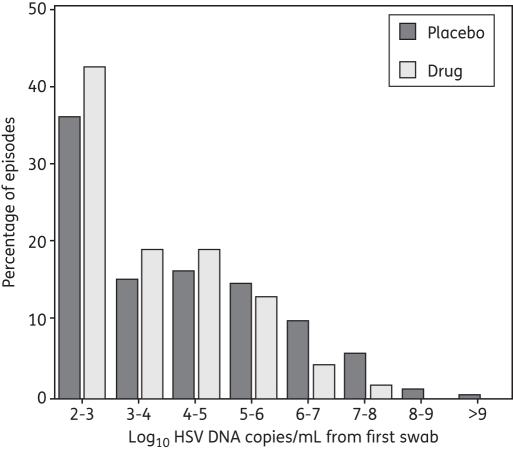
The median first positive copy number was 3.9 and 3.3 log10 HSV DNA copies/mL, respectively, for placebo versus drug therapy. We calculated median rates of expansion of 7.8 and 6.6 logs/day during the first 12 h of a shedding episode in persons off and on the drug; the mean expansion rate was 8.2 and 7.2 logs/day, respectively (P = 0.004). When participants did not take aciclovir or famciclovir, the distribution of the first positive values was similar at each of the three strata between 103 and 106 HSV DNA copies, suggesting linear exponential growth during this phase of episode expansion. However, a somewhat greater proportion of first positive swabs contained 102–103 HSV DNA copies when subjects were on the drug (43%) rather than off the drug (36%), perhaps suggesting that the viral expansion rate was, on average, slower during the initial hours of an episode in persons taking an antiviral medicine (Figure 5). Moreover, the first positive swab was less likely to contain ≥105 HSV DNA copies in persons taking aciclovir or famciclovir (19.7% versus 31.6%).
Figure 5.
Frequency histogram of first positive swab HSV DNA copy number/mL. Results are from 262 shedding episodes detected in 156 study subjects not receiving the study drug and 115 episodes detected in the same subjects while receiving the study drug.
The median regression slopes from initiation to episode peak for the 262 and 115 episodes were 4.9 and 4.9 logs/day (P = 0.59), compared with 7.8 and 6.6 logs/day during the first 12 h of an episode. This indicates that the viral expansion slope decreased after the first 12 h of an episode according to similar kinetics, whether or not a person was taking an antiviral agent.
Rate of shedding episode decay
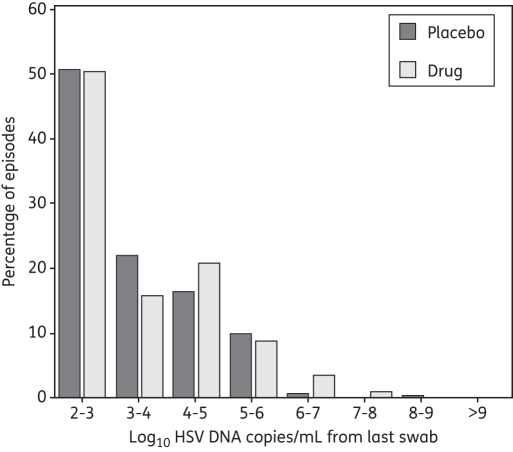
The median last positive copy number for a shedding episode was 3.0 and 2.9 log10 HSV DNA copies/mL for episodes on and off therapy, respectively. The calculated median rates of decay were −6.0 and −5.8 logs/day during the last 12 h of a shedding episode; the mean decay rates were −6.6 and −6.8 logs/day and did not differ between the two groups (P = 0.61). Frequency histograms for quantity of viral DNA in the last positive swabs were similar for persons on and off aciclovir or famciclovir, implying very similar decay kinetics for the two groups during the last 24 h of an episode (Figure 6).
Figure 6.
Frequency histogram of last positive swab HSV DNA copy number/mL. Results are from 262 shedding episodes detected in 156 study subjects not receiving the study drug and 115 episodes detected in the same subjects while receiving the study drug.
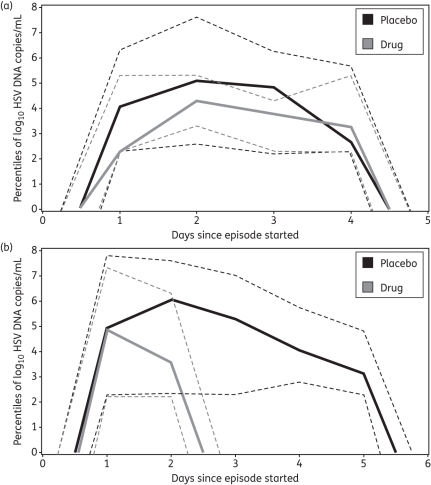
The median regression slope from peak to 12 h after the last positive swab for the 262 and 115 episodes was −2.7 and −4.6 logs/day, respectively (mean = −3.6 versus −5.2 logs/day, P < 0.001). Hence the episode exponential decay rate generally increased from the episode peak to termination in both groups, although the decay rate from peak to termination was much more rapid for persons on treatment. Episodes of comparable duration showed more rapid expansion and had a higher peak copy number off of the study drug, though the late decay rate was equivalent between the two curves (Figure 7a). The median episode on aciclovir or famciclovir twice daily was shorter in duration and had a lower peak copy number than off of antiviral medications; however, the late decay rate was again equivalent, based on the equivalent last positive swab value (Figure 7b).
Figure 7.
Kinetic differences between HSV-2 shedding episodes in persons on and off the study drug. (a) Episodes of 4 day duration (15 episodes off and 9 episodes on the drug) generated from median (continuous lines) as well as 5th and 95th percentile (broken lines) HSV DNA quantities obtained at each timepoint; first and peak genomic copy numbers were lower in persons on the drug, while last positive copy number was equivalent in the two groups. (b) Episodes of median duration (36 5 day episodes off the study drug and 25 2 day episodes on the drug) generated from median (continuous lines) as well as 5th and 95th percentile (broken lines) HSV DNA quantities obtained at each timepoint; peak genomic copy number was lower in persons on the drug, while the first and last positive copy numbers were equivalent in the two groups.
Episode decay occurred more slowly than episode expansion in both groups, although this effect was much less pronounced in persons on the drug. The median regression slope from initiation to peak was greater than the median regression slope from peak to termination in untreated persons (4.9 versus −2.7 logs/day), suggesting that, on average, episodes were composed of 36% [2.7/(2.7 + 4.9)] expansion phase and 64% decline phase. The median regression slope from initiation to peak was only slightly greater than the median regression slope from peak to termination in treated persons (4.9 versus −4.6 logs/day), suggesting that, on average, episodes consisted of 48% [4.6/(4.6 + 4.9)] expansion phase and 52% decline phase.
Episode re-expansion
Episode re-expansion (non-monotonic decline) was the most important cause of the more prolonged decline phase, particularly during longer episodes in persons not taking antiviral medication. Of 262 episodes in untreated persons, 58 (22%) had re-expansion (a decrease of at least 0.5 logs followed by an increase of at least 0.5 logs). Of 115 episodes in treated persons, 6 (5%) had re-expansion (P < 0.001). The median regression slope from peak to 12 h after the last positive swab for the 204 and 109 episodes without re-expansion was −4.6 and −4.6 logs/day, respectively. Therefore the low slope from peak to termination in persons off of antiviral medications (−2.7 logs/day) was due entirely to the 58 episodes with re-expansion.
Lesional and non-lesional episodes
The percentage of days with lesions was 3.8% (298/7926) in persons on therapy compared with 15% (1274/8584) in untreated persons (P = 0.007). A higher proportion of episodes in persons off of therapy were associated with lesions (100/262, 38%) versus (19/115, 17%) in persons on therapy (P < 0.001). Lesional episodes while on aciclovir or famciclovir were shorter (mean 3.1 versus 8.6 days, P < 0.001; median 3.0 versus 6.0 days), had a marginally lower first episode copy number (median 4.3 versus 5.3 log10 HSV DNA copy number/mL, P = 0.115) and a lower peak copy number (median 5.5 versus 6.7 log10 HSV DNA copy number/mL, P = 0.003).
Discussion
In this paper we extend our previous work by describing quantitative features of shedding episodes in persons on antiviral therapy. Our data reveal that twice-daily aciclovir and famciclovir decreased the frequency of episodes of at least 24 h duration, limited the expansion rate of episodes, limited the average peak episode copy number and, importantly, shortened the average episode duration by decreasing the likelihood of episode re-expansion 4-fold. The net effect of these kinetic differences was that persons receiving antiviral medications shed high copy numbers of virus much less commonly than when they are not receiving drug. Nevertheless, breakthrough episodes of shorter duration with high-copy shedding did occur even with twice-daily prophylactic therapy. These findings help explain why HSV-targeted antiviral medications decrease the frequency of asymptomatic shedding and recurrence,13 but do not entirely eliminate shedding or genital lesion formation. When given on a daily basis, valaciclovir decreased transmission likelihood within serodiscordant couples.14 However, even when dosed twice daily, antiviral medications did not completely eliminate high-copy shedding episodes, though the proportion of time spent above thresholds that are likely to result in transmission was considerably lower.
Our results reinforce previous conclusions that antiviral medications must be given early during recurrences to limit genital ulcer severity; these agents are most critical very soon after episode initiation when viral expansion kinetics are extremely rapid, whereas the host immune response appears to dictate viral kinetics later during episodes. We recently described 1020 heterogeneous HSV-2 shedding episodes in persons off of antiviral therapy in quantitative detail.23 We demonstrated that episodes are defined by very rapid expansion and decay phases; transition from the expansion to the decay phase is usually evident within just 1–2 days, and the episode expansion rate is already decelerating within 12 h of initiation. In the current study, the episode decay rate during the last 24 h of shedding episodes (as measured by the frequency histogram of each episode's last positive copy number) was virtually equivalent in episodes that occurred in persons on and off aciclovir or famciclovir; the slope from episode initiation to peak also did not differ between the groups, and was less than the slope of expansion during the first 12 h, which implies that immune response may have an equivalent and powerful effect early during episodes in persons who are both on and off drug therapy. Based on recent evidence of a high density of dendritic cells and HSV-specific CD4+ and CD8+ T cells in genital skin after recurrences while on antiviral therapy,15,30 mathematical model results that predict that episode severity is determined by density of immune cells at shedding episode onset31 and lack of drug resistance to antiviral agents despite long-term use,32 we surmise that episode clearance always occurs due to immune activity in the periphery, even when DNA nucleoside analogues are given. Antiviral medications, if timed early during episode expansion, limit the extent of shedding during the brief but important period before the immune response eliminates replication within infected cells.
If equivalent immune pressure is evident during episode expansion regardless of whether a person is taking prophylactic aciclovir or famciclovir, then it is somewhat counterintuitive that the slope from peak to termination is much higher overall in persons on the study drug. The explanation is that untreated persons are more likely to experience episodes with viral re-expansion, particularly during episodes with high peak copy number. Episode re-expansion is probably due to formation of secondary viral plaques, which may be evident on clinical exam as multiple ulcers, but may also occur during asymptomatic episodes, and are likely to lead to secondary peaks of virus during episodes.33 Importantly, when we analysed only episodes without re-expansion, then the rate of decay from peak to termination was equivalent in persons on and off of aciclovir or famciclovir. It is therefore an important effect of antiviral medications to keep peak viral levels below a threshold such that secondary plaques are less common.
The key limitation of our current analysis is that study subjects underwent once-daily swabbing. Clinical protocols employing swabbing every 6 h,34 in conjunction with mathematical modelling, lead to the prediction in persons off the drug that roughly 75% of episodes are missed with daily sampling.35 Episodes that are missed with daily sampling are brief, have low peak copy number and are asymptomatic. While our measured episode frequency was more than 50% lower in persons receiving scheduled antiviral medications compared with those receiving no antiviral medications, the proportion of episodes of shorter duration was higher. Therefore, if patients were to undergo continuous sampling, it is not clear whether antiviral therapy would lower episode frequency, shorten the duration of all episodes (rendering a higher proportion of episodes undetectable with once-daily swabbing) or both.
Our results do not eliminate the uncertainty regarding anatomic site of action of DNA nucleoside analogues. It is not surprising that aciclovir and penciclovir limit expansion rate, and therefore peak episode copy number during episodes, because these drugs limit viral replication in infected epithelial cells. While aciclovir and penciclovir are not known to directly impact the spread of a virus from epithelial cell to cell, it is likely that they indirectly decrease the spread based on fewer infectious particles generated by infected cells. It is less clear whether DNA nucleoside analogues enter and have activity within the dorsal root and sacral ganglia. We recently proposed that episode initiation in the periphery starts with infection of a single epithelial cell after release of virus from neurons.35 Therefore a decrease in viral release from neurons would presumably be a mechanism for decreased episode frequency. However, our current dataset does not reveal whether episode frequency would truly be decreased if we could conduct continual sampling. A related limitation is that our measure of episode rate is probably an underestimate of episode initiation rate. In the included studies, swabs of the entire genital tract, rather than swabs of a single specific reactivation region, were performed. Therefore multiple concurrent reactivations at separate anatomic sites were detected as a single shedding episode.
Although our assay is highly sensitive, we did not include positive values of <150 HSV DNA copies, based on our previous calculations of optimal cut-off values.25 Therefore we may have overestimated the viral decay rate if virus persisted at lower levels towards the end of each episode. Moreover, early during episodes, HSV may not reach the epidermal surface and swabs may lack sensitivity for low viral levels during this period, leading to overestimation of early expansion rates. Finally, by allowing for a negative or missed swab within an episode, we may have slightly overestimated episode duration and underestimated episode rate.
Our detailed quantitative analysis, when processed in concert with other recent study findings, suggests that DNA nucleoside analogues exhibit their effect early during episode expansion, but that within <24 h the immune response is having the most powerful effect on slowing the episode expansion phase and dictating rates of viral decay. The early antiviral effect is critical in decreasing the peak episode copy number, which in turn decreases the likelihood of episode re-expansion and prolonged episode duration, probably due to the diminished likelihood of secondary plaque formation. The clinical observation that early therapy is necessary to impact recurrence severity and duration supports this mechanism. Antiviral drugs also decreased the frequency of episodes of >24 h duration by approximately 50%, although breakthrough episodes were still common. As a result, persons on twice-daily dosing spend only a small proportion of time shedding ≥105 HSV DNA copies, and had fewer recurrences, but still shed virus, with local replication that occasionally reached quantities sufficient for recurrences and possibly transmission. Our data provide a quantitative framework for why DNA nucleoside analogues are useful, but only partially effective, for these indications.
Funding
This work was supported by the NIH (grant numbers P01 AI030731, R37 AI042528, K24 AI071113 and K23 AI087206). The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Transparency declarations
A. M. receives consulting fees from Immune Design Corporation, a company that develops vaccines and therapeutics, with emphasis on the prevention and treatment of infectious diseases. L. C. holds equity in and is the chairman of the Scientific Advisory Board and consults on HSV and VZV candidate vaccines for Immune Design Corporation. A. W. has received consulting fees from Astellas and Aicuris. J. T. S. and S. S.: none to declare.
Acknowledgements
This work was presented at the Infectious Diseases Society of America Meeting, Vancouver, British Columbia, Canada, 2010 (Abstract 4498).
We express gratitude to our study clinicians and study participants.
References
- 1.Elion GB, Furman PA, Fyfe JA, et al. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci USA. 1977;74:5716–20. doi: 10.1073/pnas.74.12.5716. doi:10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaeffer HJ, Beauchamp L, de Miranda P, et al. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978;272:583–5. doi: 10.1038/272583a0. doi:10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- 3.Dorsky DI, Crumpacker CS. Drugs five years later: acyclovir. Ann Intern Med. 1987;107:859–74. doi: 10.7326/0003-4819-107-6-859. [DOI] [PubMed] [Google Scholar]
- 4.Corey L, Benedetti J, Critchlow C, et al. Treatment of primary first-episode genital herpes simplex virus infections with acyclovir: results of topical, intravenous and oral therapy. J Antimicrob Chemother. 1983;12(Suppl B):79–88. doi: 10.1093/jac/12.suppl_b.79. [DOI] [PubMed] [Google Scholar]
- 5.Douglas JM, Critchlow C, Benedetti J, et al. A double-blind study of oral acyclovir for suppression of recurrences of genital herpes simplex virus infection. N Engl J Med. 1984;310:1551–6. doi: 10.1056/NEJM198406143102402. doi:10.1056/NEJM198406143102402. [DOI] [PubMed] [Google Scholar]
- 6.Mindel A, Faherty A, Carney O, et al. Dosage and safety of long-term suppressive acyclovir therapy for recurrent genital herpes. Lancet. 1988;i:926–8. doi: 10.1016/s0140-6736(88)91725-4. doi:10.1016/S0140-6736(88)91725-4. [DOI] [PubMed] [Google Scholar]
- 7.Raborn GW, McGaw WT, Grace M, et al. Treatment of herpes labialis with acyclovir. Review of three clinical trials. Am J Med. 1988;85:39–42. doi:10.1016/0002-9343(88)90361-0. [PubMed] [Google Scholar]
- 8.Reichman RC, Badger GJ, Mertz GJ, et al. Treatment of recurrent genital herpes simplex infections with oral acyclovir. A controlled trial. JAMA. 1984;251:2103–7. doi:10.1001/jama.251.16.2103. [PubMed] [Google Scholar]
- 9.Rooney JF, Straus SE, Mannix ML, et al. Oral acyclovir to suppress frequently recurrent herpes labialis. A double-blind, placebo-controlled trial. Ann Intern Med. 1993;118:268–72. doi: 10.7326/0003-4819-118-4-199302150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett BL, Tyring SK, Fife K, et al. Famciclovir treatment options for patients with frequent outbreaks of recurrent genital herpes: the RELIEF trial. J Clin Virol. 2008;43:190–5. doi: 10.1016/j.jcv.2008.06.004. doi:10.1016/j.jcv.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Bodsworth N, Bloch M, McNulty A, et al. 2-day versus 5-day famciclovir as treatment of recurrences of genital herpes: results of the FaST study. Sex Health. 2008;5:219–25. doi: 10.1071/sh08013. doi:10.1071/SH08013. [DOI] [PubMed] [Google Scholar]
- 12.Wald A, Selke S, Warren T, et al. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex Transm Dis. 2006;33:529–33. doi: 10.1097/01.olq.0000204723.15765.91. doi:10.1097/01.olq.0000204723.15765.91. [DOI] [PubMed] [Google Scholar]
- 13.Wald A, Corey L, Cone R, et al. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest. 1997;99:1092–7. doi: 10.1172/JCI119237. doi:10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350:11–20. doi: 10.1056/NEJMoa035144. doi:10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Hladik F, Woodward A, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–92. doi: 10.1038/nm.2006. doi:10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman E, Weiss H, Glynn J, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. doi:10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 17.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. doi:10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 18.Gray R, Wawer M, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. doi:10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 19.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–19. doi: 10.1016/S0140-6736(08)60920-4. doi:10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–71. doi: 10.1056/NEJMoa0800260. doi:10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–39. doi: 10.1056/NEJMoa0904849. doi:10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadinoto V, Shapiro M, Sun CC, et al. The dynamics of EBV shedding implicate a central role for epithelial cells in amplifying viral output. PLoS Pathog. 2009;5:e1000496. doi: 10.1371/journal.ppat.1000496. doi:10.1371/journal.ppat.1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiffer J, Wald A, Selke S, et al. The kinetics of mucosal herpes simplex virus-2 infection in humans: evidence for rapid viral-host interactions. J Infect Dis. 2011;204:554–61. doi: 10.1093/infdis/jir314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190:1374–81. doi: 10.1086/424519. doi:10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 25.Magaret A, Wald A, Huang M, et al. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol. 2007;45:1618–20. doi: 10.1128/JCM.01405-06. doi:10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odell PM, Anderson KM, D'Agostino RB. Maximum likelihood estimation for interval-censored data using a Weibull-based accelerated failure time model. Biometrics. 1992;48:951–9. doi:10.2307/2532360. [PubMed] [Google Scholar]
- 27.Ashley RL, Militoni J, Lee F, et al. Comparison of western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–7. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jerome KR, Huang ML, Wald A, et al. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40:2609–11. doi: 10.1128/JCM.40.7.2609-2611.2002. doi:10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corey L, Huang ML, Selke S, et al. Differentiation of herpes simplex virus types 1 and 2 in clinical samples by a real-time taqman PCR assay. J Med Virol. 2005;76:350–5. doi: 10.1002/jmv.20365. doi:10.1002/jmv.20365. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Koelle D, Cao J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. doi:10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiffer JT, Abu-Raddad L, Mark KE, et al. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc Natl Acad Sci USA. 2010;107:18973–8. doi: 10.1073/pnas.1006614107. doi:10.1073/pnas.1006614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta R, Hill E, McClernon D, et al. Acyclovir sensitivity of sequential herpes simplex virus type 2 isolates from the genital mucosa of immunocompetent women. J Infect Dis. 2005;192:1102–7. doi: 10.1086/432766. doi:10.1086/432766. [DOI] [PubMed] [Google Scholar]
- 33.Corey L, Adams HG, Brown ZA, et al. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958–72. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 34.Mark KE, Wald A, Magaret AS, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008;198:1141–9. doi: 10.1086/591913. doi:10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiffer JT, Abu-Raddad L, Mark KE, et al. Frequent release of low amounts of herpes simplex virus from neurons: results of a mathematical model. Sci Transl Med. 2009;1:7ra16. doi: 10.1126/scitranslmed.3000193. [DOI] [PMC free article] [PubMed] [Google Scholar]