Abstract
Previous studies have suggested that neonates rely heavily on innate immunity for their antimicrobial response to bacterial infections. However, the innate immune response by neonates to bacterial infection remains poorly characterized. Here, we show that in a murine model of neonatal polymicrobial sepsis, CXC ligand 10 (CXCL10) concentrations increase in the blood and peritoneum concordant with the peritoneal recruitment of granulocytes and macrophages. Additionally, CXC receptor 3 (CXCR3) expression on elicited peritoneal macrophages and granulocytes increases following sepsis. Blockade of CXCL10 worsens not only recruitment and phagocytic function of peritoneal granulocytes and macrophages but also survival. Deletion of CXCR3 also significantly increases mortality to a septic challenge. Finally, we demonstrate that the protective adjuvant effect of pretreatment with a Toll-like receptor 4 agonist to neonatal sepsis is dependent on an endogenous CXCL10 response and that pretreatment of neonates with CXCL10 can also significantly improve macrophage and granulocyte function and modestly improve outcome to polymicrobial sepsis. Together, these data suggest a critical role for CXCL10 signaling during neonatal sepsis.
INTRODUCTION
Death associated with neonatal sepsis has remained largely unchanged over the last 2 decades, and neonatal sepsis is responsible for more than 1 million deaths per year worldwide (13, 25). Mortality rates are particularly elevated in very low-birth-weight infants (<1,500 g at birth) and have been reported to be as high as 50% (12, 24). In human neonates who survive severe sepsis, developmental morbidity also has long-term social and economic consequences (1, 9, 21).
Although these stagnant outcomes closely mirror results in the adult population, there are distinct immunological differences between the adult and neonatal responses to sepsis. For example, adult mice rely on both adaptive and innate immunity for survival (10), while neonatal mice rely more heavily on their innate immune responses (10, 27). Neutrophils from neonates exhibit impaired production of neutrophil extracellular traps (NETS) and have reduced phagocytic function and reactive oxygen species (ROS) production compared to adults (27, 28). These findings highlight the increased importance of the innate immune response during sepsis in the neonate and the need to delineate mediators of neonatal innate immune modulation. The ultimate goal is not only to understand how neonates respond to severe infection but also to identify potential therapeutic targets.
We have previously shown that pretreatment of neonatal mice with a Toll-like receptor 4 (TLR4) or TLR7/8 agonist could dramatically improve outcome to subsequent polymicrobial sepsis through the improvement of innate immune function, whereas adaptive immunotherapy with a glucocorticoid-induced tumor necrosis factor alpha (TNF-α) receptor (GITR) agonistic antibody that improves outcome in adult polymicrobial sepsis failed to improve outcome in neonates (27). These results highlight the need for different immunologic approaches for the treatment of adult versus neonatal sepsis.
It is well known that TLR stimulation induces the production of multiple proinflammatory cytokines and chemokines, as well as type I (alpha and beta) interferons (2, 22, 27). These mediators serve to recruit and modulate early immunological responses to protect the host against infection. Gamma interferon-inducible protein-10 (IP-10), or CXC ligand 10 (CXCL10), is a CXC chemokine that has been shown to be produced in response to both type I (alpha and beta) and proinflammatory type II (gamma) interferons (8, 22). Many studies have linked CXCL10 to productive TH1 responses, but CXCL10 has also more recently been found to be associated with neutrophil infiltration and function (11, 19, 20). Increased CXCL10 production has been described in multiple adult infection models, and increased plasma concentrations have been associated with bacterial infection in neonates (16). Additionally, blockade of CXCL10 has been demonstrated to worsen survival in an adult murine model of experimental sepsis (15).
The present study was undertaken to examine the physiologic and pharmacologic role of CXCL10 in innate immune modulation during neonatal sepsis. We demonstrate that the influx of granulocytes and macrophages into the peritoneal cavity during polymicrobial sepsis is temporally associated with increases in peritoneal CXCL10 concentrations. Blockade of CXCL10 significantly reduces the influx and phagocytic activity of peritoneal granulocytes during polymicrobial sepsis and worsens survival. In addition to providing evidence that CXCL10 is critical for the effective recruitment of innate immune effector cells during neonatal sepsis, we demonstrate that exogenous CXCL10 can directly stimulate granulocyte and macrophage phagocytic function in vitro. Finally, to investigate the potential therapeutic role of CXCL10 in neonatal sepsis, we show that blockade of CXCL10 prevents the protective effect of pretreatment with a TLR4 agonist to polymicrobial sepsis and that pretreatment with CXCL10 prior to polymicrobial sepsis is able to modestly augment survival. These data suggest that production of CXCL10 during polymicrobial sepsis is important for the efficient recruitment and function of neonatal innate immunity and may also be a critical component of therapies designed to improve outcomes in neonatal sepsis.
MATERIALS AND METHODS
Mice.
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Florida. Six- to 8-week-old male and female wild-type or CXC receptor 3 (CXCR3)-knockout (CXCR3−/−) C57BL/6 mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and used for adult breeders. Adult mice were mated in a harem schema with one male to two females. Neonatal mice were studied at between 4 and 7 days of age.
CS model.
Polymicrobial sepsis was induced as previously described (26). Briefly, cecal contents of adult C57BL/6 mice were suspended in 5% dextrose solution at a final concentration of 80 mg/ml and injected intraperitoneally (i.p.) into 5- to 7-day-old neonates. Either a 40% lethal dose (LD40; 1 mg fecal contents/g body weight [BW]) or an LD70 (1.3 mg fecal contents/g body weight) of cecal slurry (CS), as determined from previous experiments, was used (26).
Peritoneal and serum CXCL10 concentrations.
Blood and peritoneal washes were harvested from neonates at the time points described following induction of polymicrobial sepsis. As reports have shown that heparin can bind to CXCL10 and possibly reduce its detection in blood, serum was used for these assays (4, 17). To collect peritoneal washes, the peritoneal cavity was instilled with 500 μl of cold phosphate-buffered saline (PBS) (Cellgro, Manassas, VA). The peritoneal cavity was then opened over a sterile tissue culture dish and the peritoneal wash was collected. Peritoneal washes were then centrifuged to remove debris and the supernatants were stored at −80°C. Serum and peritoneal washes were then measured for CXCL10 concentrations via enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).
Flow cytometry.
Single-cell suspensions were characterized using anti-Ly6G–allophycocyanin (APC), anti-Ly6C-peridinin chlorophyll protein-Cy5.5, anti-Gr-1–APC, anti-CD11b-phycoerythrin (PE)-Cy7, anti-CXCR3-PE, and/or anti-F4/80 APCeFluor-780 (Ebioscience, San Diego, CA) antibodies. All antibodies were purchased from Becton Dickinson (BD; Franklin Lakes, NJ) unless otherwise indicated. Samples were acquired and analyzed on an LSR II flow cytometer (BD) and analyzed via FACSDiva (BD) or FloJo (Treestar Inc., Ashland, OR) software. At least 1 × 104 live cells (SYTOX Blue negative; Invitrogen, Carlsbad, CA) were collected for analysis. Sorting was conducted on a FACSAria cell sorter (BD), and cells were sorted to >95% purity. Cytospin preparations were prepared from sorted cell populations and Wright-Giemsa stained (Sigma-Aldrich, St. Louis, Mo).
Isolation of blood, bone marrow, and splenocytes for phenotypic analysis.
Blood was obtained via intracardiac puncture of isoflurane-anesthetized neonatal mice and placed into an heparinized syringe. Spleens were harvested from neonates and then dissociated through a 70-μm-pore-size sterile filter. To harvest bone marrow, both femurs and tibias from individual animals were collected and flushed with PBS. Blood, splenocyte, and bone marrow suspensions were then subjected to erythrocyte lysis using ammonium chloride lysis solution. Cell suspensions were then stained for granulocyte (Gr-1+ or Ly6G+ CD11b+), monocyte (Ly6C+ CD11b+) or macrophage (F4/80+ CD11b+) cell surface markers and analyzed via flow cytometry (data not shown).
Harvest of peritoneal washes.
Peritoneal cell isolation was carried out in a similar manner as described previously (27). Due to the fact that peritoneal lavage samples from individual animals contained relatively few cells, peritoneal washes from three to five animals were pooled for analysis and considered to be one sample. For each time point or condition analyzed, a total of three to four peritoneal fluid samples were collected per experiment. To determine absolute numbers of innate immune effector cells, the percentage of macrophages or granulocytes within the total sample population was determined via flow cytometry, multiplied by the total sample cell number, and then divided by the total number of mice in the pooled sample. Absolute numbers represent total macrophages or granulocytes per mouse.
In vitro CXCL10 chemotaxis assay.
Splenocytes were harvested from neonates, overlaid on a Histopaque 1119 density gradient (Sigma-Aldrich, St. Louis, MO), and centrifuged. Splenocytes were then washed in PBS, resuspended in RPMI 1640 and 10% fetal bovine serum (FBS), and placed in the top well of an 8-μm Falcon Fluoroblock multiwell insert (BD). Inserts with splenocyte suspensions were then placed on top of RPMI 1640 and 10% FBS medium containing 0, 100, or 200 ng/ml of recombinant CXCL10 (R&D Systems) to establish a gradient and placed at 37°C. After 2 and 6 h of culture, bottom wells and the bottom of the insert were washed with ice-cold PBS twice and cells were scraped. Cell suspensions were counted and assayed via flow cytometry for the presence of Gr-1+ CD11b+ cells (granulocytes) and F4/80+ CD11b+ cells (macrophages).
Blockade of CXCL10.
Neonates were injected i.p. with 0.5 mg of either rabbit polyclonal anti-CXCL10 IgG (gift from S. L. Kunkel, Ann Arbor, MI) or control rabbit IgG (Fisher Scientific, Pittsburgh, PA). Specificity and activity of polyclonal rabbit anti-CXCL10 have been verified in previous studies (3). Due to the fact that the ages of the mice could vary by as much as 2 days, individual litters were split into two cohorts, those that received anti-CXCL10 and those that received control rabbit IgG, to eliminate or reduce the effect of age and/or size variability. IgG was purified from either polyclonal anti-CXCL10 antisera or control rabbit sera using protein A columns (Pierce, Rockford, IL) and then dialyzed against PBS (Cellgro) before use.
Peritoneal bacterial counts.
Animals were administered either control IgG or anti-CXCL10 2 h before cecal slurry challenge. Twelve hours following septic challenge, neonates were euthanized and injected with 500 μl of sterile PBS. Peritoneal washes were then collected, serially diluted, and cultured on blood agar plates under aerobic conditions at 37°C. Colonies were counted 18 to 24 h following culture.
Isolation of splenic macrophages and granulocytes for functional analyses.
Spleens were dissociated through a 70-μm-pore-size sterile filter into RPMI 1640 medium (Cellgro) with 10% heat-inactivated, low-endotoxin FBS and 0.5% penicillin-streptomycin (Cellgro). Suspensions were then overlaid on a Histopaque 1119 density gradient (Sigma-Aldrich) and centrifuged. Mixed cell suspensions were then washed with medium alone, and individual cell populations were assayed for phagocytosis or ROS production by flow cytometry.
In vitro stimulation with recombinant CXCL10.
Isolated splenocytes were stimulated with or without recombinant murine CXCL10 at 100 ng/ml for 4 h (R&D Systems). After incubation with CXCL10, cells were washed with PBS and subsequently assayed for phagocytic function or ROS production and phenotypically characterized via flow cytometry.
Functional analysis of splenic macrophages and granulocytes or peritoneal macrophages or granulocytes.
For phagocytosis assays, 1 × 105 splenocytes isolated via density gradient or peritoneal cell suspensions were incubated with 1 × 108 fluorescent polystyrene microspheres (Fluospheres; Invitrogen, Carlsbad, CA) for 30 min at 37°C. Cells were then stained and analyzed by flow cytometry, as described above. After cells were gated on either granulocyte (Gr-1+ CD11b+) or macrophage (F4/80+ CD11b+) populations, fluorescein isothiocyanate-positive (FITC+) cells were considered to be phagocytic. To assay for ROS production, 2 × 106 splenocytes isolated via density gradient were first stained for cell surface markers and labeled with dihydrorhodamine-1,2,3 (Invitrogen). Cells were then stimulated with 1 μM phorbol-12-myristate-13-acetate (PMA; Sigma-Aldrich) at 37°C, and aliquots were evaluated by flow cytometry at various points over a 30-min period using an LSR II flow cytometer (BD). A minimum of 1 × 104 live, nondebris cells was collected for analysis.
Pretreatment with a TLR4 agonist (bacterial LPS).
Neonatal C57BL/6 mice 4 to 6 days old received lipopolysaccharide (LPS; 1 μg/g BW i.p.; ultrapure via ion-exchange chromatography; from Escherichia coli O26:B6 [Sigma-Aldrich]) in physiologic saline 24 h before challenge with cecal slurry. Control animals received saline only i.p. 24 h before cecal slurry challenge. Total volume of injected solution was less than 50 μl.
In vivo administration of recombinant CXCL10.
Twenty nanograms of recombinant murine CXCL10 (R&D) in physiologic saline or physiologic saline alone was administered i.p. to neonatal C57BL/6 mice 5 to 7 days old. Total volume administered was 50 μl per mouse.
Statistical analyses.
Continuous variables were tested for normality and equality of variances. Differences in survival were determined by Fisher's exact test, whereas differences among groups were evaluated by either one-way analysis of variance (ANOVA) with either Dunn's or Tukey's post hoc analysis or Student's t test. Significance was determined at the 95% confidence level using a two-tailed test.
RESULTS
CXCL10 concentrations in the blood and peritoneum increase during polymicrobial sepsis.
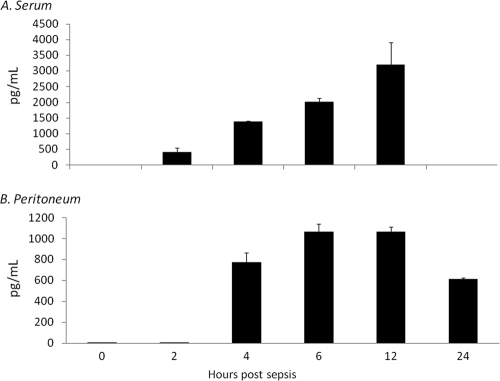
Peritoneal washes and sera were harvested from neonates at multiple time points during polymicrobial sepsis, and CXCL10 concentrations were determined. As demonstrated in Fig. 1A and B, CXCL10 serum and peritoneal concentrations increase at 6 and 12 h after induction of sepsis, respectively, but subsequently decrease by 24 h.
Fig. 1.
Serum CXCL10 concentrations increase in neonatal sepsis. Blood and peritoneal washes were collected from neonates (n = 4) at multiple time points postsepsis (LD40). Sera (A) and peritoneal washes (B) were then assayed for CXCL10 concentration (values represent means ± SDs).
Peritoneal granulocyte and macrophage kinetics follow increases in CXCL10 peritoneal concentrations.
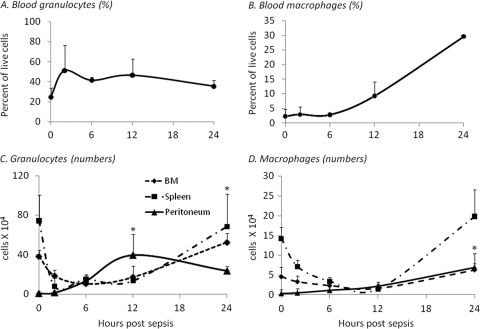
The recruitment of innate immune effector cells is important for the containment and control of bacterial infection, as well as abscess formation and localization (7). The characteristics of the adult response to sepsis have been well described but have not been fully delineated in the neonate (6, 11, 18). Blood, spleen, bone marrow, and peritoneal washes were collected at multiple time points from neonates that were exposed to an LD40 polymicrobial sepsis. Cells were then stained for granulocyte (Gr-1+ CD11b+) or macrophage (F4/80+ CD11b+) cell surface markers. As expected, granulocyte percentages increase in the blood 2 h following sepsis, whereas macrophage percentages slowly increase over the next 24 h (Fig. 2A and B). Both granulocyte and macrophage numbers increase significantly in the peritoneum within 12 and 24 h of polymicrobial sepsis, respectively (Fig. 2C and D). This correlates with a decrease in absolute numbers of granulocyte and macrophages in the bone marrow and spleen (Fig. 2C and D).
Fig. 2.
Granulocyte and macrophage kinetics in neonatal sepsis. Spleen, blood, bone marrow, and peritoneal exudates were collected from neonates at different time points after sepsis (LD40). Tissue cell suspensions were then stained for granulocytes (Gr-1+ CD11b+) (A and C) and macrophages (F4/80+ CD11b+) (B and D) and analyzed via flow cytometry. Panels A and B represent the percentage of total live cells in the blood, while panels C and D show total cell number in the spleen, bone marrow, and peritoneum (n = 4 per group per time point; values represent the means ± SDs; *, Tukey's one-way ANOVA, P < 0.01).
As the murine marker Gr-1 is comprised of two epitopes, one more specific for mature granulocytes (Ly6G) and the other more representative of monocyte populations (Ly6C), including myeloid precursors, it was unclear as to what the relative contribution of monocytes and/or mature granulocytes is to the sepsis-elicited peritoneal exudate. To address this concern, the kinetics of mature granulocyte (Ly6G+ CD11b+) and monocyte (Ly6G− Ly6C+ CD11b+) populations in blood, bone marrow, spleen, and peritoneum during neonatal sepsis were further determined. Though some heterogeneity is evident between the morphology of Gr-1+ CD11b+ versus Ly6G+ CD11b+ versus Ly6G− Ly6C+ CD11b+ cells (data not shown), mature granulocytes comprise the majority of both Gr-1+ CD11b+ and Ly6G+ CD11b+ sorted populations. In addition, the kinetics of Ly6G+ CD11b+ (granulocytes), but not Ly6G− Ly6C+ CD11b+ (immature/mature monocytes), following cecal slurry challenge are nearly identical to that of the Gr-1+ CD11b+ population (data not shown). Therefore, although the Gr-1 marker can identify both granulocyte and monocyte populations, these data indicate that the majority of cells infiltrating to the peritoneum are indeed mature granulocytes, and Gr-1+ CD11b+ cells will be referred to as granulocytes for the remainder of this report.
CXCL10 induces the recruitment of both granulocytes and macrophages in vitro.
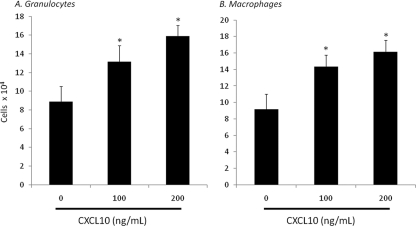
Though the data above suggest that granulocyte and macrophage recruitment to the peritoneum following sepsis is associated with increases in peritoneal CXCL10 concentration, there is little evidence to suggest that CXCL10 may directly recruit these cells following septic insult. To address this concern, splenocyte suspensions were cultured in the top well of a Fluorblock 8-μm cell culture insert and placed on top of increasing concentrations of CXCL10. As demonstrated in Fig. 3A and B, both granulocyte and macrophage numbers were significantly increased in the bottom wells of cultures containing 100 ng/ml and 200 ng/ml of recombinant CXCL10 compared to those in wells without CXCL10 after 6 h. This suggests that CXCL10 can recruit both granulocytes and macrophages directly.
Fig. 3.
Increased neonatal granulocyte and macrophage chemotaxis in response to a CXCL10 chemokine gradient. Splenocytes were harvested and cultured in the top well of an 8-μm Fluoroblock insert and placed on top of increasing concentrations of CXCL10. After 6 h, the bottom of the insert and bottom well were washed and analyzed for the presence of granulocytes and macrophages via flow cytometry (n = 6 per concentration; values represent mean ± SDs; *, Tukey's one-way ANOVA, P < 0.001).
CXCL10 neutralization worsens survival to cecal slurry peritonitis and decreases recruitment of peritoneal macrophages and granulocytes.
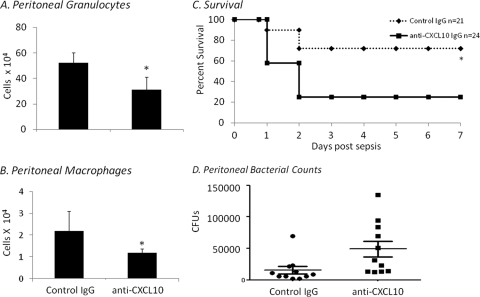
The data shown above suggest that CXCL10 may be important for the recruitment of granulocytes and macrophages to the peritoneum during polymicrobial sepsis. To further explore the requirement for CXCL10 production during neonatal sepsis, we investigated whether blockade of CXCL10 would affect the recruitment of peritoneal macrophages or granulocytes. Peritoneal exudates were examined from anti-CXCL10 antibody-treated animals 12 h following induction of polymicrobial sepsis. CXCL10 blockade significantly decreases both peritoneal macrophage and granulocyte recruitment (Fig. 4A and B).
Fig. 4.
CXCL10 blockade worsens survival and cell recruitment. Two hours prior to sepsis (LD40), neonates were administered either anti-CXCL10 IgG (squares and solid line) or control IgG (0.5 mg; diamonds and hashed line). Peritoneal washes were harvested from neonates administered anti-CXCL10 or control IgG at 12 h postsepsis. Cells were subsequently stained for granulocytes (Gr-1+ CD11b+) (A) and macrophages (F4/80+ CD11b+) (B) and analyzed by flow cytometry. The figure represents the summary of two experiments. Values represent the mean ± SDs (n = 6 each; *, Student's t test, P < 0.05; error bars indicate SDs). (C) Another cohort of animals was then monitored for survival for 7 days. The figure represents the summary of three experiments (n = 45; *, Fisher's exact test, P < 0.003). (D) Peritoneal bacterial counts were determined following sepsis in neonates that were administered either control IgG or anti-CXCL10 (n = 11 per group; values represent the mean ± SDs; *, Student's t test, P = 0.026).
To examine the overall importance of CXCL10 to sepsis response, survival of neonates to septic challenge was examined. Neonates were administered 0.5 mg of control IgG or anti-CXCL10 IgG i.p. 2 h before polymicrobial sepsis. As shown in Fig. 4C, blockade of CXCL10 significantly decreases survival to sepsis compared to controls. This was also associated with increased peritoneal bacterial counts at 12 h following sepsis in those animals administered anti-CXCL10 compared to those administered control IgG. These data suggest that CXCL10 production is critical for the appropriate recruitment of innate immune effector cells and subsequent survival to polymicrobial sepsis.
CXCR3 expression increases on peritoneal granulocytes and macrophages during septic challenge, and deletion of CXCR3 decreases peritoneal granulocyte and macrophage numbers and worsens survival to neonatal sepsis.
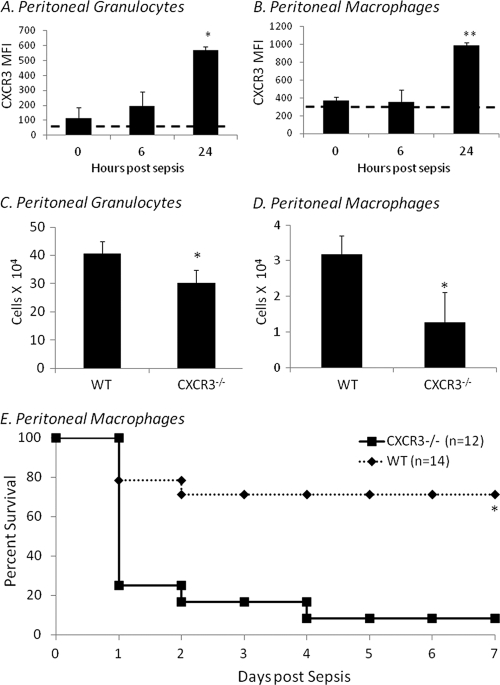
Recruitment of innate immune effector cells to the site of infection is thought to require a chemokine gradient (14). To determine if changes in the expression of the receptor for CXCL10, CXCR3, mirror the changes in CXCL10 concentrations, peritoneal washes were collected from neonates at 0, 6, and 24 h during polymicrobial sepsis. As demonstrated in Fig. 5A and B, peritoneal granulocyte and macrophage CXCR3 expression increases by 24 h postinduction of sepsis. Peritoneal granulocytes (Fig. 5A) and macrophages (Fig. 5B) from healthy mice have little to no CXCR3 expression, and CXCR3 expression increases with time after induction of sepsis, although it is delayed in time relative to peritoneal CXCL10 concentrations. Since blockade of CXCL10 was demonstrated to worsen survival to cecal slurry peritonitis, we next evaluated the requirement of CXCR3 expression for the recruitment of peritoneal granulocytes and macrophages, as well as the survival of neonates to sepsis. CXCR3−/− or wild-type mice were subjected to an LD40 septic challenge and either were killed at 12 h after septic challenge and analyzed for the presence and number of peritoneal granulocytes and macrophages or were followed for survival for 7 days. Though there were no differences in the relative percentages of peritoneal granulocytes or macrophages, there were significantly more peritoneal macrophages and neutrophils in wild-type neonates than CXCR3−/− neonates following sepsis (Fig. 5C and D). In addition, the absence of CXCR3 significantly worsened survival to sepsis (Fig. 5E). These data further support a critical role for CXCL10/CXCR3 signaling in the survival of neonates to septic challenge.
Fig. 5.
Deletion of CXCR3 significantly worsens survival to neonatal sepsis. Peritoneal granulocytes (Gr-1+ CD11b+) and macrophages (F4/80+ CD11b+) were collected from neonates at 0, 6, and 24 h postsepsis (LD40) and stained for CXCR3 expression. Isotype controls are represented as dashed lines. Mean fluorescence intensity (MFI) of CXCR3 expression in granulocytes (A) and macrophages (B) was measured by flow cytometry (mean ± SDs; *, Dunn's one-way ANOVA, P < 0.05; **, Tukey's one-way ANOVA, P < 0.001; the figure represents the summary of two experiments [n = 6]). Peritoneal washes from wild-type (WT) or CXCR3−/− neonates 12 h following an LD40 CS challenge were harvested and analyzed via flow cytometry for the presence of granulocytes (C) or macrophages (D) (n = 4 per group; values represent the means ± SDs; *, Student's t test, P < 0.05). (E) Wild-type or CXCR3−/− neonates were subjected an LD40 septic challenge and then followed for survival for 7 days. The figure represents the summary of two experiments (n = 26; *, Fisher's exact test, P < 0.002).
In vitro stimulation with CXCL10 increases phagocytic ability of neonatal splenic macrophages and granulocytes.
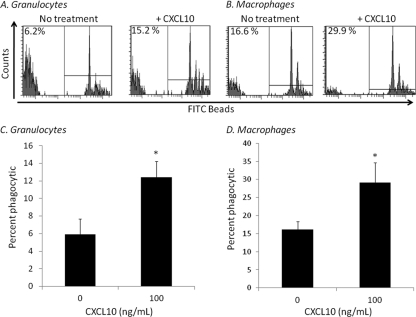
CXCL10 has been demonstrated to augment the phagocytic function of adult murine and human neutrophils (11). Therefore, in addition to recruitment, CXCL10 may play a critical role in the modulation of neonatal innate immune phagocytic function and/or reactive oxygen species production. To address this question, neonatal splenocytes from healthy neonatal mice were isolated and incubated with or without recombinant CXCL10 in vitro for 4 h. Although CXCL10 pretreatment had no effect on ROS production (data not shown), CXCL10 pretreatment significantly augmented the phagocytic function of both neonatal granulocytes and macrophages (Fig. 6A and B).
Fig. 6.
Recombinant CXCL10 improves phagocytosis in granulocytes and macrophages. Neonatal splenocytes isolated via density gradient were incubated with or without murine CXCL10 (100 ng/ml) for 4 h. Cells were then washed and incubated with FITC-labeled beads and stained for granulocyte (Gr-1+ CD11b+) (A) and macrophage (F4/80+ CD11b+) (B) cell surface markers. Cells were first gated on granulocyte or macrophage populations, and then FITC+ cells were considered phagocytic. The figure represents the summary of three experiments, and histograms are representative samples from one of the experiments (values represent the mean ± SDs; *, Student's t test, P < 0.05).
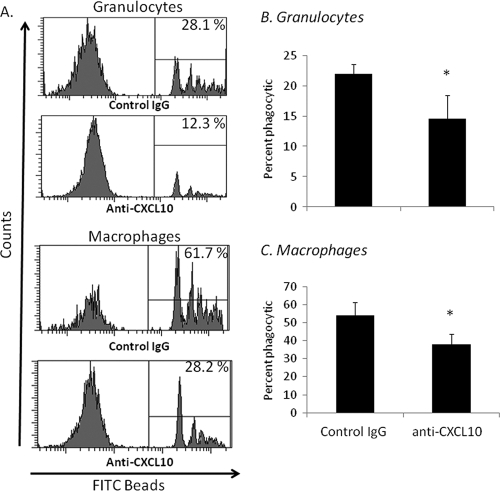
Blockade of CXCL10 worsens phagocytic ability.
To further support this proposed role in vivo, neonates were administered 0.5 mg of anti-CXCL10 or control rabbit IgG i.p. 2 h prior to sepsis. Peritoneal washes were then collected from these animals 14 h later and assayed for phagocytic uptake of FITC-labeled beads. As shown in Fig. 7, blockade of CXCL10 significantly worsens phagocytic function by both granulocytes (Fig. 7B) and macrophages (Fig. 7C). These data further support the hypothesis that CXCL10 not only functions to promote recruitment of inflammatory cells (granulocytes and macrophages) to the peritoneum during sepsis but also serves to modulate phagocytic function for efficient clearance of infection.
Fig. 7.
Inhibition of CXCL10 reduces phagocytic function of granulocytes and macrophages in vivo. Two hours prior to sepsis (LD40), neonates were administered either anti-CXCL10 or control IgG (0.5 mg) i.p. Twelve hours following sepsis (LD40), peritoneal washes were harvested and incubated with FITC-labeled latex beads and subsequently stained for granulocyte (Gr-1+ CD11b+) and macrophage (F4/80+ CD11b+) cell surface markers. Cells were first gated on granulocyte or macrophage populations, and then FITC+ cells were considered phagocytic. Histograms of representative samples (A) and the summary of two experiments for both granulocytes (B) and macrophages (C) are presented (n = 6 per group; values represent the mean ± SDs; *, Student's t test, P < 0.05).
Blockade of CXC10 inhibits adjuvant-induced protection during sepsis, and administration of CXCL10 improves outcome.
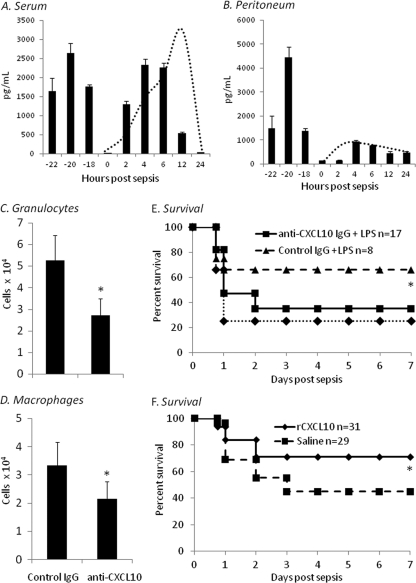
We have previously demonstrated that a 24-h pretreatment of neonates with TLR agonists confers protection to sepsis (27). This effect was associated with increased neutrophil recruitment to the peritoneum. On the basis of these observations and the data presented above, we examined whether CXCL10 was required for the observed adjuvant-induced protective effect. We first characterized the serum and intraperitoneal CXCL10 concentrations in response to LPS pretreatment (1 μg/g BW) and then induced an LD70 sepsis. As demonstrated in Fig. 8A and B, CXCL10 concentrations peaked within 4 h after LPS administration, decreased to basal levels within 24 h, and then subsequently increased again in response to sepsis.
Fig. 8.
Inhibition of CXCL10 abolishes protective adjuvant effect of a TLR4 agonist in response to sepsis, and CXCL10 administration modestly improves survival to sepsis. Sera (A) or peritoneal washes (B) were collected from neonates at multiple time points postsepsis and assayed via ELISA for the concentration of CXCL10. The dashed line represents the concentrations of CXCL10 from naïve animals following septic challenge. (C to E) Two hours prior to LPS or saline administration, animals were administered either anti-CXCL10 or control IgG (0.5 mg). Twenty-four hours following LPS administration, peritoneal washes were harvested and cells were stained for granulocytes (C) (Gr-1+ CD11b+) and macrophages (D) (F4/80+ CD11b+) and analyzed by flow cytometry. The figure represents the summary of three experiments (*, Student's t test, P < 0.05; error bars indicate SDs). (E) Twenty-four hours following LPS or saline pretreatment, neonates were challenged with an LD70 sepsis and then followed for survival. The figure represents the summary of two experiments (*, Fisher's exact test comparing control IgG+LPS and anti-CXCL10–LPS, P < 0.001). (F) Twelve hours prior to sepsis, neonates were administered either recombinant murine CXCL10 (rCXCL10; 20 ng) or physiologic saline. Following sepsis, animals were then followed for survival for 7 days. The figure represents the summary of three experiments (*, Fisher's exact test, P = 0.066).
To determine whether these increases in CXCL10 concentrations were associated with increases in peritoneal granulocytes and macrophages following adjuvant administration, neonates were injected with either 0.5 mg of control rabbit IgG or rabbit anti-CXCL10 IgG 2 h prior to the LPS pretreatment. Peritoneal washes were then harvested 24 h following LPS administration. As shown in Fig. 8C and D, blockade of CXCL10 before administration of LPS is associated with decreased recruitment of both macrophages and granulocytes to the peritoneum. To address whether this decrease in macrophage and granulocyte recruitment reduced or inhibited the adjuvant-induced protective effect to sepsis, 24 h following LPS or saline pretreatment, sepsis was induced and survival was evaluated. Surprisingly, blockade of CXCL10 2 h before LPS pretreatment completely prevents the protective effect of LPS pretreatment (Fig. 8E). Further, the effect of CXCL10 blockade appeared to be specific to LPS pretreatment and not sepsis-induced CXCL10, as blockade of CXCL10 either 2 or 22 h after LPS pretreatment did not block the protective adjuvant effect of LPS (data not shown). These data suggest that CXCL10 production is critical for the recruitment of innate immune effector cells and the subsequent protective adjuvant effect of a TLR4 agonist pretreatment.
If CXCL10 is important for the recruitment and phagocytic function of granulocytes and macrophages to the peritoneum during neonatal sepsis and LPS pretreatment improves outcome in a CXCL10-dependent manner, then pretreatment with CXCL10 may serve to improve survival to sepsis. To address this, neonates were administered either recombinant murine CXCL10 (20 ng) or saline 12 h prior to induction of neonatal sepsis and then followed for survival. As demonstrated in Fig. 8F, administration of CXCL10 modestly improves survival of neonates in response to CS challenge from 44% to 70% (P = 0.066). Though not statistically significant, these data suggest that CXCL10 may be one mediator that contributes to the improved outcome to sepsis seen with a TLR-adjuvant pretreatment schema.
DISCUSSION
Sepsis in both neonates and adults is a complex disorder that, despite advances in novel therapeutics and critical care management, is still incompletely understood. Due to the complexity of the host response to sepsis, identifying mediators that modulate innate immunity would be invaluable in the neonatal setting, as the recruitment of functional innate immune effector cells to the site of infection appears to be critical for survival to polymicrobial sepsis (27). To this end, we have identified a role for CXCL10/CXCR3 signaling during neonatal sepsis. We have demonstrated that the production of CXCL10 during neonatal sepsis not only participates in the recruitment of granulocytes and macrophages into the peritoneum (Fig. 3A and B) but also modulates their phagocytic function (Fig. 5). Additionally, we showed that ex vivo treatment of granulocytes and macrophages with CXCL10 significantly improved phagocytic function (Fig. 4), a finding consistent with what we saw with adult murine and human neutrophils (11).
Though previous work from our laboratories has demonstrated that type I interferon-dependent production of CXCL10 may be important for the function of adult neutrophils and survival to adult sepsis, type I interferons do not appear to play a significant role in the survival of neonates during polymicrobial sepsis (11, 27). Wynn et al. demonstrated that type I interferon receptor-knockout mice have similar survival to neonatal polymicrobial sepsis as wild-type mice (27). As CXCL10 can be induced by either type I or II interferons, these data suggest that though adults may rely more heavily on type I interferon-dependent CXCL10 production for survival to sepsis, neonates do not require type I interferons for survival and are clearly different in this regard.
Few studies have clearly linked CXCL10 production to either outcome from sepsis in neonates or their neutrophil and/or macrophage function. Vasquez and Soong have demonstrated an improvement in macrophage phagocytic function and a decrease in Leishmania parasite burden mediated by CXCL10 stimulation (23). Additionally, elevated plasma concentrations of CXCL10 have been associated with severe infection in human neonates (5, 16). However, no clear link between neonatal innate immune function and the role of CXCL10 has ever been described. This report provides a clear and novel mechanistic role for CXCL10 in the modulation of both granulocytes and macrophages during neonatal sepsis.
The immunomodulatory effects of CXCL10 on neonatal innate immunity suggest that therapeutics targeted to increase the production of CXCL10 may be of utility as a preventative strategy against septic insult in neonates. To support this, we have also shown that CXCL10 is a critical component of the observed benefit to survival seen with TLR4 adjuvant-induced protection to neonatal sepsis (27). Blockade with a CXCL10 antibody prevented LPS pretreatment from protecting neonatal mice from sepsis-induced lethality. However, blockade of CXCL10 as little as 2 h after LPS administration or 2 h prior to sepsis did not block the protective adjuvant effect of LPS (data not shown). Due to the fact that CXCL10 concentrations rise within 2 h after LPS administration (Fig. 7A and B), the abolition of the protective effect of TLR agonist administration appears to be due to CXCL10 blockade in response to the LPS (data not shown). However, although CXCL10 appears to be critical for this protective response, it is clear that it is more than likely working in concert, and not singularly, with other cytokines/chemokines to improve survival to sepsis. This is evidenced by the rather modest improvements in survival (25%) noted in those animals pretreated with CXCL10 and then subjected to polymicrobial sepsis (Fig. 8E).
Production of CXCL10 in the neonate during infection and sepsis appears to serve a vital role in the recruitment and phagocytic function of both granulocytes and macrophages. Further, as CXCL10 has been shown to be a critical part of LPS adjuvant-induced protection and CXCL10 pretreatment of neonates has been demonstrated to modestly improve survival to sepsis, therapeutics designed to promote or augment CXCL10 production may be efficacious in preventing mortality during neonatal sepsis.
ACKNOWLEDGMENTS
We thank Pam Lincoln for her assistance in purifying the anti-CXCL10 antibody.
This research was supported in part by grants R01 GM-097531-01, R01 GM-40586-21, and R01 GM-81923-02, awarded by the National Institute of General Medical Sciences. A.G.C., M.J.D., and K.M.K.-S. were supported by grant T32 GM 008721-10. A.G.C. was also supported by grant F32 GM 093665-01.
A.G.C., J.L.W., D.C.N., M.J.D., L.V., and K.M.K.-S. performed experiments. A.G.C., J.L.W., P.O.S., S.L.K., and L.L.M. participated in experimental design. A.G.C., J.L.W., K.E.B., S.M.W., W.H.R., C.E.M., P.A.E., and L.L.M. wrote and edited the manuscript.
We have no conflicts of interest to disclose.
Footnotes
Published ahead of print on 25 April 2011.
REFERENCES
- 1. Adams-Chapman I., Stoll B. J. 2006. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr. Opin. Infect. Dis. 19:290–297 [DOI] [PubMed] [Google Scholar]
- 2. Akira S., Takeda K. 2004. Toll-like receptor signalling. Nat. Rev. 4:499–511 [DOI] [PubMed] [Google Scholar]
- 3. Arenberg D. A., et al. 1996. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J. Exp. Med. 184:981–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campanella G. S., Lee E. M., Sun J., Luster A. D. 2003. CXCR3 and heparin binding sites of the chemokine IP-10 (CXCL10). J. Biol. Chem. 278:17066–17074 [DOI] [PubMed] [Google Scholar]
- 5. Chen H. L., Hung C. H., Tseng H. I., Yang R. C. 2009. Plasma IP-10 as a predictor of serious bacterial infection in infants less than 4 months of age. J. Trop. Pediatr. 55:103–108 [DOI] [PubMed] [Google Scholar]
- 6. Delano M. J., et al. 2007. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 204:1463–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finlay-Jones J. J., Davies K. V., Sturm L. P., Kenny P. A., Hart P. H. 1999. Inflammatory processes in a murine model of intra-abdominal abscess formation. J. Leukoc. Biol. 66:583–587 [DOI] [PubMed] [Google Scholar]
- 8. Fitzgerald K. A., et al. 2003. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the Toll adapters TRAM and TRIF. J. Exp. Med. 198:1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gray P. H., Burns Y. R., Mohay H. A., O'Callaghan M. J., Tudehope D. I. 1995. Neurodevelopmental outcome of preterm infants with bronchopulmonary dysplasia. Arch. Dis. Child. 73:F128–F134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hotchkiss R. S., et al. 1999. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J. Immunol. 162:4148–4156 [PubMed] [Google Scholar]
- 11. Kelly-Scumpia K. M., et al. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J. Exp. Med. 207:319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kermorvant-Duchemin E., Laborie S., Rabilloud M., Lapillonne A., Claris O. 2008. Outcome and prognostic factors in neonates with septic shock. Pediatr. Crit. Care Med. 9:186–191 [DOI] [PubMed] [Google Scholar]
- 13. Lukacs S. L., Schoendorf K. C., Schuchat A. 2004. Trends in sepsis-related neonatal mortality in the United States, 1985-1998. Pediatr. Infect. Dis. J. 23:599–603 [DOI] [PubMed] [Google Scholar]
- 14. Moser B., Willimann K. 2004. Chemokines: role in inflammation and immune surveillance. Ann. Rheum. Dis. 63(Suppl. 2):ii84–ii89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ness T. L., Hogaboam C. M., Strieter R. M., Kunkel S. L. 2003. Immunomodulatory role of CXCR2 during experimental septic peritonitis. J. Immunol. 171:3775–3784 [DOI] [PubMed] [Google Scholar]
- 16. Ng P. C., et al. 2007. IP-10 is an early diagnostic marker for identification of late-onset bacterial infection in preterm infants. Pediatr. Res. 61:93–98 [DOI] [PubMed] [Google Scholar]
- 17. Ranjbaran H., et al. 2006. Heparin displaces interferon-gamma-inducible chemokines (IP-10, I-TAC, and Mig) sequestered in the vasculature and inhibits the transendothelial migration and arterial recruitment of T cells. Circulation 114:1293–1300 [DOI] [PubMed] [Google Scholar]
- 18. Scumpia P. O., et al. 2006. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J. Immunol. 177:7943–7949 [DOI] [PubMed] [Google Scholar]
- 19. Singh U. P., et al. 2008. CXCL10+ T cells and NK cells assist in the recruitment and activation of CXCR3+ and CXCL11+ leukocytes during Mycobacteria-enhanced colitis. BMC Immunol. 9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh U. P., Venkataraman C., Singh R., Lillard J. W., Jr 2007. CXCR3 axis: role in inflammatory bowel disease and its therapeutic implication. Endocr. Metab. Immune Disord. Drug Targets 7:111–123 [DOI] [PubMed] [Google Scholar]
- 21. Stoll B. J., et al. 2004. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 292:2357–2365 [DOI] [PubMed] [Google Scholar]
- 22. Takeda K., Akira S. 2004. TLR signaling pathways. Semin. Immunol. 16:3–9 [DOI] [PubMed] [Google Scholar]
- 23. Vasquez R. E., Soong L. 2006. CXCL10/gamma interferon-inducible protein 10-mediated protection against Leishmania amazonensis infection in mice. Infect. Immun. 74:6769–6777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watson R. S., et al. 2003. The epidemiology of severe sepsis in children in the United States. Am. J. Respir. Crit. Care Med. 167:695–701 [DOI] [PubMed] [Google Scholar]
- 25. Wynn J. L., Neu J., Moldawer L. L., Levy O. 2009. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J. Perinatol. 29:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wynn J. L., et al. 2007. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 28:675–683 [DOI] [PubMed] [Google Scholar]
- 27. Wynn J. L., et al. 2008. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 112:1750–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yost C. C., et al. 2009. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood 113:6419–6427 [DOI] [PMC free article] [PubMed] [Google Scholar]