Abstract
Previous studies have demonstrated that the ebp operon and the ace gene of Enterococcus faecalis, encoding endocarditis- and biofilm-associated pili and an adhesin to collagen of E. faecalis, respectively, are both important in experimental urinary tract infections (UTI) and endocarditis. We have also shown that growth of E. faecalis in brain heart infusion (BHI) serum enhances Ebp pilus and Ace production and increases adherence to several host extracellular matrix proteins. Here, we report that deletion of ebpABC almost eliminated serum-elicited adherence to fibrinogen (P < 0.0001), resulted in moderate reduction in adherence to collagen (P < 0.05), and had no effect on fibronectin adherence relative to that of wild-type OG1RF. An OG1RFΔaceΔebpABC double mutant showed further reduced collagen adherence versus that of the OG1RFΔace or OG1RFΔebpABC mutants (P < 0.001). These results were corroborated by complementation and/or studies with native pilus-enriched surface extracts and a collagen-secreting 3T6 fibroblast cell line, as well as antibody inhibition. In the UTI model, both the OG1RFΔace and OG1RFΔaceΔebpABC mutants were found to be significantly attenuated compared to the wild type; however, no significant differences were observed between individual ace or ebp mutants and the OG1RFΔaceΔebpABC mutant. In summary, these data implicate the Ebp pili as having some role in collagen adherence, albeit less than that of Ace, and a very major role in fibrinogen adherence, which may explain in part the importance of these pili in experimental endocarditis. The OG1RFΔaceΔebpABC mutant was attenuated in the UTI model, although not significantly more so than the Δace or ΔebpABC mutants, suggesting involvement of other E. faecalis factors in urinary tract colonization or infection.
INTRODUCTION
Enterococcus faecalis, a common commensal of the gastrointestinal tracts of humans and animals, has emerged as an important opportunistic pathogen causing a wide variety of nosocomial infections, including infective endocarditis (IE), urinary tract infections (UTI), and bacteremia (1, 2, 16, 34). One of the problems with E. faecalis is that this species is intrinsically resistant to cephalosporins, monobactams, antistaphylococcal penicillins, and aminoglycosides and has the ability to become resistant to other antimicrobials by acquiring resistance genes or by mutating (2, 16, 32). Infecting strains usually originate from an individual's normal gastrointestinal flora and, in hospitalized patients, their endogenous flora is often replaced by multidrug-resistant E. faecalis acquired from the hospital milieu (16, 32), thus making treatment of serious E. faecalis infections difficult.
A recently recognized theme among opportunistic pathogenic bacteria is their ability to utilize host molecules during infection, similar to traditional pathogens, to facilitate bacterial colonization, entry into host cells, and, perhaps, modification of host cell signaling pathways to the pathogen's advantage (12, 42, 46). For example, emerging pathogens have either acquired or evolved surface proteins or structures to adhere to widespread host extracellular matrix (ECM) proteins (e.g., collagen, fibrinogen, fibronectin) and host cell receptors (e.g., integrins) (12, 20, 56). Some of these adhesins, such as the microbial surface components recognizing adhesive matrix molecule (MSCRAMM) (46) family proteins, also have a confirmed role in infectious processes (13, 44, 57). More recently, long filamentous appendages of Gram-positive bacteria that are covalently polymerized and attached to the peptidoglycan cell wall by the action of sortase enzymes have been described and named as pili (27, 58). Gram-positive pili generally consist of a major backbone subunit and one or more minor subunits (27). Examples of MSCRAMMs and pili of Gram-positive bacteria that mediate adherence to collagen (the most abundant protein in mammals) or fibrinogen, besides those of E. faecalis, include Cna, FnbpA, and ClfA of Staphylococcus aureus (46); Acm, Scm, and EcbA of Enterococcus faecium (11, 41, 49); Cpa component of fibronectin- and collagen-binding protein and T antigen (FCT) pili of Streptococcus pyogenes (35); and Rrg pili of Streptococcus pneumoniae (14), among others. Most of these entities have been shown to be important for the establishment and progression of bacterial infections in various animal models, including IE, UTI, and bacteremia (12, 20, 27, 46, 56–58).
The ability of E. faecalis to adhere to ECM components and to develop biofilms on medical devices (e.g., intravascular and urinary catheters) is believed to contribute to its pathogenesis (5, 8). In silico analyses indicated that E. faecalis possesses a number of MSCRAMMs with a characteristic DE variant of the immunoglobulin-like fold (51). Further studies found that E. faecalis isolates display enhanced adherence to collagen, fibrinogen, and fibronectin if grown in brain heart infusion broth supplemented with serum (BHI-S) (38). Biochemical studies with recombinant versions of MSCRAMMs and genetic analyses with mutants found the following: (i) that one of the MSCRAMMs, Ace (an adhesin to collagen from E. faecalis), mediates adherence (after growth at 46°C or in the presence of serum or collagen) to collagen types I and IV, laminin (37, 39), and dentin (15) and plays a role in infective endocarditis (55) and UTI (24); (ii) that three MSCRAMMs (EF0089, EF2505, and EF1896, renamed Fss1, Fss2, and Fss3, respectively, for E. faecalis surface proteins) mediate adherence to fibrinogen (48); and (iii) that EF1091, EF1092, and EF1093 (renamed EbpA, EbpB, and EbpC for endocarditis- and biofilm-associated pilus subunits A, B, and C) produce immunogenic and pleomorphic pili (40), which also have a role in UTI (54). Our companion paper (36) shows that the Ebp locus is ubiquitous and highly conserved, that pili are expressed by a subpopulation of cells, and that pili mediate platelet adherence. It has also been shown that secretion of Ebp pilus subunits and their processing by Bps (the sortase C of the ebp locus [19]) and SrtA are spatially coupled to specific loci on the bacterial cell surface (21), consistent with the ExPortal model proposed for streptococcal protein secretion (47).
Although our previous studies demonstrated the importance of Ebp pili for the pathogenesis of E. faecalis, the molecular mechanisms by which Ebp pili act as a virulence factor and the host targets are unknown. In an attempt to further understand these processes, we have now evaluated the role of Ebp pili in ECM protein adherence of E. faecalis by using nonpolar ebpABC deletion mutants. We next evaluated the effect of ebpABC and/or ace deletions on the ability of E. faecalis to adhere to cell lines pertinent to colonization and infection. Finally, we investigated whether an antibody mix against recombinant Ebp pilus subunits could be used to inhibit ECM adherence of E. faecalis and whether there was a combined effect of ebpABC and ace deletions on virulence using a mouse UTI model.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Wild-type E. faecalis OG1RF (4) and its deletion and complementation constructs, Escherichia coli strains, and plasmids used in this study are listed in Table S1 in the supplemental material. E. faecalis strains were routinely grown in BHI broth/agar (Difco Laboratories, Detroit, MI) and, for some experiments (see below), in BHI broth containing 40% heat-inactivated horse serum (BHI-S) (Sigma, St. Louis, MO). Tryptic soy broth (Difco) supplemented with 0.25% glucose (TSBG) was used for biofilm assays. E. coli strains were grown in Luria-Bertani (Difco) broth/agar. Antibiotics used for OG1RF and/or its derivatives were erythromycin (10 μg/ml), fusidic acid (25 μg/ml), rifampin (100 μg/ml), chloramphenicol (10 μg/ml), and gentamicin (125 μg/ml), and antibiotics used for E. coli were erythromycin (300 μg/ml), gentamicin (50 μg/ml), and kanamycin (50 μg/ml).
General techniques.
Genomic DNA was extracted using hexadecyltrimethylammonium bromide according to a previously described protocol (59). PCR primers used for amplification and sequencing are listed in Table S2 in the supplemental material. Pulsed-field gel electrophoresis (PFGE) was performed as described earlier (33). Southern and colony lysate hybridizations were carried out in high-stringency conditions with probes generated and radiolabeled as previously described (19, 52).
Creation of mutants and complementation.
The pilus-encoding ebpABC open reading frames (ORFs) of the E. faecalis ace deletion mutant (OG1RFΔace::cat; TX5467 [55]) were deleted by allelic replacement, following a previously described protocol (23). In brief, DNA fragments flanking the ebpABC region upstream (1,291 bp) and downstream (1,151 bp) were PCR amplified (see Table S2 in the supplemental material for primers), joined together by crossover PCR, digested by XbaI and PstI, and cloned into similarly digested pCJK47 (23). After transformation into E. coli EC1000 to obtain strain TX5606, the resulting plasmid, pTEX5606, was verified by sequencing and electroporated into the OG1RFΔace mutant. Single crossover colonies selected on BHI plates containing erythromycin were then screened for double crossover deletions and revertants using PheS* counterselection on MM9YEG plates supplemented with 10 mM p-Cl-Phe and 200 μg/ml of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). The OG1RFΔaceΔebpABC mutant (TX5612) was confirmed by PCR, sequencing, and PFGE.
For complementation of the OG1RFΔebpABC (7) and OG1RFΔaceΔebpABC mutants in trans, a PCR-amplified 6,764-bp ebpABC region, including its upstream ribosomal binding site (see Table S2 in the supplemental material for primers), was digested with SstI and XbaI and cloned behind the constitutive P2 promoter of similarly digested pAT392 (3). The resulting plasmid, pTEX5663, was confirmed by sequencing and then electroporated into the OG1RFΔebpABC and OG1RFΔaceΔebpABC mutants to obtain OG1RFΔebpABC(pAT392::ebpABC) (TX5662) and OG1RFΔaceΔebpABC(pAT392::ebpABC) (TX5654), respectively.
Growth curves and plasmid stability of complementation constructs.
Overnight-grown cultures of test bacteria were diluted (1:100) in BHI, BHI-S, or TSBG and grown at 37°C with gentle shaking. An optical density at 600 nm (OD600) reading was taken every hour from 0 to 12 h and then at 20 h. At intervals of 0, 6, and 10 h, the numbers of CFU were also determined by plating serial dilutions on BHI. For plasmid stability analysis, complemented and vector control strains, grown overnight with or without gentamicin, were serially diluted and plated on BHI plates. Approximately 96 colonies (per assay) were resuspended in BHI broth in microtiter plates and then replica plated onto BHI plates with and without gentamicin to screen for plasmid loss.
Biofilm assay.
A biofilm density assay was carried out for wild-type E. faecalis OG1RF and its isogenic deletion and complementation constructs, according to a previously described method (29).
RNA purification, RT-PCR, and quantitative RT-PCR.
To test bps (srtC) gene expression levels in OG1RF and in the ΔebpABC mutant during growth in BHI and BHI-S at 37°C, 150 rpm, samples were collected from mid-logarithmic phase cultures (OD600 of 0.75 for BHI culture; OD600 of 0.5 for BHI-S culture). RNA was isolated using the RNeasy minikit (Qiagen, Valencia, CA) according to the supplied protocol, but with a minor modification of 10 mg/ml lysozyme in the lysis step. RNA was treated three times with 20 U of RQ1 DNase (Promega, Madison, WI) for 30 min at 37°C. For reverse transcriptase PCR (RT-PCR), RNA was reverse transcribed with the primers listed in Table S2 in the supplemental material by using the SuperScript One-Step RT-PCR with Platinum Taq kit (Invitrogen, San Diego, CA). Reactions without reverse transcriptase were performed as controls to detect DNA contamination in the total RNA preparations.
cDNA synthesis and PCR amplification for qRT-PCR were performed by a two-step process as previously described (37). Real-time analysis was performed using the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). Primers (see Table S2 in the supplemental material), designed to produce amplicons of similar lengths, were selected using the Primer Express software (Applied Biosystems). Relative quantification of gene expression was performed using gyrB mRNA as the internal standard. The ΔΔCT method (25) was used to calculate the relative amount of specific RNA present in each sample.
Ace and Ebp pilin-specific polyclonal antibodies.
Production of rabbit polyclonal antibodies against rAce A domain (39), rEbpA, rEbpB, and rEbpC (51) was described elsewhere (39, 40). Specific antibodies were eluted from respective recombinant proteins coupled to a cyanogen bromide-activated Sepharose 4B column, according to the manufacturer's protocol (Amersham Biosciences, Piscataway, NJ).
Whole-cell ELISA.
Surface expression of Ace, EbpA, and EbpC by OG1RF and its derivatives was detected by a whole-cell enzyme-linked immunosorbent assay (ELISA), as described earlier (41). Briefly, E. faecalis cells grown for 10 h in BHI-S (with 125 μg/ml gentamicin for complementation constructs) were washed with phosphate-buffered saline (PBS), pH 7.4, and adjusted to an OD600 of 0.6 in 50 mM carbonate-bicarbonate buffer, pH 9.6, before coating onto microtiter plate wells (4HBX; Thermo Scientific, Woburn, MA). Surface expression was detected by affinity-purified polyclonal antibodies against Ace, EbpA, and EbpC (1st antibody), goat anti-rabbit F(ab′)2 fragment conjugated to alkaline phosphatase (2nd antibody) (Jackson ImmunoResearch Laboratories, West Grove, PA), and chromogenic p-nitrophenyl phosphate 1-STEP substrate (Thermo Scientific). The absorbance of each well was read at 405 nm. Antiserum raised against formalin-killed E. faecalis strain HH22 whole cells was used as a control to confirm that whole cells of all strains were bound to the microtiter plates at similar levels.
Mutanolysin cell wall extracts and Western blots.
Cell wall-anchored proteins were extracted using mutanolysin from E. faecalis OG1RF and its deletion and complementation derivatives grown in BHI-S (supplemented with 125 μg/ml gentamicin for complementation constructs) for 12 h from an initial OD600 of 0.02, as described previously (39, 40). Total protein concentrations of mutanolysin extracts were determined by the bicinchoninic acid assay (Thermo Scientific). Ten micrograms of these extracts was then analyzed by Western blotting, using affinity-purified anti-EbpA, anti-EbpC, and anti-Ace antibodies, followed by horseradish peroxidase-labeled secondary antibody [donkey anti-rabbit F(ab′)2 fragment; Jackson ImmunoResearch Laboratories] as previously described (49).
ECM protein adherence assays with bacteria.
Adherence of E. faecalis strains to collagen type I (rat tail; Sigma), fibrinogen (depleted of plasminogen, von Willebrand factor, and fibronectin) (human plasma; Enzyme Research Laboratories, South Bend, IN), fibronectin (human plasma; Enzyme Research Laboratories), and bovine serum albumin (BSA; Sigma) was determined using 35S-labeled bacteria grown in BHI-S (complementation constructs were supplemented with 125 μg/ml gentamicin) for 10 h from an initial OD600 of 0.02, as described previously (38).
Adherence inhibition assays with antibodies.
For inhibition of adherence by antibodies, 35S-labeled bacteria were preincubated with various concentrations of individual or a mixture of affinity-purified anti-EbpA, anti-EbpB, and anti-EbpC antibodies for 1 h at 37°C. Fifty microliters of these cells was then added to ECM protein-coated wells, as in the above adherence assay. Immunoglobulins from preimmune rabbit serum purified by the Immunopure (G) IgG purification kit (Pierce) were used as controls.
Binding of native Ebp pilus-enriched surface preparations of E. faecalis OG1RF to ECM proteins.
Mutanolysin surface extracts from 10 to 20 liters BHI-S-grown cells were concentrated by lyophilization, and then an Ebp pilus-enriched high-molecular-weight (HMW) surface extract fraction was obtained by ultrafiltration with a 50,000-Da molecular mass cutoff filter (Millipore, Bedford, MA) and dialysis against PBS using a 50,000-Da molecular mass cutoff membrane. Similar processing was carried out for the ΔebpABC mutant to obtain surface proteins of ≥50 kDa, to use as a control. Binding of these surface extracts to collagen types I, IV (human placenta; Sigma), and V (human placenta; Sigma), fibronectin, and BSA was tested using an ELISA-type assay previously used for recombinant proteins (41). Microwell plate (4HBX; Thermo Scientific) wells were coated with 1 μg of each ECM protein overnight at 4°C. After washing the wells three times and blocking the remaining protein-binding sites by 2% BSA and 0.1% Tween 20 in PBS (blocking buffer), 10 μg of Ebp pilus-enriched surface extracts in blocking buffer was added and incubated at room temperature for 2 h. The plates were then washed three times with 0.1% Tween 20 in PBS, and bound Ebp pili were detected by affinity-purified anti-EbpA and anti-EbpC immunoglobulins (1st antibody) and goat anti-rabbit F(ab′)2 fragments conjugated to alkaline phosphatase (2nd antibody).
Tissue culture and cell adhesion assays.
3T6 fibroblasts were cultured in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% fetal bovine serum (FBS) with 100 μg/ml gentamicin (Sigma) in a humidified 5% CO2 atmosphere at 37°C. For adhesion assays, cells in IMDM with 10% FBS, without gentamicin, were seeded into 24-well tissue culture plates at 4 × 105 cells per well in a 0.5-ml volume. The cells were incubated at 37°C in 5% CO2 to obtain a monolayer prior to the addition of BHI-S-grown E. faecalis OG1RF wild type and derivatives, resuspended in IMDM plus 0.5% Tween 80, and diluted to desired multiplicities of infection (MOI), ranging from 1 to 10. 3T6 cells were gently washed twice with IMEM without FBS. For each strain, 0.5 ml bacterial suspension was added to the wells. Plates were centrifuged (175 × g, 10 min) and incubated for 1 h at 37°C in 5% CO2. After incubation, nonadherent bacteria were withdrawn from the wells, and cell monolayers were washed three times with Hanks' balanced salt solution (HBSS; Invitrogen, Carlsbad, CA). The cell monolayers were trypsinized and subsequently lysed with HBSS plus 0.1% Triton X-100 by rigorous pipetting at room temperature. Adherent and internalized bacteria were quantified by plating serial dilutions onto BHI agar plates and counting the resulting colonies. The inoculum was plated to determine initial viable counts. Adherence percentage for each strain was calculated using the following equation: (input CFU/output CFU) × 100. All adhesion assays were performed in triplicate on three occasions.
Similarly, epithelial (Caco-2 and T24) and endothelial (HUVEC) cell lines were grown to confluent monolayers in 24-well tissue culture plates using protocols of ATCC and suppliers of respective cell lines for use in the above adherence assay.
T84 cell translocation.
Growth and maintenance of T84 cells and translocation experiments were performed according to methods described previously (61).
Mouse UTI model.
The mouse UTI monoinfection experiments were carried out by following our previously described protocols (19, 54), using inocula of 105 and 106 CFU of wild-type OG1RF and the Δace and ΔaceΔebpABC mutants. Animal experiments were performed by following a preapproved protocol and guidelines by the Animal Welfare Committee of the University of Texas Health Science Center at Houston.
Statistical analyses.
To compare adherence results between different strains to ECM proteins or cell lines and also binding between Ebp pilus-enriched surface preparations to different ECM proteins, analysis of variance (ANOVA) with Bonferroni's multiple comparison posttest or unpaired t test was used, as appropriate. Differences between biofilm formation values were evaluated by the Mann-Whitney test. The log10 CFU (geometric mean) of bacteria recovered from kidneys and bladders was analyzed for significance by unpaired t test. GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA) was used for statistical analysis.
RESULTS AND DISCUSSION
Our previous analyses of mutants of ace, fss1, and fss2 showed partial loss of serum-elicited adherence to collagen (ace) (39) and fibrinogen (fss2, fss3) (48), suggesting the presence of other E. faecalis collagen and fibrinogen binding surface adhesins. Additional findings from gene association studies (38) coupled with our observation of an increase of Ebp pili expression in BHI-S-grown E. faecalis strains (40) suggested to us that the Ebp pili (which are ubiquitously present in this species [36]) might be associated with these common phenotypes. To assess the role of Ebp pili in these adhesive functions, we have now evaluated deletion mutants, complementation constructs, recombinant proteins, and pilus-enriched surface preparations.
Adherence of Ebp pilus-deficient mutants of E. faecalis OG1RF to ECM proteins.
Initial in vitro adherence assays were carried out using our previously generated polar ebpA, ebpB, and ebpC mutants and our nonpolar bps (srtC) mutants of OG1RF (40) after growth in BHI-S. Examination of collagen type I, fibronectin, and fibrinogen adherence of these mutants revealed markedly reduced adherence to immobilized fibrinogen (86 to 89% reduction; P < 0.0001) and slightly but significantly reduced adherence to collagen type I (10 to 13% reduction; P = 0.0003) compared to that of wild-type OG1RF (see Fig. S1A and B in the supplemental material) (here, collagen type I will be referred to as “collagen” if other types of collagens were not used concurrently). In contrast, no significant difference was observed with adherence to fibronectin under these test conditions (see Fig. S1C in the supplemental material). However, these ebp mutants were polar for the downstream ebp genes and partially polar for the bps (srtC) gene, since the bps gene is cotranscribed with the ebpA-ebpC transcript in addition to an independent bps transcript (40).
To exclude a possible independent effect due to the partial loss of the Bps sortase expression and to determine the independent roles of pili and Ace in collagen adhesion and UTI pathogenesis, we recently deleted the ebpABC locus from E. faecalis OG1RF (OG1RFΔebpABC mutant, also referred to as the ΔebpABC mutant; TX5608 [7]), and here we have deleted it from the ace deletion mutant, TX5467 (OG1RFΔaceΔebpABC mutant, also referred to as the ΔaceΔebpABC mutant; TX5612) (see Table S1 in the supplemental material). Pulsed-field gel electrophoresis followed by hybridizations with respective probes and sequencing confirmed strain identity and deletion of all of the ebpA and ebpB sequences and 1,791 bp of the 1,884-bp ebpC sequence. To confirm the importance of the ebp genes, the ΔebpABC and ΔaceΔebpABC mutants were complemented in trans with a recombinant plasmid carrying the ebpABC genes or ace (see Materials and Methods) under the control of the constitutive P2 promoter of pAT392 (3); respective strains with empty pAT392 were used as negative controls (see Table S1 in the supplemental material).
(i) Growth characteristics of mutants and stability of complementation derivatives.
The OG1RFΔaceΔebpABC mutant, the previously generated OG1RFΔebpABC (7) and OG1RFΔace (55) mutants, and the parental OG1RF strain demonstrated equivalent growth kinetics as well as similar gross morphologies and chain lengths in BHI, TSBG, and BHI-S (data not shown), confirming that these genes are not important for growth in vitro. Since our experience with in trans complementation of several genes of E. faecalis and E. faecium, including ebpfm (6, 50, 55), pointed toward potential plasmid instability, we assessed loss of the complementation plasmids after growth in BHI or BHI-S with or without antibiotic selection. In contrast to our observations with other genes (6, 50, 55), the complementing plasmids were generally stable in these mutants even without gentamicin selective pressure, in both BHI and BHI-S, with an average of only 2 to 8% cells having lost the complementing plasmid or control vector; including gentamicin during growth increased plasmid retention (plasmid loss, 0 to 3%).
(ii) Effect of deletion of the ebpABC cluster on the activity of the downstream sortase gene, bps.
RT-PCR analysis using a primer pair spanning the intragenic region of bps found that the ebpABC deletion did not affect expression of bps either in BHI or in BHI-S (data not shown), unlike the ebp polar mutants in which the ebp-bps transcript was terminated at the insertion site (40). We then quantitated the bps transcripts of OG1RF and ΔebpABC from late-log-phase cells grown in BHI-S using qRT-PCR and confirmed that there was no significant difference (with a fold change of 0.998 ± 0.032) in the steady-state mRNA levels of bps. These results indicate that any change in phenotype was not due to a decrease in expression of bps.
(iii) Cell surface expression of EbpA, EbpC, and Ace by OG1RF and its isogenic ebpABC and/or ace deletion mutants and complementation derivatives.
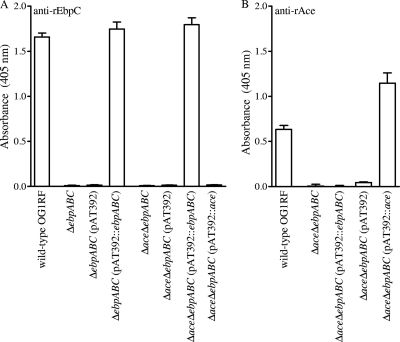
As anticipated, the ΔebpABC and ΔaceΔebpABC mutants lacked EbpABC production, and the ΔaceΔebpABC mutant also lacked Ace production (Fig. 1; see also Fig. S2 in the supplemental material). Complementation of the ebpABC locus in both of these mutants and ace in the double mutant led to surface expression of Ebp and Ace proteins in whole-cell ELISA at a level similar to or higher than that of the wild type, while no expression was detected with the empty plasmid (Fig. 1; see also Fig. S2 in the supplemental material).
Fig. 1.
Analysis of cell surface expression of EbpC and Ace by E. faecalis OG1RF, its isogenic ΔebpABC and ΔaceΔebpABC deletion mutants, and their complementation derivatives using whole-cell ELISA. (A) Surface expression of EbpC. (B) Surface expression of Ace. BHI-S-grown cells were coated onto wells of microtiter plates, and affinity-purified anti-rEbpC and anti-rAce antibodies were used for detection of cell surface expression. Bars represent the means of absorbance at 405 nm ± standard deviations (SD) from 16 wells, representing results from three independent assays.
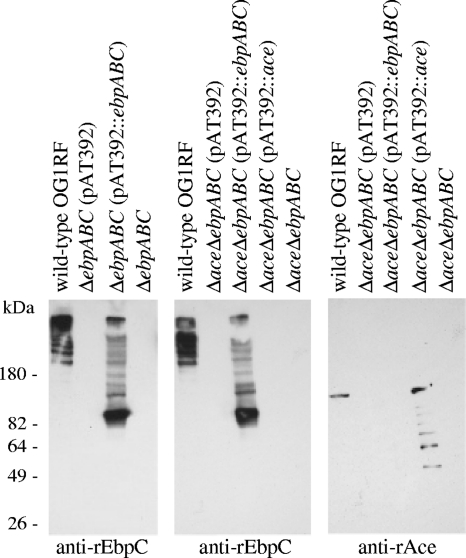
To confirm that the Ebp proteins detected on the surface of complementation constructs by whole-cell ELISA are part of polymeric pili, muramidase-derived cell wall extracts of the above constructs, separated using gradient polyacrylamide gels, were probed with affinity-purified anti-rEbpC, anti-rEbpA, or anti-rAce immunoglobulins. A ladder-like pattern of multiple HMW bands was detected using the wild-type and complemented strains for both anti-EbpC (Fig. 2, left and middle) and anti-EbpA (see Fig. S3A and B in the supplemental material) antibodies, consistent with characteristic pilus structures of Gram-positive pili. However, the HMW bands of the wild type were of sizes greater than 200 kDa, while the complemented mutants had a higher proportion of lower-molecular-weight polymers and a band corresponding to the size of the respective monomeric pilin component (Fig. 2, left and middle; see also Fig. S3A and B in the supplemental material); a similar observation was noted with E. faecium Ebpfm complementation constructs (50). We predict that the constitutively expressed Ebp proteins under the P2 promoter in the complemented deletion mutants may have overloaded the polymerization capacity of the Bps sortase, thus likely producing larger amounts of partially polymerized and unpolymerized subunits. As anticipated, a single immunoreactive band of ∼105 kDa was seen with anti-Ace immunoglobulin in surface extracts of the wild type, while the complemented strain with constitutive expression had additional degraded low-molecular-weight bands. Nonetheless, these Western blot analyses are in agreement with the whole-cell ELISA results and confirm that plasmid-carried ebpABC is polymerized into a ladderlike pattern of HMW complexes.
Fig. 2.
Western blot analysis of cell wall extracts from E. faecalis OG1RF, its ΔebpABC and ΔebpABCΔace deletion mutants, and their complementation constructs. Mutanolysin cell wall extracts were separated by gradient SDS-PAGE gels, transferred onto polyvinylidene difluoride (PVDF) membranes, and probed with affinity-purified anti-rEbpC and anti-rAce antibodies. Positions of molecular mass markers are indicated on the left.
(iv) Biofilm formation by the complementation construct.
To confirm our previous report of decreased biofilm formation by ebp mutants (7, 40) but not by the ace mutant (29), the OG1RFΔebpABC mutant and its complementation construct were evaluated in this assay. Consistent with our earlier report (7), deletion of ebpABC reduced biofilm formation by 62% (median OD570 of 0.386; interquartile range [IQR], 0.339 to 0.437) versus the wild-type (median OD570 of 1.034; IQR, 0.991 to 1.161; P = 0.0001) (see Fig. S4 in the supplemental material). Considering the inhibitory effect of gentamicin we observed on biofilm formation of E. faecium (50), the biofilm assay with complementation constructs was done without added gentamicin. Moderate but significant enhancement (∼57% increase) was observed by the complemented strain over the empty vector control (P = 0.0006); however, its biofilm production was only 62% of the wild-type level. The higher levels of unpolymerized Ebp pilin components (Fig. 2) might explain the incomplete restoration of the biofilm phenotype by the complemented strain.
Contribution of Ace and Ebp pili to adherence of E. faecalis OG1RF to immobilized collagen. (i) Studies with mutants.
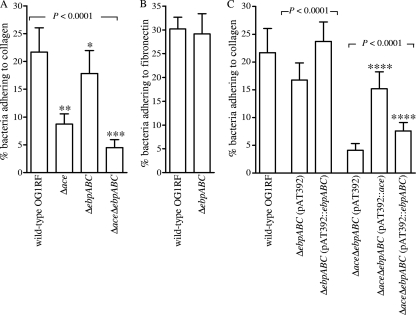
To investigate further the modest reduction in collagen adherence of the polar ebp mutants described above, the ΔebpABC and ΔaceΔebpABC mutants and their respective complementation constructs were tested for this phenotype versus wild-type OG1RF and its Δace mutant. As shown in Fig. 3A, all three mutant derivatives of OG1RF showed decreased collagen adherence (P < 0.0001 by ANOVA), with reductions ranging from 17% to 79% relative to that of wild-type OG1RF. The results for the Δace mutant are consistent with those we previously reported (55). As anticipated from the polar mutants, the decrease for the ΔebpABC mutant was small (17%) but significant (P < 0.05) by the post hoc test. The decrease seen with the double-deletion mutant versus each of the single-deletion mutants was also significant (74% decrease versus the ΔebpABC mutant and 48% decrease versus the OG1RFΔace mutant; P < 0.001 by post hoc test). Complementation of the ΔebpABC mutant with pAT392::ebpABC resulted in restoration of the collagen adherence phenotype to levels greater than or equal to that of the wild type (9% increase over wild type and 45% increase over the vector-only control; P < 0.001). Results from complementation of the Δace mutant with pAT392::ace were as reported earlier (55). Complementation of the double-deletion mutant, with ebpABC or ace, led to restoration of the adherence phenotype to similar levels as anticipated from the respective single mutants (P < 0.0001 versus respective vector-only controls). Thus, our data indicate that Ace plus the Ebp pili account for the majority of E. faecalis OG1RF's collagen adherence under the conditions tested.
Fig. 3.
Effect of ebpABC deletion and ebpABC-ace double deletion on the adherence of OG1RF to immobilized ECM proteins. (A) Adherence to collagen type I by ebp and ace mutants; (B) adherence to fibronectin by the ebp mutant; (C) adherence to collagen type I by complementation constructs. Bars represent mean percentages ± SD of 35S-labeled cells adhering to 16 ECM protein-coated wells from four independent experiments. BSA was used as a negative control. Means of adherence percentages of different strains were compared using ANOVA and Bonferroni's posttest. Means of complemented strains were compared with appropriate vector-only control strains using t test or ANOVA and Bonferroni's posttest. *, P < 0.05 versus the wild type by post hoc test; **, P < 0.001 versus the wild type and ΔebpABC mutant by post hoc test; ***, P < 0.001 versus the wild type and ΔebpABC mutant and P < 0.05 versus the Δace mutant by post hoc test; ****, P < 0.001 versus vector-only control by post hoc test.
(ii) Studies with recombinant proteins and Ebp pilus-enriched surface preparations.
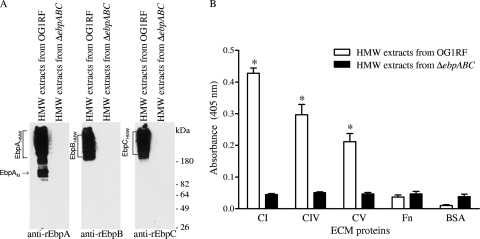
Since individual pilin components of pneumococci and group A streptococci have been documented to bind host ECM protein components (14, 17, 30, 45), we examined the potential ECM protein adhesive functions of purified rEbpA, rEbpB, and rEbpC (48). Testing of each of these recombinant Ebp proteins to a panel of individual ECM proteins in an ELISA-type solid-phase ligand binding assay showed no binding to collagen (or other ECM proteins used as potential ligands, including fibrinogen), suggesting that the collagen-binding function of pili is either due to a conformation change from the pilin subunits being incorporated into pili or possibly after being anchored as monomers onto the cell wall. To address these possibilities, HMW enriched protein surface preparations of wild-type OG1RF and the ΔebpABC mutant (Fig. 4A) were used in adherence assays and detected using either anti-rEbpA or anti-rEbpC antibodies. As seen in Fig. 4B, HMW extracts from OG1RF (but not similar extracts from the ebpABC mutant) exhibited significant binding to collagen types I, IV, and V (5- to 10-fold higher than the background level seen with HMW extracts of the ΔebpABC mutant; P < 0.0001). Binding of OG1RF HMW surface preparations to fibronectin was similar to that of the BSA control (Fig. 4B). Because our anti-rEbp immunoglobulins showed some cross-reactivity with fibrinogen coated onto the wells, we were unable to use fibrinogen as a ligand in these assays. Taken together, these results corroborate the mutant data and indicate that Ebp pili or pilus components mediate adhesion to collagen, independent of Ace.
Fig. 4.
Binding of native Ebp pilus-enriched high-molecular-weight (HMW) surface extracts to collagen types I, IV, and V and fibronectin. (A) Western blot analysis. An HMW surface extract fraction was obtained by ultrafiltration and dialysis of mutanolysin surface extracts. (B) Binding of pili from the HMW surface extract detected with an affinity-purified anti-rEbpC antibody. Bars represent means ± SD for 10 ECM protein-coated wells from up to four independent experiments. BSA was used as a negative control. Similar results obtained with anti-rEbpA antibody are not shown. Mean OD405 values from surface extracts of the wild type and the ΔebpABC mutant were compared using unpaired t test. *, P < 0.0001 versus ΔebpABC HMW surface extracts; CI, collagen type I; CIV, collagen type IV; CV, collagen type V; Fn, fibronectin; EbpAM, EbpA monomer.
Effect of ebp deletion on host cell adherence and translocation.
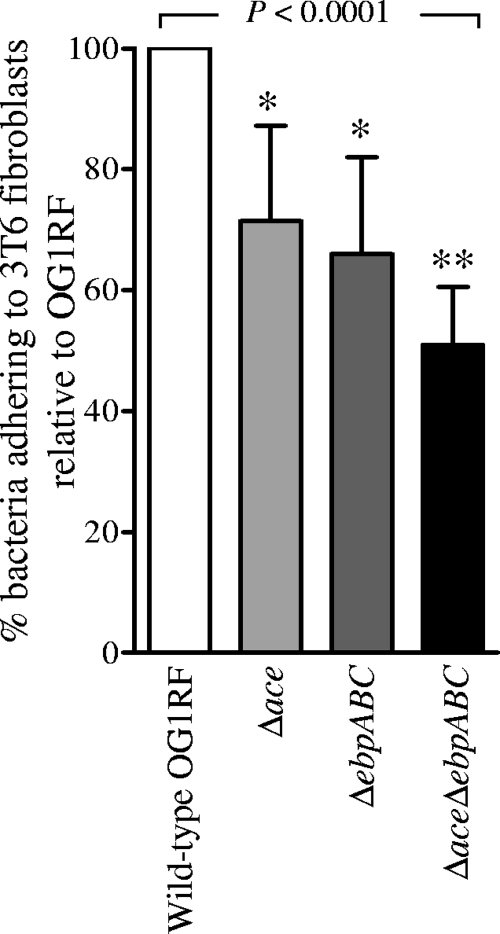
Evidence from streptococci indicates that Gram-positive pili can play a major role in adhesion to different host cells (22, 26, 28). To test whether the Ebp pili are involved in this phenotype, we investigated the ability of wild-type OG1RF and its isogenic ΔebpABC mutant to adhere to monolayers of Caco-2 cells (intestinal epithelial cells), T24 cells (human urinary bladder carcinoma cells), HUVEC cells (human umbilical vein endothelial cells), and 3T6 cells (collagen-secreting murine fibroblast cells). Our results showed no significant difference between the wild type and the ΔebpABC mutant in adherence to Caco-2 and T24 cells (data not shown). Although the ebp mutant showed reduced binding to HUVEC cells compared to that of the wild type, collagen coating of wells, essential for HUVEC cell growth, complicated the analysis. In contrast, adherence of the Δace mutant to 3T6 cells (at an MOI of 1) was 71% of the wild-type level, that of the ΔebpABC mutant was 66% of the wild-type level, and that of the ΔaceΔebpABC mutant was 51% of the wild-type level (P < 0.0001) (Fig. 5). Thus, 3T6 cell adherence data provide supporting evidence for an additive effect of Ace and Ebp pili on collagen adherence.
Fig. 5.
Adherence of OG1RF and its Δace, ΔebpABC, and ΔaceΔebpABC deletion mutants to the collagen-secreting cell line 3T6. Each bar represents mean percentages ± SD of bacteria adhering to cells coating 9 wells from three independent experiments. Data for each strain were normalized with respective data of OG1RF, which was set to 100%. Mean adherence percentages of different strains were compared using ANOVA and Bonferroni's post test. *, P < 0.001 versus the wild type by post hoc test; **, P < 0.001 versus the wild type and P < 0.01 versus the Δace mutant by post hoc test.
Based on the finding that group B streptococci utilize the major pilin protein to cross the epithelial barrier (43), we also performed transcytosis experiments with the above mutants, using a two-chamber system to assess translocation across monolayers of polarized human colon carcinoma-derived T84 cells (60, 61). Our results showed no difference between the wild-type and ebp and/or ace mutants in this assay (data not shown).
Contribution of Ebp pili to adherence of E. faecalis OG1RF to fibrinogen, a major serum and ECM component.
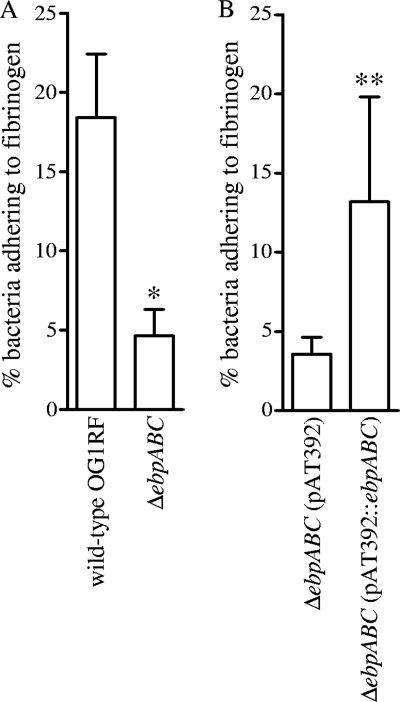
Adhesins of streptococci and staphylococci that mediate binding to fibrinogen (e.g., MSCRAMMs ClfA and FnbpA of Staphylococcus aureus) have been shown to be important for pathogenesis in experimental infective endocarditis (reviewed in reference 31). Since Ebp pili are important for E. faecalis infective endocarditis, we sought to validate our preliminary observation described above with the ebp polar mutants (see Fig. S1 in the supplemental material) and performed fibrinogen adherence studies using our nonpolar ebp deletion mutant and its complementation construct. The ebpABC deletion mutant grown in BHI-S showed a 75% decrease (P < 0.0001) in the percentage of cells adhering to fibrinogen compared to that in wild-type OG1RF (Fig. 6A). The complementation construct showed partial restoration of this phenotype (P < 0.0001), confirming that the ebp deletion is responsible for this reduced fibrinogen adherence (Fig. 6B). The greater reduction in fibrinogen adherence of the ebpABC mutant versus mutants of two other fibrinogen binding MSCRAMM-encoding genes (fss1 and fss2), which showed ≤2-fold reduction (48), raises the possibility that some of these MSCRAMMs may be displayed by Ebp pili or that pili facilitate cell contact, allowing the other adhesins to then bind fibrinogen. Another possibility is that the ebpABC deletion, which abolishes pilins and pili, leads to significant restructuring of the cell wall in the deletion mutant and may change the overall population or position of proteins displayed on the cell surface, including the fibrinogen-binding MSCRAMMs.
Fig. 6.
Effect of ebpABC deletion on the adherence of OG1RF to immobilized fibrinogen. Bars represent mean percentages ± SD of 35S-labeled cells adhering to 16 fibrinogen-coated wells from four independent experiments. BSA was used as a negative control. Mean adherence percentages of the wild type and the ΔebpABC mutant were compared using unpaired t test. *, P < 0.0001 versus wild type; **, P < 0.001 versus vector-only control.
E. faecalis adherence to fibrinogen is reduced by antibodies against Ebp pilins.
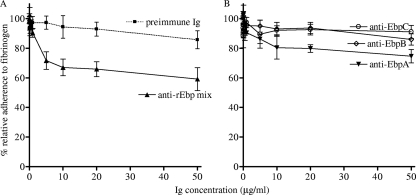
The ability of an affinity-purified anti-rEbpA, -B, and -C polyclonal antibody mix to inhibit E. faecalis adherence to fibrinogen was tested using wild-type OG1RF. The anti-EbpA, -B, and -C immunoglobulin mix at a 10-μg/ml concentration reduced adherence of OG1RF compared to preimmune immunoglobulins (25% reduction; see Fig. S5A in the supplemental material; P = 0.002). In subsequent inhibition experiments, we tested a range of concentrations (0.05 to 50 μg per ml) of this antibody mix and found that 1 μg of antibodies per ml is the minimum required concentration (exhibiting 7% reduction adherence to fibrinogen), whereas preimmune serum had no effect on adherence at this concentration (Fig. 7A). Furthermore, the inhibitory effect was concentration dependent, albeit partially (maximum 41% reduction), and showed saturation at 10 μg of antibodies per ml (Fig. 7A). We then tested immunoglobulins against each individual pilus subunit in these inhibition assays. Our results found that anti-rEbpA immunoglobulins also caused some reduction in adherence (Fig. 7B) but were less potent than the anti-rEbp antibody mix (e.g., at 5 μg of antibodies per ml, anti-rEbpA antibody caused 14% reduction, compared to anti-rEbp antibody mix, which showed 28% reduction). The difference in reduction seen between anti-rEbpA antibody and anti-rEbp antibody mix was consistent over the concentrations tested. In contrast, anti-rEbpB and anti-rEbpC antibodies had almost no effect on adherence over the range of concentrations tested. The partial inhibition that was observed may be caused by functional redundancy in the fibrinogen adherence phenotype of E. faecalis OG1RF; other possibilities that might explain the partial inhibition include a low concentration of antibodies against epitopes within the binding region of assembled pili in these polyclonals or that antigenic epitopes of the individual rEbp subunits (which did not bind fibrinogen) may be hidden in native pili. We also tested the anti-Ebp antibody mix for its effect on collagen adherence and observed little to no inhibition (see Fig. S5 in the supplemental material). The finding that anti-rEbp pilin antibodies were able to reduce adherence of E. faecalis to fibrinogen raises the possibility that these antibodies may have some potential as prophylactic agents, perhaps in combination with other protective antibodies.
Fig. 7.
Effect of anti-rEbp antibodies on adherence of OG1RF to fibrinogen. (A) Effect of anti-rEbp antibody mix. (B) Effect of individual anti-rEbpA, anti-rEbpB, and anti-rEbpC antibodies. 35S-labeled OG1RF cells were preincubated with different concentrations of individual anti-rEbp antibodies or an equal mixture of affinity-purified anti-rEbpA, anti-rEbpB, and anti-rEbpC antibodies. Data were normalized with respective data of bacteria adhering in the absence of immunoglobulins and expressed as percentages. Data points represent the means ± standard deviations (results are from two to three independent experiments). Ig, immunoglobulin.
To determine if there is a correlation between the variable fibrinogen adherence of diverse E. faecalis isolates (38) and Ebp pilus surface expression, we plotted fibrinogen adherence percentages of 22 E. faecalis strains as a function of their levels (OD405) of surface-exposed Ebp (see companion paper [36]). Regression analysis showed no correlation (r = 0.09 and r = 0.2, for EbpC and EbpA, respectively; see Fig. S6A and B in the supplemental material), perhaps explained by our previous observation that E. faecalis contains additional fibrinogen binding adhesins.
Attenuation of the Δace and ΔaceΔebpABC mutants in a murine UTI model.
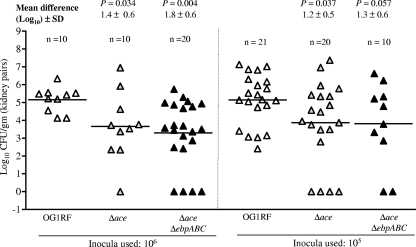
Since adherence of E. faecalis to glycoprotein (such as collagen)- and proteoglycan-rich glomerular basement membrane in the kidney could be important for causing UTIs, we (54) and others (18) previously adapted a murine UTI model using OG1 derivatives. Those results showed that OG1RF grown in BHI was less infective in the UTI model than BHI-S-grown OG1RF (53). We have also demonstrated a role for ebp pili in UTI by using pilus-deficient OG1RF (54). Recently, using another E. faecalis strain, JH2-2, and its isogenic ace mutant, Lebreton et al. (24) reported that ace is important for the ability of JH2-2 to cause infection in murine kidneys. Here, to test the effect of ace and ebp double deletion, mice were infected via intraurethral catheterization, as described earlier (54), and compared to our previous UTI results with an Ebp pilus-deficient construct of OG1RF (54); the OG1RFΔace mutant was also tested in this assay. The log10 CFU recovered from the kidney pairs of mice infected with wild-type OG1RF and its isogenic Δace and ΔaceΔebpABC mutants at 105- and 106-CFU inocula is shown in Fig. 8. A comparison of the mean log10 CFU of bacteria recovered from the kidneys showed that the differences between OG1RF and the Δace mutant (1.3 to 1.5 log10; P = 0.03 to 0.04) and between OG1RF and the ΔaceΔebpABC mutant (1.3 to 1.9 log10; P = 0.004 to 0.058) were statistically significant, while there were no significant differences between the Δace and ΔaceΔebpABC mutants (P = 0.6 and P = 0.95 with the two different inocula). Similarly, the mean CFU differences with comparable inocula between the previously published ebp allelic replacement mutant, TX5475 (54), and the ΔaceΔebpABC mutant (0.7 ± 0.8 log10 [P = 0.39] and 0.3 ± 1.1 [P = 0.77] log10 using 106- and 105-CFU inocula, respectively) were also nonsignificant. Although fewer bladders were infected than kidneys, the CFU differences between individual strains from bladders were similar to kidney infections (see Fig. S7 in the supplemental material). The modest reduction in infectivity by the ace plus ebp double deletion points toward the importance of additional factors, such as Epa polysaccharide, which we previously showed was important (9, 10, 54).
Fig. 8.
Effect of deletion of ace and double deletion of ace and ebpABC in a murine model of UTI (monoinfection using 106- and 105-CFU inocula). Data are expressed as log10 CFU/g of bacteria recovered from kidney homogenates 48 h after transurethral challenge. The log10 CFU from both kidneys were combined and averaged. Horizontal bars represent geometric means. Mean difference in CFU is given as log10 ± SD for the respective inocula. Mean log10 CFU of bacteria were analyzed for significance by unpaired t test. Results for the wild type versus ebp allelic replacement mutant TX5475 were reported in reference 54, and the CFU differences (of comparable inocula) between the ebp allelic replacement mutant and the ΔaceΔebpABC mutant were nonsignificant.
In summary, using isogenic mutants, we have identified both a fibrinogen and a collagen adhesion function for E. faecalis Ebp pili, which were shown previously to be important in experimental endocarditis and UTI models. Corroborating data from complementation, native pilus-enriched surface extracts, and a collagen-secreting cell line found that the collagen adherence phenotype of Ebp pili is independent of the previously described E. faecalis collagen-binding MSCRAMM, Ace. The almost complete elimination of fibrinogen adherence by the ebpABC mutant, while individual rEbp subunits did not bind fibrinogen, and also the observed partial inhibition by antibodies against recombinant Ebp proteins, raises different scenarios for Ebp pilus interactions with fibrinogen: (i) a direct interaction mediated only by the native form of assembled pili, independent of other fibrinogen-binding adhesins; (ii) indirect interaction (for example, Ebp pili might display other fibrinogen-binding adhesins or might initiate cell contact, thus allowing other adhesins to then bind fibrinogen); or (iii) perhaps the ebpABC deletion alters sorting of fibrinogen adhesins. The latter speculation is based in part on a recent report that showed secretion and sortase processing (of both SrtA and Bps targets) to occur together in E. faecalis (21); thus, the loss of major surface proteins, such as Ebp, might lead to asymmetric sorting and surface distribution of other fibrinogen adhesins. Our ongoing and future studies are focused on exploring such possibilities. Finally, our OG1RFΔace mutant data corroborate the previously demonstrated importance of ace in murine kidney infections using strain JH2-2 (24), and our new data from a double-deletion mutant of ace and ebp indicate no additive effect in this model. The rather modest reduction in UTI infectivity of the ace-ebp double mutant indicates the importance of additional bacterial components in this infection. Future studies of individual Ebp subunit deletion mutants will address the role of each individual pilin(s) in host cell-protein adherence, infectivity, biofilm, and pilus biogenesis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R37 AI47923 and R01 AI47923 from the Division of Microbiology and Infectious Diseases, NIAID, to B.E.M.
We thank Karen Jacques-Palaz for technical assistance.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 19 April 2011.
REFERENCES
- 1. Arias C. A., Murray B. E. 2009. Antibiotic-resistant bugs in the 21st century—a clinical superchallenge. N. Engl. J. Med. 360:439–443 [DOI] [PubMed] [Google Scholar]
- 2. Arias C. A., Murray B. E. 2008. Emergence and management of drug-resistant enterococcal infections. Expert Rev. Anti Infect. Ther. 6:637–655 [DOI] [PubMed] [Google Scholar]
- 3. Arthur M., Depardieu F., Snaith H. A., Reynolds P. E., Courvalin P. 1994. Contribution of VanY d,d-carboxypeptidase to glycopeptide resistance in Enterococcus faecalis by hydrolysis of peptidoglycan precursors. Antimicrob. Agents Chemother. 38:1899–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bourgogne A., et al. 2008. Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF. Genome Biol. 9:R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carniol K., Gilmore M. S. 2004. Signal transduction, quorum-sensing, and extracellular protease activity in Enterococcus faecalis biofilm formation. J. Bacteriol. 186:8161–8163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choudhury T., Singh K. V., Sillanpaa J., Nallapareddy S. R., Murray B. E. 2011. Importance of two Enterococcus faecium loci encoding Gls-like proteins for in vitro bile salts stress response and virulence. J. Infect. Dis. 203:1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao P., et al. 2010. Enterococcus faecalis rnjB is required for pilin gene expression and biofilm formation. J. Bacteriol. 192:5489–5498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gilmore M. S., Coburn P. S., Nallapareddy S. R., Murray B. E. 2002. Enterococcal virulence. In Gilmore M. S., Clewell D., Courvalin P., Dunny G. M., Murray B. E., Rice L.(ed.),The enterococci: pathogenesis, molecular biology, antibiotic resistance and infection control. ASM Press, Washington, DC [Google Scholar]
- 9. Guiton P. S., Hung C. S., Hancock L. E., Caparon M. G., Hultgren S. J. 2010. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infect. Immun. 78:4166–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guiton P. S., et al. 2009. Contribution of autolysin and sortase A during Enterococcus faecalis DNA-dependent biofilm development. Infect. Immun. 77:3626–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hendrickx A. P., et al. 2009. SgrA, a nidogen-binding LPXTG surface adhesin implicated in biofilm formation, and EcbA, a collagen binding MSCRAMM, are two novel adhesins of hospital-acquired Enterococcus faecium. Infect. Immun. 77:5097–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hendrickx A. P., Willems R. J., Bonten M. J., van Schaik W. 2009. LPxTG surface proteins of enterococci. Trends Microbiol. 17:423–430 [DOI] [PubMed] [Google Scholar]
- 13. Heying R., van de Gevel J., Que Y. A., Moreillon P., Beekhuizen H. 2007. Fibronectin-binding proteins and clumping factor A in Staphylococcus aureus experimental endocarditis: FnBPA is sufficient to activate human endothelial cells. Thromb. Haemost. 97:617–626 [PubMed] [Google Scholar]
- 14. Hilleringmann M., et al. 2008. Pneumococcal pili are composed of protofilaments exposing adhesive clusters of Rrg A. PLoS Pathog. 4:e1000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hubble T. S., Hatton J. F., Nallapareddy S. R., Murray B. E., Gillespie M. J. 2003. Influence of Enterococcus faecalis proteases and the collagen-binding protein, Ace, on adhesion to dentin. Oral Microbiol. Immunol. 18:121–126 [DOI] [PubMed] [Google Scholar]
- 16. Huycke M. M., Sahm D. F., Gilmore M. S. 1998. Multiple-drug-resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 4:239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Izore T., et al. 2010. Structural basis of host cell recognition by the pilus adhesin from Streptococcus pneumoniae. Structure 18:106–115 [DOI] [PubMed] [Google Scholar]
- 18. Kau A. L., et al. 2005. Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun. 73:2461–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kemp K. D., Singh K. V., Nallapareddy S. R., Murray B. E. 2007. Relative contributions of Enterococcus faecalis OG1RF sortase-encoding genes, srtA and bps (srtC), to biofilm formation and a murine model of urinary tract infection. Infect. Immun. 75:5399–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kline K. A., Dodson K. W., Caparon M. G., Hultgren S. J. 2010. A tale of two pili: assembly and function of pili in bacteria. Trends Microbiol. 18:224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kline K. A., et al. 2009. Mechanism for sortase localization and the role of sortase localization in efficient pilus assembly in Enterococcus faecalis. J. Bacteriol. 191:3237–3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Konto-Ghiorghi Y., et al. 2009. Dual role for pilus in adherence to epithelial cells and biofilm formation in Streptococcus agalactiae. PLoS Pathog. 5:e1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kristich C. J., Chandler J. R., Dunny G. M. 2007. Development of a host-genotype-independent counterselectable marker and a high-frequency conjugative delivery system and their use in genetic analysis of Enterococcus faecalis. Plasmid 57:131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lebreton F., et al. 2009. ace, which encodes an adhesin in Enterococcus faecalis, is regulated by Ers and is involved in virulence. Infect. Immun. 77:2832–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 26. Maisey H. C., Hensler M., Nizet V., Doran K. S. 2007. Group B streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. J. Bacteriol. 189:1464–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mandlik A., Swierczynski A., Das A., Ton-That H. 2008. Pili in Gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends Microbiol. 16:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manetti A. G., et al. 2007. Streptococcus pyogenes pili promote pharyngeal cell adhesion and biofilm formation. Mol. Microbiol. 64:968–983 [DOI] [PubMed] [Google Scholar]
- 29. Mohamed J. A., Huang W., Nallapareddy S. R., Teng F., Murray B. E. 2004. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 72:3658–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mora M., et al. 2005. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc. Natl. Acad. Sci. U. S. A. 102:15641–15646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moreillon P., Que Y. A., Bayer A. S. 2002. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect. Dis. Clin. North Am. 16:297–318 [DOI] [PubMed] [Google Scholar]
- 32. Murray B. E. 1990. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3:46–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. 1990. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J. Clin. Microbiol. 28:2059–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murray B. E., Weinstock G. M. 1999. Enterococci: new aspects of an old organism. Proc. Assoc. Am. Physicians 111:328–334 [DOI] [PubMed] [Google Scholar]
- 35. Nakata M., et al. 2009. Mode of expression and functional characterization of FCT-3 pilus region-encoded proteins in Streptococcus pyogenes serotype M49. Infect. Immun. 77:32–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nallapareddy S. R., et al. 2011. Conservation of Ebp-type pilus genes among enterococci and demonstration of their role in adherence of Enterococcus faecalis to human platelets. Infect. Immun. 79:2911–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nallapareddy S. R., Murray B. E. 2006. Ligand-signaled upregulation of Enterococcus faecalis ace transcription, a mechanism for modulating host-E. faecalis interaction. Infect. Immun. 74:4982–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nallapareddy S. R., Murray B. E. 2008. Role played by serum, a biological cue, in the adherence of Enterococcus faecalis to extracellular matrix proteins, collagen, fibrinogen, and fibronectin. J. Infect. Dis. 197:1728–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nallapareddy S. R., Qin X., Weinstock G. M., Hook M., Murray B. E. 2000. Enterococcus faecalis adhesin, Ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect. Immun. 68:5218–5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nallapareddy S. R., et al. 2006. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J. Clin. Invest. 116:2799–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nallapareddy S. R., Weinstock G. M., Murray B. E. 2003. Clinical isolates of Enterococcus faecium exhibit strain-specific collagen binding mediated by Acm, a new member of the MSCRAMM family. Mol. Microbiol. 47:1733–1747 [DOI] [PubMed] [Google Scholar]
- 42. Paterson G. K., Orihuela C. J. 2010. Pneumococcal microbial surface components recognizing adhesive matrix molecules targeting of the extracellular matrix. Mol. Microbiol. 77:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pezzicoli A., et al. 2008. Pilus backbone contributes to group B Streptococcus paracellular translocation through epithelial cells. J. Infect. Dis. 198:890–898 [DOI] [PubMed] [Google Scholar]
- 44. Piroth L., et al. 2008. The fibrinogen- and fibronectin-binding domains of Staphylococcus aureus fibronectin-binding protein A synergistically promote endothelial invasion and experimental endocarditis. Infect. Immun. 76:3824–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Podbielski A., Woischnik M., Leonard B. A., Schmidt K. H. 1999. Characterization of nra, a global negative regulator gene in group A streptococci. Mol. Microbiol. 31:1051–1064 [DOI] [PubMed] [Google Scholar]
- 46. Rivas J. M., Speziale P., Patti J. M., Hook M. 2004. MSCRAMM—targeted vaccines and immunotherapy for staphylococcal infection. Curr. Opin. Drug Discov. Devel. 7:223–227 [PubMed] [Google Scholar]
- 47. Rosch J. W., Caparon M. G. 2005. The ExPortal: an organelle dedicated to the biogenesis of secreted proteins in Streptococcus pyogenes. Mol. Microbiol. 58:959–968 [DOI] [PubMed] [Google Scholar]
- 48. Sillanpaa J., et al. 2009. A family of fibrinogen-binding MSCRAMMs from Enterococcus faecalis. Microbiology 155:2390–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sillanpaa J., et al. 2008. Identification and phenotypic characterization of a second collagen adhesin, Scm, and genome-based identification and analysis of 13 other predicted MSCRAMMs, including four distinct pilus loci, in Enterococcus faecium. Microbiology 154:3199–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sillanpaa J., et al. 2010. Characterization of the ebp pilus-encoding operon of Enterococcus faecium and its role in biofilm formation and virulence in a murine model of urinary tract infection. Virulence 1:236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sillanpaa J., Xu Y., Nallapareddy S. R., Murray B. E., Hook M. 2004. A family of putative MSCRAMMs from Enterococcus faecalis. Microbiology 150:2069–2078 [DOI] [PubMed] [Google Scholar]
- 52. Singh K. V., Coque T. M., Weinstock G. M., Murray B. E. 1998. In vivo testing of an Enterococcus faecalis efaA mutant and use of efaA homologs for species identification. FEMS Immunol. Med. Microbiol. 21:323–331 [DOI] [PubMed] [Google Scholar]
- 53. Singh K. V., Lewis R. J., Murray B. E. 2009. Importance of the epa locus of Enterococcus faecalis OG1RF in a mouse model of ascending urinary tract infection. J. Infect. Dis. 200:417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Singh K. V., Nallapareddy S. R., Murray B. E. 2007. Importance of the ebp (endocarditis- and biofilm-associated pilus) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J. Infect. Dis. 195:1671–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Singh K. V., Nallapareddy S. R., Sillanpaa J., Murray B. E. 2010. Importance of the collagen adhesin Ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog. 6:e1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soriani M., Telford J. L. 2010. Relevance of pili in pathogenic streptococci pathogenesis and vaccine development. Future Microbiol. 5:735–747 [DOI] [PubMed] [Google Scholar]
- 57. Speziale P., et al. 2009. Structural and functional role of Staphylococcus aureus surface components recognizing adhesive matrix molecules of the host. Future Microbiol. 4:1337–1352 [DOI] [PubMed] [Google Scholar]
- 58. Ton-That H., Marraffini L. A., Schneewind O. 2004. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim. Biophys. Acta 1694:269–278 [DOI] [PubMed] [Google Scholar]
- 59. Wilson K. 1994. Preparation of genomic DNA from bacteria, p. 2.4.1–2.4.2 In Ausubel F. M., Brent R., Kingston R. E., David D. M., Scidman J. G., Smith J. A., Struhl K. (ed.),Current protocols in molecular biology. Green Publishing Associates, Brooklyn, NY [Google Scholar]
- 60. Zeng J., Teng F., Murray B. E. 2005. Gelatinase is important for translocation of Enterococcus faecalis across polarized human enterocyte-like T84 cells. Infect. Immun. 73:1606–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zeng J., Teng F., Weinstock G. M., Murray B. E. 2004. Translocation of Enterococcus faecalis strains across a monolayer of polarized human enterocyte-like T84 cells. J. Clin. Microbiol. 42:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.