Abstract
Genetic lesions in the polyamine biosynthetic pathway of Leishmania donovani, the causal agent of visceral leishmaniasis, are conditionally lethal mutations that render the insect vector form of the parasite auxotrophic for polyamines. Recently, we have demonstrated that a Δodc L. donovani null mutant lacking ornithine decarboxylase (ODC), the rate-limiting enzyme in polyamine biosynthesis, was profoundly compromised in its ability to infect mice, indicating that ODC is essential for the infectious mammalian stage of the parasite and further validating the enzyme as a possible drug target. To assess whether other components of the polyamine biosynthetic pathway were also essential for parasite virulence, a cell line deficient in spermidine synthase (SPDSYN), the enzyme that converts putrescine to spermidine, was created by double-targeted gene replacement within a virulent L. donovani background. This Δspdsyn strain was auxotrophic for polyamines, required spermidine for growth in its insect vector form, and was adversely impacted in its ability to infect mice. These findings establish that SPDSYN, like ODC, is essential for maintaining a robust infection in mammals and indicate that pharmacologic inhibition of SPDSYN, and perhaps all components of the polyamine biosynthetic pathway, is a valid therapeutic strategy for the treatment of visceral and, potentially, other forms of leishmaniasis.
INTRODUCTION
Leishmania donovani, a digenetic protozoan parasite, is the etiological agent of visceral leishmaniasis, a devastating and invariably fatal disease when left untreated. This unicellular eukaryote lives as the extracellular, flagellated promastigote within its insect vector, the phlebotomine sandfly, and resides as the intracellular, aflagellar amastigote within the phagolysosomes of infected macrophages and other reticuloendothelial cells of the mammalian host. There is no reliable vaccine for leishmaniasis, and, consequently, drugs are the only avenue by which the disease can be treated. Regrettably, the existing collection of antileishmanial drugs is far from ideal, primarily due to their lack of selectivity toward the metabolic machinery of the parasite. Drug toxicity, as well as increased drug resistance (24, 40), reinforces the need to develop new therapeutics and to identify and validate new drug targets.
One metabolic pathway that has drawn considerable attention in proposing new antiparasitic therapies is that for the synthesis of polyamines, ubiquitous aliphatic cations (putrescine, spermidine, spermine) that are known to play critical roles in a variety of fundamental cellular processes such as growth, differentiation, and macromolecular synthesis (5, 32, 33, 57). Polyamines also act as precursors for the production of trypanothione, a molecule present in trypanosomatids that is involved in combating oxidative stress (21). The root of this interest in the polyamine biosynthesis pathway originates from the observation that dl-α-difluoromethylornithine (DFMO) has proven effective in curing West African sleeping sickness caused by Trypanosoma brucei gambiense, a protozoan parasite phylogenetically similar to Leishmania (4, 14, 20, 56, 59). DFMO is a suicide inhibitor of ornithine decarboxylase (ODC), the enzyme that catalyzes the conversion of ornithine to putrescine (39). This drug is also effective at killing other genera of protozoan parasites in vitro (1, 9, 27, 49), including Leishmania promastigotes (35, 37, 41, 49, 55), and markedly ameliorates but does not eliminate short-term L. donovani infections in mice (30, 37, 45) and hamsters (42). In addition, inhibitors of a second enzyme in the polyamine pathway, S-adenosylmethionine decarboxylase (ADOMETDC), the enzyme that produces the decarboxylated S-adenosylmethionine substrate for the spermidine synthase (SPDSYN) reaction, are also effectual antitrypanosomal agents (2, 3, 6, 8, 9, 16, 18, 58).
The polyamine biosynthetic pathway of Leishmania is comprised of four enzymes: arginase (ARG), ODC, ADOMETDC, and SPDSYN. The genes encoding all four enzymes have been cloned, and conditionally lethal gene knockouts have been created in L. mexicana and L. major (Δarg mutant only) (23, 43, 48, 52) and in L. donovani (Δodc, Δspdsyn, and Δadometdc mutants) (12, 34, 50, 51) via double-targeted gene replacement. Growth studies with the mutant promastigotes revealed that each knockout was auxotrophic for polyamines as a consequence of the gene deletion events and that this nutritional deficiency could be circumvented by propagation in medium supplemented with an appropriate source of polyamine or polyamine precursor. Thus, an intact polyamine biosynthetic pathway is essential for the viability and growth of the promastigote stage of the Leishmania parasite.
Despite the extensive genetic and biochemical characterization of the null mutants in their promastigote stage, only recently have polyamine gene functions been assessed in the infectious amastigote stage of the parasite. The evaluation of polyamine gene function in amastigotes has also been hampered by the fact that the initially characterized Δodc, Δadometdc, and Δspdsyn L. donovani knockouts were created within an attenuated wild-type strain of L. donovani that had lost its capacity to infect macrophages or rodents, therefore precluding functional evaluation of these genes as virulence determinants (34, 50, 51). Thus, an effort to reconstruct the genetic lesions in polyamine biosynthesis genes in a virulent L. donovani background was initiated.
Creation of a Δodc lesion in a wild-type clone of this virulent strain dramatically compromised the ability of the parasite to infect mouse livers and spleens (12). Moreover, episomal complementation of the chromosomal lesion in ODC restored parasite burdens in infected organs to near wild-type levels, proving that the virulence defect in the Δodc knockout was triggered by the polyamine gene deletion event (12). Recent studies also found that exogenous administration of putrescine to mice infected with Δodc L. donovani partially restored virulence in the mutant parasites (45), further substantiating that the virulence defect in the Δodc knockout is due to a lack of polyamine biosynthetic capacity and not to a secondary unknown function of ODC. The fact that a defect in the ODC gene so profoundly compromises the virulence of L. donovani suggests that other enzymes in the polyamine biosynthesis pathway might be critical virulence determinants as well.
To address the fundamental question of whether other components of the polyamine biosynthesis pathway are essential for L. donovani to trigger an infection in mammals, we have recapitulated the Δspdsyn genotype within a virulent strain of L. donovani and evaluated the capacity of the Δspdsyn mutant to infect mice. Here we report that parasite burdens in livers and spleens of mice infected with the knockout parasites are significantly lower than those in livers and spleens of mice infected with the wild-type strain. These studies demonstrate that L. donovani amastigotes require SPDSYN activity to sustain a robust infection in mice and indicate that SPDSYN and perhaps all of the enzymes in the polyamine pathway are realistic therapeutic targets for the treatment of leishmaniasis.
MATERIALS AND METHODS
Parasite cell culture.
Parasites used in this study were derived from the wild-type LdBob strain of L. donovani (29) obtained from Stephen M. Beverley (Washington University, St. Louis, MO). LdBob promastigotes were routinely cultivated at 26°C, pH 6.9, in previously described medium (29) with 10% chicken serum or 5% Serum Plus (Gibco Cell Culture, Carlsbad, CA). Wild-type parasites were cultured in the absence of supplementation, while the Δspdsyn mutants were supplemented either with 100 μM spermidine alone or with 100 μM spermidine to which 50 μg/ml hygromycin and 20 μM puromycin were added to maintain appropriate selective pressure. The “add-back” strain, Δspdsyn1(pXG-BSD-SPDSYN), was routinely propagated in 20 μg/ml blasticidin to maintain the multicopy episomal plasmid containing the full-length SPDSYN gene.
Construction of Δspdsyn knockouts.
The cloning and sequencing of SPDSYN and its adjacent flanking regions from an L. donovani cosmid library have been reported previously (50). The SPDSYN/spdsyn heterozygotes were constructed using the same drug resistance cassette, pX63-HYG-Δspdsyn, employed in the derivation of the Δspdsyn mutants in the previously described avirulent DI700 strain of L. donovani (50). The Δspdsyn null mutants were created using the drug resistance cassette pX63-PAC-Δspdsyn, which was constructed using the same methods used to construct pX63-HYG-Δspdsyn (50). These targeting vectors contain the hygromycin phosphotransferase and puromycin N-acetyltransferase markers flanked by sequences derived from the 5′ and 3′ untranslated regions of SPDSYN. Linear targeting DNAs were excised from plasmid by restriction digest with HindIII and BglII and gel purified prior to transfection as described previously (34). Wild-type L. donovani promastigotes were transfected via electroporation with linearized pX63-HYG-Δspdsyn according to standard protocols using a GenePulser XCell (Bio-Rad) system and previously described parameters (54). Transfected parasites were plated on semisolid agar containing 50 μg/ml hygromycin, colonies were expanded, and homologous recombination was confirmed by Southern blotting. One SPDSYN/spdsyn heterozygote clone was then subjected to a second round of transfection using the excised targeting construct from pX63-PAC-Δspdsyn and plated on semisolid agar containing 50 μg/ml hygromycin, 20 μM puromycin, and 100 μM spermidine. Colonies were expanded, and Southern blot analysis was employed to confirm the deletion of both wild-type SPDSYN alleles. Two independent Δspdsyn clones, Δspdsyn1 and Δspdsyn2, were chosen for further analysis.
Creation of Δspdsyn(pSPDSYN) add-back parasites.
The Δspdsyn1-knockout line was functionally complemented by transfecting a chimeric plasmid containing the L. donovani SPDSYN open reading frame ligated into the pXG-BSD leishmanial expression plasmid (28). The add-back line was selected in medium containing 20 μg/ml blasticidin and lacking polyamine and was designated Δspdsyn1(pXG-BSD-SPDSYN) according to the generally accepted genetic nomenclature for Leishmania (17).
Southern and Western blot analyses.
For Southern blot analysis, genomic DNA from logarithmic-growth-phase wild-type and Δspdsyn parasites was prepared using a DNeasy kit (Qiagen Inc.) according to the manufacturer's protocol. Genomic DNA was digested with either SalI and probed with a 1.0-kb fragment of the SPDSYN coding region or digested with XhoI and probed with a 1.2-kb fragment of the SPDSYN 5′ flanking region as described previously (50).
For Western blot analysis, parasite lysates were prepared from logarithmic-phase wild-type, Δspdsyn, and Δspdsyn1(pXG-BSD-SPDSYN) cell lines and were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (38) and blotted onto Immun-Blot polyvinylidene difluoride membranes (Bio-Rad). The membranes were probed with monospecific polyclonal antibody raised to the purified L. donovani SPDSYN (50, 53) or with commercially available anti-α-tubulin mouse monoclonal antibody (DM1A) (Calbiochem).
Growth phenotypes of Δspdsyn promastigotes.
Wild-type, Δspdsyn1, and Δspdsyn1(pXG-BSD-SPDSYN) promastigotes were inoculated at a density of 5 × 104 cells/ml into growth medium containing 5% Serum Plus and appropriate drug selections. Cultures were maintained at 26°C with 5% CO2. Δspdsyn1 parasites were starved of exogenous polyamines 2 days prior to growth phenotyping, and upon inoculation, 100 μM spermidine or 100 μM putrescine was added as appropriate. Parasites were enumerated after 4 days by hemacytometer. The inoculation density was subtracted from the final cell density, as cell growth and multiplication were of primary interest.
Infectivity studies with mice.
Groups of five 6-week-old female BALB/c mice (Charles River Laboratories) were each inoculated by tail vein injection with 5 × 106 of either wild-type, Δspdsyn1, or Δspdsyn1(pXG-BSD-SPDSYN) stationary-phase promastigotes grown in medium containing 10% chicken serum. Prior to the mouse injections, each of the three strains were passaged through mice for 10 days in order to eliminate parasites that had become attenuated in response to prolonged culture. At 4 weeks postinjection, livers and spleens were harvested and passaged through 70-μm-mesh-size cell strainers to create single-cell suspensions. To quantify parasite loads, limiting dilution assays were performed as described previously (13). Serial 4-fold dilutions of liver and spleen homogenates in 96-well microtiter plates were cultivated at 26°C with 5% CO2 in medium containing 5% Serum Plus with 100 μM spermidine supplementation for the Δspdsyn strain. Growth in individual wells was assessed after 3 weeks by visual inspection, and graphs displaying mean parasite burdens and standard deviations from five animals were constructed. Note that all mouse studies were carried out in compliance with guidelines established by the Institutional Animal Care and Use Committee at Oregon Health and Science University.
RESULTS
Molecular characterization of Δspdsyn lines in LdBob L. donovani.
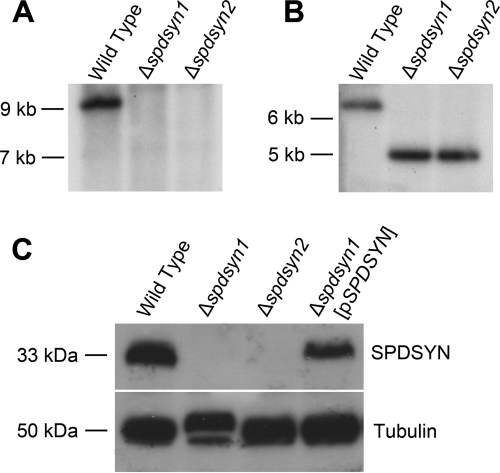
Although Δspdsyn L. donovani promastigotes have been described previously, the genetic lesion was created within an avirulent strain that precluded ascertaining whether SPDSYN was an indispensable virulence determinant (50). Thus, the Δspdsyn lesion was reconstructed in LdBob, a virulent L. donovani strain that is capable of establishing robust visceral infections in mice (11, 12, 19, 29). Two independent Δspdsyn clones were isolated after double-targeted gene replacement and designated Δspdsyn1 and Δspdsyn2. In order to authenticate the deletion of both SPDSYN alleles, Southern blot analysis was performed on genomic DNA prepared from wild-type, Δspdsyn1, and Δspdsyn2 parasites using probes to the SPDSYN gene coding region (Fig. 1A and B). Ethidium bromide staining confirmed equal loading of chromosomal DNA, and the Southern analysis demonstrated the expected genetic lesion. To further substantiate the gene knockout, expression of the SPDSYN protein was examined by Western blotting in the same three cell lines as well as an add-back line, Δspdsyn1(pXG-BSD-SPDSYN) [Δspdsyn1(pSPDSYN)] consisting of the Δspdsyn1 mutant with a multicopy episome containing the SPDSYN open reading frame (Fig. 1C). Polyclonal antisera specific for SPDSYN recognized the expected ∼33-kDa protein corresponding to the predicted SPDSYN translation product (50) in both wild-type and Δspdsyn1(pSPDSYN) lysates, while this band was not detected in extracts prepared from Δspdsyn1 and Δspdsyn2 lysates (Fig. 1C). The blot was also probed with antitubulin antibody as a loading control.
Fig. 1.
(A and B) Molecular characterization of the SPDSYN locus and SPDSYN expression in the Δspdsyn knockouts. Two micrograms of genomic DNA from wild-type, Δspdsyn1, or Δspdsyn2 parasites was digested with SalI and hybridized to a 1.0-kb fragment of the SPDSYN coding region (A) or XhoI and hybridized to a 1.2-kb fragment of the 5′ flanking region (B). Molecular size markers are indicated, and equal loading of DNA was verified via ethidium bromide staining. (C) For protein expression analysis, protein lysates from 1.0 × 106 wild-type, Δspdsyn1, Δspdsyn2, and Δspdsyn1(pXG-BSD-SPDSYN) [Δspdsyn1(pSPDSYN)] promastigotes were fractionated by SDS-PAGE, blotted, and probed with polyclonal antibodies against L. donovani SPDSYN. Tubulin expression was analyzed as a loading control.
Δspdsyn parasites are polyamine auxotrophs.
A growth assay was performed to determine the reliance of the Δspdsyn knockout cell lines on polyamines, and as anticipated, Δspdsyn1 promastigotes were auxotrophic for polyamines. While wild-type parasites had undergone approximately 10 to 11 cell doublings during the course of the growth experiment, the mutant cell line was unable to proliferate in polyamine-deficient medium (Fig. 2). Visual inspection of the Δspdsyn1 promastigotes incubated under these nonpermissive conditions at the end of the experiment revealed a few surviving parasites that were misshapen and lethargic. These parasites did not recover when maintained for longer periods of time under nonpermissive conditions. The polyamine auxotrophy of the Δspdsyn1 null mutant was circumvented genetically in the Δspdsyn1(pSPDSYN) promastigotes by complementation with the covering SPDSYN plasmid or nutritionally by supplementation of the culture medium with spermidine (Fig. 2). The polyamine auxotrophy could not, however, be bypassed by the addition of putrescine, the product of ODC and a substrate of SPDSYN (Fig. 2). The Δspdsyn2 clone displayed a growth phenotype identical to that of Δspdsyn1 but was not examined in as great of detail (data not shown).
Fig. 2.
Growth phenotype of Δspdsyn promastigotes. Wild-type, Δspdsyn1, and Δspdsyn1(pXG-BSD-SPDSYN) [Δspdsyn1(pSPDSYN)] promastigotes were incubated in their respective growth media in the absence or presence of 100 μM spermidine or 100 μM putrescine. Δspdsyn1 parasites were starved of exogenous polyamines 2 days prior to the start of the growth assay. Parasites were inoculated at 5 × 104 cells/ml, maintained at 26°C with 5% CO2, and enumerated after 4 days. Reported cell densities represent final cell densities minus inoculation densities. Final cell densities of Δspdsyn1 parasites grown under nonpermissive conditions were significantly lower than those of the initial inocula, and thus, growth was zero.
Δspdsyn L. donovani has reduced virulence in vivo.
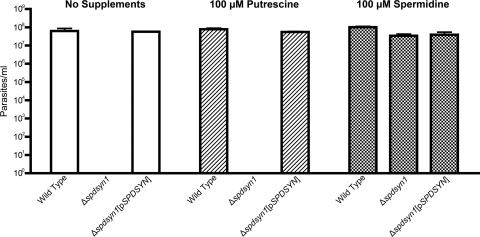
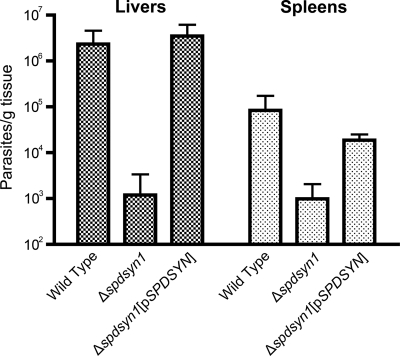
Virulence studies in BALB/c mice were carried out to determine the effects of a genetic deficiency of SPDSYN and to further characterize the SPDSYN enzyme as a potential therapeutic target. Groups of five BALB/c mice were inoculated with either wild-type, Δspdsyn1, or Δspdsyn1(pSPDSYN) promastigotes, and after 4 weeks parasite loads were quantified in livers and spleens, the two organs most affected by a visceralizing Leishmania infection. The ability of the Δspdsyn1-knockout parasites to infect mice was severely compromised (Fig. 3). The average parasite burdens in livers and spleens of mice infected with wild-type parasites were 3 and 2 orders of magnitude higher, respectively, than those in livers and spleens of mice infected with Δspdsyn parasites. The virulence defect of the knockout lines was rescued by episomal complementation with parasitemias in livers and spleens of Δspdsyn1(pSPDSYN)-infected mice equivalent to those obtained in mice inoculated with wild-type parasites (Fig. 3). It should be noted that the discrepancy in the absolute numbers of wild-type parasites between livers and spleens on a per-gram basis is typical for L. donovani infections (10, 22, 36, 44).
Fig. 3.
Parasite burdens in mice. Three separate cohorts of BALB/c mice were infected with either wild-type, Δspdsyn1, or Δspdsyn1(pXG-BSD-SPDSYN) [Δspdsyn1(pSPDSYN)] stationary-phase promastigotes as described in Materials and Methods. Mice were killed after 4 weeks, and parasite loads in liver and spleen preparations were determined by limiting dilution. The experiment was repeated three times with similar conclusions.
DISCUSSION
Previous studies with Leishmania null mutants have established that each component of the polyamine biosynthetic pathway, ARG, ODC, ADOMETDC, and SPDSYN, is indispensable for the viability and growth of the promastigote stage of the parasite (34, 48, 50–52). Whether all of these enzymes are also essential for the amastigote to maintain an infection is less clear, because virulence data with knockout strains from several species have not offered a consistent conclusion. Parasite burdens and lesion sizes of mice infected with Δarg L. mexicana (23) or Δarg L. major (43, 48) were only somewhat lower than those of mice infected with the corresponding wild-type line from which these mutants were derived. In contrast, parasite loads in mice infected with Δodc L. donovani (12) were dramatically reduced by many orders of magnitude compared to those in mice inoculated with the wild-type progenitor. Whether the differences in the impact of the genetic lesions can be ascribed to the nature of the genetic lesion, to species differences, or to differences in the cutaneous and visceral environments in which the species reside is unknown. Resolution of this fundamental question requires the evaluation of mutations of the same polyamine pathway gene in the different Leishmania species, i.e., a comparison of a Δodc mutation in L. donovani, L. mexicana, and L. major.
To establish whether null mutations in the L. donovani polyamine biosynthetic pathway other than ODC could also affect the virulence of the organism, a null mutant at the SPDSYN locus was created in a virulent wild-type background. The Δspdsyn mutation conferred polyamine auxotrophy (Fig. 2) and caused a substantial reduction in parasite burdens in mice compared to the wild type (∼3 orders of magnitude reduction in livers and 2 orders of magnitude reduction in spleens) (Fig. 3), although the extent of the virulence defect in mice was less than that previously observed for Δodc L. donovani (12).
It should be noted that livers and spleens from mice inoculated with Δspdsyn parasites retained a small population of persistent parasites, ∼103 per liver and spleen, after the 4-week infectivity experiment (Fig. 3). The mechanism of this persistence is unclear and is difficult to analyze at the molecular level since the persistent parasites can be resurrected only under permissive growth conditions in vitro (Fig. 2). One could speculate that this persistence is analogous to the situation of dying but enduring Δspdsyn parasites observed under nonpermissive growth conditions in vitro (Fig. 2) and infer that it takes >4 weeks to kill amastigotes by polyamine starvation in situ. However, whether these persistent Δspdsyn amastigotes would eventually expire is difficult to assess because BALB/c mice eventually clear L. donovani parasites, with parasitemias beginning to decrease at 4 to 8 weeks postinfection (60). A more suitable model for assessing long-term virulence of this parasite is the Syrian golden hamster, a rodent capable of mimicking the human form of visceral leishmaniasis (22). Testing Δspdsyn virulence in hamsters is a logical next step in SPDSYN target validation.
The reason for the discrepancy in the virulence deficit triggered by the Δodc and Δspdsyn lesions in an otherwise isogenic background is not known but could be ascribed to differences in the putrescine and spermidine contents of the phagolysosome in which visceral macrophages reside. It is likely that L. donovani amastigotes require both putrescine and spermidine for optimal proliferation, as has been shown for promastigotes (34). If, for example, the phagolysosomal environment contains pools of spermidine but not putrescine, then a Δodc lesion would be expected to have far more deleterious consequences to the parasite than a Δspdsyn defect, because L. donovani lacks a back-conversion pathway from spermidine to putrescine but can readily synthesize spermidine from putrescine through ODC. The conjecture that the putrescine and spermidine pools of the phagolysosome are different can be further explored by determining the virulence properties of Δadometdc L. donovani parasites, which, like Δspdsyn parasites, require spermidine but not putrescine for survival (51).
It should be noted that Δodc L. donovani promastigotes are much more susceptible to putrescine withdrawal than Δspdsyn L. donovani promastigotes are to spermidine withdrawal (personal observations). This intriguing observation is mirrored in Trypanosoma brucei, where genetic investigations showed that depletion of putrescine results in a more rapid cell death than spermidine depletion (62). Although the cellular functions of polyamines are not completely understood, it appears to be evident that putrescine is more than just a precursor for the production of spermidine, which could account for the discrepancy in the magnitude of the virulence deficit caused by the Δodc and Δspdsyn lesions.
Another explanation for the Δodc and Δspdsyn virulence discrepancy is the possibility that the amastigote form of the parasite exhibits different capabilities in transport of putrescine versus spermidine. Leishmania promastigotes express robust polyamine transport activities (7), which can account for the ability of putrescine to rescue Δodc parasites (12) and spermidine to enable Δspdsyn parasite growth (Fig. 2), but less is known about transport in the amastigote stage. It should be noted that the only leishmanial polyamine transporter that has been identified at the molecular level is enormously downregulated in the amastigote stage of the parasite (31). However, axenic amastigotes of Δodc L. donovani were able to proliferate in 200 μM putrescine (12), and Δspdsyn L. donovani axenic amastigotes survive in 200 μM spermidine (personal observation), suggesting that the polyamine transport machinery remains somewhat functional in the infectious stage of the parasite, but the extent is unknown. The crippling effects of Δodc or Δspdsyn mutations on the establishment of virulence by L. donovani, together with the at least minimally functional polyamine transport activities in the amastigote stage, suggest that the phagolysosomal compartment in which the parasite resides does not provide sufficient exogenous polyamine to bypass a genetic defect in polyamine biosynthesis.
A pairwise alignment of the L. donovani and human SPDSYN primary structures revealed an ∼56% identity (50), and the amino acids in the human crystal structure that make contact with the substrate (61) are conserved in the parasite enzyme. However, similarities in the active site or ligand-binding pockets of the L. donovani and human SPDSYNs do not preclude this enzyme from potential drug targeting. For example, DFMO, the specific ODC inhibitor used in West African sleeping sickness chemotherapy, displays selectivity for the metabolic machinery of T. brucei due to discrepant stabilities of the parasite and human ODC enzymes (25, 26, 47) and not to disparities in the affinity of DFMO for the two proteins (46). Furthermore, differential susceptibility to potential inhibitors could be caused by the presence of a parasite-specific allosteric site on the leishmanial SPDSYN or to other discrepancies in the parasite polyamine pathway, such as the lack of the mammalian back-conversion pathway that transforms spermine to spermidine (15).
The finding that an additional lesion in the polyamine biosynthetic pathway dramatically moderates L. donovani virulence implies that the virulence deficit of Δodc L. donovani is not specific to the lesion but rather is specific to a more generalized polyamine biosynthetic defect. Moreover, the fact that two independent genetic lesions in the polyamine biosynthesis pathway both cause a striking decrease in the capacity of L. donovani to infect mouse livers and spleens provides strong support that the cause of the avirulence is due to a lack of polyamine biosynthetic capacity and not to a secondary unknown function of the ODC or SPDSYN gene in the amastigote. This study demonstrates that Δspdsyn parasites are greatly reduced in their ability to infect a mammalian host and further validates additional enzymatic components of the polyamine biosynthetic pathway as potential targets for the treatment of visceral and perhaps other forms of leishmaniasis.
ACKNOWLEDGMENTS
This investigation was supported by grant AI041622 (to B.U.) from the National Institute of Allergy and Infectious Diseases and by grants 5TL1RR024159-05 and UL1RR024140 (to T.O.) from the National Institutes of Health.
Footnotes
Published ahead of print on 2 May 2011.
REFERENCES
- 1. Assaraf Y. G., Golenser J., Spira D. T., Messer G., Bachrach U. 1987. Cytostatic effect of dl-alpha-difluoromethylornithine against Plasmodium falciparum and its reversal by diamines and spermidine. Parasitol. Res. 73:313–318 [DOI] [PubMed] [Google Scholar]
- 2. Bacchi C. J., et al. 1992. Cure of murine Trypanosoma brucei rhodesiense infections with an S-adenosylmethionine decarboxylase inhibitor. Antimicrob. Agents Chemother. 36:2736–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bacchi C. J., et al. 2009. Trypanocidal activity of 8-methyl-5′-{[(Z)-4- aminobut-2-enyl]-(methylamino)}adenosine (Genz-644131), an adenosylmethionine decarboxylase inhibitor. Antimicrob. Agents Chemother. 53:3269–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bacchi C. J., McCann P. P. 1987. Parasitic protozoa and polyamines, p. 317–344In McCann P. P., Pegg A. E., Sjoerdsma A. (ed.), Inhibition of polyamine metabolism: biological significance and basis for new therapies. Academic Press, Orlando, FL [Google Scholar]
- 5. Bachrach U. 2005. Naturally occurring polyamines: interaction with macromolecules. Curr. Protein Pept. Sci. 6:559–566 [DOI] [PubMed] [Google Scholar]
- 6. Barker R. H., Jr., et al. 2009. Novel S-adenosylmethionine decarboxylase inhibitors for the treatment of human African trypanosomiasis. Antimicrob. Agents Chemother. 53:2052–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Basselin M., Coombs G. H., Barrett M. P. 2000. Putrescine and spermidine transport in Leishmania. Mol. Biochem. Parasitol. 109:37–46 [DOI] [PubMed] [Google Scholar]
- 8. Bitonti A. J., et al. 1990. Cure of Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense infections in mice with an irreversible inhibitor of S-adenosylmethionine decarboxylase. Antimicrob. Agents Chemother. 34:1485–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bitonti A. J., Dumont J. A., McCann P. P. 1986. Characterization of Trypanosoma brucei brucei S-adenosyl-l-methionine decarboxylase and its inhibition by Berenil, pentamidine and methylglyoxal bis(guanylhydrazone). Biochem. J. 237:685–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blackwell J. M. 1996. Genetic susceptibility to leishmanial infections: studies in mice and man. Parasitology. 112(Suppl.):S67–S74 [PubMed] [Google Scholar]
- 11. Boitz J. M., Ullman B. 2006. A conditional mutant deficient in hypoxanthine-guanine phosphoribosyltransferase and xanthine phosphoribosyltransferase validates the purine salvage pathway of Leishmania donovani. J. Biol. Chem. 281:16084–16089 [DOI] [PubMed] [Google Scholar]
- 12. Boitz J. M., et al. 2009. Leishmania donovani ornithine decarboxylase is indispensable for parasite survival in the mammalian host. Infect. Immun. 77:756–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buffet P. A., Sulahian A., Garin Y. J., Nassar N., Derouin F. 1995. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob. Agents Chemother. 39:2167–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burri C., Brun R. 2003. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 90(Suppl. 1):S49–S52 [DOI] [PubMed] [Google Scholar]
- 15. Casero R. A., Pegg A. E. 2009. Polyamine catabolism and disease. Biochem. J. 421:323–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang K. P., Steiger R. F., Dave C., Cheng Y. C. 1978. Effects of methylglyoxal bis(ganylhydrazone) on trypanosomatid flagellates: inhibition of growth and nucleoside incorporation in Trypanosoma brucei. J. Protozool. 25:145–149 [DOI] [PubMed] [Google Scholar]
- 17. Clayton C., et al. 1998. Genetic nomenclature for Trypanosoma and Leishmania. Mol. Biochem. Parasitol. 97:221–224 [DOI] [PubMed] [Google Scholar]
- 18. Danzin C., Marchal P., Casara P. 1990. Irreversible inhibition of rat S-adenosylmethionine decarboxylase by 5′-([(Z)-4-amino-2-butenyl]methylamino)-5′-deoxyadenosine. Biochem. Pharmacol. 40:1499–1503 [DOI] [PubMed] [Google Scholar]
- 19. Debrabant A., Joshi M. B., Pimenta P. F., Dwyer D. M. 2004. Generation of Leishmania donovani axenic amastigotes: their growth and biological characteristics. Int. J. Parasitol. 34:205–217 [DOI] [PubMed] [Google Scholar]
- 20. Docampo R., Moreno S. N. 2003. Current chemotherapy of human African trypanosomiasis. Parasitol. Res. 90(Suppl. 1):S10–S13 [DOI] [PubMed] [Google Scholar]
- 21. Fairlamb A. H., Cerami A. 1992. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 46:695–729 [DOI] [PubMed] [Google Scholar]
- 22. Garg R., Dube A. 2006. Animal models for vaccine studies for visceral leishmaniasis. Indian J. Med. Res. 123:439–454 [PubMed] [Google Scholar]
- 23. Gaur U., et al. 2007. An effect of parasite-encoded arginase on the outcome of murine cutaneous leishmaniasis. J. Immunol. 179:8446–8453 [DOI] [PubMed] [Google Scholar]
- 24. Geary T. G., Edgar S. A., Jensen J. D. 1986. Drug resistance in protozoa, p. 209–236In Campbell W. C., Rew R. S.(ed.), Chemotherapy of parasitic diseases. Plenum Press, New York, NY [Google Scholar]
- 25. Ghoda L., Phillips M. A., Bass K. E., Wang C. C., Coffino P. 1990. Trypanosome ornithine decarboxylase is stable because it lacks sequences found in the carboxyl terminus of the mouse enzyme which target the latter for intracellular degradation. J. Biol. Chem. 265:11823–11826 [PubMed] [Google Scholar]
- 26. Ghoda L., van Daalen Wetters T., Macrae M., Ascherman D., Coffino P. 1989. Prevention of rapid intracellular degradation of ODC by a carboxyl-terminal truncation. Science 243:1493–1495 [DOI] [PubMed] [Google Scholar]
- 27. Gillin F. D., Reiner D. S., McCann P. P. 1984. Inhibition of growth of Giardia lamblia by difluoromethylornithine, a specific inhibitor of polyamine biosynthesis. J. Protozool. 31:161–163 [DOI] [PubMed] [Google Scholar]
- 28. Goyard S., Beverley S. M. 2000. Blasticidin resistance: a new independent marker for stable transfection of Leishmania. Mol. Biochem. Parasitol. 108:249–252 [DOI] [PubMed] [Google Scholar]
- 29. Goyard S., et al. 2003. An in vitro system for developmental and genetic studies of Leishmania donovani phosphoglycans. Mol. Biochem. Parasitol. 130:31–42 [DOI] [PubMed] [Google Scholar]
- 30. Gradoni L., Iorio M. A., Gramiccia M., Orsini S. 1989. In vivo effect of eflornithine (DFMO) and some related compounds on Leishmania infantum preliminary communication. Farmaco 44:1157–1166 [PubMed] [Google Scholar]
- 31. Hasne M. P., Ullman B. 2005. Identification and characterization of a polyamine permease from the protozoan parasite Leishmania major. J. Biol. Chem. 280:15188–15194 [DOI] [PubMed] [Google Scholar]
- 32. Janne J., et al. 2005. Animal disease models generated by genetic engineering of polyamine metabolism. J. Cell. Mol. Med. 9:865–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Janne J., Alhonen L., Pietila M., Keinanen T. A. 2004. Genetic approaches to the cellular functions of polyamines in mammals. Eur. J. Biochem. 271:877–894 [DOI] [PubMed] [Google Scholar]
- 34. Jiang Y., et al. 1999. Ornithine decarboxylase gene deletion mutants of Leishmania donovani. J. Biol. Chem. 274:3781–3788 [DOI] [PubMed] [Google Scholar]
- 35. Kaur K., Emmett K., McCann P. P., Sjoerdsma A., Ullman B. 1986. Effects of dl-alpha-difluoromethylornithine on Leishmania donovani promastigotes. J. Protozool. 33:518–521 [DOI] [PubMed] [Google Scholar]
- 36. Kaye P. M., Gorak P., Murphy M., Ross S. 1995. Strategies for immune intervention in visceral leishmaniasis. Ann. Trop. Med. Parasitol. 89(Suppl.):75–81 [DOI] [PubMed] [Google Scholar]
- 37. Keithly J. S., Fairlamb A. H. 1987. Inhibition of Leishmania species by alpha-difluoromethylornithine, p. 749–756In Hart D. T. (ed.), Leishmaniasis: the current status and new strategies for control, vol. 163 Plenum Press, New York, NY [Google Scholar]
- 38. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 39. Metcalf B. W., et al. 1978. Catalytic irreversible inhibition of mammalian ornithine decarboxylase (E.C. 4.1.1.17) by substrate and product analogues. J. Am. Chem. Soc. 100:2551–2553 [Google Scholar]
- 40. Mishra J., Saxena A., Singh S. 2007. Chemotherapy of leishmaniasis: past, present and future. Curr. Med. Chem. 14:1153–1169 [DOI] [PubMed] [Google Scholar]
- 41. Mukhopadhyay R., Kapoor P., Madhubala R. 1996. Characterization of alpha-difluoromethylornithine resistant Leishmania donovani and its susceptibility to other inhibitors of the polyamine biosynthetic pathway. Pharmacol. Res. 34:43–46 [DOI] [PubMed] [Google Scholar]
- 42. Mukhopadhyay R., Madhubala R. 1993. Effect of a bis(benzyl)polyamine analogue, and dl-alpha-difluoromethylornithine on parasite suppression and cellular polyamine levels in golden hamster during Leishmania donovani infection. Pharmacol. Res. 28:359–365 [DOI] [PubMed] [Google Scholar]
- 43. Muleme H. M., et al. 2009. Infection with arginase-deficient Leishmania major reveals a parasite number-dependent and cytokine-independent regulation of host cellular arginase activity and disease pathogenesis. J. Immunol. 183:8068–8076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mullen A. B., Baillie A. J., Carter K. C. 1998. Visceral leishmaniasis in the BALB/c mouse: a comparison of the efficacy of a nonionic surfactant formulation of sodium stibogluconate with those of three proprietary formulations of amphotericin B. Antimicrob. Agents Chemother. 42:2722–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Olenyik T., Gilroy C., Ullman B. 2011. Oral putrescine restores virulence of ornithine decarboxylase-deficient Leishmania donovani in mice. Mol. Biochem. Parasitol. 176:109–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Osterman A., Grishin N. V., Kinch L. N., Phillips M. A. 1994. Formation of functional cross-species heterodimers of ornithine decarboxylase. Biochemistry 33:13662–13667 [DOI] [PubMed] [Google Scholar]
- 47. Phillips M. A., Coffino P., Wang C. C. 1987. Cloning and sequencing of the ornithine decarboxylase gene from Trypanosoma brucei. Implications for enzyme turnover and selective difluoromethylornithine inhibition. J. Biol. Chem. 262:8721–8727 [PubMed] [Google Scholar]
- 48. Reguera R. M., Balana-Fouce R., Showalter M., Hickerson S., Beverley S. M. 2009. Leishmania major lacking arginase (ARG) are auxotrophic for polyamines but retain infectivity to susceptible BALB/c mice. Mol. Biochem. Parasitol. 165:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reguera R. M., Fouce R. B., Cubria J. C., Bujidos M. L., Ordonez D. 1995. Fluorinated analogues of l-ornithine are powerful inhibitors of ornithine decarboxylase and cell growth of Leishmania infantum promastigotes. Life Sci. 56:223–230 [DOI] [PubMed] [Google Scholar]
- 50. Roberts S. C., et al. 2001. Genetic analysis of spermidine synthase from Leishmania donovani. Mol. Biochem. Parasitol. 115:217–226 [DOI] [PubMed] [Google Scholar]
- 51. Roberts S. C., et al. 2002. S-Adenosylmethionine decarboxylase from Leishmania donovani. Molecular, genetic, and biochemical characterization of null mutants and overproducers. J. Biol. Chem. 277:5902–5909 [DOI] [PubMed] [Google Scholar]
- 52. Roberts S. C., et al. 2004. Arginase plays a pivotal role in polyamine precursor metabolism in Leishmania. Characterization of gene deletion mutants. J. Biol. Chem. 279:23668–23678 [DOI] [PubMed] [Google Scholar]
- 53. Roberts S. C., et al. 2007. Leishmania donovani polyamine biosynthetic enzyme overproducers as tools to investigate the mode of action of cytotoxic polyamine analogs. Antimicrob. Agents Chemother. 51:438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robinson K. A., Beverley S. M. 2003. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol. Biochem. Parasitol. 128:217–228 [DOI] [PubMed] [Google Scholar]
- 55. Sanchez C. P., et al. 1997. Alpha-difluoromethylornithine-resistant cell lines obtained after one-step selection of Leishmania mexicana promastigote cultures. Biochem. J. 324(Pt 3):847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schechter P. J., Barlow J. L. R., Sjoerdsma A. 1987. Clinical aspects of inhibition of ornithine decarboxylase with emphasis on therapeutic trials of eflornithine (DFMO) in cancer and protozoan diseases, p. 345–364In McCann P. P., Pegg A. E., Sjoerdsma A. (ed.),Inhibition of polyamine metabolism: biological significance and basis for new therapies. Academic Press, Orlando, FL [Google Scholar]
- 57. Tabor C. W., Tabor H. 1984. Polyamines. Annu. Rev. Biochem. 53:749–790 [DOI] [PubMed] [Google Scholar]
- 58. Ulrich P., Grady R. W., Cerami A. 1982. The trypanocidal activity of various aromatic bisguanylhydrazones in vivo. Drug Dev. Res. 2:219–228 [Google Scholar]
- 59. Van Nieuwenhove S., et al. 1985. Treatment of gambiense sleeping sickness in the Sudan with oral DFMO (dl-alpha-difluoromethylornithine), an inhibitor of ornithine decarboxylase; first field trial. Trans. R. Soc. Trop. Med. Hyg. 79:692–698 [DOI] [PubMed] [Google Scholar]
- 60. Wilson M. E., Jeronimo S. M., Pearson R. D. 2005. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb. Pathog. 38:147–160 [DOI] [PubMed] [Google Scholar]
- 61. Wu H., et al. 2007. Structure and mechanism of spermidine synthases. Biochemistry 46:8331–8339 [DOI] [PubMed] [Google Scholar]
- 62. Xiao Y., McCloskey D. E., Phillips M. A. 2009. RNA interference-mediated silencing in ornithine decarboxylase and spermidine synthase genes in Trypanosoma brucei provides insight into regulation of polyamine biosynthesis. Eukaryot. Cell 8:747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]