Abstract
Inflammatory tissue injury and immunosuppression are the major causes of death in sepsis. Novel therapeutic targets that can prevent excessive inflammation and improve immune responses during sepsis could be critical for treatment of this devastating disease. LOX-1 (lectin-like oxidized low-density lipoprotein receptor-1), a membrane protein expressed in endothelial cells, has been known to mediate vascular inflammation. In the present study, we demonstrated that LOX-1 deletion markedly improved the survival rate in a murine model of polymicrobial sepsis. Wild-type (LOX-1+/+) and LOX-1 knockout (LOX-1−/−) mice were subjected to cecal ligation and puncture (CLP) to induce sepsis. LOX-1 deletion significantly reduced systemic inflammation and inflammatory lung injury during sepsis, together with decreased production of proinflammatory cytokines and reduced lung edema formation. Furthermore, LOX-1 deletion improved host immune responses after the induction of sepsis, as indicated by enhanced bacterial clearance. Interestingly, we were able to demonstrate that LOX-1 is expressed in neutrophils. LOX-1 deletion prevented neutrophil overreaction and increased neutrophil recruitment to infection sites after sepsis induction, contributing at least partly to increased immune responses in LOX-1 knockout mice. Our study results indicate that LOX-1 is an important mediator of inflammation and neutrophil dysfunction in sepsis.
INTRODUCTION
Sepsis often leads to multiorgan failure and death due to systemic inflammatory injury and immune suppression (7, 9). To launch efficient defense without causing inflammatory tissue damage or immune suppression, host responses must be tightly controlled. Molecular mechanisms of sepsis-induced inflammation and immune dysfunction remain largely unknown, hampering the development of therapeutics to treat this devastating disease.
LOX-1 (lectin-like oxidized low-density lipoprotein [oxLDL] receptor-1) is a membrane protein previously identified as an oxLDL receptor in endothelial cells (12). Further studies have demonstrated that LOX-1 is a multiligand receptor that also binds to C-reactive protein, apoptotic cells, bacteria, and activated platelets (12, 32, 36–38). LOX-1 has been reported to play diverse roles in proinflammatory signaling and endothelial dysfunction (12). LOX-1 expression and signaling contribute to the pathogenesis of vascular inflammation (12). NF-κB is a transcriptional regulator of proinflammatory mediators, including cytokines, chemokines, and adhesion molecules (13, 21). LOX-1 activation has been reported to induce NF-κB activation and increase the production of proinflammatory mediators TNF-α and IL-1 (5, 26, 39). The expression and function of LOX-1 in sepsis-induced inflammation have not been studied. In the present study, we employed LOX-1 knockout mice to assess the role of LOX-1 in modulating inflammatory responses in a murine model of polymicrobial sepsis.
Neutrophils are central components of the innate immune system that play a critical role in eliminating infectious agents (8, 23). However, overreactions of neutrophils may lead to tissue injury, exhaustion of neutrophil responses, and immune suppression in sepsis (1–3). Clinically, dysregulation of neutrophil function has been associated with increased mortality in sepsis patients (31, 40). Toll-like receptor (TLR) signaling is critical in neutrophil activation (4, 15). Nevertheless, neutrophil overreaction via TLR signaling is associated with inhibition of neutrophil function in sepsis (1–3). Indeed, TLR2 and TLR4 deletion has been shown to improve neutrophil chemotaxis and increase mouse survival in sepsis (1–3). Therefore, new therapeutic targets that help control neutrophil reaction could be beneficial for maintaining neutrophil function during sepsis. In the present report, we demonstrate that LOX-1 is also expressed in neutrophils and mediates sepsis-induced neutrophil dysfunction.
MATERIALS AND METHODS
Reagents.
Ultrapure lipopolysaccharide (LPS) (Escherichia coli 0111:B4) was obtained from InvivoGen (San Diego, CA). Mouse CXCL-2/MIP-2, phycoerythrin (PE)-conjugated rat anti-mouse LOX-1, and goat anti-mouse LOX-1 monoclonal antibodies were obtained from R&D Systems (Minneapolis, MN). Fluorescein isothiocyanate (FITC)–Gr-1 monoclonal antibody was obtained from eBioscience (San Diego, CA). Allophycocyanin (APC)-Ly6G, PE-Ly6G, Alexa Fluor 647-CXCR2, and FITC-CD11b antibodies were purchased from Biolegend (San Diego, CA). Phospo-p38 mitogen-activated protein kinase (MAPK) antibody was obtained from Cell Signaling (Danvers, MA).
Mice.
Mice were housed in cages with access to food and water in a temperature-controlled room with a 12-h dark/12-h light cycle. All experiments and animal care procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler. Generation of LOX-1 knockout mice has been described previously (29). Age- and sex-matched littermate mice were used in the studies. TLR4 knockout mice (TLR4−/−) and TLR2 knockout mice (TLR2−/−) were obtained from Jackson Laboratory (Bar Harbor, ME).
Sepsis model.
The cecal ligation and puncture (CLP) sepsis model was performed as described previously (30). Briefly, CLP surgery was performed on 7- to 10-week-old mice to induce sepsis. After the ligation, the cecum was punctured twice with a 20-gauge needle and gently squeezed to extrude a small amount of feces. For control animals, the same procedure was performed but without ligation and puncture of the cecum. For survival studies, mice were monitored every 12 h for 7 days after CLP surgery.
Neutrophil isolation, FACS analysis, bacterial CFU determinations, and ELISA.
Isolation of mouse neutrophils was performed as described previously (6, 14, 16). Neutrophils were >95% pure as determined by Hema-3 staining (Fisher Scientific, Pittsburgh, PA) and by Gr-1high expression analysis using flow cytometry. Fluorescence-activated cell sorter (FACS) analysis was conducted as described previously (14, 16). Bacterial CFU in the lung, blood, and peritoneal cavity were determined as described previously (11, 35). Concentrations of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) in plasma or lung homogenates were determined using enzyme-linked immunosorbent assay (ELISA) kits (Biolegend).
In vitro neutrophil phagocytosis, bactericidal, and chemotaxis assays.
In vitro neutrophil phagocytosis assays were performed as described previously (27, 44) using a Vybrant phagocytosis assay kit (Invitrogen). Briefly, neutrophils from LOX-1−/− or LOX-1+/+ littermates were isolated by density gradient centrifugation. Fluorescein-labeled E. coli particles were opsonized and incubated with neutrophils at 37°C for 1 h with gentle rotation in darkness. Cells were then washed with cold Hanks' balanced salt solution and incubated with 0.4% trypan blue to quench extracellular fluorescein-labeled bacteria. The amount of phagocytosed E. coli is expressed as the mean fluorescence intensity (MFI) of the intracellular fluorescein detected.
Neutrophil bactericidal assays were performed using E. coli as described previously (27, 44). Briefly, bacteria were opsonized with 50% pooled mouse serum and incubated with neutrophils isolated from LOX-1−/− or LOX-1+/+ littermates at a preoptimized ratio (1:1 [106/ml]) at 37°C for 1 h. Neutrophils were then lysed with water (pH 11). Serial dilutions were spread on LB agar plates and incubated at 37°C overnight. Data are expressed as numbers of E. coli CFU per plate.
Chemotaxis analysis was conducted using a 24-well plate with a transwell membrane (Corning) (5-μm pore size) as described previously (3). Briefly, neutrophils (1 × 105 cells in 100 μl of medium) were loaded on upper chamber and allowed to migrate toward medium alone or CXCL2 (30 ng/ml) in the lower chamber at 37°C with 5% CO2 for 1 h. The membrane was then fixed and stained. Neutrophils that migrated through the membrane were counted in at least 10 randomly selected fields under a light microscope.
Statistic analysis.
Data are expressed as means ± standard errors of the means (SEM). Student's t test was used for all studies unless otherwise stated. Survival curves were analyzed by log-rank tests using GraphPad Prism (GraphPad Software, San Diego, CA). Statistical significance was assigned to data with P values smaller than 0.05.
RESULTS
Increased survival of LOX-1 knockout mice in sepsis.
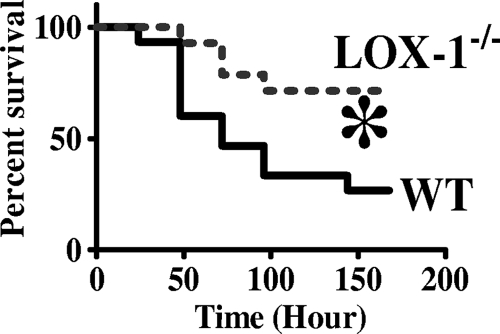
To investigate the potential role of LOX-1 in sepsis, we first examined the effect of LOX-1 deletion on sepsis-induced mortality after CLP surgery. LOX-1−/− mice exhibited a substantially higher survival rate than wild-type littermates (71% versus 27%) at 7 days after CLP (Fig. 1), suggesting that LOX-1 may modulate host responses and lead to increased death rates during sepsis.
Fig. 1.
Increased survival in LOX-1 knockout mice after the induction of sepsis. Mortality rates of wild-type mice (WT) and LOX-1 knockout mice (LOX-1−/−) were monitored after the induction of sepsis by CLP. Survival curves were analyzed by log-rank test. *, P < 0.02 versus WT (n = 15 mice per group).
LOX-1 knockout mice exhibited decreased inflammatory responses and lung injury after sepsis induction.
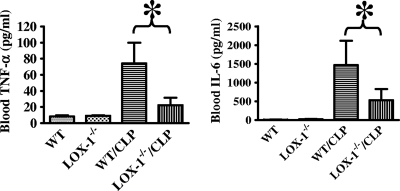
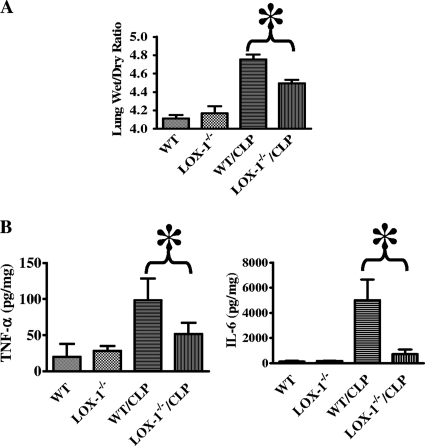
To assess the role of LOX-1 in systemic inflammatory responses in sepsis, we examined proinflammatory cytokine production in the blood of wild-type and LOX-1 knockout mice after CLP. Blood TNF-α and IL-6 production was significantly reduced in LOX-1 knockout mice after CLP compared with the results seen with wild-type littermates (Fig. 2). Inflammatory tissue injury is a major cause of death in sepsis (43). We examined lung inflammation and injury in wild-type and LOX-1 knockout mice after CLP. Our data showed that CLP-induced TNF-α and IL-6 production and lung wet/dry ratios were significantly lower for LOX-1 knockout mice than for wild-type littermates (Fig. 3).
Fig. 2.
LOX-1 deletion reduces inflammatory responses after the induction of sepsis. At 24 h after CLP, blood TNF-α and IL-1β levels in wild-type mice (WT) and LOX-1 knockout mice (LOX-1−/−) were assessed by ELISA. *, P < 0.01 (n = 7).
Fig. 3.
LOX-1 deletion attenuates lung inflammation and injury in sepsis. (A) At 24 h after CLP, lungs from LOX-1 knockout (LOX-1−/−) mice and wild-type (WT) mice were collected to examine wet/dry ratios. (B) Lung TNF-α and IL-6 levels were assessed by ELISA. *, P < 0.01 (n = 7).
LOX-1 deletion enhances bacterial clearance in sepsis.
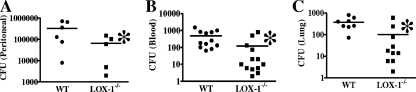
We also conducted experiments to investigate whether LOX-1 is involved in the regulation of host defense. We examined the effect of LOX-1 deletion on bacterial infection during sepsis. LOX-1 knockout mice had cecal flora similar to those seen with wild-type littermates when no surgery was performed (data not shown). Interestingly, LOX-1 knockout mice exhibited decreased bacterial CFU levels in the peritoneal cavity, blood, and lung after CLP (Fig. 4) compared with wild-type littermates, suggesting that LOX-1 deletion enhances bacterial clearance during sepsis.
Fig. 4.
LOX-1 deletion increases bacterial clearance in the peritoneal cavity, blood, and lung after the induction of sepsis. Bacterial CFU in mouse peritoneal cavity (A), blood (B), and lung (C) were examined in LOX-1 knockout (LOX-1−/−) mice and wild-type (WT) mice 24 h after CLP. *, P < 0.05 versus WT. The horizontal lines represent means.
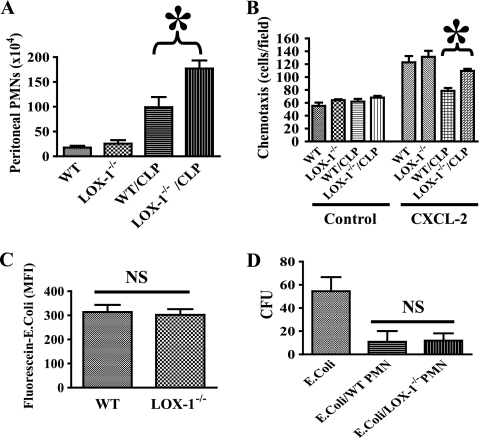
LOX-1 deletion increases neutrophil migration in sepsis.
Neutrophils are the most abundant leukocytes in circulation and play a critical role in host defense (2). Severe sepsis has been known to cause impaired neutrophil migration to the infectious focus (1–3), which leads to immune suppression and increased mortality. To assess whether LOX-1 modulates neutrophil responses during sepsis, we examined the effects of LOX-1 deletion on neutrophil function. Blood neutrophil counts in wild-type and LOX-1 knockout mice before the surgery were not significantly different (data not shown). We found that neutrophil counts in the peritoneal cavity of LOX-1−/− mice after CLP were significantly higher (Fig. 5 A) than in that of wild-type littermates. We then conducted in vitro experiments to further assess the effects of LOX-1 deletion on neutrophil chemotaxis, phagocytosis, and bactericidal activity. We demonstrated that neutrophils isolated from wild-type mice after CLP exhibited impaired chemotaxis with respect to CXCL2 (Fig. 5B), whereas neutrophils isolated from LOX-1 knockout mice were less affected. Neutrophil phagocytosis and bactericidal activity were not significantly altered by LOX-1 deletion (Fig. 5C and D). Our data suggest that LOX-1 signaling is involved in impaired neutrophil chemotaxis during sepsis.
Fig. 5.
Neutrophils in LOX-1 knockout mice exhibit increased peritoneal migration in sepsis. (A) At 6 h after CLP, peritoneal cells were collected. Neutrophil counts were determined by FACS analysis. Neutrophil numbers in the peritoneal cavity were significantly higher in LOX-1 knockout (LOX-1−/−) mice. *, P < 0.02 versus wild-type (WT)/CLP (n = 6). (B) Neutrophils were isolated from whole blood 2 h after CLP. Chemotaxis of neutrophils for CXCL2 was examined. *, P < 0.01. (C) For phagocytosis assays, FITC-labeled and heat-killed E. coli bacteria were incubated with blood neutrophils isolated from LOX-1 knockout (LOX-1−/−) or WT littermates. Following quenching of extracellular E. coli with trypan blue, uptake of E. coli was analyzed by FACS. NS, not significant (n = 8). (D) Bacterial killing by neutrophils isolated from LOX-1 knockout (LOX-1−/−) or WT littermates was determined. NS, not significant (n = 8). PMN, polymorphonuclear leukocytes.
LOX-1 is expressed in neutrophils.
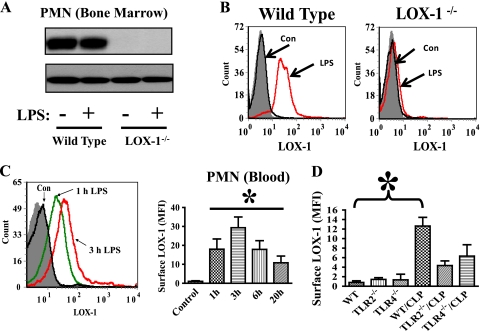
LOX-1 is a membrane protein with a single transmembrane domain (12). To explore the direct role of LOX-1 in neutrophil function, we examined whether LOX-1 is expressed in neutrophils. We detected high basal LOX-1 protein expression in neutrophils in wild-type mice (Fig. 6 A and B). Interestingly, LPS challenge did not increase LOX-1 total protein expression (Fig. 6A). However, LOX-1 surface (membrane) expression, which was shown by FACS to label only cell surface LOX-1, was quickly and markedly increased after the challenge by LPS (Fig. 6B and C). LOX-1 surface expression was also upregulated by the TLR2 agonist LTA (lipoteichoic acid), but the effect of LTA was less potent than that of LPS (data not shown). Our data indicate that LOX-1 is presynthesized and stored in neutrophils and can be quickly released from the intracellular store to the cell surface after LPS challenge. Neutrophil LOX-1 expression and the specificity of LOX-1 antibodies were further confirmed using LOX-1 knockout mice. No LOX-1 protein or surface expression was observed in LOX-1−/− neutrophils (Fig. 6A and B). We also assessed whether LOX-1 surface expression is modulated during sepsis. FACS analysis showed that LOX-1 surface expression was increased in neutrophils of wild-type mice after CLP surgery (Fig. 6D). TLR2−/− and TLR4−/− neutrophils exhibited lower LOX-1 surface expression than wild-type neutrophils after the induction of sepsis (Fig. 6B), suggesting that both TLR2 and TLR4 activation can mediate neutrophil LOX-1 surface expression during sepsis.
Fig. 6.
LOX-1 is expressed in neutrophils. (A) Wild-type mice were challenged with LPS (2.5 mg/kg of body weight by intraperitoneal injection). Immunoblotting analysis was performed to examine LOX-1 protein expression in isolated neutrophils (PMN) at 2 h after LPS challenge. No LOX-1 expression was detected in neutrophils of LOX-1−/− mice with or without LPS challenge. The data also showed that LOX-1 total protein expression in wild-type neutrophils was not affected by LPS challenge. (B) FACS was conducted to examine LOX-1 surface expression in blood neutrophils of LOX-1 knockout (LOX-1−/−) or wild-type littermate mice at 2 h after LPS challenge (2.5 mg/kg by intraperitoneal injection). Representative histograms of isotype IgG staining (filled gray) and LOX-1 staining of neutrophils from control mice (Con) and LPS-challenged mice (LPS) are shown. (C) Representative histograms of LOX-1 staining in neutrophils (Gr-1high blood cells) from control mice (Con [challenged by phosphate-buffered saline]), mice challenged by LPS for 1 h (1 h LPS) and 3 h (3 h LPS), or PE-isotype IgG control mice (filled gray). The bar graph shows neutrophil LOX-1 surface expression at different time points after LPS challenge. Data represent mean fluorescence intensity (MFI). MFI values were calculated by subtracting isotype control antibody staining from LOX-1 antibody staining. *, P < 0.05 versus control. (D) At 6 h after CLP, LOX-1 surface expression in blood neutrophils (Ly6G+) of wild-type (WT), TLR2 knockout (TLR2−/−), and TLR4 knockout (TLR4−/−) mice was analyzed by FACS. MFI values were calculated by subtracting isotype control antibody staining from LOX-1 antibody staining. *, P < 0.03 versus WT (n ≥ 6).
LOX-1 deletion inhibits neutrophil overreaction and CXCR2 downregulation in sepsis.
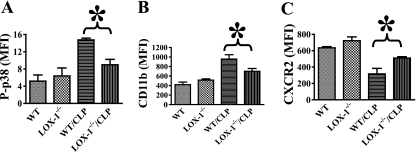
Our finding of LOX-1 expression in neutrophils implies that LOX-1 may possess a novel function in neutrophils. To test this possibility, we examined whether LOX-1 deletion could affect neutrophil activation after the induction of sepsis. CLP induced neutrophil activation in wild-type mice, as demonstrated by increased neutrophil p38 MAPK phosphorylation and CD11b surface expression. Interestingly, neutrophil p38 MAPK phosphorylation and CD11b surface expression during sepsis were significantly lower in LOX-1 knockout mice (Fig. 7 A and B), indicating that LOX-1 deletion may prevent neutrophil overreaction in sepsis.
Fig. 7.
LOX-1 deletion prevents p38 MAPK phosphorylation, CD11b surface expression, and CXCR2 downregulation after the induction of sepsis. At 2 h after CLP-induced sepsis, p38 MAPK phosphorylation (A) and CD11b (B) and CXCR2 (C) surface expression in blood neutrophils (Ly6G+) of wild-type (WT) and LOX-1−/− mice were examined by FACS. Data represent mean fluorescence intensity (MFI). MFI values were calculated by subtracting isotype control antibody staining from phosphorylated p38, CD11b, or CXCR2 antibody staining. *, P < 0.03 (n ≥ 4).
Chemokine receptor CXCR2 is a key mediator of neutrophil migration (2, 3). Neutrophil overreaction during severe sepsis has been known to induce CXCR2 downregulation and impaired neutrophil migration (1–3). We examined whether reduced neutrophil activation after the induction of sepsis in LOX-1 knockout mice could lead to improved CXCR2 surface expression. LOX-1 deletion prevented the downregulation of neutrophil CXCR2 surface expression during sepsis (Fig. 7C), which is consistent with the increased neutrophil migration and chemotaxis seen both in vivo and in vitro (Fig. 5), suggesting that LOX-1 deletion helps maintain a better CXCR2-mediated neutrophil response.
DISCUSSION
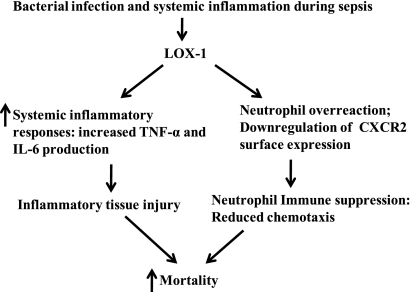
The results from our study indicate that LOX-1 signaling aggravates inflammatory responses and mediates immune suppression after the induction of sepsis (Fig. 8), likely contributing to the increased mortality seen in a mouse model of polymicrobial sepsis. Sepsis contains two pathophysiological phases, systemic inflammation and immune suppression (2, 10, 33). An ideal treatment for sepsis-induced multiorgan dysfunction would be able to prevent inflammatory tissue injury but maintain immune responses (2, 10, 33). Therefore, the finding that LOX-1 signaling modulates both the inflammatory and immune responses during sepsis is very intriguing.
Fig. 8.
Schematic presentation of the role of LOX-1 in sepsis. LOX-1 signaling aggravates inflammatory responses and causes neutrophil overreaction, which leads to increased inflammatory tissue injury and neutrophil suppression and contributes to high mortality in sepsis.
Production and upregulation of proinflammatory mediators such as TNF-α and IL-6 contribute to sepsis-induced inflammatory tissue injury (17, 20). In our studies, LOX-1 deletion prevented TNF-α and IL-6 production and lung injury in sepsis. The role of LOX-1 in the development of inflammation-driving cardiovascular diseases has been well established (24). LOX-1 blockade was able to prevent proinflammatory and prooxidant responses in endothelial cells and reduce atherogenesis in mouse atherosclerosis models (18, 45). LOX-1 signaling has been shown to mediate the production of proinflammatory cytokines TNF-α and IL-1 (39). Our studies suggest that LOX-1 also plays a role in sepsis-induced inflammatory responses.
Immune suppression has been a major problem for sepsis patients (2, 25, 33). It hampers bacterial clearance and causes further exaggeration of tissue damage. Immune suppression also makes sepsis patients more susceptible to secondary infection. A high mortality rate has been reported for sepsis patients with secondary bacterial pneumonia (2, 25, 33). Sepsis has been known to suppress neutrophil function, including chemotaxis and signaling (2, 3), likely due to overreaction and exhaustion of neutrophils (1–3). Our studies showed that LOX-1 deletion prevented neutrophil overreaction in sepsis and allowed better control of neutrophil responses during sepsis, leading to increased neutrophil recruitment at the sites of infection and increased bacterial clearance.
CXCR2 surface expression in neutrophils can regulate a wide variety of cellular responses, including respiratory burst, degranulation, integrin activation, and migration (2, 3, 41). Downregulation of CXCR2 surface expression due to neutrophil overreaction during sepsis leads to impaired neutrophil migration (1–3), which is a major factor in sepsis-induced neutrophil suppression. p38 MAPK activation has been reported to mediate CXCR2 downregulation in neutrophils (19, 22, 42). LOX-1 deletion prevented p38 MAPK activation in neutrophils, possibly contributing to the inhibition of CXCR2 downregulation and improved neutrophil migration in LOX-1 knockout mice during sepsis.
Neutrophil modulation appears to be involved in both inflammatory and immune dysfunction in sepsis (7, 25, 33, 34). Proinflammatory cytokines and chemokines induce neutrophil activation and migration to the sites of infection (34). On the other hand, neutrophil overreaction, impaired neutrophil chemotaxis, and altered Toll-like receptor (TLR) signaling are associated with immunosuppression in sepsis (1–3). Our present study specifically examined LOX-1 expression and function in neutrophils. However, since LOX-1 is also expressed in endothelial cells and macrophages (12, 28), we cannot exclude the possibility that LOX-1 deletion may also provide the observed beneficial effects for those cells. Apparently, more studies are required to characterize the function of LOX-1 in other cell types during sepsis. Nevertheless, our studies indicate that better-controlled neutrophil responses in LOX-1 knockout mice contribute, at least in part, to the improved immune responses in sepsis. Further studies are warranted to explore the underlying mechanisms. For example, identifying potential ligands and receptors that may interact with LOX-1 in neutrophils could advance our current knowledge of neutrophil function and the pathogenesis of sepsis.
Footnotes
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Alves-Filho J. C., de Freitas A., Russo M., Cunha F. Q. 2006. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit. Care Med. 34:461–470 [DOI] [PubMed] [Google Scholar]
- 2. Alves-Filho J. C., de Freitas A., Spiller F., Souto F. O., Cunha F. Q. 2008. The role of neutrophils in severe sepsis. Shock 30(Suppl. 1):3–9 [DOI] [PubMed] [Google Scholar]
- 3. Alves-Filho J. C., et al. 2009. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc. Natl. Acad. Sci. U. S. A. 106:4018–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aomatsu K., et al. 2008. Toll-like receptor agonists stimulate human neutrophil migration via activation of mitogen-activated protein kinases. Immunology 123:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Apte R. N., et al. 2006. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 25:387–408 [DOI] [PubMed] [Google Scholar]
- 6. Boxio R., Bossenmeyer-Pourie C., Steinckwich N., Dournon C., Nusse O. 2004. Mouse bone marrow contains large numbers of functionally competent neutrophils. J. Leukoc. Biol. 75:604–611 [DOI] [PubMed] [Google Scholar]
- 7. Buras J. A., Holzmann B., Sitkovsky M. 2005. Animal models of sepsis: setting the stage. Nat. Rev. Drug Discov. 4:854–865 [DOI] [PubMed] [Google Scholar]
- 8. Craig A., Mai J., Cai S., Jeyaseelan S. 2009. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect. Immun. 77:568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Decker T. 2004. Sepsis: avoiding its deadly toll. J. Clin. Invest. 113:1387–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Jong H. K., van der Poll T., Wiersinga W. J. 2010. The systemic pro-inflammatory response in sepsis. J. Innate Immun. 2:422–430 [DOI] [PubMed] [Google Scholar]
- 11. Deng J. C., et al. 2006. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J. Clin. Invest. 116:2532–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn S., et al. 2008. The lectin-like oxidized low-density-lipoprotein receptor: a pro-inflammatory factor in vascular disease. Biochem. J. 409:349–355 [DOI] [PubMed] [Google Scholar]
- 13. Everhart M. B., et al. 2006. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J. Immunol. 176:4995–5005 [DOI] [PubMed] [Google Scholar]
- 14. Fu J., Naren A. P., Gao X., Ahmmed G. U., Malik A. B. 2005. Protease-activated receptor-1 activation of endothelial cells induces protein kinase Calpha-dependent phosphorylation of syntaxin 4 and Munc18c: role in signaling p-selectin expression. J. Biol. Chem. 280:3178–3184 [DOI] [PubMed] [Google Scholar]
- 15. Gao H., Leaver S. K., Burke-Gaffney A., Finney S. J. 2008. Severe sepsis and Toll-like receptors. Semin. Immunopathol. 30:29–40 [DOI] [PubMed] [Google Scholar]
- 16. Gao X. P., et al. 2007. Blockade of class IA phosphoinositide 3-kinase in neutrophils prevents NADPH oxidase activation- and adhesion-dependent inflammation. J. Biol. Chem. 282:6116–6125 [DOI] [PubMed] [Google Scholar]
- 17. Goodman R. B., Pugin J., Lee J. S., Matthay M. A. 2003. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 14:523–535 [DOI] [PubMed] [Google Scholar]
- 18. Hu C., et al. 2007. LOX-1 deletion alters signals of myocardial remodeling immediately after ischemia-reperfusion. Cardiovasc. Res. 76:292–302 [DOI] [PubMed] [Google Scholar]
- 19. Juffermans N. P., et al. 2000. Expression of the chemokine receptors CXCR1 and CXCR2 on granulocytes in human endotoxemia and tuberculosis: involvement of the p38 mitogen-activated protein kinase pathway. J. Infect. Dis. 182:888–894 [DOI] [PubMed] [Google Scholar]
- 20. Kamochi M., et al. 1999. P-selectin and ICAM-1 mediate endotoxin-induced neutrophil recruitment and injury to the lung and liver. Am. J. Physiol. 277:L310–L319 [DOI] [PubMed] [Google Scholar]
- 21. Kang J. L., et al. 2001. Genistein prevents nuclear factor-kappa B activation and acute lung injury induced by lipopolysaccharide. Am. J. Respir. Crit. Care Med. 164:2206–2212 [DOI] [PubMed] [Google Scholar]
- 22. Khandaker M. H., et al. 1998. CXCR1 and CXCR2 are rapidly down-modulated by bacterial endotoxin through a unique agonist-independent, tyrosine kinase-dependent mechanism. J. Immunol. 161:1930–1938 [PubMed] [Google Scholar]
- 23. Koh A. Y., Priebe G. P., Ray C., Van Rooijen N., Pier G. B. 2009. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect. Immun. 77:5300–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li D., et al. 2002. Statins modulate oxidized low-density lipoprotein-mediated adhesion molecule expression in human coronary artery endothelial cells: role of LOX-1. J. Pharmacol. Exp. Ther. 302:601–605 [DOI] [PubMed] [Google Scholar]
- 25. Lyn-Kew K., Standiford T. J. 2008. Immunosuppression in sepsis. Curr. Pharm. Des. 14:1870–1881 [DOI] [PubMed] [Google Scholar]
- 26. Matsunaga T., Hokari S., Koyama I., Harada T., Komoda T. 2003. NF-kappa B activation in endothelial cells treated with oxidized high-density lipoprotein. Biochem. Biophys. Res. Commun. 303:313–319 [DOI] [PubMed] [Google Scholar]
- 27. McGovern N. N., et al. 2011. Hypoxia selectively inhibits respiratory burst activity and killing of Staphylococcus aureus in human neutrophils. J. Immunol. 186:453–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehta J. L., Chen J., Hermonat P. L., Romeo F., Novelli G. 2006. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc. Res. 69:36–45 [DOI] [PubMed] [Google Scholar]
- 29. Mehta J. L., et al. 2007. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ. Res. 100:1634–1642 [DOI] [PubMed] [Google Scholar]
- 30. Moreno S. E., et al. 2006. IL-12, but not IL-18, is critical to neutrophil activation and resistance to polymicrobial sepsis induced by cecal ligation and puncture. J. Immunol. 177:3218–3224 [DOI] [PubMed] [Google Scholar]
- 31. Muller Kobold A. C., et al. 2000. Leukocyte activation in sepsis; correlations with disease state and mortality. Intensive Care Med. 26:883–892 [DOI] [PubMed] [Google Scholar]
- 32. Oka K., et al. 1998. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 95:9535–9540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reddy R. C., Chen G. H., Tekchandani P. K., Standiford T. J. 2001. Sepsis-induced immunosuppression: from bad to worse. Immunol. Res. 24:273–287 [DOI] [PubMed] [Google Scholar]
- 34. Reddy R. C., Standiford T. J. 2010. Effects of sepsis on neutrophil chemotaxis. Curr. Opin. Hematol. 17:18–24 [DOI] [PubMed] [Google Scholar]
- 35. Renckens R., et al. 2006. Matrix metalloproteinase-9 deficiency impairs host defense against abdominal sepsis. J. Immunol. 176:3735–3741 [DOI] [PubMed] [Google Scholar]
- 36. Shih H. H., et al. 2009. CRP is a novel ligand for the oxidized LDL receptor LOX-1. Am. J. Physiol. Heart Circ. Physiol. 296:H1643–H1650 [DOI] [PubMed] [Google Scholar]
- 37. Shimaoka T., et al. 2001. Lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) supports cell adhesion to fibronectin. FEBS Lett. 504:65–68 [DOI] [PubMed] [Google Scholar]
- 38. Shimaoka T., et al. 2001. LOX-1 supports adhesion of Gram-positive and Gram-negative bacteria. J. Immunol. 166:5108–5114 [DOI] [PubMed] [Google Scholar]
- 39. Shin H. K., Kim Y. K., Kim K. Y., Lee J. H., Hong K. W. 2004. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation 109:1022–1028 [DOI] [PubMed] [Google Scholar]
- 40. Tavares-Murta B. M., et al. 2002. Failure of neutrophil chemotactic function in septic patients. Crit. Care Med. 30:1056–1061 [DOI] [PubMed] [Google Scholar]
- 41. Tsai W. C., et al. 2000. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 68:4289–4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van den Blink B., et al. 2004. P38 mitogen activated protein kinase is involved in the downregulation of granulocyte CXC chemokine receptors 1 and 2 during human endotoxemia. J. Clin. Immunol. 24:37–41 [DOI] [PubMed] [Google Scholar]
- 43. van der Poll T., Meijers J. C. 2010. Systemic inflammatory response syndrome and compensatory anti-inflammatory response syndrome in sepsis. J. Innate Immun. 2:379–380 [DOI] [PubMed] [Google Scholar]
- 44. Van Ziffle J. A., Lowell C. A. 2009. Neutrophil-specific deletion of Syk kinase results in reduced host defense to bacterial infection. Blood 114:4871–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu X., Gao X., Potter B. J., Cao J. M., Zhang C. 2007. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler. Thromb. Vasc. Biol. 27:871–877 [DOI] [PubMed] [Google Scholar]