Abstract
The etiology of inflammatory bowel disease is not completely known, but it is influenced by the presence of normal gut microflora as well as yet-unrecognized pathogens. The anaerobic, Gram-negative bacterial species Fusobacterium nucleatum is a common resident of the human mouth and gut and varies in its pathogenic potential. In this study, we demonstrate that highly invasive F. nucleatum isolates derived from the inflamed guts of Crohn's disease patients evoked significantly greater MUC2 and tumor necrosis factor alpha (TNF-α) gene expression than minimally invasive strains isolated from the noninflamed gut in human colonic epithelial cells and in a rat ligated colonic loop model of infection. Only live F. nucleatum induced mucin secretion and TNF-α expression in direct contact with and/or during invasion of colonic cells. In rat colons, mucin secretion was augmented in response to a highly invasive F. nucleatum isolate but was unaffected by treatment with a minimally invasive strain. Taken together, these studies reveal that F. nucleatum may represent a challenging pathogen in the etiology of gut inflammatory diseases and highlight the importance of different pathotypes of candidate bacterial species in disease pathogenesis.
INTRODUCTION
Inflammatory bowel disease (IBD) is an umbrella term for a group of chronic, relapsing/remitting disorders of the gastrointestinal (GI) tract that includes ulcerative colitis (UC) and Crohn's disease (CD). The precise etiology of IBD is unknown, but loss of mucosal homeostasis is widely believed to be a key player (13, 27). Mucosal homeostasis is intricately linked with the community of microbial residents of the gut, the so-called gut microbiota. Both animal (22, 33, 42) and clinical (11, 14, 39) studies support the view that changes in the density and composition of the intestinal microbiota (dysbiosis) lead to initiation and perpetuation of IBD, although it is not known whether this imbalance is driven by functional alteration of the commensal gut microbial population or introduction of pathogenic species.
Loss of mucosal homeostasis in IBD is attributed, in part, to damage of mucosal barriers leading to dysregulated mucosal immunity. Alteration in mucin secretion and/or the expression of mucin genes could be a causative factor in barrier compromise, as secreted polymeric mucin forms a physical barrier between luminal antigens and effector immune cells (13). Thus, interference with mucin integrity is a potential strategy whereby pathogenic bacteria in the gut might gain an advantage over the host. The non-spore-forming, anaerobic, Gram-negative bacterial species Fusobacterium nucleatum is both a normal inhabitant of the human mouth and gut and a recognized opportunistic pathogen implicated in inflammatory diseases of both the mouth, such as periodontitis, and the gut, such as appendicitis and IBD (36, 40, 43). F. nucleatum, as a species, is highly heterogeneous, and it has been shown that some strains behave as pathogens while others are more benign; however, the basis for these differences is currently not known (18, 19).
In this study we examined the effects of eight different F. nucleatum isolates derived from the human gut on the perturbation of gut mucosal barrier functions. Invasive strains were recovered from the inflamed mucosae of CD patients, whereas minimally invasive isolates were recovered from the healthy gut mucosae of patients undergoing colon cancer screens. To define a potential role for F. nucleatum as an invasive pathogen, we studied the effect of infection by the isolated F. nucleatum strains, as well as F. nucleatum ATCC 25586T (a known invasive F. nucleatum type strain [23]), on mucin gene expression and secretion in a high-mucin-producing human colonic cell line model of invasion (7). In addition, the effect of invasion by three well-defined strains (EAVG_002, EAVG_003, and ATCC 25586T) was examined for mucin secretagogue activity and Muc2 expression in rat colonic loops.
MATERIALS AND METHODS
Isolation and preparation of bacteria.
Patient clinical histories and anatomical locations of the eight F. nucleatum strains used in this study are shown in Table 1. Strain ATCC 25586T was obtained as a Culti-loop culture (Remel) and revived according to the manufacturer's instructions for use as a control invasive strain in our assays. The phenotypic information as well as further details of sample collection and isolation for each strain are given elsewhere (41). Bacteria were stored at −80°C in TSBsupp (Trypticase soy broth [Oxoid] supplemented with 5 μg/ml hemin and 1 μl/ml menadione [both from Sigma]) containing 25% (vol/vol) glycerol. Strains were grown anaerobically at 37°C in 5 ml of TSBsupp to stationary phase in a Bug Box anaerobic chamber (Ruskinn Technology Inc.) for use in tissue culture invasion experiments.
Table 1.
Patient data and status of Fusobacterium nucleatum isolates
| F. nucleatum isolate | Patient gender | Patient age (yr) at time of procedure | Disease status or reason for colonoscopya | Site of biopsyb |
|---|---|---|---|---|
| EAVG_001 | Female | 53 | IBS | AC |
| EAVG_002 | Male | 45 | CD (active) | SCc |
| EAVG_003 | Male | 60 | CCS | NSC |
| EAVG_005 | Female | 19 | CD | SCc |
| EAVG_012 | Female | 59 | CCS | AC |
| EAVG_014 | Male | 59 | CCS | R |
| EAVG_016 | Female | 54 | CD (active) | ACc |
| EAVG_018 | Male | 45 | CD (active) | TIc |
IBS, irritable bowel syndrome; CD, Crohn's disease; CCS, colon cancer screen.
AC, ascending colon; SC, sigmoid colon; NSC, nonspecific colon (collection site not recorded); R, rectum; TI, terminal ileum.
The sample was taken from inflamed tissues.
In vitro mucin expression and secretion studies with LS 174T cells.
The human colonic adenocarcinoma cell line LS 174T was obtained from the American Type Culture Collection (Manassas, VA) and cultured in minimal essential medium (Gibco BRL Life Technologies, Burlington, Canada) containing 10% fetal calf serum (HyClone Laboratories, Logan, UT), 100 U/ml penicillin, 100 mg/ml streptomycin sulfate, and HEPES at 37°C in a humidified 5% CO2 incubator. Cells were grown in plastic 24-well plates to 80% confluence. To evaluate the concentration-dependent effect of the tested bacterial strains on mucin secretion by LS 174T cells, 4 different inoculums corresponding to 4 × 106, 8 × 106, 1.6 × 107, and 3.2 × 107 CFU/ml over a 4 h period were used. The controls used in these studies were no stimulus as a negative control and 10 μmol/liter phorbol 12-myristate 13-acetate (PMA), a known mucin secretagogue, as a positive control (6). To elucidate the effects of different bacterial strains on the expression profiles of various mucin genes and important proinflammatory cytokines, quantitative real-time PCR (qPCR) analysis was performed using RNA isolated from LS 174T cells incubated with the bacterial strains and controls. Cells were collected after addition of 1 ml of TRIzol reagent to each well. RNA was isolated using the manufacturer's instructions. Based on the data from real-time PCR analysis (see below for methods) of mucin and proinflammatory cytokine gene expression by the different bacterial strains, we choose two strains for further experiments on mucin secretion, one showing high (EAVG_002) and one showing low (EAVG_003) mucin (MUC2) and proinflammatory cytokine (tumor necrosis factor alpha [TNF-α]) expression. From preliminary gene expression studies, we found that an inoculum of 1.6 × 107 CFU/ml was most effective (data not shown).
To elucidate the effect of F. nucleatum strains on mucin secretion, mucin from LS 174T cells (80% confluent) was metabolically labeled with 2 μCi/ml [3H]glucosamine in fresh medium for 24 h. Cells were then thoroughly washed with warm medium, and strains EAVG_002 and EAVG_003 at their effective concentration of 1.6 × 107 were incubated with the labeled cells for 4 h. After 4 h of exposure, cell supernatants from each condition were collected in 1.5-ml microcentrifuge tubes and centrifuged at 1,000 × g for 10 min to remove cellular debris. The cell-free supernatant was reserved, and the 3H-labeled secreted glycoproteins were precipitated with equal volumes of 10% trichloroacetic acid (TCA) and 1% phosphotungstic acid (PTA) (Sigma Chemical Co.) overnight at 4°C. The TCA/PTA pellet was isolated by centrifugation (15,000 × g for 10 min), solubilized in phosphate-buffered saline (PBS), and neutralized to pH 7.0 to 7.4 with 0.1 mol/liter NaOH. Ten milliliters of scintillation cocktail (UniverSol) was added to 100-μl samples, and the total 3H-labeled glycoproteins secreted were determined in a scintillation counter. To confirm the identity of the high-molecular-weight mucin following incubation with the different F. nucleatum strains, the secreted 3H-labeled glycoprotein was subjected to Sepharose 4B (S4B) (Sigma) column chromatography, which separates mucin from nonmucin glycoprotein based on the high molecular weight of mucin (9). Neutralized samples were applied to a column that had been previously equilibrated with 0.01 mol/liter Tris-HCl. Fractions (30 to 40 fractions of 1 ml each) were collected, and the 3H activity of each fraction was determined by liquid scintillation counting. The column was calibrated using the following standards: blue dextran (BD) (2,000 kDa), thyroglobulin (TG) (669 kDa), and bovine serum albumin (BSA) (67 kDa) (Bio-Rad). The high-molecular-weight 3H-labeled mucins that eluted in the void volume (V0; fractions 10 to 15) were expressed as percentage change over the values obtained from negative control (Dulbecco's PBS [DPBS] treated). In addition to infections with live bacteria, the effect of heat-killed bacteria and that of bacterial culture supernatant on in vitro mucin secretion by LS 174T cells was also determined following 4 h of incubation. In brief, bacteria were heat killed at 95°C for 10 min (conditions that rendered 100% of the bacterial sample nonviable), and bacterial culture supernatant was prepared by centrifugation of a freshly grown culture at 6,000 × g for 10 min to remove bacterial cells, followed by filtration through a 0.20-μm syringe filter. Regardless of the conditions, the secreted 3H-labeled mucin produced in response to the bacterial strains was quantified by S4B chromatography as described above.
Bacterial invasion assays in Caco-2 and LS 174T cells.
Bacterial cultures were grown to stationary phase and normalized by adjusting the absorbance at 600 nm to 1.0. Prepared cultures were added to Caco-2 and/or LS 174T cells grown on collagen-coated coverslips at a multiplicity of infection (MOI) of 200:1 (bacterial cells/colonic cells). Cells were incubated at 37°C with 5% CO2. Infections were stopped at indicated time points by gentle washing with sterile PBS and fixation in 2.5% paraformaldehyde.
Differential immunofluorescence staining.
Fixed cells were blocked overnight in 10% normal goat serum (NGS) (Sigma) in 1 ×PBS. Polyclonal antisera EAV_AS1 (rabbit polyclonal, reactive to ATCC 25586T), EAV_AS2 (rat polyclonal, reactive to EAVG_003), and EAV_AS3 (rat polyclonal, reactive to EAVG_002) were diluted 1/200 in 10% NGS in PBS and applied to fixed cells for 30 min at 37°C. Cells were washed in 1× PBS, and secondary anti-rabbit Alexa 350 or anti-rat Cy5 antibody was then diluted to 1/200, added to cells, and incubated for 30 min at room temperature. Cells were gently washed with 1× PBS and then permeabilized for 20 min with 0.5% Triton X-100 in 10% NGS-PBS. A second round of primary antibody was then applied to the cells as described above, and following a wash step, secondary anti-rabbit or anti-rat Cy3 diluted to 1/500 was added to the cells and allowed to incubate for a further 30 min at room temperature. Following thorough washing, coverslips were mounted onto slides with Mowiol (Sigma) and examined using a Leica DM 5000B microscope. Samples were examined using a Hamamatsu ImagEM EM-CCD digital camera, and images were acquired and deconvolved using Volocity 4 software (Improvision).
In vivo mucin expression and secretion studies in rat colonic loops.
Rat colonic loops was used to assess the direct effect of different strains of F. nucleatum on mucin secretion (45). Sprague-Dawley rats, 4 to 5 weeks old, were fasted for 24 h and then injected intraperitoneally with 20 μCi of [3H]glucosamine in 0.5 ml of DPBS (pH 7.2). Rats were left for 3 h to allow for metabolic labeling of newly synthesized mucin. Animals were anesthetized with pentobarbital sodium (35 mg/kg), the abdomen cavity was opened, and colons were flushed with warm DPBS to eliminate stool before surgical ligation. Ligated colonic loops, 1 cm in length, were made with black-braided nylon, leaving the mesenteric blood vessels intact. The colon 1 cm distal from the cecum and the entire rectum were not used. Immediately following ligation, loops were inoculated with 1 ml of each bacterial strain containing 1.6 × 107 CFU/ml. Controls used for mucin secretion included DPBS as a negative control and 1 × 106 Entamoeba histolytica (clone HM1-IMSS) live log-phase trophozoites in DPBS as a positive control (7). Following 4 h of incubation, rats were killed and one small piece of tissue from each animal was immediately saved in TRIzol reagent for RNA preparation and was processed for real-time PCR expression analysis. 3H-labeled mucus glycoproteins from the ligated loops were gently removed by scraping and the secreted proteins placed in 1 ml of DPBS, vortexed on high speed for 10 min, and then clarified by centrifugation (1,000 × g for 10 min). Samples for mucin secretion studies were processed as described above. Results are expressed as percent change from the values obtained from negative-control (DPBS-treated) animals. In parallel experiments done with nonradiolabeled animals, colonic tissues (1 cm) were taken from each animal, fixed in 10% neutral buffered formalin, and embedded in paraffin. Tissue sections 6 μm in thickness were stained with periodic acid-Schiff (PAS) reagent to visualize mucus glycoprotein from goblet cells and those secreted in the lumen.
qPCR analysis.
Quantitative real-time PCR (qPCR) analysis was performed using RNA isolated from rat colonic tissue samples (in vivo study) and LS 174T cells (in vitro study) challenged with the bacterial strains and different controls. RNA isolation was performed using TRIzol (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized from 2 μg of extracted RNA using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen). qPCR for mucin genes (MUC1, MUC2, and MUC3), intestinal trefoil factor/trefoil factor 3 (ITF/TFF-3), resistin-like molecule β (RELM-β), and proinflammatory cytokines (TNF-α and interleukin-1β [IL-1β]) was performed using SYBR green super mix (Qiagen) in a Rotor-Gene detection system (Corbette Research). Primer sequences used in the study are given in Table 2. Each qPCR was performed in triplicate and normalized with the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) housekeeping gene. The results were analyzed using the 2−ΔΔCT method (30).
Table 2.
Sequences of human and rat primers, annealing temperatures, and product sizes
| Species and genea | Primer | Sequence | Annealing temp (°C) | Product size (bp) |
|---|---|---|---|---|
| Human | ||||
| MUC1 | Forward | CAGCCAGCGCCTGCCTGAAT | 60 | 137 |
| Reverse | TGCGGGTTTAGGGGCTGTGG | |||
| MUC2 | Forward | GGGGACAGTGGCTGCGTTCC | 60 | 145 |
| Reverse | CGGGGCAGGGCAGGTCTTTG | |||
| MUC3 | Forward | GCAGCTGTTGGGGCTCCTCG | 60 | 194 |
| Reverse | GAGAGGCGAGCTGGGGGACA | |||
| TFF-3 | Forward | AACCGGGGCTGCTGCTTTG | 60 | 92 |
| Reverse | GAGGTGCCTCAGAAGGTGC | |||
| RELM-β | Forward | CCCTTCTCCAGCTGATCAAC | 60 | 218 |
| Reverse | CCACGAACCACAGCCATA | |||
| IL-1β | Forward | ACGCTCCGGGACTCACAGCA | 60 | 163 |
| Reverse | TGAGGCCCAAGGCCACAGGT | |||
| TNF-α | Forward | CAGAGGGAAGAGTTCCCCAG | 55 | 325 |
| Reverse | CCTTGGTCTGGTAGGAGACG | |||
| GAPDH | Forward | TGACGCTGGGGCTGGCATTG | 60 | 143 |
| Reverse | GGCTGGTGGTCCAGGGGTCT | |||
| Rat | ||||
| Muc1 | Forward | GAGTGAATATCCTACCTACCAC | 58 | 319 |
| Reverse | TTCACCAGGCTAACGTGGTGAC | |||
| Muc2 | Forward | GCCAGATCCCGAAACCA | 55 | 170 |
| Reverse | TATAGGAGTCTCGGCAGTCA | |||
| Muc3 | Forward | AACTTCCAGCCCTCCCTAAG | 50 | 254 |
| Reverse | GCTTCCAGCATCGTCTCTCT | |||
| TFF-3 | Forward | TTTGACTCCAGCATCCCA | 50 | 236 |
| Reverse | CGCAATTAGAACAGCCTTG | |||
| RELM-β | Forward | TTCCTTCTCTCGCTGATGGT | 55 | 231 |
| Reverse | GCAGTGGCAAGTAGTTCCAT | |||
| IL-1β | Forward | CACCTCTCAAGCAGAGCACAG | 59 | 79 |
| Reverse | GGGTTCCATGGTGAAGTCAAC | |||
| TNF-α | Forward | AAATGGGCTCCCTCTCATCAGTT | 59 | 111 |
| Reverse | TCTGCTTGGTGGTTTGCTACGAC | |||
| GAPDH | Forward | TGACAACTCCCTCAAGATTGTCA | 60 | 121 |
| Reverse | GGCATGGACTGTGGTCATGA |
MUC or Muc, mucin; TFF-3, trefoil factor 3; RELM, resistin-like molecule, IL, interleukin; TNF, tumor necrosis factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis.
Results are expressed as means ± standard deviations (SD). Significant differences between controls and the other strains were determined using the Kruskal-Wallis test with Dunn's posttest to compare specific groups. The choice of a nonparametric test (Kruskal-Wallis test) instead of a parametric test (analysis of variance [ANOVA]) was based on the fact that at least one of the groups in all but one of the comparisons was non-Gaussian. To maintain consistency, the Kruskal-Wallis test was used for all comparisons. All statistical analyses were performed using Graph Pad Instat software. P values of >0.05 were considered significant.
RESULTS
F. nucleatum infection of LS 174T cells induces mucin and TNF-α gene expression.
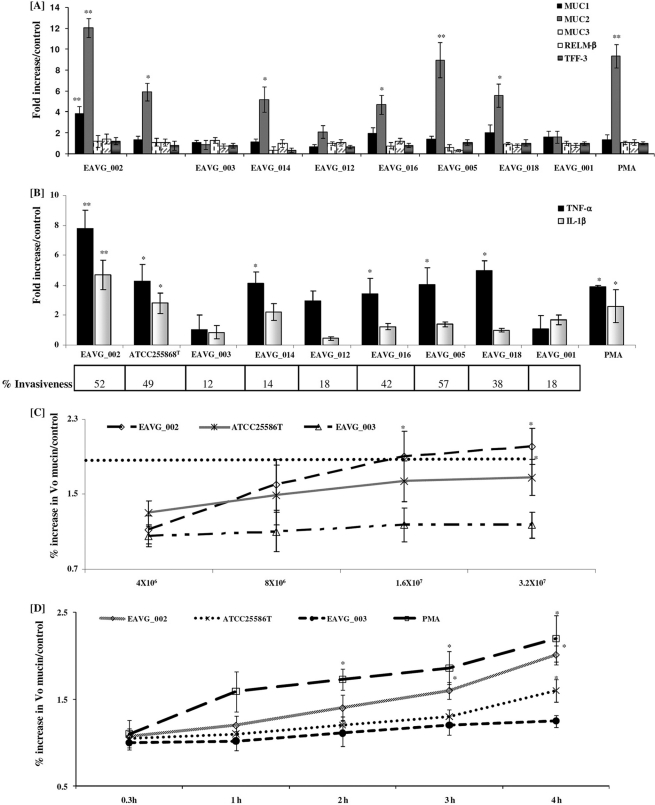
To determine the invasive potential of the F. nucleatum isolates derived from the inflamed mucosae of CD patients compared to that of isolates recovered from the healthy gut mucosae of control patients undergoing colon cancer screening (Table 1), bacterial invasion of the mucin-producing colonic cell line LS 174T was analyzed by determining mucin gene (MUC1, MUC2, and MUC3) and proinflammatory cytokine (TNF-α and IL-1β) expression. As a reference, we compared the expression data to those for the known invasive F. nucleatum ATCC 25586T strain (23). As shown in Fig. 1A, F. nucleatum strains EAVG_014, EAVG_016, EAVG_018, ATCC 25586T, EAVG_005, and EAVG_002 significantly increased MUC2 gene expression, by 5-, 5-, 6-, 6-, 9-, and 12-fold, respectively, in comparison to the PBS-treated control group. F. nucleatum strains EAVG_003, EAVG_001, and EAVG_012 did not induce significant MUC2 gene expression. There was a strong correlation between MUC2 gene expression from F. nucleatum strains derived from inflamed gut biopsy specimens and that from strains derived from healthy tissue. In particular, F. nucleatum strains EAVG_002 and EAVG_005, from inflamed tissue, elicited MUC2 gene expressions as high as that with the positive control PMA (10-fold). Curiously, only EAVG_002, derived from a patient with active CD, showed a marginal upregulation of MUC1 gene expression (4-fold). None of the other strains tested or the positive control PMA showed any significant increases in MUC1 and MUC3 gene expression (Fig. 1A).
Fig. 1.
F. nucleatum infection induces mucin and proinflammatory cytokine gene expression in LS 174T colonic cells. (A) Effects of F. nucleatum isolates on MUC1 to -3 gene expression and goblet cell innate defense mediators. (B) Effects of F. nucleatum isolates and PMA on proinflammatory cytokine gene expression. The percent invasiveness into Caco-2 cells was determined using immunofluorescence microscopy and was calculated as (number of invaded cells in a field of view)/(total number of cells in the same field) × 100 (see Materials and Methods for details). (C) Concentration-dependent effect of F. nucleatum infection on the release of high-molecular-weight mucin (S4B V0 mucin/controls; see Materials and Methods for details) after 4 h. The dotted horizontal line represents the fold increase in mucin gene expression induced by the positive control PMA. (D) Time-dependent increase in high-molecular-weight V0 mucin secretion by F. nucleatum isolates (1.6 × 107 CFU/ml). *, P < 0.05; **, P < 0.01.
F. nucleatum is a known proinflammatory pathogen (20, 29, 43), and it was of interest to determine if the F. nucleatum strains also stimulated the proinflammatory cytokines TNF-α and IL-1β. As shown in Fig. 1B, ATCC 25586T, EAVG_014, EAVG_005, EAVG_018, and EAVG_002 significantly increased TNF-α mRNA expression, by 4-, 4-, 4-, 5-, and 8-fold, in comparison to that in the PBS-treated cells. Interestingly, the proinflammatory mediator IL-1β was only marginally upregulated by EAVG_002 and ATCC 25586T (5- and 3-fold, respectively) in comparison to the control group (Fig. 1B). The positive control PMA showed 4- and 3-fold increases in TNF-α and IL-1β expression after 4 h of incubation. To determine if there was a correlation between MUC2 and TNF-α mRNA expression and colonic cell invasion, we determined F. nucleatum invasiveness into Caco-2 cells (expressed as percent internalized bacteria) by immunofluorescence microscopy. As shown in Fig. 1B, the percent F. nucleatum invasiveness strongly correlated with MUC2 and TNF-α gene expression. The most invasive F. nucleatum strains showed the highest MUC2 and TNF-α gene expression. Based on these data, the following F. nucleatum strains were chosen for all subsequent studies: EAVG_002 (highly invasive and with high MUC2, TNF-α, and IL-1β expression), EAVG_003 (minimally invasive and with no MUC2 gene or proinflammatory cytokine induction), and ATCC 25586T (a known invasive F. nucleatum type strain with high MUC2 and TNF-α expression).
EAVG_002 and EAVG_003 infection of LS 174T cells induces mucin secretion in a dose- and time-dependent manner.
For these studies we used the human colonic cell line LS 174T, which secretes mucin constitutively and in response to mucin secretagogues, including the colonic pathogen E. histolytica (6, 7, 21). LS 174T cells were infected separately with 3 different doses of the F. nucleatum strains, representing multiplicities of infection (MOIs) of 40, 80, and 160 to one LS 174T cell. Cells were incubated for 4 h, and then cell culture supernatant was collected and mucin secretion evaluated. For these assays, PMA (10 μmol/liter) was used as a positive control and PBS treatment as a negative control. As shown in Fig. 1C, there was a dose-dependent increase in the secretion of high-molecular-weight V0 (void volume by Sepharose 4B column chromatography) mucin in response to increasing MOIs for EAVG_002 and to a lesser extent for ATCC 25586T, whereas no significant response was seen with EAVG_003. To determine if F. nucleatum infection resulted in a time-dependent increase in mucin secretion, studies were carried out between 0.3 h and 4 h using the highest tested MOI (Fig. 1D). Both EAVG_002 and ATCC 25586T showed a temporal increase in mucin secretion over 4 h, although the response toward EAVG_002 was more pronounced whereas the response to ATCC 25586T was delayed but became significant after 4 h of incubation. No significant change in mucin secretion was seen for EAVG_003. As expected, treatment with PMA resulted in a significant increase in mucin secretion at all time points tested (Fig. 1D).
Bacterial pathogens use a plethora of mechanisms to affect host cell functions, including the secretion of exotoxins either indirectly or directly through specialized secretion systems (37). Bacterial cell wall components themselves can also affect host gene expression through interaction with host receptor molecules such as Toll-like receptors (15). To determine whether the host cell response to F. nucleatum was driven either directly or indirectly by the bacterium, we examined mucin secretion in response to the two invasive F. nucleatum strains using dead (heat-killed) bacterial cells as well as cell-free bacterial culture supernatant. Neither heat-killed bacterial cells nor bacterium-free culture supernatants had any measurable effects on mucin secretion in LS 174T cells (data not shown), implying that bacterium-host cell contact and/or invasion is a necessary first step to evoke mucin secretion.
Visualization of invasion of LS 174T cells in response to F. nucleatum infection.
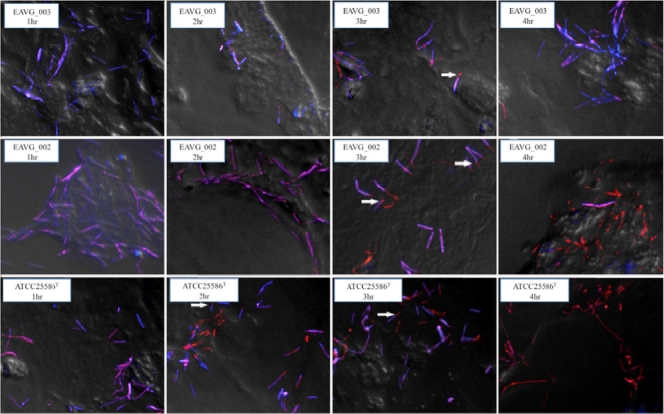
F. nucleatum is an invasive bacterial species and has been shown to penetrate colonic epithelial cells in vitro (23) as well as human appendix tissues in vivo (43). To determine whether the temporal pattern of mucin secretion noted above correlated with bacterial invasion, we visualized invasion of LS 174T cells over a time course of infection from 1 to 4 h using immunofluorescence microscopy and a differential staining technique. Figure 2 shows representative deconvolved images of the F. nucleatum cells taken over this time course and overlaid onto phase-contrast images of LS 174T cells. Under these conditions, bacterial cells external to the cell are colored purple, whereas those that have invaded are colored orange. At 1 h postinfection, no bacterial invasion was seen for any tested strain. However, by 4 h, all bacterial strains demonstrated some degree of invasion: EAVG_003 showed a limited level of invasion, with most bacterial cells found outside the host cell; in contrast, EAVG_002 and ATCC 25586T cells were found almost exclusively inside host cells at this time point. We also noted changes in the rate of invasion by EAVG_002 and ATCC 25586T. The latter strain invaded earlier than the former; by 2 h postinoculation approximately 50% of ATCC 25586T cells had invaded, whereas no invasion was noted for EAVG_002 until 3 h.
Fig. 2.
Time-dependent invasion of LS 174T cells by EAVG_002, EAVG_003, or ATCC 25586T. Bacterial cells were differentially stained using fluorescently labeled polyclonal antibodies, and merged images of fluorescent bacteria were deconvolved and overlaid on a snapshot phase-contrast image of the host cells. Internalized bacteria are stained orange, while those external to the host cell are stained purple. Arrows indicate examples of bacterial cells undergoing end-on invasion by the zipper mechanism; i.e., these cells are shown half internalized. All studied F. nucleatum isolates were invasive, but EAVG_003 is only minimally invasive. Differences in cell morphology between strains have been previously reported (37).
F. nucleatum infection stimulates mucin secretion in rat colon.
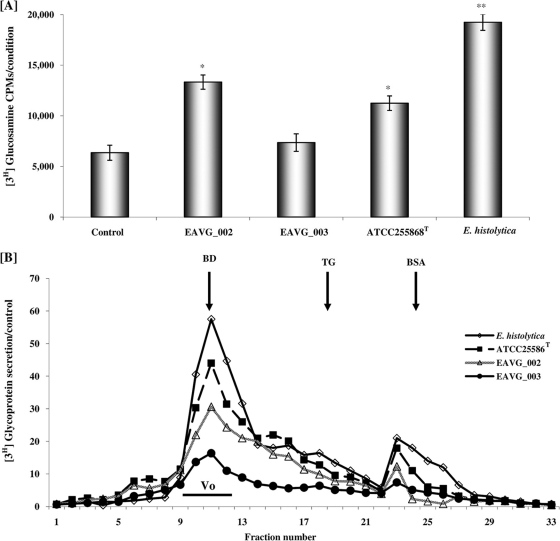
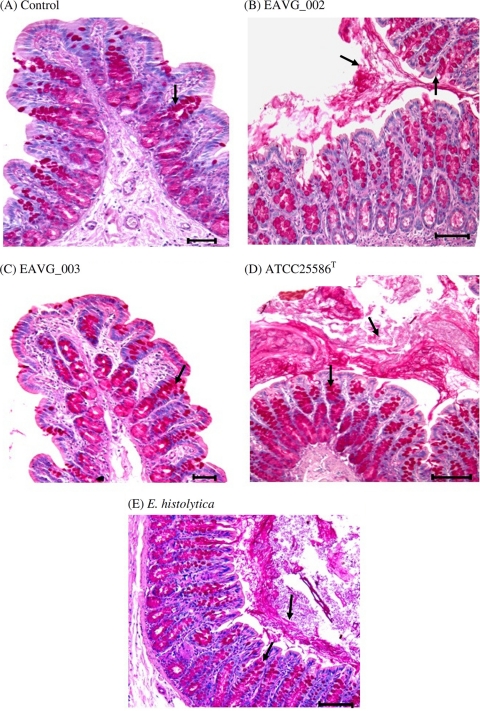
To determine whether the F. nucleatum strains could stimulate mucin secretion in vivo, rat colonic mucin was metabolically labeled with [3H]glucosamine and mucin secretion in response to F. nucleatum was quantified after 4 h of stimulation. As shown in Fig. 3A, colonic loops challenged with F. nucleatum strains EAVG_002, ATCC 25586T, and EAVG_003 resulted in 2-, 2-, and 1-fold increases, respectively, in total secreted luminal [3H]glycoproteins in comparison to DPBS-treated control animals. A known mucin secretagogue, E. histolytica, showed a 3-fold increase in comparison to the negative control. When glycoproteins were analyzed by S4B column chromatography, which separates high-molecular-weight mucins from low-molecular-weight nonmucin components, rat colons inoculated with EAVG_002 showed the most prominent increase in high-molecular-weight V0 mucin secretion over controls (44%), followed by ATCC 25586T (30%) and EAVG_003 (16%). As expected, the positive-control amoebae showed a 58% increase in mucin secretion over DPBS-treated animals (Fig. 3B). By histological analysis using periodic acid-Schiff (PAS) reagent to visualize stored and secreted mucin, colonic loops inoculated with F. nucleatum strains EAVG_002 and ATCC 25586T showed intense mucus secretion from goblet cells in the crypts with formation of mucus plugs in the lumen (Fig. 4B and D) compared to the PBS-inoculated loops (Fig. 4A). Predictably, the noninvasive strain, EAVG_003 showed no mucus secretagogue activity or mucus exudates in the lumen (Fig. 4C). The well-known mucin secretagogue E. histolytica showed high mucin secretion in the lumen and acute mucin secretagogue activity in goblet cells (Fig. 4E).
Fig. 3.
Effects of F. nucleatum infection on total glycoprotein and mucin secretion in rat colonic loops. (A) Secretion of [3H]glucosamine-labeled total glycoproteins in colonic loops in response to different F. nucleatum isolates and the positive control, E. histolytica. (B) Sepharose 4B column chromatography profiles of secreted [3H]glucosamine-labeled mucin and nonmucin glycoproteins. The column was calibrated with the molecular weight markers as indicated by the arrows (see Materials and Methods) and the mucin eluted in the void volume (V0) as indicated. *, P < 0.05; **, P < 0.01.
Fig. 4.
Histological analysis of luminal and goblet cell mucin in colonic loops challenged with F. nucleatum isolates and E. histolytica. (A) Representative periodic acid-Schiff reagent-stained colonic section from a control animal inoculated with PBS at 4 h. The arrow indicates mucin-filled goblet cells (magenta). (B) EAVG_002 inoculated into a colonic loop and incubated for 4 h, demonstrating intense mucin secretion into the lumen and mucus streaming from the crypts (arrows). (C) Representative section from a colonic loop inoculated with EAVG_003, showing mucin-filled goblet cells (arrow) with little or no secreted mucin in the lumen. (D) Representative image of a section of colonic loop inoculated with ATCC 25586T, showing a dense mucus plug in the lumen resulting from intense mucus secretagogue activity and goblet cells filled with mucin in the deep crypts (arrows). (E) Representative image of a section of a colonic loop inoculated with E. histolytica (positive control), showing intense mucus secretagogue activity with dense luminal mucin as well as significant mucin secretion from the crypt goblet cells (arrows). Scale bars represent 25 μm.
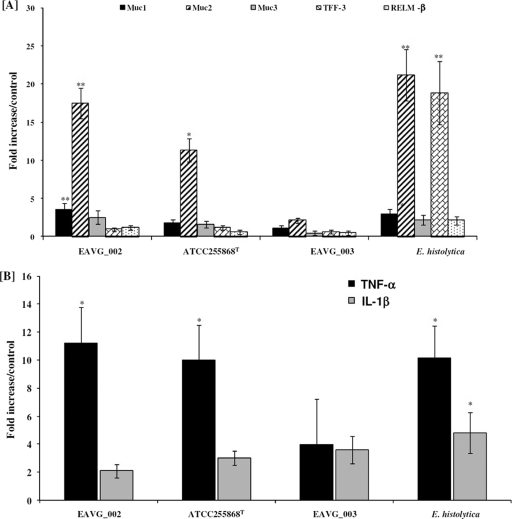
EAVG_002 and ATCC 25586T, but not EAVG_003, upregulate Muc2 and TNF-α gene expression in the colon.
To determine if F. nucleatum infection can stimulate Muc2 as well as other goblet cell innate host defense mediators in rat colon, mRNA was isolated from colonic loops and analyzed for host gene expression in situ by qPCR. As depicted in Fig. 5A, the increased mucin protein secretion observed in EAVG_002- and ATCC 25586T-infected animals (Fig. 3) was concomitant with 12- and 18-fold increases in Muc2 gene expression, respectively, in comparison to that in DPBS-treated controls. However, no significant changes in the expression pattern of the genes encoding the goblet cell markers TFF-3 and RELM-β were observed. Expression levels of the Muc1 gene were also significantly upregulated with EAVG_002 compared to the negative-control group, although this change was not as marked as that for Muc2 expression. EAVG_003 infections had no effect on the genes tested. In contrast, rat colons challenged with live E. histolytica consistently showed increased levels of Muc1 and Muc2, similar to those for EAVG_002, and in addition showed a significant increase in expression of the TFF-3 gene (Fig. 5A). It was of interest to determine if the F. nucleatum strains also modulated the proinflammatory cytokines TNF-α and IL-1β in rat colon. As shown in Fig. 5B, treatment with EAVG_002 and ATCC 25586T, but not EAVG_003, significantly upregulated the expression of TNF-α (11- and 10-fold, respectively), similarly to the positive control, E. histolytica. Interestingly, no change in the expression of the IL-1β gene was observed; levels of expression of this gene were identical to those in the negative-control tissues.
Fig. 5.
Effects of F. nucleatum infection on mucin and goblet cell mediators and proinflammatory cytokine genes in rat colonic loops. (A) Effects of F. nucleatum isolates and E. histolytica on Muc1 to -3 gene expression and goblet cell innate host defense mediators (TFF-3 and RELM-β). (B) Effects of F. nucleatum isolates and E. histolytica on proinflammatory cytokine gene expression in rat colonic tissues. *, P < 0.05; **, P < 0.01.
DISCUSSION
The exact cause of IBD is not yet understood, although recent advances have demonstrated that dysregulation in the expression of mucin and proinflammatory cytokine genes is a hallmark of disease (8, 16). There is also an established role for the gut microflora in the promotion of IBD exacerbation (1). Over the past decade, several bacterial pathogens have been implicated as contributing or even causal factors in IBD, including Mycobacterium paratuberculosis (5), adherent-invasive E. coli (38), and Fusobacterium varium (32, 34). We have previously shown an increased recovery of highly invasive isolates of F. nucleatum from the gastrointestinal mucosae of CD patients compared to those of control colon cancer screen patients (40). In the present study, we investigated whether invasive isolates of F. nucleatum could alter innate host defenses through mucin and cytokine gene dysregulation using two separate models of infection, a mucin-secreting colonic cell line and ligated rat colonic loops. F. nucleatum is a highly heterogeneous species with demonstrated differences in virulence between strains, which do not correlate with current efforts to identify F. nucleatum subspecies (10, 19). In this study, 9 isolates that we and others have previously characterized were used (40). Based on the results of MUC2, TNF-α, and IL-1β mRNA expression studies, we chose 3 of these isolates for further study: strain EAVG_002 (a highly invasive isolate from inflamed mucosal tissue taken from the colon of a CD patient), strain EAVG_003 (a minimally invasive strain originally isolated from healthy mucosal tissue from the colon of a colon cancer screen patient) (41), and strain ATCC 25586T (a well-characterized, invasive F. nucleatum type strain that has been demonstrated to evoke proinflammatory activity in both the gut and the mouth) (23). We hypothesized that the more invasive F. nucleatum isolates, EAVG_002 and ATCC 25586T, would elicit higher levels of mucin secretion and proinflammatory cytokines than the less invasive isolate, EAVG_003, and our results clearly demonstrate that this was the case. Moreover, we saw a strong concordance in the results using both in vitro and in vivo models of invasion, validating our findings and indicating that F. nucleatum infection could be a contributing factor in altering innate host defenses by causing mucin disruption and triggering host inflammatory responses in the gut.
Recent studies have demonstrated that 2 strains of a pathogenic species related to F. nucleatum, F. varium, originally isolated from UC patients, adhered to and invaded cultured human colonic epithelial cells and significantly upregulated the expression of TNF-α mRNA (35). Our results similarly show that invasive strains of F. nucleatum had a significant effect on the induction of TNF-α and IL-1β mRNA expression levels in vitro, whereas noninvasive strains did not. Our data clearly indicate that invasiveness of F. nucleatum directly coincides with increased expression of the proinflammatory cytokine TNF-α. The lack of concordance in the expression profiles of the two proinflammatory cytokines TNF-α and IL-1β was surprising. In particular, we have seen that a highly significant increase in the expression of one cytokine coincides with a not-as-significant increase in the expression of the other cytokine, due to an unknown mechanism.
Our findings also indicate that infection with more invasive strains of F. nucleatum leads to the upregulation of MUC1 (4-fold) and MUC2 (12- to 15-fold) both in vivo and in vitro. Similar to the results for TNF-α expression, an increase in the expression of MUC2 coincided with F. nucleatum invasiveness. Of the 9 F. nucleatum strains evaluated in this study, 5 strains, all of which had been isolated from IBD patients, were highly invasive and elicited expression of MUC2 and TNF-α in vitro to various degrees. The only notable exception was strain EAVG_014, which is a less invasive strain but yet showed increased expression of both MUC2 and TNF-α. This is interesting, as phylogenetically EAVG_014 clusters away from the other strains tested and groups instead with the F. nucleatum subsp. vincentii ATCC 49256 type strain. In the studies of metabolically labeled mucin secretion, there was increased secretion of mucin in response to the invasive strains EAVG_002 and ATCC 25586T under both in vitro and in vivo conditions. It has been previously demonstrated that an increase in mucin production in the gut is a part of the sequelae of events that follow an inflammatory response, presumably to enhance the epithelial barrier during the acute stages of injury (2, 4, 17, 25, 26). The role of upregulated mucin secretion as a result of infection with F. nucleatum is not yet known. It is possible that more invasive F. nucleatum isolates upregulate mucin genes and that the subsequent increased mucin secretion promotes the formation of a patchy or irregular mucus gel layer. Speculatively, this in turn could provoke microbial dysbiosis in the gut. It is also feasible that substantial upregulation of mucin genes in the gut upon invasion of the host intestinal epithelia by F. nucleatum leads to rapid depletion of mucin stores from goblet cells, resulting in later breaches of the mucus gel layer. We also evaluated the expression profiles of other two goblet cell mediators, RELM-β and TFF-3. RELM-β plays a critical role in the maintenance of colonic barrier function and GI innate immunity (24). It has been previously shown that recombinant RELM-β can evoke a significant increase in MUC2 gene expression in HT29-C1.16E cells, as well as an increase in Muc2 secretion by murine intestinal goblet cells (28). In the same study, pretreatment of mice with RELM-β was shown to significantly attenuate trinitrobenzene sulfonic acid (TNBS)-induced colitis (28). Another goblet cell marker, TFF-3, has been shown to protect epithelial cells by contributing in mucosal defense (3). In TFF-3-deficient mice, epithelial restitution is absent in the colon and oral administration of dextran sulfate sodium salt (DSS) induces death associated with extensive colitis (31). In our study, TFF-3 was used as a marker of gut epithelial injury, and in this context the expression of both TFF-3 and RELM-β can be closely linked (44). We were surprised not to find any significant change in either of these two markers in our experiments, indicating that MUC2 can be regulated independently of RELM-β and TFF-3.
We used a colonic epithelial cell line to demonstrate that the effects of infection with highly invasive F. nucleatum isolates on mucin secretion in vitro occurred in a dose- and time-dependent fashion and correlated with the invasive phenotype of the bacteria. Interestingly, EAVG_002 showed a delayed invasion phenotype, with invasion noted only at the 3-h time point as assessed by differential immunofluorescence microscopy, in comparison to ATCC 25586T, which could be seen inside host cells by 2 h postinfection. Despite this, mucin secretion at the 2-h time point was still greater for cells infected with EAVG_002 than for those infected with ATCC 25586T. It is not yet known whether adhesion and/or invasion per se is responsible for initiation of mucin gene upregulation in this model. It is possible that the adhesion and subsequent release of effector molecules into the host cell may promote both invasion of the bacterium and host cell gene transcription. In support of this notion, examination of the F. nucleatum genome has revealed the presence of a type V secretion system (12), and it is tempting to speculate that this could be involved in bacterial virulence in this model. Whatever the case, the effects on host cell mucin secretion and cytokine gene upregulation that we observed seem to be dependent on the presence of live bacteria, since neither heat-killed F. nucleatum nor F. nucleatum culture supernatant was sufficient to elicit the observed effects.
In summary, our study has demonstrated that invasive F. nucleatum strains isolated from the human gut differ in their abilities to induce changes in expression of MUC2 and TNF-α as well as mucin secretion. The significance of these findings remains to be explored, but they clearly illustrate that current efforts to identify pathogenic species in the gut environment should be extended to consider different pathotypes within the same species.
ACKNOWLEDGMENTS
This work was supported by a grant from the Canadian Institute for Health Research (CIHR) (K.C.) and a Grant in Aid of Research from the Crohn's and Colitis Foundation of Canada (E.A.-V.), as well as additional support through the CCFC Chair of IBD Research at the University of Calgary, Keith Sharkey (J.S. and E.A.-V.). P.D. is funded by a Canadian Association of Gastroenterology-Astra Zeneca-CIHR Research and Fellowship Award. J.S. is funded by a CIHR doctoral scholarship award.
Footnotes
Published ahead of print on 2 May 2011.
REFERENCES
- 1. Abraham C., Cho J. H. 2009. Inflammatory bowel disease. N. Engl. J. Med. 361:2066–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahn D. H., et al. 2005. TNF-alpha activates MUC2 transcription via NF-kappaB but inhibits via JNK activation. Cell Physiol. Biochem. 15:29–40 [DOI] [PubMed] [Google Scholar]
- 3. Andoh A., Kinoshita K., Rosenberg I., Podolsky D. K. 2001. Intestinal trefoil factor induces decay-accelerating factor expression and enhances the protective activities against complement activation in intestinal epithelial cells. J. Immunol. 167:3887–3893 [DOI] [PubMed] [Google Scholar]
- 4. Andrianifahanana M., Moniaux N., Batra S. K. 2006. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim. Biophys. Acta 1765:189–222 [DOI] [PubMed] [Google Scholar]
- 5. Behr M. A., Kapur V. 2008. The evidence for Mycobacterium paratuberculosis in Crohn's disease. Curr. Opin. Gastroenterol. 24:17–21 [DOI] [PubMed] [Google Scholar]
- 6. Belley A., Chadee K. 1999. Prostaglandin E(2) stimulates rat and human colonic mucin exocytosis via the EP(4) receptor. Gastroenterology 117:1352–1362 [DOI] [PubMed] [Google Scholar]
- 7. Belley A., Keller K., Grove J., Chadee K. 1996. Interaction of LS174T human colon cancer cell mucins with Entamoeba histolytica: an in vitro model for colonic disease. Gastroenterology 111:1484–1492 [DOI] [PubMed] [Google Scholar]
- 8. Brown S. J., Mayer L. 2007. The immune response in inflammatory bowel disease. Am. J. Gastroenterol. 102:2058–2069 [DOI] [PubMed] [Google Scholar]
- 9. Chadee K., Keller K., Forstner J., Innes D. J., Ravdin J. I. 1991. Mucin and nonmucin secretagogue activity of Entamoeba histolytica and cholera toxin in rat colon. Gastroenterology 100:986–997 [DOI] [PubMed] [Google Scholar]
- 10. Citron D. M. 2002. Update on the taxonomy and clinical aspects of the genus Fusobacterium. Clin. Infect. Dis. 35:S22–27 [DOI] [PubMed] [Google Scholar]
- 11. Contractor N. V., et al. 1998. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J. Immunol. 160:385–394 [PubMed] [Google Scholar]
- 12. Desvaux M., Khan A., Beatson S. A., Scott-Tucker A., Henderson I. R. 2005. Protein secretion systems in Fusobacterium nucleatum: genomic identification of type 4 piliation and complete type V pathways brings new insight into mechanisms of pathogenesis. Biochim. Biophys. Acta 1713:92–112 [DOI] [PubMed] [Google Scholar]
- 13. Dharmani P., Chadee K. 2008. Biologic therapies against inflammatory bowel disease: a dysregulated immune system and the cross talk with gastrointestinal mucosa hold the key. Curr. Mol. Pharmacol. 1:195–212 [DOI] [PubMed] [Google Scholar]
- 14. Dianda L., et al. 1997. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am. J. Pathol. 150:91–97 [PMC free article] [PubMed] [Google Scholar]
- 15. Eckmann L. 2006. Sensor molecules in intestinal innate immunity against bacterial infections. Curr. Opin. Gastroenterol. 22:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Einerhand A. W., et al. 2002. Role of mucins in inflammatory bowel disease: important lessons from experimental models. Eur. J. Gastroenterol. Hepatol. 14:757–765 [DOI] [PubMed] [Google Scholar]
- 17. Enss M. L., et al. 2000. Proinflammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Inflamm Res. 49:162–169 [DOI] [PubMed] [Google Scholar]
- 18. Gharbia S. E., Shah H. N., Lawson P. A., Haapasalo M. 1990. Distribution and frequency of Fusobacterium nucleatum subspecies in the human oral cavity. Oral Microbiol. Immunol. 5:324–327 [DOI] [PubMed] [Google Scholar]
- 19. Gmur R., Munson M. A., Wade W. G. 2006. Genotypic and phenotypic characterization of fusobacteria from Chinese and European patients with inflammatory periodontal diseases. Syst. Appl. Microbiol. 29:120–130 [DOI] [PubMed] [Google Scholar]
- 20. Gmur R., Wyss C., Xue Y., Thurnheer T., Guggenheim B. 2004. Gingival crevice microbiota from Chinese patients with gingivitis or necrotizing ulcerative gingivitis. Eur. J. Oral Sci. 112:33–41 [DOI] [PubMed] [Google Scholar]
- 21. Gottke M., Chadee K. 1996. Exogenous nitric oxide stimulates mucin secretion from LS174T colonic adenocarcinoma cells. Inflamm. Res. 45:209–212 [DOI] [PubMed] [Google Scholar]
- 22. Gui G. P., et al. 1997. Two-year-outcomes analysis of Crohn's disease treated with rifabutin and macrolide antibiotics. J. Antimicrob. Chemother. 39:393–400 [DOI] [PubMed] [Google Scholar]
- 23. Han Y. W., et al. 2000. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect. Immun. 68:3140–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hogan S. P., et al. 2006. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. J. Allergy Clin. Immunol. 118:257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iwashita J., et al. 2003. mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-alpha through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol. Cell Biol. 81:275–282 [DOI] [PubMed] [Google Scholar]
- 26. Kim Y. D., et al. 2002. Interleukin-1beta induces MUC2 gene expression and mucin secretion via activation of PKC-MEK/ERK, and PI3K in human airway epithelial cells. J. Korean Med. Sci. 17:765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knight P., Campbell B. J., Rhodes J. M. 2008. Host-bacteria interaction in inflammatory bowel disease. Br. Med. Bull. 88:95–113 [DOI] [PubMed] [Google Scholar]
- 28. Krimi R. B., et al. 2008. Resistin-like molecule beta regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflamm. Bowel Dis. 14:931–941 [DOI] [PubMed] [Google Scholar]
- 29. Liu H., Redline R. W., Han Y. W. 2007. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J. Immunol. 179:2501–2508 [DOI] [PubMed] [Google Scholar]
- 30. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 31. Mashimo H., Wu D. C., Podolsky D. K., Fishman M. C. 1996. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 274:262–265 [DOI] [PubMed] [Google Scholar]
- 32. Minami M., et al. 2009. Seroprevalence of Fusobacterium varium in ulcerative colitis patients in Japan. FEMS Immunol. Med. Microbiol. 56:67–72 [DOI] [PubMed] [Google Scholar]
- 33. Oberhelman H. A., Jr., Kohatsu S., Taylor K. B., Kivel R. M. 1968. Diverting ileostomy in the surgical management of Crohn's disease of the colon. Am. J. Surg. 115:231–240 [DOI] [PubMed] [Google Scholar]
- 34. Ohkusa T., et al. 2003. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut 52:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohkusa T., et al. 2009. Commensal bacteria can enter colonic epithelial cells and induce proinflammatory cytokine secretion: a possible pathogenic mechanism of ulcerative colitis. J. Med. Microbiol. 58:535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Polak D., et al. 2009. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J. Clin. Periodontol. 36:406–410 [DOI] [PubMed] [Google Scholar]
- 37. Popoff M. R. 2005. Bacterial exotoxins. Contrib. Microbiol. 12:28–54 [DOI] [PubMed] [Google Scholar]
- 38. Rolhion N., Darfeuille-Michaud A. 2007. Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflamm. Bowel Dis. 13:1277–1283 [DOI] [PubMed] [Google Scholar]
- 39. Sellon R. K., et al. 1998. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 66:5224–5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strauss J., et al. 13 January 2011. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm. Bowel Dis. [Epub ahead of print] doi:10.1002/ibd.21606 [DOI] [PubMed] [Google Scholar]
- 41. Strauss J., White A., Ambrose C., McDonald J., Allen-Vercoe E. 2008. Phenotypic and genotypic analyses of clinical Fusobacterium nucleatum and Fusobacterium periodonticum isolates from the human gut. Anaerobe 14:301–309 [DOI] [PubMed] [Google Scholar]
- 42. Sutherland L., et al. 1991. Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut 32:1071–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Swidsinski A., et al. 2011. Acute appendicitis is characterized by local invasion with Fusobacterium nucleatum/necrophorum. Gut 60:34–40 [DOI] [PubMed] [Google Scholar]
- 44. Taupin D., Podolsky D. K. 2003. Trefoil factors: initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 4:721–732 [DOI] [PubMed] [Google Scholar]
- 45. Tse S. K., Chadee K. 1992. Biochemical characterization of rat colonic mucins secreted in response to Entamoeba histolytica. Infect. Immun. 60:1603–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]