Abstract
The type VI secretion system (T6SS) is recognized as an important virulence mechanism in several Gram-negative pathogens. In Vibrio cholerae, the causative agent of the diarrheal disease cholera, a minimum of three gene clusters—one main cluster and two auxiliary clusters—are required to form a functional T6SS apparatus capable of conferring virulence toward eukaryotic and prokaryotic hosts. Despite an increasing understanding of the components that make up the T6SS apparatus, little is known about the regulation of these genes and the gene products delivered by this nanomachine. VasH is an important regulator of the V. cholerae T6SS. Here, we present evidence that VasH regulates the production of a newly identified protein, VasX, which in turn requires a functional T6SS for secretion. Deletion of vasX does not affect export or enzymatic function of the structural T6SS proteins Hcp and VgrG-1, suggesting that VasX is dispensable for the assembly of the physical translocon complex. VasX localizes to the bacterial membrane and interacts with membrane lipids. We present VasX as a novel virulence factor of the T6SS, as a V. cholerae mutant lacking vasX exhibits a phenotype of attenuated virulence toward Dictyostelium discoideum.
INTRODUCTION
Vibrio cholerae is the marine bacterium responsible for the diarrheal disease cholera. Multiple cholera pandemics have been caused by the O1 serogroup of V. cholerae, and recently, O139 has emerged as a new pandemic strain (11). The principal virulence factors postulated to be essential for the pandemic spread of strains belonging to these serogroups are cholera toxin (CT) and the toxin-coregulated pilus (TCP) (13, 17). Non-O1 and non-O139 serogroups of V. cholerae are also capable of causing disease, in some cases in the absence of CT and TCP (10). In 1968, the O37 serogroup strain V52 was responsible for an outbreak of cholera-like diarrheal illness in Sudan, with 460 cases leading to 125 deaths (42). The genome of V52 carries both CT and TCP genes, but whether this strain produces CT and TCP in vivo has not yet been determined. V52 possesses a constitutively active type VI secretion system (T6SS) that confers virulence toward phagocytic cells, including the social amoeba Dictyostelium discoideum and murine macrophages (34). Pandemic strains of V. cholerae also possess the full complement of T6SS genes, but it is currently unclear how the T6SS is activated and how this system contributes to human disease.
Six distinct secretion systems in Gram-negative bacteria provide means to translocate proteins across the bacterial inner and outer membranes (12). The recently discovered T6SS is predicted to structurally resemble the cell-puncturing device of T4 bacteriophage and to function similarly to the T3SS and T4SS, which transfer proteins directly from the microbe into host cells (20, 33, 34). Recent work has demonstrated that the T6SS can target prokaryotic as well as eukaryotic organisms (18, 25, 37).
The T6SS gene clusters include several conserved genes that encode needle apparatus components (4, 20, 28, 33), inner and outer membrane proteins (2, 24, 29, 34), and transcriptional regulators (3, 6, 28). V. cholerae utilizes the transcriptional activator VasH to control the expression of T6SS genes, such as hcp, for which there are two alleles (VCA0017 and VC1415) in the Vibrio genome (34). VasH is encoded by the large T6SS cluster (VCA0107 to VCA0123) and is predicted to activate sigma factor σ54-dependent transcription (6, 34). Another noteworthy protein is virulence-associated secretion protein K (VasK). VasK is a T6SS inner membrane protein that, in Agrobacterium tumefaciens, has a role in hydrolyzing nucleoside triphosphates for the T6SS machine assembly or secretion of T6SS substrates (24).
The T6SS translocon complex shares homology with the T4 bacteriophage puncturing device (20, 33) and minimally consists of the hemolysin-coregulated protein (Hcp) and valine-glycine repeat (VgrG) proteins. Hcp hexamers form nanotubes in vitro with an internal channel diameter of 4 nm; the nanotubes are predicted to be decorated with VgrG trimeric tips (4, 20, 28, 33). Because Hcp and VgrGs are codependent for export from the bacterial cell (33), it was hypothesized that these proteins form a structural translocon complex through which effector proteins transit en route to the host cell cytoplasm (4, 20, 35).
It is important to discern between “exported,” “secreted,” and “translocated” proteins. In this report, we define exported proteins such as Hcp and the VgrGs as structural constituents of the T6SS which are transported out of the bacterium to assemble as a needle-like injectosome on the bacterial surface. Secreted proteins are those dependent on the structural apparatus to exit the cell. Finally, translocated proteins are effectors, which are secreted through the injectosome directly into host cells.
Although rapid progress in understanding the contribution of the structural components that make up the physical T6SS apparatus is being made, little is known about the regulation of the genes encoding them. A better understanding of T6SS gene regulation will allow us to determine when and where this virulence mechanism is engaged during V. cholerae's complex life cycle and to assess whether and how the T6SS contributes to the diarrheal disease cholera. A vasH mutant is unable to export Hcp and displays a phenotype of attenuated virulence toward amoebae and mammalian macrophages (34), demonstrating a central role for VasH in T6SS-mediated virulence. In this study, we report the identification of a VasH-regulated, 121-kDa virulence factor designated VasX, whose T6SS-dependent transport contributes to the virulent behavior of V. cholerae.
MATERIALS AND METHODS
Strains and culture conditions.
D. discoideum AX3 was grown in shaking liquid culture in HL5 medium (40) at 22°C. A V. cholerae V52 derivative strain lacking hlyA, hapA, and rtxA was the background strain (34) for all T6SS deletion mutants and was used as the wild-type strain in all experiments. Escherichia coli strains DH5α and SM10λpir were used for cloning and mating purposes, respectively. All bacterial strains were grown in LB broth at 37°C. Murine RAW 264.7 macrophages, a gift from Hanne Ostergaard (University of Alberta), were maintained at 37°C in a 5% CO2 atmosphere in Eagle's minimum essential media (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum, 4 mM l-glutamine, 1 mM sodium pyruvate, 100 units·ml−1 penicillin, and 0.1 mg·ml−1 streptomycin (Sigma-Aldrich, St. Louis, MO).
Secretion profiles.
Overnight cultures of bacteria were diluted 1:100 in 3 ml LB broth (containing the appropriate antibiotics) and grown to an optical density at 600 nm (OD600) of ∼0.8. Bacteria were pelleted, and the supernatant was filter sterilized through Millipore 0.22-μm low-protein-binding polyvinylidene fluoride (PVDF) syringe filters. Supernatant proteins were precipitated with 20% trichloroacetic acid (TCA) for 15 min at 4°C and washed twice with acetone to remove residual TCA. Proteins were resuspended in 50 μl of 1× SDS-protein lysis buffer (40% glycerol, 0.24 M Tris-HCl, pH 6.8, 8% SDS, 0.04% bromophenol blue, 5% β-mercaptoethanol) and boiled for 10 min. Bacterial pellet fractions were resuspended in 1× SDS-protein lysis buffer and boiled for 10 min. Samples were then subjected to SDS-PAGE (10% acrylamide) and analyzed by silver staining or immunoblotting using anti-VasX (this study; diluted 1:100), anti-DnaK (Stressgen, Plymouth Meeting, PA; diluted 1:10,000), and anti-Hcp (23; diluted 1:500) antibodies. The polyclonal antibody against VasX was generated by New England Peptide Inc. (Gardner, MA). Briefly, a synthetic peptide, comprising amino acids 271-VAKRTKAIGDETQQHKMQMAELTRT-295 of VasX was prepared and conjugated to keyhole limpet hemocyanin. The conjugated peptide was injected into rabbits (New Zealand White), boosted twice, and then tested on crude cell extracts from V. cholerae strain V52 by Western blotting to ensure antibody specificity. Secondary antibodies included goat anti-mouse IgG horseradish peroxidase (HRP) (Santa Cruz Biotechnology Inc., Santa Cruz, CA; diluted 1:3,000) and goat anti-rabbit IgG HRP (Sigma-Aldrich; diluted 1:3,000).
Mass spectrometry and secondary structure prediction analysis.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis was performed at the University of Alberta Mass Spectrometry Facility (Department of Chemistry). In-gel proteins were reduced with 4 mM dithiothreitol and carbidomethylated with 10 mM iodoacetamide followed by tryptic digestion overnight with 0.06 μg·μl−1 modified bovine trypsin (Promega, Madison, WI) at 30°C. Peptides were subjected to LC-MS/MS analysis on a NanoAcquity ultraperformance liquid chromatography system (Waters, Milford, MA) coupled with a Q-TOF Premier mass spectrometer (Waters, Milford, MA). Obtained MS/MS data were analyzed by PEAKS proteomic software (Bioinformatics Solutions Inc., Waterloo, Ontario, Canada). Secondary structure prediction was performed using the HHpred server (38) and the Phyre server (5, 19).
In-frame deletions and complementation.
In-frame deletion of T6SS genes was performed as described by Metcalf et al. (26). A vasX deletion mutant was created using primers A-KOvasX (5′-GGATCCCTTCTTTAGATTCTATTG-3′), B-KOvasX (5′-ACATTTAACCTTTTCCTACATTGGGATTACTCATCCTT-3′), C-KOvasX (5′-AAGGATGAGTAATCCCAATGTAGGAAAAGGTTAAATGT-3′), and D-KOvasX (5′-GGATCCTTATTATGAATACTCTTC-3′). PCR products resulting from primer combinations A-KOvasX/B-KOvasX and C-KOvasX/D-KOvasX were stitched together by overlapping PCR. The resulting knockout construct for vasX was digested with BamHI and cloned into the suicide plasmid pWM91 (26).
Plasmid pBAD24-vasX was constructed for arabinose-inducible vasX expression. vasX was amplified by PCR from the V52 genome by using primers 5′-vasX (5′-GGGGTACCCATGAGTAATCCCAATCAAGCT-3′) and 3′-vasX (5′-GCTCTAGATTAACCTTTTCCTACAACGAG-3′). The resulting PCR product was digested with KpnI and XbaI and subcloned into plasmid pBAD24 (15). vasH was amplified from the V52 genome by using primers 5′-vasH (5′-GAATTCACCATGAGTCAATGGCTGGCG-3′) and 3′-vasH (5′-TCTAGATCATGGGGTTTTGATCTC-3′). The resulting PCR product was subcloned into the TOPO-TA vector pCR2.1 and transformed into the E. coli strain Top10. pCR2.1-vasH was then digested by using EcoRI and XbaI, and vasH was cloned into pBAD24.
RNA isolation and quantitative RT-PCR.
Shaking bacterial cultures were grown at 37°C to mid-logarithmic stage in the absence or presence of 0.1% arabinose. Total mRNA was extracted by using an RNeasy minikit (Qiagen, Germantown, MD) according to the manufacturer's instructions. One microgram of RNA from each sample was subjected to DNase I (Invitrogen, Carlsbad, CA) treatment to remove residual DNA, followed by a reverse transcription reaction using SuperScript III reverse transcriptase (Invitrogen). Semiquantitative real-time PCR (RT-PCR) was performed with PerfeCTa SYBR green FastMix (Quanta, Gaithersburg, MD) and primers against vasX (vasX-F [5′-TCCTTTACCCAAAGCGGATCGACA-3′] and vasX-R [5′-GCCATCACGCAGTTGCCTTAAAGT-3′]) and ompW (OmpW-F [5′-GGACTTGCTGCTAACGTTGGCTTT-3′] and OmpW-R [5′-CCTGCTTTGTAGGTTGCCGTTGTT-3′]) as an internal control. The threshold cycle (CT) values were determined by Mastercycler ep realplex (Eppendorf, Mississauga, ON), and the fold change was calculated using the 2−ΔΔCT method (22a).
D. discoideum plaque assays.
Plaque assays were performed as described previously (34) with the following exceptions: the incubation period was increased to 4 days to allow for an increase in plaque size and, where applicable, arabinose was added to a final concentration of 0.1% to induce vasX expression. Pictures of plaques were generated using a Kodak Gel Logic 200 imaging system and Kodak 1D v3.6 software. Statistical significance was determined using Tukey's multiple comparison test (95% confidence interval).
In vivo actin cross-linking.
Actin cross-linking experiments were performed as described previously (33). Briefly, murine RAW 264.7 macrophages were seeded into 24-well tissue culture plates at a density of 1 × 105 cells per well 18 h prior to infection. Two hours prior to infection, the penicillin-streptomycin-containing medium was aspirated from cells and the cells were washed twice in prewarmed phosphate-buffered saline (PBS). Macrophages were then incubated for 2 h in antibiotic-free medium. Cells were infected with V52 and the given T6SS mutants at a multiplicity of infection (MOI) of 10 for 2 h at 37°C. Cells were harvested by centrifugation and resuspended in 50 μl of lysis buffer. Ten microliters of each sample was analyzed by Western blotting using actin primary antiserum (Santa Cruz Biotechnology Inc.; diluted 1:500) and goat anti-rabbit IgG-HRP secondary antibody (Sigma-Aldrich; diluted 1:3,000).
Subcellular fractionation.
The subcellular fractionation method was adapted from Sandkvist et al. (36). V52 transformed with pBAD24 (15) was grown to late logarithmic phase and pelleted by centrifugation at 3,000 ×g for 20 min. The pellet was resuspended in LB broth with 1 mg·ml−1 polymyxin B and incubated at room temperature for 20 min in order to release periplasmic contents. Permeabilized V52 (lacking periplasmic content) was pelleted at 3,000 × g for 20 min at room temperature. The resulting supernatant was collected as the periplasmic fraction. Permeabilized V52 was washed with 5 ml LB broth and pelleted at 3,000 × g for 20 min at room temperature. The pellet was resuspended in 4 ml PBS and passed twice through a French pressure cell at 8,000 lb/in2. Unbroken cells were pelleted, and the membrane/cytosolic fraction was retained. Membrane and cytosolic fractions were separated by centrifugation of the resulting supernatant in a Beckman L-30 Optima ultracentrifuge at 100,000 × g for 30 min at 4°C in an SW55 Ti swinging bucket rotor. Membranes were washed once in 5 ml PBS and pelleted at 100,000 × g for 30 min at 4°C in an SW55 Ti rotor. Protein concentrations of all fractions were determined using the Pierce bicinchoninic acid (BCA) protein assay. Twelve micrograms of protein per well was loaded on SDS-PAGE gel for Western blot analysis using anti-Hcp (23), anti-VasX (this study; diluted 1:100), anti-beta-lactamase (Abcam, Cambridge, MA; diluted 1:200), anti-OmpU (Santa Cruz Biotechnology, Inc.; diluted 1:200), and anti-DnaK (Stressgen; diluted 1:10,000) primary antibodies. Secondary antibodies included goat anti-rabbit IgG-HRP (Sigma-Aldrich; diluted 1:3,000), goat anti-mouse IgG-HRP (Santa Cruz Biotechnology Inc.; diluted 1:3,000), and donkey anti-goat-HRP (Santa Cruz Biotechnology Inc.; diluted 1:3,000).
Cloning, expression, and purification of recombinant VasX.
The full-length vasX gene was cloned from a Gateway entry vector (Harvard Institute of Proteomics clone identification no. VcCD00020120) into the pET-DEST42 expression vector in frame with a C-terminal V5 and 6×His fusion for IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible high-level expression. The resulting plasmid, pET-DEST42-vasX, was transformed into E. coli BL21 Star (DE3) (Invitrogen), which encodes the T7 RNA polymerase required for expression from the T7 promoter. The E. coli BL21 Star (DE3)/pET-DEST42-vasX culture was incubated at 30°C to an OD600 of ∼0.5, induced with 0.1 mM IPTG, and harvested 4 h postinduction by centrifugation at 5,000 × g.
Primers vasX-pET28a-F (5′-GTTGGCTAGCATGAGTAATCCCAATCAAGCTGC-3′) and vasXPH-pET28a-R (5′-GGTTGCTCGAGTTAATCCTGCTCAGCTGGCGTC-3′) were used to amplify the N-terminal 200 residues of VasX for cloning into the pET28a vector (Novagen) using the restriction sites NheI and XhoI to place the fragment in frame with an N-terminal 6×His tag. The construct, pET28a-PH, was transformed into E. coli BL21 Star (DE3) and grown at 25°C to an OD600 of ∼0.9, induced by the addition of 10 μM IPTG for 16 to 20 h, and harvested by centrifugation at 5,000 × g.
Bacterial pellets were resuspended in 20 mM Tris-HCl, pH 8.0, 500 mM NaCl, and 20 mM imidazole supplemented with 1 mM phenylmethylsulfonyl fluoride and lysed by three passes through a French pressure cell at 18,000 lb/in2. Lysates were cleared of insoluble cell debris by centrifugation at 20,000 × g and filtered through a 0.45-μm PVDF filter (Millipore). His-tagged proteins were purified using the AKTAdesign Basic system (GE Healthcare, Piscataway, NJ) mounted with a HisTrap FF Crude (GE Healthcare) column using an elution gradient of 20 mM to 500 mM imidazole. The purified protein fractions were dialyzed against PBS (pH 7.4), and the concentration was determined by A280.
PIP Strip immunoblots.
PIP Strips (Echelon Biosciences, Salt Lake City, UT) were incubated with full-length VasX, or the VasX fragment containing residues 1 to 200 [VasX(1–200)], according to the manufacturer's recommendations. Briefly, the strips were blocked with 3% bovine serum albumin (BSA) dissolved in phosphate-buffered saline containing 0.1% Tween 20 (PBS-T) for 30 min at room temperature and incubated with the target protein at 2 μg·ml−1 in 3% BSA–PBS-T for 1 h at room temperature. As a positive control, purified VasX protein was spotted directly onto the nitrocellulose membrane prior to blocking. Purified E. coli (strain 0127:B8) lipopolysaccharide (LPS) was purchased from Sigma-Aldrich (St. Louis, MO) and was spotted onto the nitrocellulose membrane in an amount (100 picomoles) equimolar to the various lipids. Bound proteins were detected with mouse anti-6×His (Clontech, Mountain View, CA; diluted 1:1,000) primary antibody and an anti-mouse IgG-HRP secondary antibody (Santa Cruz Biotechnology Inc.; diluted 1:3,000).
Multilamellar vesicle pulldown.
Multilamellar vesicles were prepared as described previously (1). Briefly, membrane lipid preparations (Avanti Polar Lipids, Alabaster, AL) were transferred to methanol-washed glass tubes, dried under a nitrogen stream, and rehydrated in 500 μl of PBS for 3 min at room temperature. Samples were vortexed for 3 min, yielding a suspension of 5 mM multilamellar vesicles (MLV). Eighty microliters of purified full-length VasX, VasX(1–200), or BSA at a concentration of 60 μg·ml−1 was added to 20 μl of MLV, gently mixed, and incubated for 5 min at room temperature. Fifty microliters of this mixture was centrifuged for 30 min at 13,000 × g at 4°C to pellet MLV-protein complexes. Fractions from each sample (total, pellet, or supernatant) were mixed with 1× SDS-protein lysis buffer, boiled, and separated by SDS-PAGE. Proteins were visualized by staining with Coomassie blue R-250 stain.
RESULTS
Identification of VasX.
In an attempt to identify secreted/exported V. cholerae proteins that require VasH for expression, we used a complemented vasH-null mutant of V. cholerae (V52ΔvasH) that carries the plasmid pBAD24-vasH with vasH under the control of an arabinose-inducible promoter. We compared SDS-PAGE protein profiles from culture supernatants in which bacteria were grown in the presence or absence of arabinose. Supernatants of strains grown in the presence of arabinose revealed multiple bands absent from supernatants of V. cholerae bacteria grown in the absence of arabinose (Fig. 1A). Given that V52ΔvasH alone does not secrete Hcp (34), restoration of Hcp secretion to wild-type levels upon vasH induction indicated proper complementation (Fig. 1A and data not shown). In addition to Hcp, an ∼120-kDa band appeared when VasH expression was induced. The 100- to 140-kDa range from each lane was excised and subjected to in-gel trypsin digestion followed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. Mass spectrometry led to the identification of a protein with a molecular mass of 121 kDa in supernatants of VasH-producing bacteria that was not present in supernatants of bacteria lacking VasH. We named the 121-kDa protein VasX (VCA0020). In addition to VasX, other proteins present only in supernatants of vasH-induced bacteria were identified by mass spectrometry, and these proteins included VgrG-1, VgrG-3, and Hcp (Fig. 1B). Surprisingly, even though VgrG-2 is encoded directly upstream of vasX, it was not identified from culture supernatants when vasH was overexpressed. The VasX protein is encoded on the V. cholerae small chromosome, downstream of two T6SS genes, hcp-2 and vgrG-2 (VCA0017 and VCA0018, respectively) (Fig. 1C). The location of vasX suggests that this gene belongs to a T6SS gene cluster and may play an integral role in T6SS-mediated virulence. The NCBI denotes VasX as a hypothetical protein, and BLASTP analysis revealed that VasX homologs are present in Gram-negative bacteria such as Pseudomonas syringae, Photobacterium damselae, and Aeromonas hydrophila. The hypothetical gene VCA0019 is located between vgrG-2 and vasX and also belongs to this T6SS gene cluster, but its role in virulence remains to be determined.
Fig. 1.
Identification of VasX. (A) A V. cholerae vasH mutant carrying plasmid pBAD24-vasH that allows arabinose-inducible expression of vasH was grown in the absence or presence of 0.1% arabinose. Total protein of culture supernatants was resolved by 10% SDS-PAGE and visualized by silver staining. The 100- to 140-kDa regions from both lanes were excised and subjected to LC-MS/MS analysis. (B) Proteins identified by mass spectrometry as present in culture supernatant after vasH expression was induced. Swiss ID, Swiss-Prot identification number. (C) VasX belongs to a T6SS satellite gene cluster on the V. cholerae small chromosome immediately downstream of hcp-2, vgrG-2, and VCA0019.
The hidden Markov model homology search program HHpred (38) identified an N-terminal pleckstrin homology (PH) domain (E = 0.5; P = 5E−5) near the N terminus of VasX. Although the E values identified by HHpred are large, further protein structure prediction of the candidate PH domain (amino acids 73 to 166) in VasX by the Phyre server (5, 19) also indicated a canonical PH domain structure consisting of two β-sheets followed by an α-helix (21). Since PH domain-containing proteins are frequently involved in signal transduction in eukaryotic cells, these predicted structural features in VasX raise the possibility that it may imitate host cell proteins by utilizing its PH domain to attach itself to host cell structures such as biological membranes.
VasX production depends on the σ54-dependent transcriptional activator VasH.
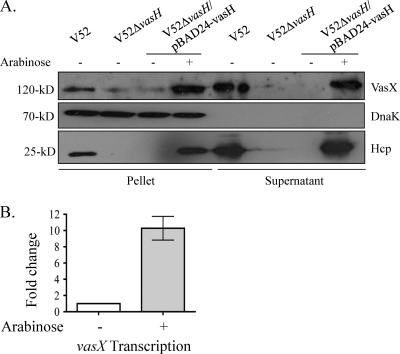
Our finding that VasX was present exclusively in supernatants of VasH-producing V. cholerae (Fig. 1A) suggests that VasH regulates the production of VasX. This hypothesis is supported by our observations that VasX cannot be detected in V52ΔvasH culture supernatants by Western blotting with VasX antibody and that VasX levels are significantly reduced in pellet fractions (Fig. 2A). Importantly, DnaK, a cytoplasmic heat shock protein (22), was present only in bacterial pellet samples, indicating that the bacteria were intact, and thus, VasX is actively secreted and not released by cell lysis.
Fig. 2.
VasX expression depends on the T6SS regulator VasH. (A) Western blot demonstrating that trans expression of vasH restores production of both Hcp and VasX. Bacterial cultures were grown to late logarithmic phase in the presence or absence of 0.1% arabinose. Pellet and supernatant fractions were separated and subjected to 10% SDS-PAGE followed by Western blotting with anti-VasX, -Hcp, and -DnaK antibodies. Results are representative of three independent experiments. (B) VasH regulates the expression of vasX. V52ΔvasH carrying pBAD24-vasH to allow arabinose-controlled complementation was grown to mid-logarithmic phase in the presence or absence of arabinose. VasX transcript levels were determined by RT-PCR using the gene encoding the V. cholerae outer membrane protein OmpW (ompW) as an internal control. Results are representative of three experimental data sets.
To investigate the relationship between VasH and the physically linked genes hcp and vasX (as depicted in Fig. 1C), we used Western blotting with polyclonal antibodies to detect Hcp and VasX in the complemented vasH deletion mutant. No Hcp and significantly reduced levels of VasX could be detected in pellets or concentrated supernatants when vasH was not expressed; however, upon induction of vasH expression, Hcp and VasX production within the cell and export/secretion into culture supernatants was restored (Fig. 2A). These data indicate that VasH plays an integral role in regulating both Hcp and VasX, supporting our hypothesis that hcp-2, vgrG-2, VCA0019, and vasX belong to the same T6SS regulon.
To further validate our data presented in Fig. 2A, we used semiquantitative RT-PCR to measure the vasX transcript levels in the complemented V52ΔvasH strain relative to the transcription of the outer membrane protein OmpW. It was previously demonstrated that the presence of arabinose does not affect the expression of OmpW (30). When vasH expression was induced with arabinose, vasX transcript levels increased ∼10-fold (Fig. 2B). We propose that VasH acts directly at the promoter located immediately upstream of the T6SS satellite cluster encoding Hcp-2, VgrG-2, and VasX in V. cholerae V52. Our hypothesis is supported by work from Bernard et al., who recently demonstrated that VasH from V. cholerae O395 binds to promoters found upstream of the core T6SS gene cluster as well as promoters upstream of the satellite gene clusters (6).
VasX secretion is dependent on the T6SS proteins Hcp, VasK, and VgrG-2.
Since VasX depends on the T6SS regulator VasH for expression, we hypothesized that the T6SS structural complex is required for VasX secretion. To test this, we employed the isogenic T6SS deletion mutants V52Δhcp-1,2 (V52 with hcp-1 and hcp-2 deleted), V52ΔvgrG-1, V52ΔvgrG-2, V52ΔvgrG-3, and V52ΔvasK (34). As shown in Fig. 3A, VasX was secreted by wild-type V52, V52ΔvgrG-1, and V52ΔvgrG-3 but not by V52ΔvasK, V52Δhcp-1,2, or V52ΔvgrG-2. Furthermore, the secretion of VasX matched the export pattern of Hcp. VgrG-2 was the only one of the three VgrG proteins absolutely required for the export and secretion of both Hcp and VasX (Fig. 3A). The absence of VasX in culture supernatants was not due to a failure to produce VasX within the cell, as VasX was present in bacterial pellet samples of all T6SS mutants tested (with the exception of the in-frame deletion mutant V52ΔvasX) (Fig. 3A).
Fig. 3.
Secretion of VasX is T6SS dependent. (A) Western blot of VasX and Hcp in culture supernatants and bacterial pellets. Bacterial cultures were grown to late logarithmic phase; supernatant and pellet fractions were isolated and subjected to Western blotting using the indicated antibody types (listed to the left of the blot). Molecular masses are shown to the right of both blots. Results are representative of at least three experimental data sets. (B) VasX is not required for T6SS-mediated cross-linking of murine macrophage actin. RAW 264.7 macrophages were infected at a multiplicity of infection of 10 for 2 h. Cell lysates were subjected to SDS-PAGE followed by Western blotting using an antiactin antibody. Molecular mass markers are shown to the left of the blot. The hcp deletion strain V52Δhcp-1,2 has both chromosomal copies of hcp deleted. Results are representative of at least three experimental data sets.
Interestingly, deletion of vasX did not affect the production/export of Hcp (Fig. 3A), and thus, VasX does not appear necessary for the formation of the T6SS structural apparatus. Aside from Hcp nanotubes, VgrG protein trimers are also important factors for producing a functional T6SS cell-puncturing complex (33, 34). Therefore, we tested whether VasX was required for VgrG-1-mediated cross-linking of host cell actin. To determine whether host cell actin is cross-linked by V52ΔvasX, murine RAW 264.7 macrophages were infected with the wild type, V52Δhcp-1,2, V52ΔvasK, V52ΔvgrG-1, or V52ΔvasX for 2 h. Following infection, cells were collected, lysed, and resolved by SDS-PAGE followed by Western blotting using an antiactin antibody. Actin cross-linking was visualized by a “laddering” effect of actin bands, as monomeric units of G-actin are covalently attached to one another, forming higher-molecular-weight structures (33). As seen in Fig. 3B, only wild-type V52 and V52ΔvasX strains were capable of cross-linking RAW 264.7 cell actin. Thus, deletion of vasX did not affect the assembly or the enzymatic function of the T6SS physical translocon complex. Taken together, we conclude from these data that VasK, Hcp, and VgrG-2 are required for VasX secretion and that VasX has the same transport dependencies as Hcp. Furthermore, VasX is not imperative for the formation of a functional T6SS translocon complex.
VasX localizes to the bacterial membrane.
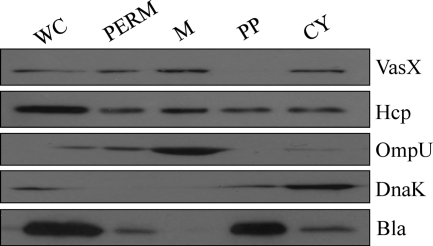
To investigate the localization of VasX and Hcp within the bacterium, we performed subcellular fractionation experiments. V52 was transformed with the β-lactamase-encoding plasmid pBAD24 for subcellular fractionation experiments to use β-lactamase as a periplasmic control. Other fractionation controls included DnaK (cytoplasm) and OmpU (membrane). As shown in Fig. 4, Hcp localized to all cellular compartments, whereas VasX localized specifically to the cytoplasmic and membrane fractions. It should be noted that Hcp was present in the periplasm at high abundance, while VasX could not be detected in this compartment.
Fig. 4.
VasX localizes to the bacterial membrane. V52/pBAD24 was grown to mid-logarithmic phase and subjected to subcellular fractionation. Various fractions (whole cell [WC], permeabilized V52 [PERM], membrane [M], periplasm [PP], and cytosol [CY]) were separated by SDS-PAGE followed by Western blotting with anti-VasX, -Hcp, -OmpU (membrane control), -DnaK (cytosol control), and -β-lactamase (Bla; periplasm control) primary antibodies. Results are representative of three independent experiments.
VasX is required for virulence toward the host model Dictyostelium discoideum.
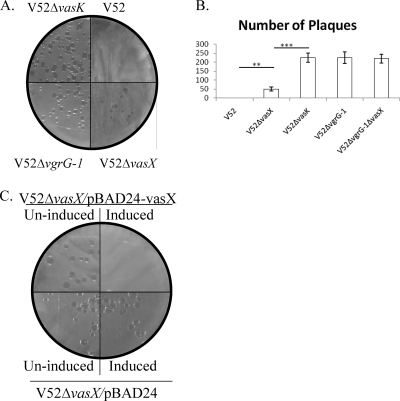
We examined whether VasX is essential for virulence toward eukaryotes by using the D. discoideum host model system (9, 32, 39). In this plaque assay, V. cholerae was mixed with D. discoideum and plated on SM/5 nutrient agar (40), which is able to support the growth of bacteria but not the amoebae. To survive, amoebae must prey on the bacteria, resulting in plaque formation in the bacterial lawn. However, V. cholerae strains expressing a functional T6SS, such as V52, are virulent toward amoebae and can resist predation, thus preventing plaque formation (34). Importantly, T6SS-mediated virulence toward D. discoideum results in a loss of viable amoebae, as demonstrated by Pukatzki et al. (34). We performed a plaque assay with V52ΔvasX to determine whether this strain lost its virulence toward D. discoideum. As shown in Fig. 5A, no plaques were observed on plates with wild-type V52; however, plaques developed after a 4-day incubation with V52ΔvasX, V52ΔvasK, and V52ΔvgrG-1. As shown in Fig. 5B, the number of plaques in a lawn of V52ΔvasX was significantly less than those in lawns of V52ΔvasK and V52ΔvgrG-1 (P value < 0.001). V52ΔvgrG-1 exhibited a stronger plaque phenotype than V52ΔvasX; however, both VasX and VgrG-1 are required for T6SS-mediated virulence toward D. discoideum. To determine if VgrG-1 and VasX act synergistically, we created a vgrG-1 vasX double-knockout strain (V52ΔvgrG-1ΔvasX) and tested its plaque assay phenotype. As shown in Fig. 5B, the number of plaques in a lawn of V52ΔvgrG-1ΔvasX was not significantly different from that in a lawn of V52ΔvgrG-1 but did differ significantly from the number in a lawn of V52ΔvasX. Therefore, although VasX is important for T6SS-mediated virulence toward D. discoideum, VasX and VgrG-1 do not appear to act synergistically.
Fig. 5.
VasX is required for virulence toward D. discoideum. (A) Plaque assay comparing strains V52, V52ΔvasK, V52ΔvasX, and V52ΔvgrG-1. Bacteria were mixed with approximately 500 amoebae, spread on nutrient SM/5 agar, and incubated at 22°C for 4 days to allow for plaque formation. Results are representative of three independent experiments. (B) Graphical representation comparing plaques in lawns of V52 and other T6SS mutants, including V52ΔvasX, V52ΔvasK, V52ΔvgrG-1, and V52ΔvgrG-1ΔvasX. Bacteria and amoebae were mixed on SM/5 nutrient agar and incubated at 22°C for 4 days to allow for plaque formation. Results are representative of three experimental data sets. ***, P value < 0.001; **, P value < 0.01. (C) trans expression of vasX restores virulence toward D. discoideum. V52ΔvasX/pBAD24-vasX and the plasmid control (V52ΔvasX/pBAD24) were mixed with approximately 500 amoebae, spread on nutrient SM/5 agar (with or without 0.1% arabinose), and incubated for 4 days at 22°C to allow for plaque formation. All results are representative of at least three experimental data sets.
To verify that the ΔvasX phenotype is caused solely by the absence of vasX, we cloned vasX into the plasmid pBAD24 (15) downstream of an arabinose-inducible promoter (pBAD24-vasX) and introduced this plasmid into V52ΔvasX (resulting in strain V52ΔvasX/pBAD24-vasX) for complementation experiments. Overexpression of vasX in trans results in VasX secretion that is comparable to wild-type levels (data not shown). To confirm that VasX was responsible for the plaque-forming phenotype, plaque assays were carried out with V52ΔvasX/pBAD24-vasX under inducing (0.1% arabinose) and noninducing (absence of arabinose) conditions. As shown in Fig. 5C, plaques developed when vasX was not expressed; however, no plaques developed under inducing conditions. Transformation of V52ΔvasX with pBAD24 (plasmid control) did not affect the plaque phenotype in the absence or presence of arabinose. Furthermore, arabinose had no effect on the plaque phenotype in a lawn of the avirulent Klebsiella pneumoniae (data not shown), indicating that arabinose had no adverse effects on the survival of D. discoideum. Taken together, these complementation experiments confirm that the attenuated virulence of the vasX mutant was due to the removal of vasX and not to another variable such as an effect on downstream genes (polarity), indicating a role for VasX in T6SS-mediated virulence toward D. discoideum.
VasX interacts with membrane lipids via its PH domain.
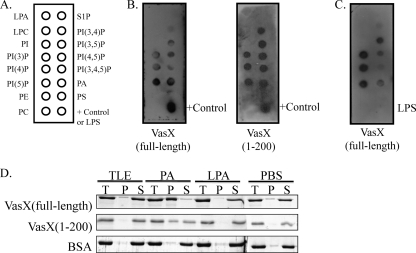
To test whether VasX can bind membrane lipids, we performed far-Western blotting using nitrocellulose membranes spotted with a variety of membrane lipids (Fig. 6A). Lipid-bound membranes were probed with purified VasX to determine its ability to interact with cell membrane phospholipids. As shown in Fig. 6B (left panel), VasX interacts with membrane phospholipids that bear a phosphorylated head group and two acyl chains, namely, phosphatidic acid (PA) and each of the phosphatidylinositol phosphates (PIP). However, VasX does not bind phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylcholine (PC), lysophosphatidic acid (LPA), lysophosphocholine (LPC), or sphingosine-1-phosphate (S1P). We also tested whether the first 200 residues of VasX, which encode the putative PH domain, retained the ability to bind membrane lipids. As shown in Fig. 6B (right panel), VasX(1–200) exhibited the same lipid-binding pattern as full-length VasX. The positive control indicated in Fig. 6B represents purified protein (either full-length or truncated VasX) spotted directly onto the membrane.
Fig. 6.
VasX binds phosphoinositides but not LPS. (A) Schematic representation of the various biological membrane lipids present on PIP Strips, including phosphatidic acid (PA), phosphatidyl inositol (PI), phosphatidylinositol phosphate (PIP), phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylcholine (PC), lysophosphatidic acid (LPA), lysophosphocholine (LPC), and sphingosine-1-phosphate (S1P). (B and C) Purified full-length VasX or a truncated version consisting of residues 1 to 200 (containing the PH domain) was used to probe PIP Strips for lipid binding. Bound protein was detected using anti-6×His primary antibody and anti-mouse-HRP secondary antibody. The positive control (+) included purified VasX spotted directly onto the PIP Strip membrane. In panel C, E. coli LPS was spotted in place of the positive control on the PIP Strip. Results are representative of three independent experiments. (D) MLV pulldown of purified recombinant VasX and the N-terminal fragment of VasX(1–200). Full-length VasX, VasX(1–200), and BSA (negative control) were mixed with MLV of total liver extract (TLE), phosphatidic acid (PA), lysophosphatidic acid (LPA), or PBS (as a technical control) and divided into total (T), pellet (P), and supernatant (S) fractions by centrifugation. The partitioning of protein into each fraction was visualized by SDS-PAGE and Coomassie blue staining. Results are representative of three independent experiments.
Because subcellular fractionation experiments indicated that VasX localizes to the bacterial membrane, we tested whether VasX possessed the ability to bind bacterial lipopolysaccharide (LPS). Purified E. coli LPS (Sigma-Aldrich, St. Louis, MO) was spotted onto a PIP Strip at the same concentration as each of the membrane lipids on the strip (100 picomoles). In a separate experiment, the presence of LPS on the membrane was confirmed using an anti-lipid A polyclonal antibody (Abcam, Cambridge, MA) (data not shown). As shown in Fig. 6C, full-length VasX retained the ability to bind PA and each PIP as demonstrated in Fig. 6B; however, VasX did not bind to bacterial LPS.
To determine whether these interactions also occurred in aqueous solution, multilamellar vesicles (MLV) of PA, LPA, and a natural mixture of bovine total liver lipid extracts (TLE) (which does not contain a significant amount of PA or PIPs) were prepared in phosphate-buffered saline (PBS) and mixed with purified full-length VasX, VasX(1–200), or BSA (negative control). These large lipid vesicles, and any associated proteins, can be pelleted by centrifugation, creating total (T), pellet (P), and supernatant (S) fractions for SDS-PAGE analysis. As shown in Fig. 6D, VasX was found in the pellet (lipid pulldown) fractions when mixed with PA but not when mixed with TLE, LPA, or PBS. Similarly, the truncated version of VasX, containing the predicted PH domain, showed the same pattern of lipid-binding as full-length VasX in MLV experiments. As expected, BSA was not pulled down with any of the membrane lipids tested. These results suggest that VasX interacts with phosphorylated membrane lipids via its PH domain.
DISCUSSION
In this study, we identified and characterized the virulence factor VasX and demonstrated its requirement for T6SS-mediated virulence toward D. discoideum. The production of VasX required the presence of the σ54 activator VasH, since VasX was significantly reduced in V52ΔvasH pellet fractions, and trans complementation of V52ΔvasH restored Hcp export and VasX secretion into culture supernatants (Fig. 2A). Because VasH regulates production of Hcp-2 (Fig. 2A) and VasX is encoded directly downstream of hcp-2, we speculate that hcp-2, vgrG-2, and vasX are included in a σ54-regulated T6SS regulon. The fact that VasH (encoded in the large T6SS gene cluster) was essential for the production of Hcp and VasX, both of which are encoded by a small auxiliary cluster, suggests that expression of genes within the large cluster supersedes that of the auxiliary gene clusters.
VasX secretion requires a functional T6SS, as V. cholerae mutants lacking the T6SS genes vasK, vgrG-2, and hcp-1,2 failed to secrete VasX into culture supernatants (Fig. 3A). In contrast, vgrG-1 and vgrG-3 mutants retained the ability to secrete VasX (Fig. 3A). The dependence of VasX secretion on VasK and Hcp was consistent with recent reports that VasK and Hcp are important for T6SS machine assembly and nanotube formation, respectively (4, 24). The observation that only VgrG-2 and not VgrG-1 or VgrG-3 was required for VasX secretion suggests that VgrGs differ in aspects other than their C-terminal effector domains. Furthermore, V52ΔvgrG-1 secreted less VasX than V52ΔvgrG-3. This suggests a VgrG protein hierarchy for the secretion of VasX into culture supernatants. Interestingly, the secretion of VasX from different T6SS mutants corresponds with the export of Hcp from the same strains. This implies a secretory relationship between VasX and Hcp and indicates that the Hcp nanotube is required for VasX secretion from the bacterium. Importantly, a polar effect of the vgrG-2 deletion was not responsible for the lack of secreted VasX, as VasX was present in V52ΔvgrG-2 pellets (Fig. 3A). We hypothesize that VgrG proteins possessing C-terminal extensions, such as the actin cross-linking domain of VgrG-1, are required exclusively for in vivo infections in order to provide an anchoring mechanism for T6SS effector delivery into the host cell. Therefore, VasX in vitro secretion requires only the core protein VgrG-2, which is also required to export Hcp into culture supernatants. Alternatively, the absence of VasX in V52ΔvgrG-2 supernatants might have been due to the lack of Hcp export from the cell. Both V52ΔvgrG-1 and V52ΔvgrG-3 retain the ability to export Hcp (33) and are therefore able to secrete VasX. As a consequence, VasX may strictly rely on Hcp for secretion from the bacterium.
Results of our plaque assays with D. discoideum indicated that VasX is required for virulence toward amoebae. The number of plaques that formed in V52ΔvasX lawns was significantly less than the numbers of plaques that developed in lawns of V52ΔvasK, V52ΔvgrG-1, and V52ΔvgrG-1ΔvasX. Even though VgrG-1 was dispensable for VasX secretion in vitro (Fig. 3A) and VasX is not required for the actin cross-linking activity of VgrG-1, both VgrG-1 and VasX are required for virulence toward amoebae (Fig. 5A and B) (23). Since V52ΔvgrG-1 and V52ΔvgrG-1ΔvasX have a more severe plaque-forming phenotype than V52ΔvasX, we conclude that V52ΔvgrG-1 has a dominant plaque phenotype and VasX and VgrG-1 do not appear to act synergistically in T6SS-mediated virulence.
Our bioinformatics analysis suggested that VasX possesses a PH domain (5, 19, 38). PH domains are defined by 100- to 200-amino-acid stretches that create a structural superfold typically implicated in binding phosphoinositides on cellular membranes (21). We demonstrated that VasX and a truncated version of VasX consisting of residues 1 to 200 (encompassing the putative PH domain) bind to membrane lipids, including various phosphatidylinositol phosphates and phosphatidic acid, by using two independent methods (Fig. 6). Because inositol phosphates are rarely found in bacteria (27), we postulate that the PH domain of VasX has a role in binding to host membrane lipids. Interference with host cell phosphoinositide metabolism and signaling by pathogenic bacteria is not uncommon and has been previously demonstrated for other enteric bacterial pathogens (8, 16, 31).
VasX is not required for the export of the T6SS apparatus component Hcp (Fig. 3A) or the enzymatic function of VgrG-1 (i.e., actin cross-linking) (Fig. 3B). In contrast, VasX requires Hcp and VgrG-2 for secretion into the extracellular milieu (Fig. 3A). Subcellular fractionation experiments identified VasX only in membrane and cytosolic fractions, while Hcp localizes to all bacterial compartments, including the periplasm. This allows us to speculate that VasX passes through the Hcp nanotube and bypasses the periplasm en route out of the bacterium, where it associates with the bacterial envelope. VasX detected in culture supernatants may be the result of being sloughed from the bacterial surface along with other T6SS structural proteins, such as Hcp and VgrG.
Far-Western blots indicated that VasX binds phosphoinositides but not bacterial LPS. Although we cannot rule out that VasX binds to other structurally related biolipids in the bacterial envelope, we speculate that VasX associates with the T6SS apparatus to contact host membranes and function as an effector protein. VasX may be translocated into the host cell via the structural T6SS apparatus, where it binds phosphoinositides, the classical substrates of PH domains, on the inner leaflet of eukaryotic cell membranes (7, 14). Alternately, V52 may possess a mechanism to alter the location of host phosphoinositides to the outer leaflet of the cell membrane in a mechanism similar to that described for the gastric pathogen Helicobacter pylori (41). We postulate that during host infection, VasX uses its PH domain to bind host membrane lipids and disrupt host cell signaling, leading to T6SS-mediated toxicity.
ACKNOWLEDGMENTS
We thank Richard Friedman, Richard Kessin, Bart Hazes, and Tracy Raivio for helpful discussions. We also thank Joanne Simala-Grant, Mark Peppler, Kim Ellison, Marcia Craig, and Daniele Provenzano for critically reviewing the manuscript. We thank Rosemary Cornell (Simon Fraser University) for providing lipids and Susanne Lingrell (Dennis Vance's laboratory, University of Alberta) for technical assistance. We acknowledge the Dicty Stock Center for providing the D. discoideum AX3 strain used in this study.
Work in S.U.P.'s laboratory is supported by the Canadian Institute for Health Research Operating Grant MOP-84473 and Alberta Innovates—Health Solutions (funded by the Alberta Heritage Foundation for Medical Research Endowment Fund).
Footnotes
Published ahead of print on 9 May 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Arnold R. S., Cornell R. B. 1996. Lipid regulation of CTP: phosphocholine cytidylyltransferase: electrostatic, hydrophobic, and synergistic interactions of anionic phospholipids and diacylglycerol. Biochemistry 35:9917–9924 [DOI] [PubMed] [Google Scholar]
- 2. Aschtgen M. S., Bernard C. S., De Bentzmann S., Lloubes R., Cascales E. 2008. SciN is an outer membrane lipoprotein required for type VI secretion in enteroaggregative Escherichia coli. J. Bacteriol. 190:7523–7531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aubert D. F., Flannagan R. S., Valvano M. A. 2008. A novel sensor kinase-response regulator hybrid controls biofilm formation and type VI secretion system activity in Burkholderia cenocepacia. Infect. Immun. 76:1979–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ballister E. R., Lai A. H., Zuckermann R. N., Cheng Y., Mougous J. D. 2008. In vitro self-assembly of tailorable nanotubes from a simple protein building block. Proc. Natl. Acad. Sci. U. S. A. 105:3733–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennett-Lovsey R. M., Herbert A. D., Sternberg M. J., Kelley L. A. 2008. Exploring the extremes of sequence/structure space with ensemble fold recognition in the program Phyre. Proteins 70:611–625 [DOI] [PubMed] [Google Scholar]
- 6. Bernard C. S., Brunet Y. R., Gavioli M., Lloubes R., Cascales E. 4 March 2011. Regulation of type VI secretion gene clusters by {sigma}54 and cognate enhancer binding proteins. J. Bacteriol. doi:10.1128/JB.00029-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bretscher M. S. 1972. Asymmetrical lipid bilayer structure for biological membranes. Nat. New Biol. 236:11–12 [DOI] [PubMed] [Google Scholar]
- 8. Broberg C. A., Zhang L., Gonzalez H., Laskowski-Arce M. A., Orth K. 2010. A Vibrio effector protein is an inositol phosphatase and disrupts host cell membrane integrity. Science 329:1660–1662 [DOI] [PubMed] [Google Scholar]
- 9. Cosson P., et al. 2002. Pseudomonas aeruginosa virulence analyzed in a Dictyostelium discoideum host system. J. Bacteriol. 184:3027–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dziejman M., et al. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc. Natl. Acad. Sci. U. S. A. 99:1556–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faruque S. M., Nair G. B., Mekalanos J. J. 2004. Genetics of stress adaptation and virulence in toxigenic Vibrio cholerae. DNA Cell Biol. 23:723–741 [DOI] [PubMed] [Google Scholar]
- 12. Filloux A., Hachani A., Bleves S. 2008. The bacterial type VI secretion machine: yet another player for protein transport across membranes. Microbiology 154:1570–1583 [DOI] [PubMed] [Google Scholar]
- 13. Finkelstein R. A., LoSpalluto J. J. 1969. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J. Exp. Med. 130:185–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordesky S. E., Marinetti G. V. 1973. The asymetric arrangement of phospholipids in the human erythrocyte membrane. Biochem. Biophys. Res. Commun. 50:1027–1031 [DOI] [PubMed] [Google Scholar]
- 15. Guzman L. M., Belin D., Carson M. J., Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernandez L. D., Hueffer K., Wenk M. R., Galan J. E. 2004. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science 304:1805–1807 [DOI] [PubMed] [Google Scholar]
- 17. Herrington D. A., et al. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hood R. D., et al. 2010. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7:25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelley L. A., Sternberg M. J. 2009. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4:363–371 [DOI] [PubMed] [Google Scholar]
- 20. Leiman P. G., et al. 2009. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc. Natl. Acad. Sci. U. S. A. 106:4154–4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lemmon M. A. 2004. Pleckstrin homology domains: not just for phosphoinositides. Biochem. Soc. Trans. 32:707–711 [DOI] [PubMed] [Google Scholar]
- 22. Lindquist S. 1986. The heat-shock response. Annu. Rev. Biochem. 55:1151–1191 [DOI] [PubMed] [Google Scholar]
- 22a. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 23. Ma A. T., McAuley S., Pukatzki S., Mekalanos J. J. 2009. Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells. Cell Host Microbe 5:234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma L. S., Lin J. S., Lai E. M. 2009. An IcmF family protein, ImpLM, is an integral inner membrane protein interacting with ImpKL, and its walker a motif is required for type VI secretion system-mediated Hcp secretion in Agrobacterium tumefaciens. J. Bacteriol. 191:4316–4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. MacIntyre D. L., Miyata S. T., Kitaoka M., Pukatzki S. 2010. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl. Acad. Sci. U. S. A. 107:19520–19524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Metcalf W. W., et al. 1996. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1–13 [DOI] [PubMed] [Google Scholar]
- 27. Morita Y. S., et al. 2010. Stress-induced synthesis of phosphatidylinositol 3-phosphate in mycobacteria. J. Biol. Chem. 285:16643–16650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mougous J. D., et al. 2006. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312:1526–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mueller R. S., Beyhan S., Saini S. G., Yildiz F. H., Bartlett D. H. 2009. Indole acts as an extracellular cue regulating gene expression in Vibrio cholerae. J. Bacteriol. 191:3504–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nandi B., Nandy R. K., Sarkar A., Ghose A. C. 2005. Structural features, properties and regulation of the outer-membrane protein W (OmpW) of Vibrio cholerae. Microbiology 151:2975–2986 [DOI] [PubMed] [Google Scholar]
- 31. Niebuhr K., et al. 2002. Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S.flexneri effector IpgD reorganizes host cell morphology. EMBO J. 21:5069–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pukatzki S., Kessin R. H., Mekalanos J. J. 2002. The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium discoideum. Proc. Natl. Acad. Sci. U. S. A. 99:3159–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pukatzki S., Ma A. T., Revel A. T., Sturtevant D., Mekalanos J. J. 2007. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. U. S. A. 104:15508–15513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pukatzki S., et al. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. U. S. A. 103:1528–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pukatzki S., McAuley S. B., Miyata S. T. 2009. The type VI secretion system: translocation of effectors and effector-domains. Curr. Opin. Microbiol. 12:11–17 [DOI] [PubMed] [Google Scholar]
- 36. Sandkvist M., Hough L. P., Bagdasarian M. M., Bagdasarian M. 1999. Direct interaction of the EpsL and EpsM proteins of the general secretion apparatus in Vibrio cholerae. J. Bacteriol. 181:3129–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwarz S., et al. 2010. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog. 6:pii:e1001068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Söding J., Biegert A., Lupas A. N. 2005. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33:W244–W248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Solomon J. M., Rupper A., Cardelli J. A., Isberg R. R. 2000. Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions. Infect. Immun. 68:2939–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sussman M. 1987. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 28:9–29 [DOI] [PubMed] [Google Scholar]
- 41. Wandler A. M., Parthasarathy R., Guillemin K. 2010. A greasy foothold for Helicobacter pylori. Cell Host Microbe 7:338–339 [DOI] [PubMed] [Google Scholar]
- 42. Zinnaka Y., Carpenter C. C., Jr 1972. An enterotoxin produced by noncholera vibrios. Johns Hopkins Med. J. 131:403–411 [PubMed] [Google Scholar]