Abstract
In areas where Plasmodium falciparum is endemic, pregnancy is associated with accumulation of infected red blood cells (RBCs) in the placenta, a condition referred to as placental malaria (PM). Infants born to PM-positive mothers are at an increased risk of malaria, which is putatively related to the transplacental passage of parasite-derived antigens, with consequent tolerization of the fetal immune system. Here we addressed the impact of PM on the regulation of neonatal T cell responses. We found that the frequency of regulatory CD25+ CD127−/low Foxp3+ CD4+ T cells was significantly decreased in neonates born to mothers with high levels of P. falciparum-induced placental inflammation, consisting mainly of primigravid mothers. However, at the individual level, the ratio between regulatory and effector (CD25+ CD127+ Foxp3−) CD4+ T cells was unaffected by PM. In addition, parasite-induced CD4+ T cell activation and production of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and IL-10 were strongly reduced in neonates born to PM-positive mothers. Thus, our results show that active PM at delivery is associated with a marked suppression of P. falciparum-specific cellular neonatal immune responses, affecting secretion of both pro- and anti-inflammatory cytokines. Additionally, our results suggest that, as in adults, effector and regulatory CD4+ T cell populations are tightly coregulated in all neonates, irrespective of the maternal infection status.
INTRODUCTION
Malaria remains the most important parasitic disease in the world. In 2008, there were an estimated 247 million malaria cases, causing nearly 1 million deaths, with 85% of them being of children under 5 years living in sub-Saharan Africa (36). Acquiring a better knowledge of the factors that contribute to this elevated susceptibility of the youngest children to malaria is thus still a priority.
Several epidemiological studies have shown that Plasmodium falciparum infection during pregnancy can be considered a risk factor for malaria in the offspring (8, 18, 21, 23, 26, 30). A particular feature of P. falciparum infection in pregnant women is the accumulation of infected red blood cells (iRBC) within the placenta, which is referred to as placental malaria (PM). iRBC adhere to placental cells of fetal origin, which often results in densities of parasites higher than those in peripheral blood. Accumulation of leukocytes, including monocytes, in the placental intervillous spaces and the local production of proinflammatory cytokines also occur in response to infection and are associated with low birth weight and maternal anemia (10). Primigravid mothers living in areas of endemicity are the population most at risk of developing PM. They have more frequent and severe placental infections, characterized by higher placental parasitemia and inflammation, than multigravid women (10). This is likely because primigravid women lack protective antibody- and cell-dependent immunity to placenta-adherent P. falciparum strains (reviewed in references 10 and 27).
PM is associated with an increased risk of anemia and infection in infancy (8, 18, 21, 26, 30). Paradoxically, however, children of P. falciparum-infected primigravid mothers are reported to be at lower risk of infection during infancy than those born either to P. falciparum-infected multigravid or to uninfected primigravid mothers (26, 30). The basis for the modified susceptibility to malaria of infants born to mothers with PM thus far remains unclear. PM can be associated with a decreased integrity of the placental barrier that could potentially lead to congenital transfer or could facilitate the passage of parasite-derived soluble molecules (9). Recent data indeed suggest that antibody-dependent transfer of parasite antigens could occur in vivo (24). Moreover, P. falciparum-specific B and T cells have been detected in cord blood mononuclear cells (CBMC) from neonates born to mothers living in areas where malaria is endemic, suggesting that exposure to parasite antigens occurs in utero (17, 22, 23, 25). Given the fact that the fetal immune system is prone to tolerance, it could be expected that in utero encounter with parasite antigens would lead to tolerogenic responses associated with an anti-inflammatory cytokine profile. However, both pro- and anti-inflammatory cytokines are produced by CBMC in response to P. falciparum antigens in vitro, with Th1-type responses more often reported in neonates born to primigravid infected mothers (17, 22, 25). These quite heterogeneous profiles of neonatal parasite-specific cytokine responses may be explained by the immaturity of the fetal immune system related to the gestational age of mothers when infection occurs and also by the dose of antigen to which the fetus is exposed (reviewed in reference 5). Nevertheless, when addressing more specifically neonates born to mothers with PM at delivery, a decreased Th1 response and a bias toward the production of interleukin-10 (IL-10) by CBMC in response to P. falciparum antigens have been reported (7, 16, 21). Moreover, and in line with tolerance induction, depletion of CD25+ CD4+ cells led to an increased P. falciparum-specific gamma interferon (IFN-γ) response by CBMC of neonates born to mothers with PM (4, 6), and addition of IL-2 and IL-15 to CBMC led to a recovery of parasite-induced proliferation in some neonates (21). Taken together, these data support the idea that PM affects the balance between effector and regulatory P. falciparum-specific responses in the neonate in favor of the latter.
In adults, an increase in anti-inflammatory responses commonly associated with regulatory T cells, such as transforming growth factor β (TGF-β), has been associated with a less efficient control of P. falciparum parasitemia (34). In addition, high numbers of CD25high CD4+ T cells in peripheral blood of patients correlated with an increased risk of clinical malaria (32). Also, it was proposed that the higher resistance to malaria of Fulani people, an ethnic group from West Africa, could be related to a functional deficit of regulatory T cells (33). Thus, in malaria, as in many infectious diseases, the balance between effector and regulatory responses seems to be a key factor in the outcome of the infection and disease (3, 12, 14, 28).
Here we studied the impact of PM on this balance in the neonate. For this purpose, we characterized the immunological cellular profiles of neonates born to mothers with or without PM at delivery in southern Benin, where P. falciparum malaria is endemic. We measured the frequencies of naive versus memory CD4 T cells and of effector versus regulatory Foxp3-expressing neonatal CD4 T cells. We also analyzed short-term P. falciparum-specific pro- and anti-inflammatory cytokine responses of CBMC after coculture with live parasites, an experimental setup that more accurately reflects the physiology of the in vivo immune response.
MATERIALS AND METHODS
Study site, study population, and sample collection.
This study took place in Cotonou, located in the coastal southern part of Benin, where malaria transmission is perennial (with two peaks during the rainy seasons, April to July and mid-September to November) and P. falciparum is the principal malaria species. The study was conducted at the Hôpital de la Mère et de l'Enfant Lagune (HOMEL) in Cotonou and at the Health Center of Houenoussou in the suburb of Cotonou. Samples were collected from August to December 2007 and from June to September 2008. Written informed consent was obtained from mothers before inclusion, and the study was approved by the Ethics Committee of the Faculty of Health Sciences of the Abomey-Calavi University, Benin.
Pregnant women responded to a questionnaire designed to capture personal information (age, gravidity, malaria history during pregnancy, prophylaxis for malaria during pregnancy, and use of bed nets). When available, the information was verified in the mother's own medical records. When the HIV status was known, HIV-infected women were excluded. Pregnancies with problems, twins, and preterm births were also excluded. To identify women infected with P. falciparum, a rapid diagnostic test (RDT) for P. falciparum HRPII (Cypress Diagnostics, Langdorp, Belgium) was performed before delivery. In addition, blood smear examination did not give evidence of other malaria species. All women identified with a P. falciparum infection were given a standard treatment course of quinine after delivery. For each infected woman, an RDT-negative control woman was enrolled sequentially. Control women were selected when declaring that they had no malaria episode during pregnancy and were matched for age (±2 years) and gravidity (±1) with PM-positive mothers. Characteristics of the study population are summarized in Table 1.
Table 1.
Characteristics of the study population
| Characteristic | Value for group |
||
|---|---|---|---|
| All (n = 54) | Placental malaria negative (n = 25) | Placental malaria positive (n = 29) | |
| Mean (SD) age of mothers, yr | 25 (5.7) | 24 (5.2) | 25 (6.1) |
| No. primigravid mothers/no. multigravid mothers | 25/29 | 10/15 | 15/14 |
| No. with bed net use/no. without bed net use | 53/1 | 25/0 | 28/1 |
| No. receiving SP-IPTp/no. not receiving SP-IPTpa | 50/4 | 24/1 | 26/3 |
Sulfadoxine-pyrimethamine (SP) was implemented as the intermittent preventive treatment for malaria in pregnancy (IPTp) in Benin in 2006.
Immediately after delivery, 20 to 40 ml of blood from the umbilical cord was recovered in sodium heparin Vacutainer tubes (BD Biosciences). Placental blood smears and impression smears of the maternal side of placental tissue were made. Samples of peripheral blood from healthy Beninese adults living in the same area were obtained from the Blood Bank of the Centre National Hospitalo-Universitaire Hubert Koutoukou-Maga in Cotonou.
Determination of P. falciparum infection status of the mothers.
For each RDT-positive woman, microscopic examination of Giemsa-stained placental smears was carried out to determine parasite density (expressed as the percentage of iRBC detected in a total of 500 RBC), as well as the number of monocytes containing malarial pigment in 20 high-power fields. RDT-positive women with an active placental infection at delivery, defined by the presence of iRBC or iRBC plus monocytes containing malarial pigment, were included in the PM-positive group. The absence of iRBC and monocytes containing pigment was verified on placental smears before mothers were included in the PM-negative group. Parasitological characteristics of PM-positive mothers are summarized in Table 2.
Table 2.
Placental parasitemia and inflammation in PM-positive mothers
| Condition | Mean level (interquartile)a in: |
P valueb | ||
|---|---|---|---|---|
| All PM-positive mothers | Primigravid mothers (n = 15) | Multigravid mothers (n = 14) | ||
| Placental parasitemia | 4.52 (9.79) | 9 (7.98) | 3.39 (14.25) | 0.4321 |
| Placental inflammation | 11 (25) | 27 (37.75) | 3 (13) | 0.0056 |
Parasitemia is expressed as the percentage of iRBC among total RBC (iRBC plus uRBC) on placental smears. Inflammation is expressed as the number of monocytes containing malarial pigment per 20 high-powered fields on placental smears.
For primigravid group compared to multigravid group (Mann-Whitney U test).
Plasma collection and isolation of CBMC and PBMC.
Cord and adult peripheral blood samples were centrifuged at 500 × g at room temperature (RT) for 15 min, and plasma samples were aliquoted and stored at −80°C until use. Cord blood mononuclear cells (CBMC) and peripheral blood mononuclear cells (PBMC) were isolated on Ficoll-Hypaque (Pharmacia Uppsala, Sweden) and washed twice with sterile phosphate-buffered saline (PBS)–3% fetal bovine serum (FBS) before cells were counted.
Flow cytometry analyses.
For surface staining, cells were stained for 20 min at 4°C in the dark with different monoclonal antibodies (MAbs) specific for surface markers (MAbs were purchased from BD Biosciences unless otherwise specified): anti-CD25–fluorescein isothiocyanate (FITC) (M-A251), anti-CD127–phycoerythrin (PE) (hIL-7R-M21), anti-CD4–peridinin chlorophyll protein (PerCP) (SK3), anti-CCR7–PE (150503; R&D Systems), anti-CD45RA–biotin (HI100), or anti-CD45RO–biotin (UCHL1). Cells were then washed with PBS–3% FBS. Biotinylated MAbs were revealed with streptavidin-allophycocyanin (APC) (BD Biosciences). Anti-Foxp3–APC intranuclear staining was performed according to the manufacturer's protocol (PCH101; eBiosciences). For intracellular staining of cytokines, cells were fixed in PBS containing 2% paraformaldehyde (Sigma) for 30 min at RT in the dark. Cells were then washed with PBS–3% FBS before being incubated in PermWash solution (BD Biosciences) for 30 min at RT in the dark with different antibodies coupled to PE and purchased from BD Biosciences: rat IgG1 or mouse IgG1 isotype control MAbs, anti-IL-10 MAb (JES3-19F1), anti-IFN-γ MAb (B27), or anti-TNF-α MAb (Mab11). Cells were then washed with PermWash and PBS–3% FBS before data acquisition on a 4-color FACSCalibur (BD Biosciences) with the CellQuestPro software.
Live Plasmodium falciparum-infected RBC.
The preparation and cryopreservation of iRBC and uninfected RBC (uRBC) have been described in detail elsewhere (15). Briefly, highly purified populations of late trophozoite/schizont-infected red blood cells were prepared from asynchronous cultures of asexual P. falciparum at high parasitemia (15 to 20%) by density gradient centrifugation, washed, counted, and cryopreserved as aliquots prior to use in stimulation assays with CBMC/PBMC. Cryopreserved aliquots of mock-cultured uRBC were prepared in the same way.
Coculture with live P. falciparum-infected RBC.
Fresh CBMC or PBMC were cultured in 24-well plates at a concentration of 1 × 106 cells/ml of complete medium (RPMI 1640 containing 25 mM HEPES, supplemented with 2% heat-inactivated FBS, 2 mM l-Gln, 100 IU penicillin-streptomycin, and 1 mM sodium pyruvate). Frozen P. falciparum-iRBC were rapidly thawed with complete medium and immediately added to CBMC to reach a final ratio of 1 CBMC to 1 iRBC. The same protocol was applied to control CBMC cultured with uRBC. After 24 h of culture, an aliquot of culture supernatant was harvested and stored at −80°C. After 35 h of culture, monensin (Golgi Stop; BD Biosciences) (used according to the manufacturer's instructions) and 10 μg/ml of brefeldin A (Sigma Aldrich) were added to the wells. Five hours later, supernatants were harvested, aliquoted, and stored at −80°C. Cells were then washed once in PBS–3% FBS before performing surface and intracellular staining.
Multiplex analysis of supernatant cytokine concentrations.
Concentrations of IFN-γ, IL-6, IL-2, IL-10, IL-4, and TNF-α in culture supernatants were determined with the Milliplex kit from Millipore. Supernatants were diluted 1:5 and 1:10. The thresholds of sensitivity were as follows IFN-γ, 0.64 pg/ml; IL-2, 3.2 pg/ml; IL-4, 3.2 pg/ml; IL-6, 3.2 pg/ml; IL-10, 0.64 pg/ml; and TNF-α, 0.64 pg/ml. Samples were analyzed by immunoassay using flowmetric Luminex Bio-plex technology (Bio-Rad).
Statistical analyses.
For statistical analyses of differences between two groups, we used the nonparametric Mann-Whitney U test. For analyses of associations between continuous variables, we used the nonparametric Spearman rank correlation test. Statistical analyses were done with Prism 4.0 (GraphPad Software, Inc.), and a P value of <0.05 was considered significant.
RESULTS
PM does not alter the frequencies of naive and memory neonatal CD4 T cells.
Since the neonatal immune system is essentially naive, we asked whether we could detect signs of cell activation in the circulating CD4 T cell compartment in neonates born to PM-positive mothers. We assessed by flow cytometry the expression of CD45RA, CD45RO, and CCR7 by CD4+ T cells in CBMC (see Fig. S1A in the supplemental material). We found no difference in the frequency of naive (CD45RA+) or memory (CD45RO+) CD4+ T cells between neonates born to PM-negative and PM-positive mothers (see Fig. S1B in the supplemental material). Furthermore, the frequencies of central memory (CCR7+ CD45RA−) and effector memory (CCR7− CD45RA+) CD4+ T cells were also similar in the two groups (data not shown). There was also no difference in the frequencies of CD45RA+ or CD45RO+ CD4+ T cells in neonates born to either primigravid or multigravid mothers with or without PM (see Fig. S1C in the supplemental material). Finally, we found no association between the levels of placental parasitemia or placental inflammation and the frequency of CD45RA+ or CD45RO+ neonatal CD4 T cells (data not shown; see Fig. S1D in the supplemental material).
Placental inflammation is associated with a lower frequency of Treg cells in neonates.
It has been reported that neonates born to PM-positive mothers had increased frequencies of circulating CD25high CD4+ T cells (6). To better discriminate effector T (Teff) from regulatory T (Treg) cells, we determined by flow cytometry the frequencies of these two populations (defined by the phenotypes CD25+ CD127+ Foxp3− and CD25+ CD127−/low Foxp3+, respectively) among CD4+ T cells on freshly isolated CBMC (Fig. 1A) (19, 31). The frequencies of these two CD4 T cell subsets were similar in neonates born to PM-negative and PM-positive mothers (Fig. 1B). When these groups were discriminated according to the mother's gravidity, there was also no significant difference in the frequency of Teff cells (Fig. 1C, left panel). In contrast, for neonates born to primigravid mothers, the frequency of Treg cells tended to be lower in the PM-positive group, although the differences were of borderline significance (Fig. 1C, right panel) (P = 0.069 by Mann-Whitney U test). However, no such trend was observed in neonates born to multigravid mothers (Fig. 1C, right panel).
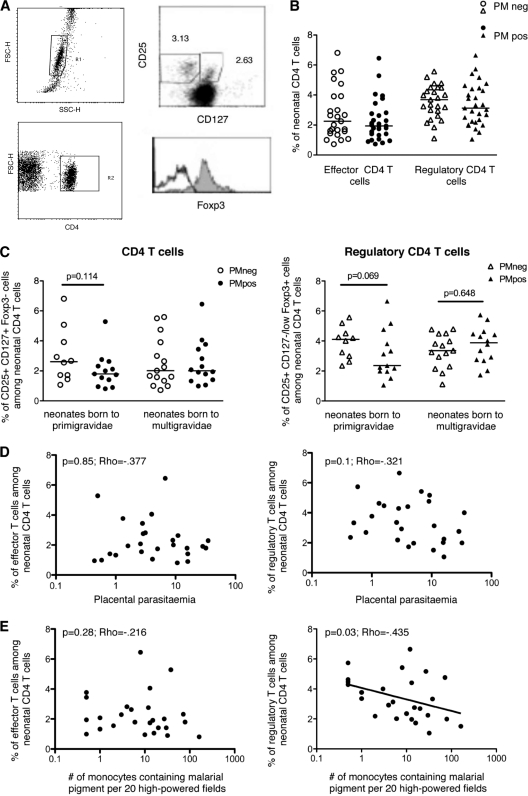
Fig. 1.
P. falciparum-associated placental inflammation and, to a lesser extent, mother's gravidity are associated with a lower frequency of regulatory T cells in the neonate. (A) FACS dot plot and histogram showing a representative analysis. Among lymphocytes (upper left panel), CD4+ T cells were selected (lower left panel) and analyzed for the expression of CD25 and CD127 (upper right panel), together with Foxp3 (lower right panel) among effector CD4+ CD25+ CD127+ (empty histogram) and regulatory CD4+ CD25+ CD127−/low (filled histogram) T cells. Numbers represent percentages. (B) Percentages of effector (CD25+ CD127+ Foxp3−) and regulatory (CD25+ CD127−/low Foxp3+) cells among neonatal CD4+ T cells were determined by FACS analysis on fresh CBMC from neonates born to PM-negative (n = 25) and PM-positive (n = 29) mothers. Individual (symbols) and median (bars) values are shown. (C) The same results are shown according to mothers' gravidity (primigravid women, n = 10 PM negative [PMneg] and n = 13 PM positive [PMpos]; multigravid women, n = 15 PMneg and n = 14 PMpos). Individual (symbols) and median (bars) values are shown. Significance was determined by the Mann-Whitney U test. (D and E) Placental parasitemia (D) and P. falciparum-induced placental inflammation (defined as the number of monocytes containing malarial pigment) (E) were determined on placental smears of PM-positive women and plotted against the frequencies of neonatal effector (left panels) or regulatory (right panels) CD4+ T cells (n = 26). Significance was determined by the Spearman correlation test.
For neonates born to PM-positive mothers, we found no correlation between the frequency of Teff cells and levels of placental parasitemia or placental inflammation (Fig. 1D and E, left panels). Interestingly, while the frequency of Treg cells did not vary according to placental parasitemia (Fig. 1D, right panel), it decreased significantly as the level of placental inflammation increased (Fig. 1E, right panel).
Thus, the only significant alteration we detected was the low frequency of Treg cells in cases of PM with high placental inflammation at delivery.
Impact of PM on the systemic balance between regulatory and effector T cells.
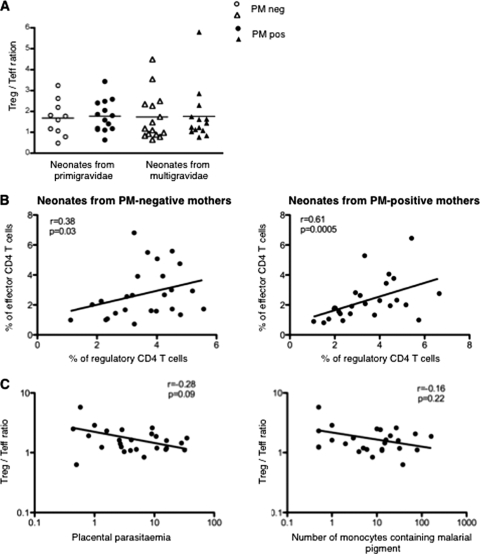
When considering the global Treg/Teff cell ratio, we found no difference between neonates born to primigravid or multigravid women with or without PM (Fig. 2A). This suggested that neonates with the lowest frequency of Treg cells also had the lowest frequency of Teff cells. Indeed, a positive correlation was found between frequencies of Treg and Teff cells in neonates born to PM-negative or PM-positive mothers (Fig. 2B). Interestingly, the Treg/Teff cell ratio did not vary according to the levels of placental parasitemia or placental inflammation (Fig. 2C). Thus, in each individual, the Treg/Teff cell ratio is apparently kept stable whatever the infection status of the mother.
Fig. 2.
Placental malaria does not alter the Treg/Teff cell ratio in neonates. (A) Ratio of the frequencies of regulatory CD4+ CD25+ CD127−,low Foxp3+ cells to effector CD4+ CD25+ CD127+ Foxp3− cells in neonates according to mother's parity and PM (n = 10 for PMneg and n = 13 for PMpos primigravid women; n = 15 for PMneg and n = 14 for PMpos multigravid women). Individual (symbols) and median (bars) values are shown. (B) Correlation between frequencies of Treg and Teff cells in neonates from PM-negative mothers (left panel, n = 25) and PM-positive mothers (right panel, n = 26). (C) Treg/Teff cell ratio according to placental parasitemia (left panel, n = 26) and P. falciparum-induced placental inflammation (defined as the number of monocytes containing malarial pigment) (right panel, n = 26). P and Rho values were determined with the Spearman correlation test.
Absence of pro- and anti-inflammatory P. falciparum-specific responses in neonates born to PM-positive mothers.
Next, we asked whether PM could be associated with a modified neonatal cellular immune response to P. falciparum. We addressed this question using live parasites to more closely reflect the in vivo parasite-induced immune response. Freshly isolated CBMC were cultured in the presence of P. falciparum-iRBC or control uninfected RBC (uRBC) at a ratio of 1. PBMC from healthy Beninese adults living in the same area were cultured under similar conditions. A representative fluorescence-activated cell sorter (FACS) analysis of CD25 and CD4 expression after 40 h of coculture is shown in Fig. 3A. Strikingly, the frequency of CD4+ T cells expressing high levels of CD25 was significantly increased after coculture with iRBC in CBMC of neonates born to PM-negative mothers but not in those of neonates born to PM-positive mothers or in adult individuals (P = 0.0002 and P = 0.12, respectively, by Mann-Whitey U test) (Fig. 3B, bottom panel). No such differences were observed with respect to the frequency of cells expressing intermediate level of CD25 (Fig. 3B, top panel).
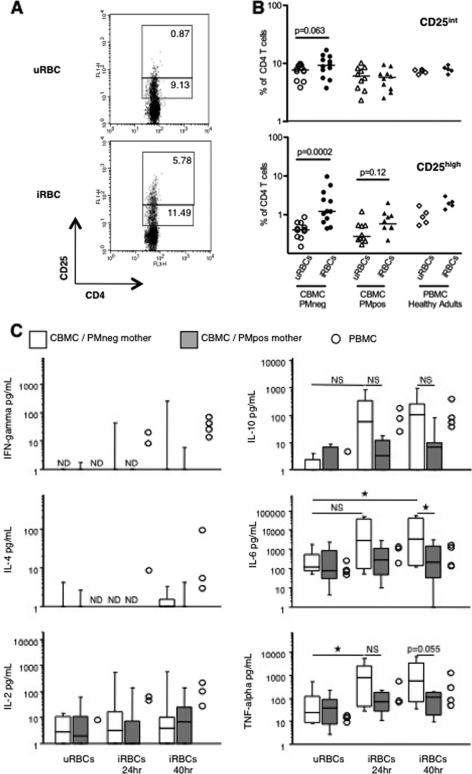
Fig. 3.
Lack of P. falciparum-induced-CD25high CD4+ T cells and reduced production of cytokines following culture with live parasites in CBMC from neonates born to PM-positive mothers. (A) Representative FACS dot plots showing the expression of CD25 on neonatal CD4+ T cells after 40 h of culture in the presence of uRBC or iRBC. Percentages of CD25high and CD25int cells among CD4+ T cells are shown. (B) Frequencies of CD25int and CD25high cells among neonatal CD4+ T cells from all CBMC samples cultured in the presence of uRBC or iRBC and segregated according to PM. Data obtained with PBMC from 5 healthy adult Beninese donors are shown for comparison. Results are expressed as individual (symbols) and median (bars) values. Significance was determined by the Mann-Whitney U test. (C) Supernatants were collected at 24 h and 40 h of culture with uRBC or iRBC, and concentrations of IFN-γ, IL-4, IL-2, IL-10, IL-6, and TNF-α were determined by multiplex analysis. Results are expressed as box plots showing the medians with 25th and 75th percentiles and whiskers for 10th and 90th percentiles, except for PBMC, which are shown as individual values. For panels B and C, n = 12 CBMC samples from neonates born to PMneg mothers, n = 11 CBMC samples from neonates born to PMpos mothers, and n = 4 PBMC samples. NS, not significant; ★, P < 0.05 by Mann-Whitney U test.
We also quantified IFN-γ, IL-6, IL-2, IL-10, IL-4, and TNF-α in culture supernatants at 24 h and 40 h of coculture. We observed that very few CBMC of neonates born to either PM-negative or PM-positive mothers produced detectable levels of IFN-γ or IL-4 in response to live iRBC and that there was no iRBC-specific IL-2 production above the uRBC-induced background (Fig. 3C, left panels). Of note, CBMC can produce IFN-γ in response to live parasites, since we detected IFN-γ-producing CD25+ CD4+ T cells among 2 CBMC samples out of 22 (both were from PM-negative mothers) by intracellular staining (not shown). By comparison, P. falciparum-specific production of these 3 cytokines was observed in supernatants of PBMC (Fig. 3C, left panels).
P. falciparum-specific production of IL-10 was observed in cultures of CBMC from neonates born to PM-negative mothers (although the median IL-10 concentration was not significantly different from that obtained after culture with uRBC) but not in those from neonates born to PM-positive mothers (Fig. 3C, right panels). In contrast, P. falciparum-specific IL-10 production was clearly detected in cultures of PBMC. By intracellular staining, IL-10-producing CD4+ CD25+ cells were detected in only 1 sample of PBMC out of 4 but in none of the 22 CBMC samples (not shown), which may reflect the difficulty in detecting these cells or the fact that this cytokine is produced by another cell type.
IL-6 and TNF-α concentrations significantly increased in response to live parasites in cultures of CBMC from neonates born to PM-negative mothers and in cultures of PBMC but not in cultures of CBMC from neonates born to PM-positive mothers (Fig. 3C, right panels). TNF-α-producing CD25+ CD4+ T cells were detected in 7 out of 22 CBMC samples, all from neonates of PM-negative mothers, and in 4 out of 4 PBMC samples (not shown).
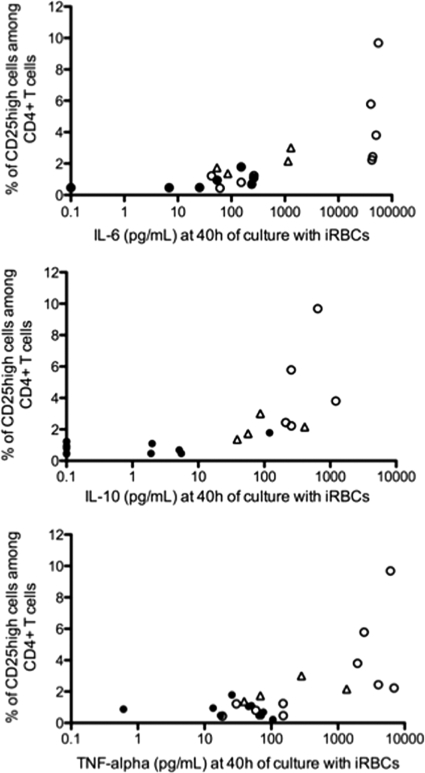
Interestingly, the presence of P. falciparum-induced CD25high CD4+ T cells among CBMC and PBMC correlated positively with the highest IL-6, IL-10, and TNF-α responses to the parasite (P = 0.001, P = 0.007, and P = 0.046 for IL-6, IL-10, and TNF-α, respectively, by Spearman correlation test) (Fig. 4).
Fig. 4.
The presence of P. falciparum-induced CD25high CD4+ T cells among CBMC correlates with the highest IL-6, IL-10, and TNF-α responses to live parasites. Frequencies of P. falciparum-induced CD25high CD4+ T cells determined after 40 h of in vitro culture with live parasites were plotted against IL-6 (n = 16), IL-10 (n = 17), and TNF-α (n = 20) concentrations (iRBC-uRBC) measured in supernatants. Empty circles, neonates from PM-negative mothers; filled circles, neonates from PM-positive mothers; triangles, healthy Beninese adults.
Of note, no difference was found in the activation or cytokine response of CD4 T cells from neonates born to PM-positive and PM-negative mothers in response to polyclonal T cell activation with anti-CD3 and anti-CD28 MAbs (see Fig. S2A and B in the supplemental material).
DISCUSSION
In this study we observed that the ex vivo frequencies of naive CD45RA+, memory CD45RO+, and effector CD25+ CD127+ Foxp3− CD4+ T cells were similar in CBMC of neonates born to PM-positive and PM-negative mothers, whatever the maternal gravidity and the level of placental parasitemia or inflammation. These results are consistent with those recently published by Flanagan et al. (13), who found no difference in the frequency of CD4+ CD25+ Foxp3+ T cells between neonates born to PM-negative and PM-positive mothers. We did not detect any increase in the ex vivo frequency of CD25high CD4+ T cells in neonates born to PM-positive mothers as reported by others (6). However, in CBMC of those born to primigravid women with PM, the frequency of regulatory CD25+ CD127−/low Foxp3+ CD4+ T cells was lower than that in CBMC of those born to primigravid women without PM, whereas no such difference was found in CBMC of neonates born to multigravid women with and without PM. In the same context, the degree of placental inflammation was inversely related to the frequency of neonatal Treg cells, although no association was found with the placental parasitemia. These two findings are coherent, since primigravid women displayed far higher levels of placental inflammation than multigravid women, while placental parasitemia did not differ according to gravidity. This implies that the local P. falciparum-induced placental immune response may be a more critical determinant of the impact of PM on the neonatal immune system than the mere presence of parasites. Few studies have simultaneously addressed the relative contributions of maternal gravidity and/or the local placental P. falciparum-induced immune response to the relationship between PM and neonatal immune responses, but it has been proposed that infants born to primigravid mothers are the group with the most exposure to P. falciparum antigens in utero (22). Indeed, CBMC produce different cytokines in response to blood-stage antigens that reflect both the presence of PM and the mother's gravidity, suggesting that the neonatal parasite-specific immune response may be dependent on the degree (magnitude/duration) of fetal exposure to P. falciparum antigens (22). Moreover, P. falciparum infection occurring late in gestation has been proposed to have the highest impact on the neonatal immune system and more precisely on the neonatal innate immune response (1). Our results are consistent with this and further suggest that, in areas with stable transmission, neonates from PM-positive primigravid mothers may represent a separate group with respect to their regulatory T cell compartment.
Across individuals, the relative frequency of CD4 Treg and Teff cells in CBMC was maintained around a similar ratio regardless of the mother's gravidity and level of placental parasitemia or inflammation. These findings are consistent with those of Flanagan et al. (13) and support the idea that both populations of T cells are affected concomitantly and are coregulated (29), even where reduced Treg cell frequencies (in CBMC of newborns from mothers with active PM and high P. falciparum-induced placental inflammation at delivery) were observed. The latter decrease could be a consequence of cell death or, more plausibly, their migration to fetal lymphoid tissues.
In adults living in areas of endemicity, malaria clinical episodes have been associated with an increase in Treg cell frequency (32, 33), and in malaria-naive individuals, an increase in markers commonly associated with responses of Treg cells (such as CD25high, Foxp3, and TGF-β) has been associated with a less efficient control of parasitemia (34; reviewed in reference 28). Here, in CBMC, we saw a trend in the opposite direction and, in addition, that effector and regulatory T cells are in balanced equilibrium. Our data are thus much more in line with those of Finney et al., who reported a homeostatic regulation of these two T cell populations in malaria-exposed adults and children (11). Hence, our data suggest that these findings can be extended to neonates.
In the context of CBMC T cell activity after in vitro stimulation, we found similar responses to polyclonal activation regardless of maternal infection status, confirming the lack of any intrinsic defect of neonatal T cells (2). The P. falciparum-specific neonatal immune response was characterized most strikingly by the marked increase in CD25high CD4+ T cells seen in CBMC of neonates born to PM-negative mothers, with no such change in CBMC of neonates born to PM-positive mothers. Scholzen and colleagues recently reported the appearance of CD25high CD4+ T cells, comprising a mixture of effector-like Foxp3int and regulatory-like Foxp3high cells, among PBMC of malaria-naive donors after coculture with live iRBC (29). These data are consistent with the marked increase in CD25high cell frequency that we observed among CBMC from PM-negative neonates. It would also be highly interesting to determine whether the same CD25high Foxp3int and CD25high Foxp3high cells are generated among CMBC.
PBMC from healthy Beninese adults produced all 6 cytokines tested, thus showing that the cytokine response to live parasites is diverse in malaria-immune adults, as reported for malaria-naive adults (35). Surprisingly, among the CBMC cytokine responses, production of IFN-γ in response to iRBC was negligible, while the IL-2 response, although present, did not exceed the levels induced by uRBC. These particular profiles were unaffected by maternal infection status. Although not identical in some aspects of their design and/or technical parameters, other studies have reported relatively frequent induction of these two Th1-type cytokines in CBMC, with some reporting higher frequencies of such responses among CBMC from those born to primigravid and/or PM-positive mothers (4, 6, 7, 22, 25). Independent studies in two different settings have identified an important role for IL-10 in suppressing parasite antigen-specific responses in CBMC of those born to PM-positive mothers (6, 7, 21), but our data do not reveal differences in the capacity of CBMC to produce IL-10 according to maternal infection status that would explain the absence of Th1-type cytokine activity. Moreover, our data suggest that production of IL-10, as well as that of the proinflammatory cytokines IL-6 and TNF-α, is suppressed in CBMC of neonates born to PM-positive mothers. These results are striking but are consistent with our ex vivo observation that the Teff/Treg cell ratio is preserved in neonates from PM-positive mothers, suggesting that both cell subsets are affected concomitantly by PM. These results are also in accordance with the comparatively poor capacity for Toll-like receptor (TLR) ligand-mediated TNF-α responses reported by Adegnika and colleagues (1) and with the data published by Flanagan et al., who found that PM has no significant effect on day 3 malaria-specific IFN-γ or IL-10 levels in cord blood culture supernatants (13). In addition, Mackroth et al. recently reported that PM-induced suppression of the P. falciparum-specific neonatal immune response is independent of IL-10 and TGF-β (20). Of note, we cannot exclude that we may have obtained different results if using field isolates for in vitro stimulation of CBMC and PBMC.
Induction of CD25high CD4+ T cells by live P. falciparum in PBMC from malaria-naive adults was shown to be dependent on IL-2, IL-10, and TGF-β and partly on the interaction with major histocompatibility complex (MHC) class II molecules (29). The comparatively low frequency of CD25high CD4+ T cells among CBMC from neonates born to PM-positive mothers may be due to the lack of such an upstream cytokine response.
To summarize, our data show that P. falciparum-induced placental inflammation has a strong impact on Treg cell frequency in neonates born to primigravid women, suggesting that these neonates may represent a separate group with respect to their regulatory T cell compartment, thus deserving more extended immunological studies. Besides this, our results show that PM has no effect on the homeostasis of regulatory and effector CD4 T cells in a given individual. This supports the idea that these two populations are coregulated in neonates, as well as in adults (11, 29). We also show that the in vitro response of CBMC to live P. falciparum iRBC differs in terms of activation and cytokine secretion between neonates born to PM-negative and -positive mothers. Moreover, our data argue in favor of a broad suppression of the P. falciparum-specific immune response at birth, independent of IL-10, and affecting both the effector and the regulatory arms of the neonatal immune system. It remains to be determined whether this hyporesponsiveness is transient or persists after birth and whether it is restricted to P. falciparum antigens or not.
Supplementary Material
ACKNOWLEDGMENTS
We thank the maternity staff from the Hôpital de la Mère et l'Enfant Lagune (HOMEL) for their support and D. Vodounon, M. Hounkonnou, N. Magnonfinon, and D. Moussa for their collaboration. We also thank Bernadette Gandonoun, Vénérande Capo-Chichi, Claire Degnonvi, and the midwives and nurses from Houenoussou Health Center for their great job with sample collection. We are grateful to all the women who participated to the study. We also thank Pépin Kounou for his irreplaceable logistical and technical help and also Jacqueline Millet, Gilles Cottrell, and Valérie Briand for their advice with statistical analyses. We are grateful to Julien Zuber and Hélène Dujardin for their technical advice on regulatory T cells and to Marga van de Vegte-Bolmer for provision of P. falciparum-infected red blood cells.
This work was supported by the IMEA, NATIXIS, and the French Ministry of Research (FSP REFS). V.S. was a postdoctoral fellow of the Conseil Régional d'Ile-de-France (convention no. 3486).
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 25 April 2011.
REFERENCES
- 1. Adegnika A. A., et al. 2008. Pregnancy-associated malaria affects Toll-like receptor ligand-induced cytokine responses in cord blood. J. Infect. Dis. 198:928–936 [DOI] [PubMed] [Google Scholar]
- 2. Adkins B., Leclerc C., Marshall-Clarke S. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553–564 [DOI] [PubMed] [Google Scholar]
- 3. Belkaid Y., Rouse B. T. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353–360 [DOI] [PubMed] [Google Scholar]
- 4. Bisseye C., et al. 2009. Plasmodium falciparum infection of the placenta impacts on the T helper type 1 (Th1)/Th2 balance of neonatal T cells through CD4(+)CD25(+) forkhead box P3(+) regulatory T cells and interleukin-10. Clin. Exp. Immunol. 158:287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broen K., Brustoski K., Engelmann I., Luty A. J. 2007. Placental Plasmodium falciparum infection: causes and consequences of in utero sensitization to parasite antigens. Mol. Biochem. Parasitol. 151:1–8 [DOI] [PubMed] [Google Scholar]
- 6. Brustoski K., et al. 2006. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J. Infect. Dis. 193:146–154 [DOI] [PubMed] [Google Scholar]
- 7. Brustoski K., et al. 2005. IFN-gamma and IL-10 mediate parasite-specific immune responses of cord blood cells induced by pregnancy-associated Plasmodium falciparum malaria. J. Immunol. 174:1738–1745 [DOI] [PubMed] [Google Scholar]
- 8. Cornet M., et al. 1998. Prevalence and risk factors for anemia in young children in southern Cameroon. Am. J. Trop. Med. Hyg. 58:606–611 [DOI] [PubMed] [Google Scholar]
- 9. Crocker I. P., et al. 2004. Syncytiotrophoblast degradation and the pathophysiology of the malaria-infected placenta. Placenta 25:273–282 [DOI] [PubMed] [Google Scholar]
- 10. Duffy P. E. 2007. Plasmodium in the placenta: parasites, parity, protection, prevention and possibly preeclampsia. Parasitology 134:1877–1881 [DOI] [PubMed] [Google Scholar]
- 11. Finney O. C., Nwakanma D., Conway D. J., Walther M., Riley E. M. 2009. Homeostatic regulation of T effector to Treg ratios in an area of seasonal malaria transmission. Eur. J. Immunol. 39:1288–1300 [DOI] [PubMed] [Google Scholar]
- 12. Finney O. C., Riley E. M., Walther M. 2010. Regulatory T cells in malaria—friend or foe? Trends Immunol. 31:63–70 [DOI] [PubMed] [Google Scholar]
- 13. Flanagan K. L., et al. 2010. The effect of placental malaria infection on cord blood and maternal immunoregulatory responses at birth. Eur. J. Immunol. 40:1062–1072 [DOI] [PubMed] [Google Scholar]
- 14. Hansen D. S., Schofield L. 2010. Natural regulatory T cells in malaria: host or parasite allies? PLoS Pathog. 6:e1000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hartgers F. C., et al. 2009. Responses to malarial antigens are altered in helminth-infected children. J. Infect. Dis. 199:1528–1535 [DOI] [PubMed] [Google Scholar]
- 16. Ismaili J., et al. 2003. Plasmodium falciparum infection of the placenta affects newborn immune responses. Clin. Exp. Immunol. 133:414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. King C. L., et al. 2002. Acquired immune responses to Plasmodium falciparum merozoite surface protein-1 in the human fetus. J. Immunol. 168:356–364 [DOI] [PubMed] [Google Scholar]
- 18. Le Hesran J. Y., et al. 1997. Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am. J. Epidemiol. 146:826–831 [DOI] [PubMed] [Google Scholar]
- 19. Liu W., et al. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203:1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mackroth M. S., et al. 2011. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J. Immunol. 186:2780–2791 [DOI] [PubMed] [Google Scholar]
- 21. Malhotra I., et al. 2009. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med. 6:e1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malhotra I., et al. 2005. Distinct Th1- and Th2-type prenatal cytokine responses to Plasmodium falciparum erythrocyte invasion ligands. Infect. Immun. 73:3462–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malhotra I., et al. 2008. Fine specificity of neonatal lymphocytes to an abundant malaria blood-stage antigen: epitope mapping of Plasmodium falciparum MSP1(33). J. Immunol. 180:3383–3390 [DOI] [PubMed] [Google Scholar]
- 24. May K., et al. 2009. Antibody-dependent transplacental transfer of malaria blood-stage antigen using a human ex vivo placental perfusion model. PLoS One 4:e7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Metenou S., Suguitan A. L., Jr., Long C., Leke R. G., Taylor D. W. 2007. Fetal immune responses to Plasmodium falciparum antigens in a malaria-endemic region of Cameroon. J. Immunol. 178:2770–2777 [DOI] [PubMed] [Google Scholar]
- 26. Mutabingwa T. K., et al. 2005. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2:e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rogerson S. J., Hviid L., Duffy P. E., Leke R. F. G., Taylor D. W. 2007. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect. Dis. 7:105–117 [DOI] [PubMed] [Google Scholar]
- 28. Scholzen A., Minigo G., Plebanski M. 2009. Heroes or villains? T regulatory cells in malaria infection. Trends Parasitol. 26:16–25 [DOI] [PubMed] [Google Scholar]
- 29. Scholzen A., Mittag D., Rogerson S. J., Cooke B. M., Plebanski M. 2009. Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta. PLoS Pathog. 5:e1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schwarz N. G., et al. 2008. Placental malaria increases malaria risk in the first 30 months of life. Clin. Infect. Dis. 47:1017–1025 [DOI] [PubMed] [Google Scholar]
- 31. Seddiki N., et al. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203:1693–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Todryk S. M., et al. 2008. Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4+ CD25high T cells susceptibility in Kenyans. PLoS One 3:e2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Torcia M. G., et al. 2008. Functionnal deficit of T regulatory cells in Fulani, an ethnic group with low susceptibility to Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. U. S. A. 105:646–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walther M., et al. 2005. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 23:287–296 [DOI] [PubMed] [Google Scholar]
- 35. Walther M., et al. 2006. Innate immune responses to human malaria: heterogeneous cytokine responses to blood-stage Plasmodium falciparum correlate with parasitological and clinical outcomes. J. Immunol. 177:5736–5745 [DOI] [PubMed] [Google Scholar]
- 36. WHO 2008. World malaria report 2008. WHO, Geneva, Switzerland [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.