Abstract
Clostridium difficile is the causative agent of primary and recurrent antibiotic-associated diarrhea and colitis in hospitalized patients. The disease is caused mainly by two exotoxins, TcdA and TcdB, produced by the bacteria. Recurrent C. difficile infection (CDI) constitutes one of the most significant clinical issues of this disease, occurs in more than 20% of patients after the first episode, and may be increasing in frequency. However, there is no well-established animal model of CDI relapse currently available for studying disease pathogenesis, prevention, and therapy. Here we report the establishment of a conventional mouse model of recurrence/relapse CDI. We found that the primary episode of CDI induced little or no protective antibody response against C. difficile toxins and mice continued shedding C. difficile spores. Antibiotic treatment of surviving mice induced a second episode of diarrhea, while a simultaneous reexposure of animals to C. difficile bacteria or spores elicited a full spectrum of CDI similar to that of the primary infection. Moreover, mice treated with immunosuppressive agents were prone to more severe and fulminant recurrent disease. Finally, utilizing this model, we demonstrated that vancomycin only delayed disease recurrence, whereas neutralizing polysera against both TcdA and TcdB completely protected mice against CDI relapse. In conclusion, we have established a mouse relapse CDI model that allows for future investigations of the role of the host immune response in the disease's pathogenesis and permits critical testing of new therapeutics targeting recurrent disease.
INTRODUCTION
Clostridium difficile, a Gram-positive, anaerobic, and spore-forming bacterium, is an etiologic agent of pseudomembranous colitis and accounts for a quarter of all cases of antibiotic-associated diarrhea (10). With the recent emergence of hypervirulent antibiotic-resistant strains, the incidence of C. difficile-associated diarrhea and intestinal inflammatory disease (collectively designated CDI) has increased significantly in both North America and Europe, causing lengthy hospitalizations and substantial morbidity and mortality (24, 26). CDI is now considered an important reemerging disease.
C. difficile produces metabolically dormant spores that are excreted from infected patients. The infectious spores persist in the environment and are highly resistant to commonly used disinfectants. Spores survive exposure to gastric acidity and germinate in the gut. The use of antibiotics that spare C. difficile but suppress the intestinal microbiota allows C. difficile to proliferate and produce two exotoxins, TcdA and TcdB, which cause intestinal tissue damage and inflammation. Therefore, antibiotic exposure is the most significant risk factor for the diseases (6). CDI ranges from mild diarrhea to life-threatening fulminant colitis (5, 8, 26). In addition to gastrointestinal disease, systemic complications of infection like ascites (15), pleural effusion (7, 38), hepatic abscess (30), and renal failure (11) have also been reported. Standard treatment for CDI is use of the antibiotic metronidazole or vancomycin, although neither of these antibiotics is fully effective (37), and an estimated 20 to 35% of those who appear cured by the initial treatment develop a second episode of the disease (4, 34). The rate of occurrence of further episodes of CDI in patients who have already had one recurrence can be more than 50% (27), and a subset of patients will have multiple recurrences. Recurrent CDI is not always due to infection with the same strain. A new strain was found in 33 to 56% of recurrent episodes (3, 18, 28, 33, 36). Important factors for the development of recurrent CDI include persistent disruption of the intestinal microflora, continuation of antimicrobial therapy, an inadequate antitoxin antibody response, and advanced age. Other factors were also reported to contribute to the recurrence of CDI such as long hospital stays and concomitant receipt of antacid medications (16). Recurrent CDI is a frustrating condition because it is not only difficult to treat but may affect patients for months or even years (17).
CDI has been studied in a number of animal models, including hamsters, guinea pigs, rabbits, rats, germfree mice, conventional mice, and germfree piglets (1, 12, 13, 20, 29, 32). The hamster model has been traditionally widely used, but recently developed mouse and piglet CDI models more closely resemble the disease symptoms in humans (9, 32). Hamsters are extremely sensitive to C. difficile, develop clinical signs of CDI rapidly, and die within 2 to 3 days of infection (25). Therefore, this model does not represent the usual course and spectrum of CDI in human beings. The model was also explored to study relapses (2, 19), but because the animals are treated with antibiotics right after the bacterial inoculation and develop only one episode of clinic disease in these studies, they are not true relapses. In this study, we describe the establishment of a conventional mouse model of CDI relapse/recurrence, which mimics the clinical symptoms of recurrent disease in humans.
MATERIALS AND METHODS
Animals.
C57BL/6 mice (5 to 6 weeks old) were purchased from Jackson Laboratory. All mice used in the experiments were housed in groups of 5 per cage under the same conditions. Food, water, bedding, and cages were autoclaved. All procedures involving animals were conducted under protocols approved by the Institutional Animal Care and Use Committee.
Preparation of inoculum.
C. difficile UK1, an epidemic strain (kindly provided by Dale Gerding), was isolated during a 2006 outbreak at Stoke-Mandeville Hospital in the United Kingdom. Sporulation of the C. difficile UK1 strain was induced on BHIS agar as described previously (31). Briefly, an overnight C. difficile culture in BHIS medium was diluted in fresh medium to an optical density at 600 nm of 0.2. A 150-μl portion of this suspension was spread onto 5 ml BHIS agar in each well of a six-well tissue culture dish. The culture was incubated anaerobically for 4 to 7 days to induce sporulation. The spores were washed off the plates with phosphate-buffered saline (PBS). The spore suspension was then heated at 60°C for 20 min to kill vegetative cells. The spore suspension was stored at 4°C, and the spore concentration was determined by serial dilution. In some experiments, vegetative bacteria of laboratory strain C. difficile VPI 10463 were also used for challenge as described previously (9).
Assessment of C. difficile challenge doses.
The experimental design of different challenge doses of C. difficile UK1 spores is shown in Fig. 1 A. To establish a suitable dose for C. difficile UK1 spores, mice were fed with an antibiotic mixture followed by intraperitoneal (i.p.) injection of clindamycin as described previously (9). Three different doses of spores (104, 105, and 106 CFU) were used for challenge via gavage. Mice were observed daily for the duration of the experiment for the presence of diarrhea and other symptoms. Weights were measured every day. Animals judged to be in a moribund state were euthanized, and tissue samples from the intestine were taken for histopathology analysis.
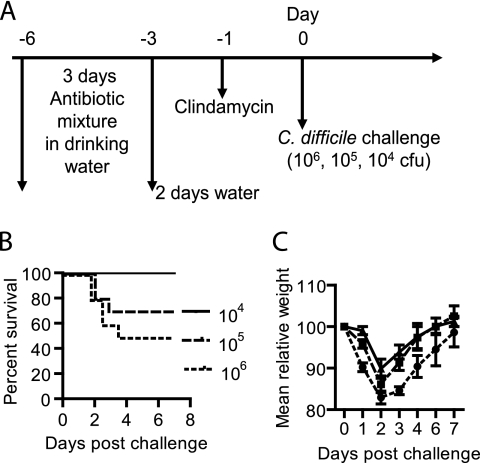
Fig. 1.
Development of primary CDI after C. difficile spore challenge. (A) Experimental design of different challenge doses of C. difficile UK1 spores. After antibiotic cocktail treatment, groups of mice were orally challenged with different doses of C. difficile UK1 spores by gavage: 104 (solid line), 105 (dashed line), and 106 (dotted line) CFU (n = 10). Mice were monitored for signs of disease and euthanized when they became moribund. (B) Kaplan-Meier survival plot of mice infected with different doses of C. difficile spores. (C) Mean relative weights of surviving mice. Relative weight is based on the weight on day 0. Error bars show means ± standard errors.
Establishment of the CDI relapse model in mice.
The experimental scheme of relapse/recurrence CDI models is illustrated in Fig. 2 A. Mice were initially challenged with 106 CFU of C. difficile UK1 spores after antibiotic cocktail treatment (9). Thirty days later, mice that had recovered from primary CDI were divided into 4 groups. Group 1 mice were i.p. injected with 3 consecutive doses of clindamycin (10 mg/kg per dose per day) without a C. difficile spore challenge. Group 2 mice were rechallenged with 106 CFU of C. difficile UK1 spores after the same antibiotic cocktail treatment as the primary infection. Group 3 was the same as group 2, except that the mice also received dexamethasone in their drinking water (100 mg/liter) for 8 consecutive days and one subcutaneous injection (1 μg/mouse) prior to reinfection. Group 4 mice were rechallenged with 106 CFU of C. difficile VPI10463 vegetative cells after receiving the same antibiotic mixture treatment as for the primary infection. Also included was a control group (group 5) which was treated with the antibiotic cocktail and dexamethasone without C. difficile exposure. Animals were observed for CDI symptoms such as weight loss, diarrhea, hunched posture, and death.
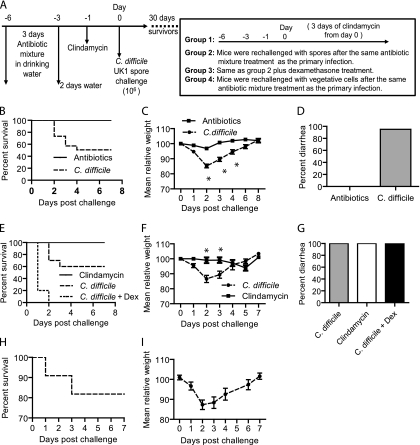
Fig. 2.
Induction of recurrence/relapse CDI in mice. (A) Experimental scheme of relapse/recurrence CDI models. Thirty days after the initial infection, surviving mice were divided into 4 groups. Group 1 (n = 9), clindamycin only; group 2 (n = 10), antibiotic cocktail plus spore challenge; group 3 (n = 10), antibiotic cocktail plus dexamethasone (Dex) plus spore challenge; group 4 (n = 10), strain VPI10463 challenge. A control group (group 5, n = 10), which was treated with antibiotic cocktail and dexamethasone without C. difficile exposure, was also included. Data on the survival (B), weight loss (C), and diarrhea (D) of mice treated with antibiotics only (n = 10, solid line) or treated with antibiotics and then given an initial spore challenge (n = 85, dashed line) are shown. Also shown are data on deaths (E), weight loss (F), and diarrhea (G) in mice with recurrent CDI (group 1, solid line; group 2, dashed line; group 3, dotted line; all of the mice in group 5 appeared normal, without weight loss or death, and their data are not shown here for clarity). Data on the survival (H) and weight loss (I) of mice challenged with strain VPI10463 are shown at the bottom. Relative weight is based on the weight on day 0. The data shown are means ± standard errors, and asterisks show significant differences between the two groups.
The vancomycin treatment-induced relapse experimental scheme is illustrated in Fig. 3 A. In group 1, mice (n = 10) were orally administered vancomycin for 9 consecutive days (50 mg/kg/day via gavage) starting on day 2 after C. difficile challenge. Group 2 was the same as group 1, except that mice were rechallenged with 106 CFU of C. difficile UK1 spores on day 11 (after withdraw of vancomycin treatment). Mice were monitored for symptoms of disease.
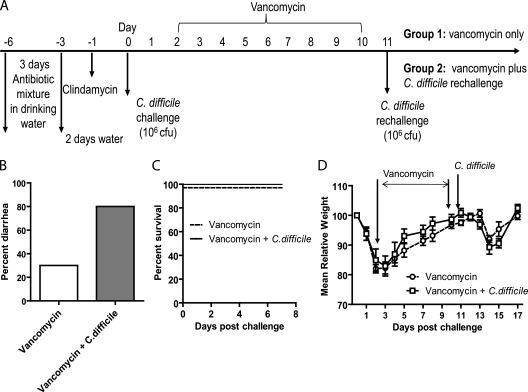
Fig. 3.
Induction of relapse by vancomycin treatment. (A) Experimental scheme. Group 1 mice (n = 10) were orally administered vancomycin for 9 consecutive days starting on day 2 after C. difficile challenge; group 2 received the same treatment as group 1 plus a C. difficile rechallenge on day 11 (after withdraw of vancomycin treatment). Data on the diarrhea (B), survival (C), and weight loss (D) of the two groups of mice are shown.
Treatment of relapse CDI.
For the experimental design of treating CDI relapse/recurrence to examine the therapeutic effects of vancomycin or antibodies on relapse disease, see Fig. 7A. One month after primary infection, survivors were rechallenged with 106 CFU of C. difficile UK1 spores. For treatment, mice were i.p. injected once with 100 μl of anti-TcdA and anti-TcdB polysera from alpaca (a domesticated species of South American camelid) or the same volume of presera (sera collected prior to immunization of alpaca with glucosyltransferase-deficient holotoxin aTcdA or aTcdB) as a control at 4 h postinfection. Vancomycin treatment (50 mg/kg/day via gavage) was started on the day of the rechallenge and was continued for 5 days. Animals were monitored for CDI symptoms.
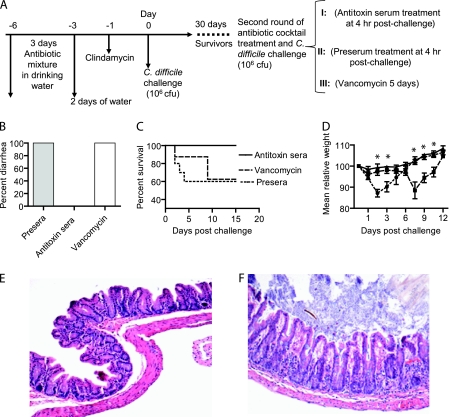
Fig. 7.
Antitoxin serum but not vancomycin treatment prevents recurrent CDI. (A) Scheme of experimental design for treating relapse/recurrence CDI. Groups: I (n = 10), antitoxin polyserum treatment (solid lines); II (n = 10), presera as a control (dotted lines); III (n = 8), vancomycin treatment (dashed lines). Mouse diarrhea (B), deaths (C), and weight loss (D) were monitored. Asterisks show the significant differences between groups I and II and groups I and III. (E, F) Representative histology of ceca from antitoxin serum-treated mice showed intact mucosa, no edema or inflammation (E; magnification, ×100), or occasionally mild inflammation without edema with only mild changes in the mucosa (F; magnification, ×100).
Fecal cytotoxicity.
After the primary and secondary challenges with C. difficile spores, feces were collected and dissolved in an equal volume (g/ml) of sterile PBS containing protease inhibitor cocktail and the supernatants were collected after centrifugation and stored at −80°C. To measure toxin-mediated cytotoxicity in fecal samples, the supernatants were filtered and serially diluted before addition to CT26 monolayers, and cell rounding was observed under a phase-contrast microscope. Toxin titers were defined as the highest dilution to cause 100% cell rounding after 24 h of incubation. Goat anti-TcdA and -TcdB polysera (Techlab Inc.) were used to determine the specific activity caused by C. difficile toxins.
Bacterial culture.
Fecal samples were dissolved in an equal volume of sterile PBS. Samples were serially diluted and plated on TCCFA (taurocholate-cefoxitin-cycloserine-fructose agar) plates and cultured anaerobically for 24 to 48 h at 37°C. The Remel PRO Disc test (Remel, Lenexa, KS) was used to confirm the presence of C. difficile by following the manufacturer's instructions. For strain typing, DNA was isolated and digested with HindIII. The fragments were separated on 0.7% agarose, and DNA fragment patterns were compared with those of the C. difficile strains used for infection.
Antibody titers and neutralizing activity.
IgG titers in sera and IgA titers in feces were measured by standard enzyme-linked immunosorbent assay against purified recombinant holotoxins. Positive control sera were obtained from mice immunized with glucosyltransferase-deficient holotoxins aTcdA and aTcdB (generated in our laboratory). To assess in vitro neutralizing activities of the serum samples, we used a mouse intestinal epithelial cell line, CT26, which is sensitive to both TcdA and TcdB. The serum neutralizing titer is defined as the maximum dilution that still blocks cell rounding induced by toxin at a given concentration. This given concentration is 4 times the minimum dose of the toxin that causes all CT26 cells to round after a 24-h exposure to the toxin. Wild-type TcdA at 1.25 ng/ml or TcdB at 0.0625 ng/ml causes rounding of 100% of CT26 cells after 24 h of toxin exposure. Therefore, TcdA at 5 ng/ml or TcdB at 0.25 ng/ml was mixed with serially diluted serum samples and then applied to CT26 cells and cell rounding was observed under a phase-contrast microscope after 24 h of incubation.
Histopathological analysis.
Histopathological analysis was performed to evaluate mucosal damage and inflammation induced by the toxins. Resected colon or cecum tissues were fixed in 4% formaldehyde buffered with PBS and then embedded in paraffin. Deparaffinized 6-μm-thick sections were stained with hematoxylin and eosin for histological analysis.
Statistical analysis.
Data were subjected to Kaplan-Meier survival analysis, analysis of variance, and t-test analysis using the StatView statistical software program (Abacus Concepts, Berkeley, CA). Results are expressed as means ± standard errors unless otherwise indicated.
RESULTS
The severity of CDI depends on the C. difficile challenge dose.
CDI most likely initiates from and is transmitted through spores. In recent years, the incidence and severity of CDI have sharply increased, in part due to outbreaks caused by the hypervirulent strains of type NAP1/BI/027. We therefore chose to challenge mice with C. difficile UK1 (an epidemic NAP1/BI/027 strain isolated from a patient) spores to mimic the disease occurrence in humans. The experimental scheme is shown in Fig. 1A. Mice were challenged with different doses of spores. Challenge with 106 CFU of spores led to 50% of the mice becoming moribund by day 4 (Fig. 1B), and all of the mice developed diarrhea within 2 to 3 days postchallenge. The mortality and diarrhea rates were 30% (Fig. 1B) and 70%, respectively, for those challenged with 105 CFU of spores. All mice survived a challenge with 104 CFU of spores (Fig. 1B), while 30% of the mice developed diarrhea. Figure 1C shows the mean relative weight of all surviving mice and indicates a sharp decline within 2 to 3 days postinfection. After day 3 or 4, the surviving mice showed clinical recovery from diarrhea and other signs of CDI, began to gain weight, and by day 6 had returned to a normal weight. Based on these data, a C. difficile dose of 106 CFU was chosen as a challenge that would cause severe CDI in a substantial proportion of mice but not universal lethality.
Development of recurrence/relapse CDI in mice.
The experimental scheme is illustrated in Fig. 2A. In the primary infection, 85 mice were challenged with 106 CFU of C. difficile UK1 spores after antibiotic mixture and clindamycin administration. A control group of mice were treated with antibiotic cocktail only without C. difficile infection. Animals were monitored for death (Fig. 2B), weight loss (Fig. 2C), and diarrhea (Fig. 2D). In the C. difficile-infected group, 81 mice (95%) developed diarrhea and lost weight and 42 (50%) of these mice were moribund (Fig. 2B). The remaining 39 surviving mice eventually recovered from diarrhea, weight loss, and other signs of CDI. Four mice did not develop any signs of CDI and were excluded from subsequent experiments. All mice treated with the antibiotic cocktail alone did not develop any signs of disease, although the treatment reduced mouse weight slightly (Fig. 2C).
To induce relapse/recurrent CDI, 39 mice that had recovered from primary CDI were divided into 4 groups (Fig. 2A). A control group (group 5) was included which was treated with the antibiotic cocktail and dexamethasone without C. difficile exposure. In group 1, three doses of clindamycin injection induced all 9 mice to develop diarrhea, which quickly resolved in 1 or 2 days and they survived (Fig. 2E). Mice lost weight but not significantly (Fig. 2F). In group 2, all 10 mice surviving the primary infection developed CDI symptoms, including diarrhea (Fig. 2G) and weight loss (Fig. 2F), after a spore rechallenge. Forty percent of the mice were moribund (Fig. 2E), and their weight change showed a pattern similar to that seen after the primary C. difficile challenge. Recurrent CDI occurs more often in immunocompromised individuals (16, 17). Therefore, in group 3, we used dexamethasone to suppress the mouse immune system, mimicking one of the immunocompromised situations. All dexamethasone-treated mice (n = 10) developed severe diarrhea (data not shown) and became moribund (Fig. 2E) after a rechallenge with C. difficile. All of the mice in group 5 appeared normal without diarrhea or death. These results indicate that immunosuppressed mice were more susceptible to severe disease and death. Recurrent CDI is not always due to reinfection with the same bacterial strain as in the primary infection (16). Therefore, we investigated whether a different strain of C. difficile induced relapse CDI after mice recovered from primary C. difficile UK1 infection. In group 4, 10 mice were challenged with vegetative cells of C. difficile strain VPI10463 after treatment with the antibiotic cocktail and all of them developed diarrhea and lost weight (Fig. 2I) in a pattern similar to that seen after the primary infection (Fig. 2C), with 20% mortality (Fig. 2H).
To more closely mimic what occurs in humans treated for CDI, 2 groups of mice were orally administered vancomycin for 9 consecutive days after they experienced clinical symptoms of CDI (2 days postinfection), and one of the two groups was rechallenged with C. difficile UK1 spores after withdraw of vancomycin (Fig. 3A). Vancomycin treatment did not reduce the diarrhea rate or other clinical symptoms associated with the initial C. difficile infection (data not shown), possibly because mice usually start to recover at around day 3 postinfection even without any treatment intervention. Three days after withdraw of vancomycin, 30% of the mice developed mild diarrhea (Fig. 3B) but recovered 1 day later and all of the mice survived (Fig. 3C). Mice lost weight but not as severely as in the initial infection (Fig. 3D). Rechallenge with C. difficile after vancomycin treatment increased the rate of diarrhea to 80% (Fig. 3B) but did not induce any deaths (Fig. 3C) or significant weight loss compared with the group treated with vancomycin alone (Fig. 3D). These results suggest that vancomycin treatment is not enough to fully disrupt intestinal microflora, allowing C. difficile to induce severe disease in mice.
The primary and relapse CDIs exhibit similar intestinal diseases.
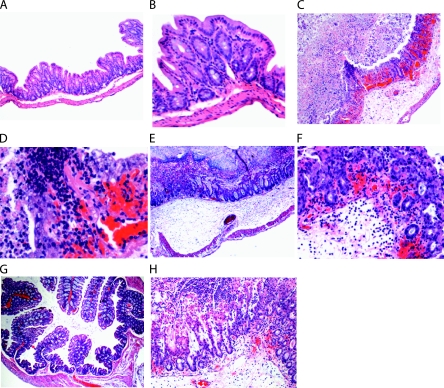
We investigated whether recurrent CDI in mice displays an intestinal histopathology similar to that seen in the primary disease. Histology shows that intestinal sections from mice with relapse CDI exhibited mild-to-severe necrotizing typhylitis and colitis. A remarkable result was the consistency and extent of submucosal to transmural (all layers) edema (Fig. 4). Cecal tissues from mice treated with an antibiotic cocktail alone appeared normal (Fig. 4A and B), while tissues from mice with recurrent CDI showed extensive ulceration, hemorrhage, neutrophilic infiltration, and edema (Fig. 4C and D). Histological examination of ceca obtained from mice with primary CDI showed similar findings (Fig. 4E and F). The colons of infected mice showed marked disruption of the mucosa and significant inflammation, congestion, and hemorrhage (Fig. 4H) in comparison with those of noninfected mice (Fig. 4G). Mouse small intestines were not significantly affected in either primary or recurrent CDI (data not shown).
Fig. 4.
Histology of mouse intestinal tissues. Magnifications: A, ×100; B, ×200. Cecum tissue from a mouse treated with antibiotic cocktail only shows intact mucosa. Slight separation of the submucosa is a sectioning artifact. Magnifications: C, ×100; D, ×400. Cecum from a mouse with relapse CDI that became moribund displays extensive ulceration, hemorrhage, neutrophilic infiltration, and edema. (E, F) Cecal tissue from a mouse with severe primary CDI shows transmural inflammation, loss of superficial mucosal surface (erosions), an expanded submucosal layer (edema), and extravasated red blood cells (hemorrhage) with neutrophilic infiltrates. Magnifications: E, ×40; F, ×200. (G) Colonic tissue from a mouse treated with antibiotic cocktail alone shows a normal mucosal structure (magnification, ×40). (H) Colonic tissue from a mouse with relapse CDI that became moribund exhibits marked disruption of the mucosa and significant inflammation, congestion, and hemorrhage (magnification, ×100).
Presence of C. difficile bacteria and toxins in feces.
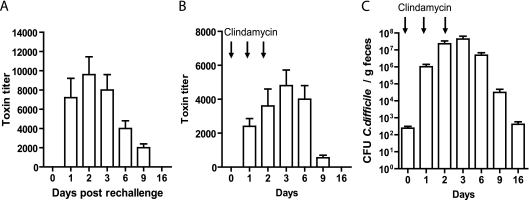
Fecal samples from mice that experienced relapse CDI were examined for the presence of bacteria and toxins. In C. difficile rechallenge-induced relapse, the presence of the toxins was detected in mouse feces from day 1 to day 9 postinfection (Fig. 5 A), with peak cytotoxicity seen on days 2 and 3. Toxins were also detected in the feces of clindamycin-treated mice (Fig. 5B) but at a level significantly lower than that in mice rechallenged with C. difficile (Fig. 5A). We further examined C. difficile bacterial colonization and found that three consecutive doses of clindamycin induced a high level of C. difficile spore shedding at around day 3 (Fig. 5C) and mice continued to shed spores over a 30-day experimental period, which is in agreement with the recent finding (23).
Fig. 5.
C. difficile spore and toxin shedding in the feces of mice with recurrent CDI. The experimental scheme is illustrated in Fig. 2A. Shown are toxin levels in feces from mice experiencing a relapse induced by antibiotic treatment and a C. difficile rechallenge (A) or by clindamycin treatment alone (B) and C. difficile spore shedding in mice treated with clindamycin (C).
Immune response to C. difficile infection in mice.
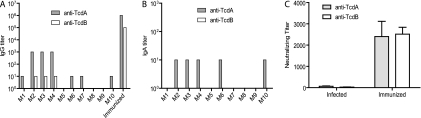
Antitoxin IgG titers in sera and IgA titers in feces were measured on days 15 and 30 after primary infection to assess the host immune response to the toxins. All surviving mice had no or low levels of IgG against TcdA or TcdB on both days 15 and 30 (data for day 15 are not shown), whereas mice immunized with both glucosyltransferase-deficient holotoxins (aTcdA and aTcdB) developed a significant antitoxin IgG response (Fig. 6 A). Fecal IgA against TcdA and TcdB was also determined and showed a pattern similar to that of IgG levels in sera (Fig. 6B). The serum titers of neutralizing antibodies against TcdA and TcdB were barely detectable in C. difficile-infected mice compared with those in sera from aTcdA- and aTcdB-immunized mice (Fig. 6C).
Fig. 6.
Mouse IgG and IgA responses after C. difficile infection. Mouse serum and fecal samples (n = 10, M1 to M10) were collected 30 days after an initial spore challenge. Serum IgG titers (A), fecal IgA titers (B), and serum neutralizing titers (C) were measured. Gray and open bars show anti-TcdA and anti-TcdB levels, respectively. Immunization of glucosyltransferase-deficient holotoxins A (aTcdA) and B (aTcdB) induced higher levels of IgG. “Immunized” represents sera from mice immunized with aTcdA and aTcdB. “Infected” represents sera from mice infected with C. difficile.
Neutralizing antibodies to TcdA and TcdB, but not vancomycin, prevent relapse CDI.
We examined the therapeutic effects of vancomycin and antitoxin polysera on recurrent/ relapse CDI in mice. The experimental design is illustrated in Fig. 7 A. Twenty-eight mice that recovered from primary CDI were challenged with C. difficile spores and then divided into 3 groups. All mice in the preserum-treated group developed diarrhea (Fig. 7B), and 40% of them were moribund within 4 days postinfection (Fig. 7C), whereas antibody-treated mice showed no signs of disease during the entire course of the experiment. In the vancomycin-treated group, one mouse died on day 2 postinfection and the remaining 7 mice did not develop CDI symptoms during therapy. However, 2 days after vancomycin treatment was discontinued, all 7 mice developed severe diarrhea (Fig. 7B) and 2 of them died. In total, about 40% of the mice in the vancomycin-treated group died (Fig. 7C).
Figure 7D shows the mean relative weight of all surviving mice (up to when they became moribund). The preserum-treated mice showed significant weight loss from day 1 and reached their lowest weight on day 2. Surviving mice showed recovery from diarrhea and other signs of CDI and began to gain weight by day 3 to 4. By day 7, these mice had returned to a normal weight. The vancomycin-treated group had slight weight loss during treatment but developed signs of CDI after discontinuation of the therapy. By day 7 (2 days after vancomycin treatment), significant weight loss was evident, and body weight reached the lowest level on day 8, but thereafter the surviving mice gradually recovered (Fig. 7D). This result is similar to that reported in the hamster model, except that the disease occurred faster in mice after withdraw of vancomycin treatment (19). In the antibody-treated group, mice did not lose weight (Fig. 7D) or develop diarrhea (Fig. 7B) during or after therapy. Therefore, antibodies against the two toxins, but not vancomycin, prevented relapse CDI, which is also similar to what occurred in hamsters (19).
On necropsy, the intestines of the antibody-treated mice appeared normal (data not shown). Histology of the ceca from antibody-treated mice showed intact mucosa, without apparent edema or inflammation (Fig. 7E and F). However, mice that were moribund after discontinuation of vancomycin had severe cecal mucosal damage and inflammation (data not shown).
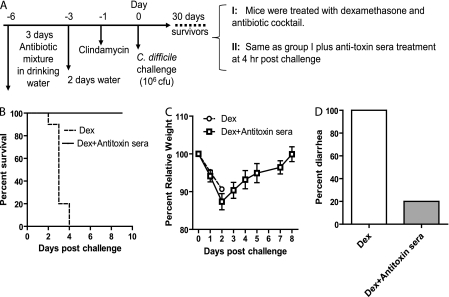
Since the dexamethasone-treated mice are more susceptible to severe relapse CDI and all of them died (Fig. 2E), we investigated whether passive antibody treatment can prevent fulminant and fatal CDI in these mice. Surviving mice from the first episode of C. difficile infection were administered dexamethasone, rechallenged with C. difficile spores, and then divided into 2 groups with or without antitoxin polyserum therapy, respectively (Fig. 8 A). As expected, all mice without antibody treatment developed severe CDI symptoms and were moribund (Fig. 8B and C). In contrast, all mice treated with a single dose of antitoxin antibodies survived (Fig. 8B) even though they lost weight (Fig. 8C). Only 20% of these mice developed mild diarrhea (Fig. 8D) but recovered 1 day later. Thus, neutralizing antibodies against the two toxins prevent mice from developing fulminant relapse CDI.
Fig. 8.
Antitoxin antibodies prevent mice from fulminant relapse CDI. (A) Scheme of the experimental design. (B) Kaplan-Meier survival plot of mice with or without antitoxin antibody treatment. Also shown are data on weight loss (C) and diarrhea (D) in the two groups of mice (n = 10). Dex, dexamethasone.
DISCUSSION
Recurrence/relapse is a significant issue faced by clinicians in the management of CDI patients. In this study, we reported the establishment of a conventional mouse model of CDI relapse/recurrence. We believe that this model provides a much-needed tool to investigate the pathogenesis and host immune response to recurrent CDI and to evaluate new strategies of interventions against the disease.
Recurrent/relapse CDI exhibits a disease profile similar to that of the first episode of CDI. Primary infection with 106 C. difficile UK1 spores routinely caused more than 95% of the animals to develop diarrhea, with a mortality rate of around 50%. After antibiotic exposure and rechallenge of survivors with the same dose of spores 1 month later, mice developed relapse CDI with morbidity and mortality rates comparable to those of primary CDI. Similar histopathology was also evident in the intestines of mice that experienced the first and second episodes of CDI, both of which resemble the CDI pathology in humans. The most consistent and significant inflammatory lesions were observed in the cecum, with occasional inflammation in the colon but seldom in the small intestine. Mice developed mild-to-severe typhylitis and colitis. In addition to intestinal inflammation and injury, a portion of the mice developed acute and systemic fatal disease and died within 2 to 3 days after rechallenge. The toxins may be associated with the development of the systemic disease since the presence of toxin was detected in sera from these mice using an ultrasensitive immunocytotoxicity assay we have described previously (14; data not shown).
Although the mechanisms whereby recurrent CDI occurs have not been completely understood, the intimate association of CDI with prior or concurrent antibiotic use indicates that disruption of normal intestinal microflora and a decrease in colonization resistance are critical factors in the development of recurrent CDI (17). Factors other than endogenous microflora may also contribute to recurrent CDI. Immune responses to C. difficile and/or its toxins are likely to play a role. Investigators previously have reported an association between inadequate immune responses to TcdA and recurrent CDI (21, 22, 35). Patients with recurrent CDI were shown to have significantly lower serum levels of IgG antibody against TcdA (21). Recurrent CDI in humans can be induced by the same or different C. difficile strains (17). Our model mimics these features of relapse CDI in humans in the following ways. First, we treated mice with an antibiotic cocktail before the primary and secondary infections to disrupt the normal microflora; second, the toxin-neutralizing antibodies were low or absent prior to recurrent CDI; and third, the relapse/recurrent CDI can be induced by challenging with either the same strain of C. difficile that caused the initial infection or a different strain. Despite these similarities, there is a significant difference in the induction of relapse between mice and humans. In patients, the standard treatment with vancomycin or metronidazole is often associated with a high rate of relapse CDI and the disease severity ranges from mild diarrhea to fulminant colitis and death. Vancomycin treatment of mice resulted in only a mild disease, whereas the antibiotic cocktail induced CDI with a full spectrum of severity seen in CDI patients. The mouse gut microflora may be very different from that of humans, and vancomycin alone may not be enough to precipitate severe CDI in mice.
Using this model, we demonstrated that antitoxin antibodies against both toxins, but not vancomycin, prevent recurrent CDI. After a single dose of antibody treatment, mice have not developed any signs of recurrent CDI for months until the completion of the experiments. On necropsy, the treated mice showed no gross signs of intestinal inflammation (data not shown). Histology of the ceca from antibody-treated mice showed intact mucosa, no inflammation, or occasionally slight changes in the mucosa but without edema. Vancomycin treatment, on the other hand, only delayed the onset of recurrent CDI. All mice developed severe recurrent disease after discontinuation of the antibiotic treatment. The mouse relapse CDI model thus allows us to evaluate the new treatment strategies against recurrent CDI.
We found that dexamethasone-treated mice were more susceptible to severe and fulminant disease, indicating an important role of the immune response in preventing recurrent CDI. Consistent with this finding, immunocompromised individuals are more likely to develop severe C. difficile-associated disease and relapse (16, 17). It is likely that specific elements of the immune system are compromised in such individuals, making them susceptible to more severe disease and relapse, although further studies are necessary to address this hypothesis.
ACKNOWLEDGMENTS
The project described here was supported by NIH grants R01AI088748, R01DK084509, and K01DK076549.
We thank Joseph Sorg for providing C. difficile UK1 spores.
Footnotes
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Abrams G. D., Allo M., Rifkin G. D., Fekety R., Silva J., Jr 1980. Mucosal damage mediated by clostridial toxin in experimental clindamycin-associated colitis. Gut 21:493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babcock G. J., et al. 2006. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect. Immun. 74:6339–6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barbut F., Petit J. C. 2000. Epidemiology, risk factors and prevention of Clostridium difficile nosocomial infections. Pathol. Biol. (Paris) 48:745–755 [PubMed] [Google Scholar]
- 4. Barbut F., et al. 2000. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J. Clin. Microbiol. 38:2386–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartlett J. G. 2002. Clinical practice.Antibiotic-associated diarrhea. N. Engl. J. Med. 346:334–339 [DOI] [PubMed] [Google Scholar]
- 6. Bartlett J. G. 2006. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann. Intern. Med. 145:758–764 [DOI] [PubMed] [Google Scholar]
- 7. Boaz A., et al. 2000. Pseudomembranous colitis: report of a severe case with unusual clinical signs in a young nurse. Dis. Colon Rectum 43:264–266 [DOI] [PubMed] [Google Scholar]
- 8. Borriello S. P. 1998. Pathogenesis of Clostridium difficile infection. J. Antimicrob. Chemother. 41(Suppl. C):13–19 [DOI] [PubMed] [Google Scholar]
- 9. Chen X., et al. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992 [DOI] [PubMed] [Google Scholar]
- 10. Cloud J., Kelly C. P. 2007. Update on Clostridium difficile associated disease. Curr. Opin. Gastroenterol. 23:4–9 [DOI] [PubMed] [Google Scholar]
- 11. Cunney R. J., Magee C., McNamara E., Smyth E. G., Walshe J. 1998. Clostridium difficile colitis associated with chronic renal failure. Nephrol. Dial. Transplant. 13:2842–2846 [DOI] [PubMed] [Google Scholar]
- 12. Czuprynski C. J., Johnson W. J., Balish E., Wilkins T. 1983. Pseudomembranous colitis in Clostridium difficile-monoassociated rats. Infect. Immun. 39:1368–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fekety R., et al. 1979. Antibiotic-associated colitis: effects of antibiotics on Clostridium difficile and the disease in hamsters. Rev. Infect. Dis. 1:386–397 [DOI] [PubMed] [Google Scholar]
- 14. He X., et al. 2009. An ultrasensitive rapid immunocytotoxicity assay for detecting Clostridium difficile toxins. J. Microbiol. Methods 78:97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jafri S. F., Marshall J. B. 1996. Ascites associated with antibiotic-associated pseudomembranous colitis. South. Med. J. 89:1014–1017 [DOI] [PubMed] [Google Scholar]
- 16. Johnson S. 2009. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J. Infect. 58:403–410 [DOI] [PubMed] [Google Scholar]
- 17. Johnson S. 2009. Recurrent Clostridium difficile infection: causality and therapeutic approaches. Int. J. Antimicrob. Agents 33(Suppl. 1):S33–S36 [DOI] [PubMed] [Google Scholar]
- 18. Johnson S., Adelmann A., Clabots C. R., Peterson L. R., Gerding D. N. 1989. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J. Infect. Dis. 159:340–343 [DOI] [PubMed] [Google Scholar]
- 19. Kink J. A., Williams J. A. 1998. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect. Immun. 66:2018–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knoop F. C. 1979. Clindamycin-associated enterocolitis in guinea pigs: evidence for a bacterial toxin. Infect. Immun. 23:31–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kyne L., Warny M., Qamar A., Kelly C. P. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357:189–193 [DOI] [PubMed] [Google Scholar]
- 22. Kyne L., Warny M., Qamar A., Kelly C. P. 2000. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 342:390–397 [DOI] [PubMed] [Google Scholar]
- 23. Lawley T. D., et al. 2009. Antibiotic treatment of Clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect. Immun. 77:3661–3669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loo V. G., et al. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442–2449 [DOI] [PubMed] [Google Scholar]
- 25. Lyerly D. M., Saum K. E., MacDonald D. K., Wilkins T. D. 1985. Effects of Clostridium difficile toxins given intragastrically to animals. Infect. Immun. 47:349–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDonald L. C., et al. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433–2441 [DOI] [PubMed] [Google Scholar]
- 27. McFarland L. V., Elmer G. W., Surawicz C. M. 2002. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am. J. Gastroenterol. 97:1769–1775 [DOI] [PubMed] [Google Scholar]
- 28. O'Neill G. L., Beaman M. H., Riley T. V. 1991. Relapse versus reinfection with Clostridium difficile. Epidemiol. Infect. 107:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pawlowski S. W., et al. 2010. Murine model of Clostridium difficile infection with aged gnotobiotic C57BL/6 mice and a BI/NAP1 strain. J. Infect. Dis. 202:1708–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sakurai T., et al. 2001. Liver abscess caused by Clostridium difficile. Scand. J. Infect. Dis. 33:69–70 [DOI] [PubMed] [Google Scholar]
- 31. Sorg J. A., Sonenshein A. L. 2010. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J. Bacteriol. 192:4983–4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steele J., Feng H., Parry N., Tzipori S. 2010. Piglet models of acute or chronic Clostridium difficile illness. J. Infect. Dis. 201:428–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang-Feldman Y., Mayo S., Silva J., Jr., Cohen S. H. 2003. Molecular analysis of Clostridium difficile strains isolated from 18 cases of recurrent Clostridium difficile-associated diarrhea. J. Clin. Microbiol. 41:3413–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tonna I., Welsby P. D. 2005. Pathogenesis and treatment of Clostridium difficile infection. Postgrad. Med. J. 81:367–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Warny M., Vaerman J. P., Avesani V., Delmee M. 1994. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect. Immun. 62:384–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilcox M. H., Fawley W. N., Settle C. D., Davidson A. 1998. Recurrence of symptoms in Clostridium difficile infection—relapse or reinfection?. J. Hosp. Infect. 38:93–100 [DOI] [PubMed] [Google Scholar]
- 37. Zar F. A., Bakkanagari S. R., Moorthi K. M. L. S. T., Davis M. B. 2007. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin. Infect. Dis. 45:302–307 [DOI] [PubMed] [Google Scholar]
- 38. Zwiener R. J., Belknap W. M., Quan R. 1989. Severe pseudomembranous enterocolitis in a child: case report and literature review. Pediatr. Infect. Dis. J. 8:876–882 [DOI] [PubMed] [Google Scholar]