Abstract
As a novel family of cell surface receptors, triggering receptors expressed on myeloid cells (TREMs) play an important role in inflammatory responses. However, the role of TREMs in the ocular immune system remains unknown. In this study, we examined the expression and function of TREM-1 in Pseudomonas aeruginosa keratitis, one of the most common sight-threatening ocular diseases. TREM-1 was significantly increased in human corneas after P. aeruginosa infection. Consistent with TREM-1 expression at the human ocular surface, TREM-1 levels (mRNA and protein) were also elevated in the infected corneas of C57BL/6 (B6) mice at 1, 3, and 5 days postinfection. To determine whether TREM-1 dictates the outcome of P. aeruginosa keratitis in susceptible mice, TREM-1 signaling in B6 mice was blocked with a soluble mTREM-1/Fc fusion protein. The results indicated that blockade of TREM-1 reduced the severity of corneal disease, polymorphonuclear neutrophil infiltration, Th1/proinflammatory cytokine expression and Toll-like receptor (TLR) activation but enhanced the production of Th2 cytokines, murine β-defensin 2 (mBD2), single Ig interleukin-1R-related molecule (SIGIRR), and ST2. Furthermore, we also used agonistic anti-mTREM-1 antibody to activate TREM-1 signaling in B6 mice and found that TREM-1 activation resulted in worsened disease and earlier corneal perforation in infected B6 mouse corneas and elevated production of proinflammatory cytokines and TLR signaling molecules but reduced expression of mBD2, SIGIRR, and ST2. To the best of our knowledge, this study provides the first evidence that TREM-1 functions as an inflammatory amplifier in P. aeruginosa keratitis by modulating TLR signaling and Th1/Th2 responses.
INTRODUCTION
Pseudomonas aeruginosa is one of the most common bacterial pathogens that cause sight-threatening corneal infection, especially in extended-wear contact lens users (39). Clinically, P. aeruginosa keratitis progresses rapidly and results in inflammatory epithelial edema, stromal infiltration, corneal ulceration, and often tissue destruction and vision loss (12). Experimental P. aeruginosa challenge usually induces corneal perforation in susceptible C57BL/6 (B6) mice at 5 days postinfection (p.i.) (15). Studies using this murine model have provided substantial information about the ocular immune response to bacterial infection (12). Both bacterial virulence factors and the host immune response contribute to the pathogenesis of corneal disease after infection (9, 12, 17), but treating bacterial infection with antibiotics commonly does not prevent ocular pathology (10). This is primarily because immunopathological processes have been triggered by pattern recognition receptors such as Toll-like receptors (TLRs) and proceed even if viable bacteria are cleared from the cornea (12). Activation of TLR signaling initiates a variety of inflammatory events, such as infiltration of inflammatory cells (e.g., polymorphonuclear neutrophils [PMNs] and monocytes/macrophages) (13, 20, 21, 27), as well as the production of inflammatory cytokines (e.g., tumor necrosis factor alpha [TNF-α], macrophage inflammatory protein 2 [MIP-2], interleukin-1β [IL-1β], gamma interferon [IFN-γ], IL-6, and IL-12) (12, 22). In bacterial keratitis, the TLR-induced inflammatory mediators promote bacteria clearance and induce tissue repair; however, if unbalanced or uncontrolled, they may amplify the host inflammatory response, leading to tissue damage and corneal perforation (12, 24).
The disease outcome of P. aeruginosa keratitis is largely affected by the balance of activation versus inhibition of TLR signaling. Several negative regulators of TLR signaling, including single Ig IL-1R-related molecule (SIGIRR), ST2, SOCS1, and IRAK-M, have been identified over the past several years (6, 31, 38). Our previous studies provide evidence that SIGIRR and ST2 promote host resistance to P. aeruginosa infection by downregulating TLR signaling and production of proinflammatory cytokines (18, 19). Although compelling evidence suggests that both TLR activation and negative regulation play a critical role in bacterial keratitis (12, 14), little is known about TLR positive regulation, which amplifies the host immune response. In this regard, triggering receptors expressed on myeloid cells (TREMs) are emerging as a new extended family of receptors that regulate both innate and adaptive immune responses at an early stage of the host response to bacterial infection (5, 8, 25).
TREM-1 is a recently identified activating receptor expressed at high levels on PMNs and a subset of CD14+ monocytes/macrophages that infiltrate infected tissue (4). Recent studies demonstrated that expression of TREM-1 was strongly upregulated in PMNs and monocytes/macrophages by extracellular bacteria such as P. aeruginosa and Staphylococcus aureus, as well as by lipopolysaccharide (LPS) stimulation (5, 29). Although the endogenous TREM-1 ligand remains unknown, costimulation with TLR ligands such as LPS synergistically enhances the sustained secretion of proinflammatory cytokines/chemokines such as TNF-α and IL-1β (5) and may also result in prolonged survival of PMNs and monocytes at the inflammatory site (7, 32). On the other hand, blockade of TREM-1 with a soluble mTREM-1/IgG fusion protein protects mice against LPS-induced septic shock and microbial sepsis caused by live Escherichia coli (5). This blockade reduces the TREM-1-mediated inflammatory response but still allows sufficient control of the bacterial infection by downregulating the production of proinflammatory cytokines, as well as the total number of infiltrating PMNs and macrophages (5). Taken together, these studies suggest that TREM-1 functions as an amplifier of TLR signaling and host inflammation.
Since nothing is known about the role of TREM-1 in the cornea (or in the eye), this study is the first to investigate the expression and role of TREM-1 in P. aeruginosa keratitis. Our data provide compelling evidence that TREM-1 is significantly enhanced in both human and mouse corneas after P. aeruginosa infection and amplifies corneal inflammation by modulating TLR signaling and Th1/Th2-type immune responses.
MATERIALS AND METHODS
Patients and tissue specimens.
P. aeruginosa keratitis patients at the Zhongshan Ophthalmic Center (Sun Yat-sen University, Guangzhou, China) from January 2010 to December 2010 were included. Criteria for inclusion were clinically diagnosed P. aeruginosa keratitis and experimental confirmation by microbial culture of corneal scrapings. Based upon the infection time, these patients were divided into three groups (each with 8 patients) as follows: group 1, at 1 to 6 days p.i., 6 male, 2 female, 27 to 60 years old; group 2, at 7 to 13 days p.i., 5 male, 3 female, 19 to 71 years old; group 3, at 14 to 30 days p.i., 5 male, 3 female, 22 to 76 years old. Corneal scrapings were collected prior to the first treatment at the Zhongshan Ophthalmic Center and analyzed by using real-time reverse transcription (RT)-PCR. Controls were normal corneal tissues remaining after corneal transplantation. Donor corneas were procured within 6 h of the donor's death, kept in a special storage reagent at low temperature to maintain biological activity, and confirmed to be free of any detectable prior pathologic conditions. For the use of these clinical materials for research purposes, the patient's consent and approval from the Institutional Research Ethics Committee were obtained.
Ocular infection and clinical examination.
Eight-week-old female B6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). P. aeruginosa strain ATCC 19660 (cytotoxic) was used for ocular infection. The left cornea of each mouse was infected as described before (18, 19, 41). Eyes were examined at 1 day p.i. and/or at the times described below to ensure that the mice were similarly infected and to monitor disease. Corneal disease was graded using the following established scale: 0, clear or slight opacity partially or fully covering the pupil; +1, slight opacity partially or fully covering the anterior segment; +2, dense opacity partially or fully covering the pupil; +3, dense opacity covering the entire anterior segment; +4, corneal perforation or phthisis. A clinical score was recorded for each mouse after infection for statistical comparison of disease severity, and photography with a slit lamp was used to illustrate the disease response. Animals were treated humanely and in compliance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Real-time RT-PCR.
Total RNA was isolated from individual corneas for analysis (as indicated below) using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations and quantitated by using NanoDrop 2000C spectrophotometers (ThermoScientific, West Palm Beach, FL). One microgram of total RNA was reverse transcribed to produce cDNA, and then the cDNA was amplified using SYBR green Master Mix (Bio-Rad, Hercules, CA) as suggested by the manufacturer. The primer sequences used to amplify the human and mouse TREM-1 genes are as follows. hTREM-1, 5′-AGT CCC CAG GAT CAT ACT AGA AGA C-3′ (forward) and 5′-AGG CTG GTA GAT CAC ACA CTG ATA C-3′ (reverse); mTREM-1, 5′-TCC TAT TAC AAG GCT GAC AGA GCG TC-3′ (forward) and 5′-AAG ACC AGG AGA GGA AAC AAC CGC-3′ (reverse). Other primers used in real-time PCR were either the primers described previously (18, 19, 41) or primers purchased from SABiosciences, Frederick, MD (mouse IFN-γ, IL-4, and IL-5). Quantitative real-time RT-PCRs were performed using the CFX96 Real-Time PCR System (Bio-Rad). Relative mRNA levels were calculated after normalization to β-actin.
Western blot analysis.
Whole corneas (n = 5/group/time) were collected and pooled at each time point from normal uninfected and infected B6 mouse eyes at 1, 3, and 5 days p.i. Pooled corneas were lysed and homogenized using a 1-ml glass tissue homogenizer in 1× sample buffer (2% SDS, 10% glycerol, 5% 2-mercaptoethanol). Debris was pelleted by centrifugation for 5 min at 7,500 rpm, and the protein concentration of the supernatant was determined by the Quick Start Bradford protein assay (Bio-Rad). Twenty micrograms of corneal protein was loaded onto each respective lane, separated by 10% SDS-PAGE, and then transferred to a supported polyvinylidene difluoride membrane (Bio-Rad). After blocking in a 5% solution of nonfat dry milk prepared with PBST (1× phosphate-buffered saline [PBS] containing 0.05% Tween 20; Bio-Rad), blots were incubated with primary goat-anti-mouse TREM-1 antibody (Ab; 0.2 μg/ml; R&D Systems, Minneapolis, MN) at 4°C overnight, washed three times for 15 min each with PBST, followed by secondary IRDye 800CW donkey anti-goat IgG (H + L) Ab (1:5,000; LI-COR Biosciences, Lincoln, NE) for 1 h at room temperature, and detected using the Odyssey Infrared Imaging System (LI-COR Biosciences) by following the manufacturer's protocol.
Blockade of TREM-1.
In vivo use of soluble mTREM-1/Fc fusion protein for blockade has been described by others (5). The soluble mTREM-1/Fc protein competes with cell surface TREM-1 to bind with TREM-1 ligand, thereby inhibiting the activation of TREM-1 signaling. For the studies described herein, mTREM-1/IgG fusion protein or appropriate IgG/Fc controls (R&D Systems, Minneapolis, MN) were injected subconjunctivally (1 μg in 5 μl PBS per cornea) into the left eyes of B6 mice (n = 5/time/treatment) 1 day before infection. Then, at 1 and 3 days p.i., each mouse was injected intraperitoneally with an additional 10 μg of mTREM-1/IgG fusion protein or IgG/Fc controls diluted in 100 μl PBS. According to the manufacturer's instructions, the blocking activity of mTREM-1/Fc protein was determined by its effect on TNF-α production.
Activation of TREM-1.
In vivo use of agonistic mTREM-1 Ab has been described by others (5, 25). The mTREM-1 Ab links to TREM-1 molecules simultaneously and aggregates them together, thereby inducing the activation of downstream protein kinases. For the studies described here, mouse TREM-1 affinity-purified polyclonal Ab or appropriate IgG controls (R&D) were injected subconjunctivally (5 μg in 5 μl PBS per cornea) into the left eyes of B6 mice (n = 5/time/treatment) at 1 day before infection. Then, at 1 day p.i., each mouse was injected intraperitoneally with an additional 100 μg of mTREM-1 Ab or IgG controls diluted in 100 μl PBS. According to the manufacturer's instructions, the agonistic activity of anti-TREM-1 Ab was determined by its effect on TNF-α production.
Bacterial plate counts.
Corneas from mTREM-1/Fc fusion protein-treated versus IgG/Fc control-treated mice (at 1 and 5 days p.i.) or agonistic mTREM-1 Ab-treated versus IgG control-treated B6 mice (at 1 and 3 days p.i.) were collected (n = 5/group/time), and the numbers of viable bacteria were determined. Individual corneas were homogenized in sterile water containing 0.85% (wt/vol) NaCl and 0.25% bovine serum albumin. Serial 10-fold dilutions of the samples were plated on Pseudomonas isolation agar (BD Difco Laboratories, Sparks, MD) in triplicate, and plates were incubated overnight at 37°C. Results are reported as the mean number (105) of CFU per cornea ± the standard error of the mean (SEM).
Myeloperoxidase (MPO) assay.
An MPO assay was used to quantitate PMN numbers in the corneas of B6 mice treated with mTREM-1/Fc fusion protein versus those treated with the IgG/Fc control or those of B6 mice treated with agonistic mTREM-1 Ab versus those treated with the IgG control. Infected corneas (n = 5/group/time) were homogenized in 1.0 ml of 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (Sigma, St. Louis, MO). Samples were freeze-thawed four times and centrifuged at 13,000 rpm for 10 min. A 0.1-ml volume of the supernatant was added to 2.9 ml of 50 mM phosphate buffer containing 16.7 mg/100 ml o-dianisidine dihydrochloride (Sigma) and 0.0005% hydrogen peroxide. The change in absorbance at 460 nm was monitored for 5 min at 30-s intervals, and the results were expressed as units of MPO per cornea. One unit of MPO activity is equivalent to 2 × 105 PMNs (40).
Enzyme-linked immunosorbent assay (ELISA).
Cytokine protein levels were selectively tested using ELISA kits (R&D). Corneas from mTREM-1/Fc protein-, agonistic mTREM-1 Ab-, or control-treated B6 mice were individually collected (n = 5/group/time) at the indicated times. Corneas were homogenized in 0.5 ml of PBS with 0.1% Tween 20. All samples were centrifuged at 13,000 rpm for 5 min, and an aliquot of each supernatant was assayed in duplicate for MIP-2, TNF-α, IL-6, and IL-1β proteins in accordance with the manufacturer's instructions. The reported sensitivities of these assays are <1.5 pg/ml for MIP-2, <5.1 pg/ml for TNF-α, 1.3 to 1.8 pg/ml for IL-6, and <3.0 pg/ml for IL-1β.
Statistical analysis.
The difference between the clinical scores of two groups at each time point was tested by the Mann-Whitney U test. An unpaired, two-tailed Student t test was used to determine the statistical significance of differences in viable real-time RT-PCR, Western blot assay, ELISA, bacterial plate count, and MPO assay results. Differences were considered significant at P < 0.05.
RESULTS
TREM-1 expression was significantly increased in human corneas after P. aeruginosa infection.
To determine whether TREM-1 mediates ocular inflammation, TREM-1 expression was tested in P. aeruginosa-infected (clinical specimen) versus normal human corneas. PCR data (Fig. 1) showed that TREM-1 was significantly enhanced in human corneas after P. aeruginosa infection. The expression levels of TREM-1 in P. aeruginosa-infected corneas were approximately 50-fold higher at 1 to 6 days p.i., 260-fold higher at 7 to 13 days p.i., and 900-fold higher at 14 to 30 days p.i., compared with those in uninfected normal corneas (P < 0.01, P < 0.001, and P < 0.001, respectively), indicating that TREM-1 expression dynamically accompanied the disease progression in P. aeruginosa keratitis.
Fig. 1.
Expression of TREM-1 in human corneas. TREM-1 mRNA levels were examined in normal and P. aeruginosa-infected human corneas at 1 to 6 days p.i. (group 1), 7 to 13 days p.i. (group 2), and 14 to 30 days p.i. (group 3). Data are the mean ± SEM with 8 patients/group.
TREM-1 expression was upregulated in mouse corneas after P. aeruginosa infection.
To further explore the role of TREM-1 in bacterial keratitis, a well-characterized and accepted murine model of P. aeruginosa keratitis was used to mimic human ocular infection. Similar to the human TREM-1 expression pattern, both mRNA (Fig. 2 A) and protein (Fig. 2B, Western blot assay, and C, integrated density value) levels of mouse TREM-1 were significantly upregulated in the infected B6 mouse corneas in a time-dependent manner and peaked at 5 days p.i. (all P < 0.001, compared with normal controls).
Fig. 2.
Expression of TREM-1 in B6 mouse corneas. TREM-1 mRNA levels (A) were examined in normal (N) uninfected and infected B6 mouse corneas at 1, 3, and 5 days p.i. Data are the mean ± SEM and represent three individual experiments, each with 5 animals/time. TREM-1 protein expression in B6 mouse corneas before and after P. aeruginosa infection was further examined using Western blot analysis (B). Equivalent protein (20 μg) was loaded in each lane. (C) Band intensity was quantitated and normalized to the β-actin control. Data for the Western blot assay represent one of three similar experiments each using 5 pooled corneas/time. IDV, integrated density value.
Blockade of TREM-1 reduced corneal disease after P. aeruginosa infection.
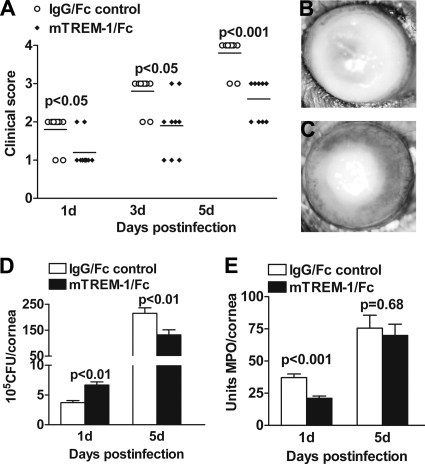
Since TREM-1 (mRNA and protein) expression was significantly upregulated in B6 mouse corneas after P. aeruginosa infection, the next series of in vivo studies were designed to determine whether blockade of TREM-1 promotes host resistance to P. aeruginosa infection. B6 mice were subconjunctivally and intraperitoneally injected with soluble mTREM-1/Fc fusion protein or the IgG/Fc control. Clinical scores (Fig. 3 A) showed that mTREM-1/Fc-treated mice exhibited reduced disease levels at 1, 3, and 5 days p.i. (P < 0.05, P < 0.05, and P < 0.001, respectively). Representative photographs (taken with a slit lamp) of mTREM-1/Fc (Fig. 3C)-treated versus IgG/Fc control (Fig. 3B)-treated mice at 5 days p.i. are provided. By 5 days p.i., the corneas of mTREM-1/Fc-treated mice consistently displayed less-severe disease (grade +2/+3), whereas most of the IgG/Fc control-treated corneas were perforated (grade +4).
Fig. 3.
Blockade of TREM-1-promoted host resistance to P. aeruginosa infection. Clinical scores (A) indicate statistically significant differences at 1, 3, and 5 days p.i. between mTREM-1-blocked and control mice. Representative photographs of infected eyes at 5 days p.i. display less opacity in mTREM-1/Fc-treated mice (C) than in control-treated mice (B). Bacterial loads (D) and PMN infiltration (E) were measured in the two groups by bacterial plate counts and MPO assay, respectively. Data are the mean ± SEM and represent two individual experiments, each with 5 animals/group/time.
Therefore, we further assessed the effect of TREM-1 blockade on the bacterial load, PMN infiltration, and disease pathogenesis. Bacterial plate counts were used to detect viable bacteria in the infected corneas of mTREM-1/Fc versus control-treated mice at 1 and 5 days p.i. Results are shown in Fig. 3D. After blocking of TREM-1, the bacterial load was first elevated at 1 day p.i. (P < 0.01) and then downregulated at 5 days p.i. (P < 0.01), compared with that of controls. While PMN infiltration (Fig. 3E, shown as MPO activity) was reduced in mTREM-1/Fc- versus control-treated mice at 1 day p.i. (P < 0.001), no change was shown between the two groups at 5 days p.i.
Blockade of TREM-1 decreased Th1 responses and TLR activation but increased Th2 responses and mBD2 production.
To ascertain the mechanism by which TREM-1 modulates the immune response, mRNA levels of selected Th1/proinflammatory and Th2 cytokines were analyzed by real-time RT-PCR in the infected corneas of mTREM-1/Fc- and control-treated B6 mice at 5 days p.i. Compared with controls, blockade of mTREM-1 significantly downregulated Th1/proinflammatory cytokines, including IFN-γ, IL-1β, and IL-6 (Fig. 4 A, all P < 0.01), but upregulated Th2 cytokines such as IL-4, IL-5, and IL-10 (Fig. 4B, P < 0.05, P < 0.01, and P < 0.05, respectively).
Fig. 4.
Cytokine production after blocking of TREM-1. RT-PCR data demonstrated that at 5 days p.i., treatment with mTREM-1/Fc fusion protein led to downregulation of Th1 cytokines (A) and TLR signaling molecules (C) but upregulation of Th2 cytokines (B), as well as mBD2, SIGIRR, and ST2 (D), compared with controls. ELISA data (E and F) also showed that blockade of TREM-1 significantly reduced the expression levels of proinflammatory cytokine proteins, including MIP-2, IL-1β, TNF-α, and IL-6, at 5 days p.i. Data are the mean ± SEM and represent two individual experiments, each with 5 animals/group/time.
Moreover, TLR2, TLR4, TLR5, TLR9, MyD88, mBD2, SIGIRR, and ST2 mRNA expression levels were also tested by real-time RT-PCR in mTREM-1/Fc- versus control-treated B6 mouse corneas at 5 days p.i. (Fig. 4C and D). TLR2, TLR4, and MyD88 mRNA levels were significantly downregulated (P < 0.001, P < 0.001, and P < 0.01, respectively), whereas mBD2, SIGIRR, and ST2 were upregulated (P < 0.05, P < 0.05, and P < 0.001, respectively) in the infected corneas after blocking of mTREM-1. For TLR5 and TLR9, no difference in mRNA expression was detected between the two groups (data not shown). In addition, ELISA data (Fig. 4E and F) also showed that blockade of TREM-1 significantly reduced the protein expression levels of proinflammatory cytokines, including MIP-2, IL-1β, TNF-α, and IL-6, at 5 days p.i., compared to the control treatment.
Activation of TREM-1 accelerated disease progression after P. aeruginosa infection.
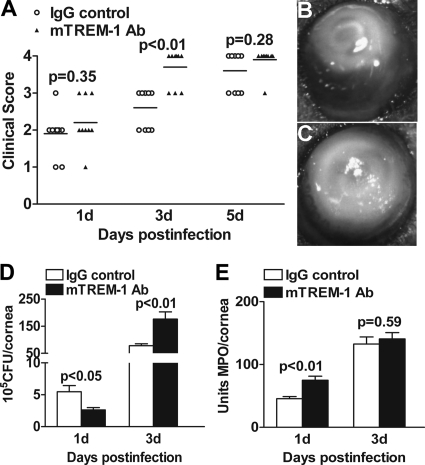
To further determine the role of TREM-1 in P. aeruginosa-induced keratitis, agonistic mTREM-1 Ab was used to cross-link and activate TREM-1 in B6 mice. Clinical score data (Fig. 5 A) showed that mTREM-1 Ab versus control-treated mice displayed an increased disease level and earlier corneal perforation at 3 days p.i. (P < 0.01). Representative photographs (taken with a slit lamp) of mTREM-1 Ab (Fig. 5C) versus IgG control (Fig. 5B)-treated mice at 3 days p.i. are provided. By 3 days p.i, control-treated mice displayed a consistent disease level (grade +2/+3), whereas most mTREM-1 Ab-treated corneas were perforated (grade +4).
Fig. 5.
Cross-linking activation of TREM-1 accelerated the disease progression of bacterial keratitis. Clinical scores (A) indicate statistically significant difference at 3 days p.i. between agonistic mTREM-1 Ab- and control-treated mice. Representative photographs of infected eyes at 3 days p.i. display a worsened disease response and earlier corneal perforation in mTREM-1 Ab-treated mice (C) versus control-treated mice (B). Bacterial loads (D) and PMN infiltration (E) were measured in the two groups by bacterial plate counts and MPO assay, respectively. Data are the mean ± SEM and represent two individual experiments, each with 5 animals/group/time/assay.
Moreover, to determine the role of TREM-1 in disease pathogenesis, bacterial loads (Fig. 5D) and PMN infiltration (Fig. 5E) were tested in the infected corneas at 1 and 3 days p.i. After TREM-1 activation, the bacterial load was first downregulated at 1 day p.i. (P < 0.05) and then upregulated at 3 days p.i. (P < 0.01), whereas PMN infiltration was enhanced at 1 day p.i. only (P < 0.01) and no change was detected at 3 days p.i.
Activation of TREM-1 enhanced Th1 responses and TLR activation but reduced production of Th2 cytokines and mBD2.
To further explore the regulatory mechanism of TREM-1 in the immune response, the expression levels (protein or mRNA) of selected proinflammatory cytokines and TLR signaling molecules were tested by ELISA and real-time RT-PCR, respectively. ELISA data indicated that at 3 days p.i., treatment with agonistic mTREM-1 Ab significantly upregulated the protein expression levels of MIP-2, TNF-α, IL-6, and IL-1β (Fig. 6 A to D, all P < 0.01), compared with control treatment. No difference in their expression between the two groups was detected at 1 day p.i. Meanwhile, real-time RT-PCR data (Fig. 7 A to F) suggested that mRNA levels of TLR2, TLR4, and MyD88 were significantly enhanced in agonistic mTREM-1 Ab- versus control-treated corneas at 3 days p.i. (P < 0.05, P < 0.001, and P < 0.001 for TLR2, TLR4, and MyD88, respectively), while no change was shown at 1 day p.i. In addition, at both 1 and 3 days p.i., agonistic mTREM-1 Ab versus control treatment resulted in reduced expression of mRNA for mBD2, SIGIRR, and ST2 (mBD2, P < 0.001 and P < 0.05; SIGIRR, P < 0.01 and P < 0.001; ST2, P < 0.01 and P < 0.01 [at 1 and 3 days p.i., respectively]), whereas no change in TLR5 and TLR9 expression between the two groups was detected (data not shown).
Fig. 6.
Cross-linking activation of TREM-1 enhanced proinflammatory cytokine production. After treatment with agonistic mTREM-1 Ab, MIP-2 (A), TNF-α (B), IL-6 (C), and IL-1β (D) protein expression levels were significantly increased at 3 days p.i. No difference between the two treatments was found at 1 day p.i. Data are the mean ± SEM and represent two individual experiments with 5 mice/group/time.
Fig. 7.
Production of TLR signaling molecules after cross-linking activation of TREM-1. After treatment with agonistic mTREM-1 Ab, TLR2 (A), TLR4 (B), and MyD88 (C) mRNA expression levels were significantly increased at 3 days p.i., while mBD2 (D), SIGIRR (E), and ST2 (F) mRNA expression levels were downregulated at both 1 and 3 days p.i., compared with those of controls. Data are the mean ± SEM and represent two individual experiments with 5 mice/group/time.
DISCUSSION
TREM-1 is a novel cell surface receptor that has recently been implicated as a key molecule in the development of innate and adaptive immune responses. Expression of TREM-1 is detected on a variety of immune cells, such as PMNs, macrophages, and monocytes (4, 11, 33), and strongly elevated after bacterial infection (5, 28). Studies have demonstrated that activation of TREM-1 alters the dynamics of pulmonary IRAK-M expression and improves the host defense during pneumococcal pneumonia (25). TREM-1 activation also enhances the production of TNF-α, IL-6, and MIP-2, as well as PMN infiltration (25). However, nothing is known regarding the role of TREM-1 in the eye.
Studies described herein demonstrated that TREM-1 expression was significantly enhanced in P. aeruginosa-infected versus control uninfected human corneas. Controls were obtained from cadaver eyes, which were procured within 6 h of the donor's death and kept in a special storage reagent at low temperature to maintain biological activity. Since normal corneas are avascular tissue, cytotoxic materials released during death hardly affect the cornea. Also, donors were confirmed to be free of any detectable prior pathological conditions. Thus, the cadaver corneas could reflect normal physiological conditions of human corneas. It should be noted that these P. aeruginosa-infected cornea samples were obtained from both male and female patients 19 to 76 years old. Despite the sex and age variations, our results indicate that the TREM-1 induction levels are associated largely with the infection period and disease severity. Furthermore, experimental murine models of P. aeruginosa keratitis also showed that TREM-1 expression was significantly induced in infected mouse corneas, which is consistent with other studies showing that TREM-1 is strongly elevated after bacterial infection (5, 29). The induction of TREM-1 at both human and mouse ocular surfaces was dynamically increased with disease progression, indicating the potential association of this molecule with P. aeruginosa keratitis. Our in vivo study showed that blockade of TREM-1 promoted host resistance to P. aeruginosa corneal infection, while activation of TREM-1 accelerated the disease progression, indicating that TREM-1 contributes to the disease progression of P. aeruginosa keratitis.
The outcome of P. aeruginosa keratitis is largely determined by the balance of Th1- versus Th2-type immune responses (12). While Th1-related proinflammatory cytokines such as IFN-γ, IL-2, IL-12, and TNF-α promote the inflammatory response to eliminate invading pathogens (12, 34), Th2-related cytokines, including IL-4, IL-5, IL-10, and IL-13, counter the tissue-damaging effects of Th1-type responses (12, 34). Previous studies showed that sustained IL-12-driven IFN-γ production contributes to corneal perforation in B6 mice (16) and IL-1β and MIP-2 play a critical role in ocular bacterial infection by modulating the PMN influx (20, 35). In this study, TREM-1 significantly elevated the production of Th1 (IFN-γ) and proinflammatory cytokines (IL-1β, IL-6, MIP-2, and TNF-α) but reduced the expression of Th2 cytokines (IL-4, IL-5, and IL-10), indicating that the pathological contribution of TREM-1 in P. aeruginosa infection is probably disruption of the Th1/Th2 balance.
The modulation of these Th1 and Th2 cytokines is closely associated with TLR signaling. Studies using a pneumococcal pneumonia model indicated that TREM-1 enhanced the production of proinflammatory cytokines by reducing the expression of IRAK-M (a negative TLR regulator) (25). Our previous studies showed that other negative TLR regulators such as SIGIRR and ST2 also play an essential role in host resistance against bacterial infection (18, 19). SIGIRR promotes the host defense against P. aeruginosa infection by downregulating the Th1 response, as well as IL-1R1 and TLR4 signaling (19), while ST2 enhances the Th2 response and resistance to P. aeruginosa keratitis (18). In this study, TREM-1 amplified the TLR signaling in response to P. aeruginosa infection by downregulating the expression of SIGIRR and ST2. TREM-1 elevated the expression of TLR2, TLR4, and MyD88, while TLR5 and TLR9 expression was not affected (data not shown). Interestingly, other studies have demonstrated that TREM-1 expression is enhanced by activating TLR2 and TLR4 but not TLR3, TLR7/8, or TLR9 (23, 43, 44). Taken together, these findings suggested that TREM-1 may be associated with TLR2 and TLR4 but not other TLRs. Although the molecular mechanism of the interaction between TREM-1 and TLR2/4 remains unclear, we speculate that SIGIRR, ST2, and the MyD88 adaptor-like (Mal) protein may play important roles in the cross talk between TREM-1 and TLR2/4 signaling. SIGIRR inhibits TLR signaling by forming a complex with MyD88 (31, 37), ST2 functions as a sequestrator of MyD88 and Mal (6, 26), and Mal specifically participates in the MyD88-dependent pathways shared by TLR2 and TLR4 but not other TLRs (1, 30, 36). Thus, we hypothesize that in ocular infection, TREM-1 may cross talk with TLR2 and TLR4 signaling via a MyD88/Mal-dependent pathway.
We also found that TREM-1 reduced the production of mBD2, an antimicrobial peptide produced mainly by epithelial cells. Studies indicated that mBD2 is required in both innate and adaptive immunity for direct killing of invading pathogens, modulation of the production of inflammatory cytokines, and induction of the maturation of dendritic cells (2, 3, 41). Our previous studies using the same murine P. aeruginosa keratitis model indicated that silencing of mBD2 led to the upregulation of TLR2 and TLR4, while TLR5 and TLR9 were unaffected (41, 42). By reducing TLR2/TLR4 signaling, mBD2 decreased the bacterial load, PMN infiltration, and proinflammatory cytokine production, thereby promoting host resistance to corneal P. aeruginosa infection (41). Thus, TREM-1 may indirectly enhance the TLR2/TLR4-mediated inflammatory response via inhibition of mBD2 production.
In addition, inflammatory cells such as PMNs also play an important role in the inflammatory response to ocular P. aeruginosa infection. In this study, PMN infiltration was reduced by TREM-1 blockade and enhanced by TREM-1 activation at an early time (1 day p.i.), while no difference was detected at a later time (3 or 5 days p.i.). These data suggested that TREM-1 functions as an amplifier of the early inflammatory response partially through PMN infiltration. This result is consistent with others showing that TREM-1 activation leads to earlier pulmonary inflammation by enhancing PMN infiltration (25).
Interestingly, both blockade and activation studies indicated that TREM-1 downregulated the bacterial load at an early time (1 day p.i.) but upregulated it at a later time (3 or 5 days p.i.). We hypothesize that the bacterial load shift may be caused by an early enhancement of PMN infiltration and a late reduction of mBD2 production. In other words, at the early infection stage, PMNs were first recruited to the infection site to remove the invading pathogens. Thus, activation of TREM-1 overall reduced the number of viable bacteria by enhancing PMN influx, while at the late stage, since there was little difference in PMN recruitment between TREM-1-activated and control mice, the bacterial load change depended largely on another antibacterial component, mBD2. TREM-1 activation inhibited mBD2 production, thereby potentially enhancing the bacterial load to overcome the early effects of PMNs.
In summary, our study provides direct evidence that in both human and mouse corneas, TREM-1 expression was significantly enhanced after P. aeruginosa infection in a time-dependent manner. Blockade of TREM-1 reduced disease severity, whereas activation of TREM-1 accelerated disease progression. Mechanistically, TREM-1 seems to amplify Th1 and the proinflammatory responses but attenuate Th2 cytokines and mBD2 production by modulating TLR2 and TLR4 signaling. To our knowledge, this is the first study showing the role of TREM-1 in the eye, which may provide a promising target for the clinical treatment of ocular diseases such as P. aeruginosa keratitis.
ACKNOWLEDGMENTS
This investigation was supported by National Natural Science Foundation of China grants U0832006 (X.H.) and 30972763 (X.H.), the Guangdong Recruitment Program of Creative Research Groups (X.H.), the Midwest Eye Bank (X.H.), and NIH/NEI grants R01 EY019021 (X.H.) and P30 EY04068 (L.D.H.).
Footnotes
Published ahead of print on 9 May 2011.
REFERENCES
- 1. Akira S., Takeda K. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511 [DOI] [PubMed] [Google Scholar]
- 2. Biragyn A., et al. 2008. Murine beta-defensin 2 promotes TLR-4/MyD88-mediated and NF-kappaB-dependent atypical death of APCs via activation of TNFR2. J. Leukoc. Biol. 83:998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biragyn A., et al. 2002. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science 298:1025–1029 [DOI] [PubMed] [Google Scholar]
- 4. Bouchon A., Dietrich J., Colonna M. 2000. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164:4991–4995 [DOI] [PubMed] [Google Scholar]
- 5. Bouchon A., Facchetti F., Weigand M. A., Colonna M. 2001. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410:1103–1107 [DOI] [PubMed] [Google Scholar]
- 6. Brint E. K., et al. 2004. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat. Immunol. 5:373–379 [DOI] [PubMed] [Google Scholar]
- 7. Cohen J. 2001. TREM-1 in sepsis. Lancet 358:776–778 [DOI] [PubMed] [Google Scholar]
- 8. Colonna M. 2003. TREMs in the immune system and beyond. Nat. Rev. Immunol. 3:445–453 [DOI] [PubMed] [Google Scholar]
- 9. Cowell B. A., et al. 1998. An ocular strain of Pseudomonas aeruginosa is inflammatory but not virulent in the scarified mouse model. Exp. Eye Res. 67:347–356 [DOI] [PubMed] [Google Scholar]
- 10. Engel L. S., et al. 1995. Effectiveness of specific antibiotic/steroid combinations for therapy of experimental Pseudomonas aeruginosa keratitis. Curr. Eye Res. 14:229–234 [DOI] [PubMed] [Google Scholar]
- 11. Ferat-Osorio E., et al. 2008. The increased expression of TREM-1 on monocytes is associated with infectious and noninfectious inflammatory processes. J. Surg. Res. 150:110–117 [DOI] [PubMed] [Google Scholar]
- 12. Hazlett L. D. 2004. Corneal response to Pseudomonas aeruginosa infection. Prog. Retin. Eye Res. 23:1–30 [DOI] [PubMed] [Google Scholar]
- 13. Hazlett L. D. 2002. Pathogenic mechanisms of P. aeruginosa keratitis: a review of the role of T cells, Langerhans cells, PMN, and cytokines. DNA Cell Biol. 21:383–390 [DOI] [PubMed] [Google Scholar]
- 14. Hazlett L. D. 2005. Role of innate and adaptive immunity in the pathogenesis of keratitis. Ocul. Immunol. Inflamm. 13:133–138 [DOI] [PubMed] [Google Scholar]
- 15. Hazlett L. D., McClellan S., Kwon B., Barrett R. 2000. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Invest. Ophthalmol. Vis. Sci. 41:805–810 [PubMed] [Google Scholar]
- 16. Hazlett L. D., Rudner X. L., McClellan S. A., Barrett R. P., Lighvani S. 2002. Role of IL-12 and IFN-gamma in Pseudomonas aeruginosa corneal infection. Invest. Ophthalmol. Vis. Sci. 43:419–424 [PubMed] [Google Scholar]
- 17. Hazlett L. D., Zelt R., Cramer C., Berk R. S. 1985. Pseudomonas aeruginosa induced ocular infection. A histological comparison of two bacterial strains of different virulence. Ophthalmic Res. 17:289–296 [DOI] [PubMed] [Google Scholar]
- 18. Huang X., Du W., Barrett R. P., Hazlett L. D. 2007. ST2 is essential for Th2 responsiveness and resistance to Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci. 48:4626–4633 [DOI] [PubMed] [Google Scholar]
- 19. Huang X., Hazlett L. D., Du W., Barrett R. P. 2006. SIGIRR promotes resistance against Pseudomonas aeruginosa keratitis by down-regulating type-1 immunity and IL-1R1 and TLR4 signaling. J. Immunol. 177:548–556 [DOI] [PubMed] [Google Scholar]
- 20. Kernacki K. A., Barrett R. P., Hobden J. A., Hazlett L. D. 2000. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. J. Immunol. 164:1037–1045 [DOI] [PubMed] [Google Scholar]
- 21. Kernacki K. A., Barrett R. P., McClellan S., Hazlett L. D. 2001. MIP-1alpha regulates CD4+ T cell chemotaxis and indirectly enhances PMN persistence in Pseudomonas aeruginosa corneal infection. J. Leukoc. Biol. 70:911–919 [PubMed] [Google Scholar]
- 22. Kernacki K. A., Goebel D. J., Poosch M. S., Hazlett L. D. 1998. Early cytokine and chemokine gene expression during Pseudomonas aeruginosa corneal infection in mice. Infect. Immun. 66:376–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knapp S., et al. 2004. Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J. Immunol. 173:7131–7134 [DOI] [PubMed] [Google Scholar]
- 24. Kwon B., Hazlett L. D. 1997. Association of CD4+ T cell-dependent keratitis with genetic susceptibility to Pseudomonas aeruginosa ocular infection. J. Immunol. 159:6283–6290 [PubMed] [Google Scholar]
- 25. Lagler H., et al. 2009. TREM-1 activation alters the dynamics of pulmonary IRAK-M expression in vivo and improves host defense during pneumococcal pneumonia. J. Immunol. 183:2027–2036 [DOI] [PubMed] [Google Scholar]
- 26. Liew F. Y., Liu H., Xu D. 2005. A novel negative regulator for IL-1 receptor and Toll-like receptor 4. Immunol. Lett. 96:27–31 [DOI] [PubMed] [Google Scholar]
- 27. McClellan S. A., Huang X., Barrett R. P., van Rooijen N., Hazlett L. D. 2003. Macrophages restrict Pseudomonas aeruginosa growth, regulate polymorphonuclear neutrophil influx, and balance pro- and anti-inflammatory cytokines in BALB/c mice. J. Immunol. 170:5219–5227 [DOI] [PubMed] [Google Scholar]
- 28. Murakami Y., et al. 2006. Induction of triggering receptor expressed on myeloid cells 1 in murine resident peritoneal macrophages by monosodium urate monohydrate crystals. Arthritis Rheum. 54:455–462 [DOI] [PubMed] [Google Scholar]
- 29. Murakami Y., Kohsaka H., Kitasato H., Akahoshi T. 2007. Lipopolysaccharide-induced up-regulation of triggering receptor expressed on myeloid cells-1 expression on macrophages is regulated by endogenous prostaglandin e2. J. Immunol. 178:1144–1150 [DOI] [PubMed] [Google Scholar]
- 30. O'Neill L. A., et al. 2003. Mal and MyD88: adapter proteins involved in signal transduction by Toll-like receptors. J. Endotoxin Res. 9:55–59 [DOI] [PubMed] [Google Scholar]
- 31. Qin J., Qian Y., Yao J., Grace C., Li X. 2005. SIGIRR inhibits interleukin-1 receptor- and Toll-like receptor 4-mediated signaling through different mechanisms. J. Biol. Chem. 280:25233–25241 [DOI] [PubMed] [Google Scholar]
- 32. Radsak M. P., Salih H. R., Rammensee H. G., Schild H. 2004. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J. Immunol. 172:4956–4963 [DOI] [PubMed] [Google Scholar]
- 33. Schenk M., Bouchon A., Seibold F., Mueller C. 2007. TREM-1-expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J. Clin. Invest. 117:3097–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schulze-Koops H., Kalden J. R. 2001. The balance of Th1/Th2 cytokines in rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 15:677–691 [DOI] [PubMed] [Google Scholar]
- 35. Thakur A., Barrett R. P., McClellan S., Hazlett L. D. 2004. Regulation of Pseudomonas aeruginosa corneal infection in IL-1 beta converting enzyme (ICE, caspase-1) deficient mice. Curr. Eye Res. 29:225–233 [DOI] [PubMed] [Google Scholar]
- 36. Verstak B., et al. 2009. MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. J. Biol. Chem. 284:24192–24203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wald D., et al. 2003. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 4:920–927 [DOI] [PubMed] [Google Scholar]
- 38. Wang J., Hu Y., Deng W. W., Sun B. 2009. Negative regulation of Toll-like receptor signaling pathway. Microbes Infect. 11:321–327 [DOI] [PubMed] [Google Scholar]
- 39. Wilhelmus K. R. 1987. Review of clinical experience with microbial keratitis associated with contact lenses. CLAO J. 13:211–214 [PubMed] [Google Scholar]
- 40. Williams R. N., Paterson C. A., Eakins K. E., Bhattacherjee P. 1982. Quantification of ocular inflammation: evaluation of polymorphonuclear leucocyte infiltration by measuring myeloperoxidase activity. Curr. Eye Res. 2:465–470 [DOI] [PubMed] [Google Scholar]
- 41. Wu M., McClellan S. A., Barrett R. P., Hazlett L. D. 2009. Beta-defensin-2 promotes resistance against infection with P. aeruginosa. J. Immunol. 182:1609–1616 [DOI] [PubMed] [Google Scholar]
- 42. Wu M., McClellan S. A., Barrett R. P., Zhang Y., Hazlett L. D. 2009. Beta-defensins 2 and 3 together promote resistance to Pseudomonas aeruginosa keratitis. J. Immunol. 183:8054–8060 [DOI] [PubMed] [Google Scholar]
- 43. Youssef R. E., et al. 2009. The role of Toll-like receptors (TLR-2 and -4) and triggering receptor expressed on myeloid cells 1 (TREM-1) in human term and preterm labor. Reprod. Sci. 16:843–856 [DOI] [PubMed] [Google Scholar]
- 44. Zheng H., et al. 2010. MYD88-dependent and -independent activation of TREM-1 via specific TLR ligands. Eur. J. Immunol. 40:162–171 [DOI] [PubMed] [Google Scholar]