Abstract
Carbohydrate mimicry between Campylobacter jejuni lipooligosaccharides (LOS) and host neural gangliosides plays a crucial role in the pathogenesis of Guillain-Barré syndrome (GBS). Campylobacter jejuni LOS may mimic various gangliosides, which affects the immunogenicity and the type of neurological deficits in GBS patients. Previous studies have shown the interaction of LOS with sialic acid-specific siglec receptors, although the functional consequences remain unknown. Cells that express high levels of siglecs include dendritic cells (DCs), which are crucial for initiation and differentiation of immune responses. We confirm that α2,3-sialylated GD1a/GM1a mimic and α2,8-sialylated GD1c mimic LOS structures interact with recombinant Sn and siglec-7, respectively. Although the linkage of the terminal sialic acid of LOS did not regulate expression of DC maturation markers, it displayed clear opposite expression levels of interleukin-12 (IL-12) and OX40L, molecules involved in DC-mediated Th cell differentiation. Accordingly, targeting DC-expressed siglec-7 with α2,8-linked sialylated LOS resulted in Th1 responses, whereas Th2 responses were induced by targeting with LOS containing α2,3-linked sialic acid. Thus, our data demonstrate for the first time that depending on the sialylated composition of Campylobacter jejuni LOS, specific Th differentiation programs are initiated, possibly through targeting of distinct DC-expressed siglecs.
INTRODUCTION
Infection with Campylobacter jejuni usually causes uncomplicated gastroenteritis; however, in rare cases this infection can lead to the Guillain-Barré syndrome (GBS). GBS is a postinfectious immune-mediated disorder of the peripheral nerves and nerve roots. Molecular mimicry between lipooligosaccharides (LOS) present on the cell wall of C. jejuni and gangliosides found in the human nervous system is thought to play a critical role in the pathogenesis of C. jejuni-related GBS (2). The terminal glycans of both gangliosides and LOS are composed of α2,3-linked or α2,8-linked sialic acids. In patients with C. jejuni-related GBS, cross-reactive antibodies against these bacterial structures are induced during infection. The specificity of the antibodies is highly associated with the type of neurological deficits.
Sialic acids are a wide family of carbohydrates, consisting of a nine-carbon backbone (4). They are distinguished from other monosaccharides, which are composed of 5 or 6 carbon sugars. Sialic acids are often found at the outer ends of surface-exposed oligosaccharide chains attached to proteins and lipids to provide the oligosaccharide with a negative charge. In addition, in this terminal position they serve as ligands for lectins to mediate cell-cell interactions (27). Cst-II has been identified as the sialyltransferase responsible for the sialylation pattern of C. jejuni-expressed LOS, acting in a bifunctional manner by catalyzing the formation of both α2,3- and α2,8-linked sialic acids (16). Loss of sialic acid expression on LOS by cst-II mutant strains resulted in lower dendritic cell (DC) activation and subsequent DC-mediated B cell responses (30). The ganglioside mimics of GD1a/GM1a and GD1c expressed by C. jejuni LOS are both sialylated. Interestingly, the GD1a/GM1a mimics of C. jejuni LOS that express terminal α2,3-linked sialic acids are associated with pure motor forms of GBS (25), whereas GD1c mimics of LOS exposing terminal α2,8-linked sialic acids are associated with GBS with ophthalmoplegia (18). Thus, the sialylation pattern of C. jejuni LOS could be an important pathogen-related factor for the induction of GBS.
Sialic acid-binding Ig-like lectins (siglecs) are the best-characterized I-type lectins involved in the recognition of sialic acids (3). Siglecs are expressed predominantly on cells of the immune system (12), whereby different leukocyte subsets express their own variety of siglecs. Siglecs are categorized into two subsets, the evolutionarily well-conserved group consisting of sialoadhesin (Sn, or siglec-1), CD22 (siglec-2), MAG (siglec-4), and siglec-15 and the rapidly evolving CD33-related siglecs (siglec-3, -5, -7, -8, -9, -10, -11, -14, and -16) (13). All siglecs have an unique glycan binding specificity, depending mainly on the linkage of the sialic acid and the underlying glycan (48). Siglecs are thought to play a role in both positive and negative regulation of immune responses (12, 33). CD33-related siglecs mainly act as negative immunoregulators via their ITIM motifs. In contrast, siglec-14, -15, and -16 lack ITIMs and interact with DAP-12, an ITAM-containing receptor. Sn, on the other hand, lacks known signaling motifs (13).
Previous studies have demonstrated that inhibitory siglecs interact with sialylated pathogens such as Neisseria meningitidis and group B streptococci with the potential to subvert immune responses (6, 10, 26). LOS of C. jejuni strains mimicking GD1a/GM1a were shown to interact with Sn (21), whereas siglec-7 bound to LOS structures mimicking gangliosides with terminal α2,8-linked sialic acids such as GD1c (6). Sn has a preference for clustered oligosaccharides terminating in Neu5Acα2,3Gal (α2,3-sialylated glycans) (13). The CD33-related inhibitory receptor siglec-7 has an unusual preference for α2,8-disialylated structures over α2,3- and α2,6-sialylated glycans (5, 49). These α2,8-disialylated epitopes are mainly found on gangliosides (37).
DCs are professional antigen-presenting cells that are important for the initiation and differentiation of immune responses. Like other immune cells, DCs express a variety of siglecs on their surface, including siglec-7 (48). Sn can be induced on immature monocyte-derived DCs following exposure to rhinovirus (28), most likely via production of alpha interferon (IFN-α), which is a potent inducer of Sn expression on monocytes (50). Immature DCs reside in the tissue, where they sense pathogens. Upon pathogen recognition, DCs migrate to the lymph node, where they arrive as fully mature DCs to promote the polarization of naïve T cells to T helper 1 (Th1) cells, T helper 2 (Th2) cells, Th17 cells, or regulatory T cells (7, 14, 42). Classically, Th1 cells are critical for immunity to intracellular organisms, whereas Th2 cells convert immunity to extracellular pathogens and are the major mediators of class switching in B cells (34, 51).
Because of their siglec expression and important immune regulatory role, we hypothesized that different LOS structures of C. jejuni isolates from GBS patients would differentially influence human DC biology. We show here that the interaction of DCs with both GD1a/GM1a LOS mimics and GD1c LOS mimics induced DC maturation to similar levels. However, the GD1a/GM1a mimic induced a more pronounced Th2 skewing, whereas the GD1c mimic induced DC-mediated Th1 responses. Thus, our data demonstrate for the first time that the sialylated composition of C. jejuni LOS structures determines DC-mediated T helper cell polarization in a manner consistent with siglec-dependent modulation.
MATERIALS AND METHODS
Ethics statement.
The study was approved by the VU University Medical Center, Amsterdam, and the Commissie Wetenschappelijk Onderzoek by written consent. Written informed consent was obtained from the healthy donors for the use of blood samples.
Human dendritic cells.
Monocytes were isolated from the blood of healthy donors (Sanquin, Amsterdam, Netherlands) through Ficoll gradient centrifugation and positive selection of CD14+ cells using magnetic activated cell sorting (MACS) (Miltenyi Biotec, CA). Isolated monocytes (purity, >99%) were cultured in RPMI 1640 (Invitrogen, Gibco, CA) supplemented with 10% fetal calf serum (FCS) (BioWhittaker, Walkersville, MD), 1,000 U/ml penicillin (BioWhittaker, Walkersville, MD), 1,000 U/ml streptomycin (BioWhittaker, Walkersville, MD), and 2 mM glutamine (BioWhittaker, Walkersville, MD) in the presence of interleukin-4 (IL-4) (500 U/ml; Biosource, CA) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (800 U/ml; Biosource, CA) for 7 days (43). Anti-DC-SIGN stainings confirmed that >99% of our cells were DCs. Maturation was induced by treatment with 10 ng/ml Escherichia coli lipopolysaccharide (LPS) (Sigma-Aldrich, MO) for 24 h. In order to cleave off sialic acids from the cell surface, DCs (5 × 106/ml) were preincubated with Vibrio cholerae neuraminidase (Roche) (2.5 × 10−2 U/ml) for 1 h at 37°C in TSM (20 mM Tris-HCl, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2) (40). Sialic acid-specific lectin stainings confirmed complete removal of sialic acids.
Bacterial strains and LOS purification.
Four C. jejuni strains (GB2, GB11, GB16, and GB19) were isolated from different GBS patients as described previously (21). The strains were cultured as reported previously (21), and LOS was purified by hot phenol-water extraction as described previously (24). Mutagenesis of the cst-II target gene was described previously (17), and the corresponding cst-II mutants derived from GB11 (Cst-II KO) or derived from GB2 (GB2 Cst-II KO) express a truncated LOS outer core due to the absence of sialic acids, thus lacking ganglioside mimicry (46). GB19 LOS was incubated with Vibrio cholerae neuraminidase (Roche) overnight at 37°C to remove sialic acids, followed by inactivation of neuraminidase for 30 min at 80°C (GB19 neuraminidase). Purified LOS yields were determined by silver staining and mass spectrometry (18, 30).
Siglec-Fc binding.
Nunc MaxiSorp 96-well enzyme-linked immunosorbent assay (ELISA) plates (Nalge Nunc International, Rochester, NY) were coated overnight at 4°C with 20 μg/ml LOS. As a negative control for siglec binding, the plates where coated with the nonsialylated sugar GalNAc coupled to polyacrylamide (PAA), whereas as a positive control the wells were coated with α2,3-sialyllactose, α2,6-sialyllactose, and α2,8-linked di-, tri-, or polysialic acid coupled to polyacrylamide (20 μg/ml; Lectinity, Moscow, Russia) in phosphate-buffered saline (PBS). Wells were blocked for 30 min at 37°C with TSM with 1% bovine serum albumin (BSA) (Calbiochem). For soluble siglecs to bind their sialylated ligands, a precomplexed conformation is required; therefore, Sn-Fc and siglec-7-Fc (10 μg/ml) were precomplexed with goat anti-human Fc-peroxidase (PO) (0.5 μg/ml) in TSM with 1% BSA for 60 min. at room temperature. This mixture, consisting of siglec-Fc proteins complexed with anti-human Fc antibodies, was added to the wells coated with LOS or control glycans and incubated for 60 min at room temperature. Tetramethylbenzidine (TMB) substrate was added to the wells, and binding of the precomplexed siglec-Fc proteins to their ligands was analyzed by measuring the optical density at 450 nm.
Flow cytometry.
For antibody stainings, DCs (5 × 104/well) were incubated with 10 μg/ml 7D2 (20) (anti-Sn), S7.5A (35) (anti-siglec-7), or phycoerythrin (PE)-labeled anti-OX40L (BD Biosciences, CA) for 45 min at room temperature (RT) in PBS with 1% BSA. Cells were washed in PBS, and for siglec stainings the cells were counterstained with a secondary fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (Zymed, CA) for 30 min at RT in PBS with 1% BSA. Stainings were analyzed by flow cytometry (FACSCalibur; BD Biosciences, CA).
For maturation assays, DCs (5 × 104/well) were incubated with PE-labeled antibodies against human CD80 (BD Biosciences, CA), CD83 (IOtest, Newcastleton, United Kingdom), and CD86 and MCH II (both from BD Biosciences, CA) in PBS for 45 min at 4°C. After washing, binding was analyzed by flow cytometry (FACSCalibur; BD Biosciences, CA).
Glycan binding assay.
DCs (5 × 104/well) were pretreated for 45 min at 37°C with blocking antibodies to human siglec-7 (clone 7.7a) (35), followed by a 30-min incubation at 37°C with 10 μg/ml biotinylated PAA attached to galactose, α2,3-sialyllactose, α2,6-sialyllactose, and di- or tri-linked α2,8-sialic acids (Lectinity, Moscow, Russia) in TSM with 1% BSA. Cells were washed in TSM and stained with Alexa 488-labeled streptavidin (Invitrogen, CA) for 30 min at 37°C in TSM with 1% BSA. After washing, binding was analyzed by flow cytometry (FACSCalibur; BD Biosciences, CA).
TLR4 activation.
HEK293-TLR4/MD2 cotransfectants (31) were grown in T75 flasks in RPMI 1640 (Invitrogen, Gibco, CA) supplemented with 10% FCS (BioWhittaker, Walkersville, MD), 10,000 U/ml penicillin (BioWhittaker, Walkersville, MD), 10,000 U/ml streptomycin (BioWhittaker, Walkersville, MD), 10,000 U/ml glutamine (Sigma-Aldrich, MO), and G418 (1 mg/ml). A total of 105 cells in 100 μl RPMI were plated into 96-well flat-bottom plates and stimulated with LOS for 24 h. E. coli LPS served as positive control for Toll-like receptor 4 (TLR4) activation. Subsequently, supernatants were analyzed for IL-8 production by ELISA according to the manufacturer's guidelines (Biosource, CA).
Real-time PCR.
Cells were lysed and mRNA was isolated using an mRNA Capture kit (Roche, Basel, Switzerland). cDNA was synthesized using the Reverse Transcription System kit (Promega, WI) according to the manufacturer's guidelines. Oligonucleotides were designed by using the computer software Primer Express 2.0 (Applied Biosystems) and synthesized by Invitrogen Life Technologies (Invitrogen, Breda, Netherlands). Real-time PCR analysis was performed as previously described with the SYBR green method in an ABI 7900HT sequence detection system (Applied Biosystems), using GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as an endogenous reference (15).
Cytokine ELISA.
IL-12p70 ELISA was performed as described previously (44). In short, monoclonal antibody (MAb) 20C2 was applied overnight to Nunc MaxiSorp 96-well ELISA plates (Nalge Nunc International, Rochester, NY). The plates were blocked for 30 min at 37°C with PBS supplemented with 1% BSA, and the supernatants together with MAb C8.6-biotin (BD biosciences) were added to the plates. IL-12p70 was detected with a streptavidin-PO conjugate, and optical density was measured at 450 nm.
T helper cell differentiation assay.
The T helper cell assay was performed as described before (14). Briefly, monocytes were isolated from healthy blood donors, and DCs were generated as described before. At day 6, maturation was induced by culturing the cells with 10 ng/ml LOS for 2 days. At day 8, DCs were harvested and washed. Maturation of the DCs was tested by flow cytometric analysis of CD86 expression. DCs (5 × 103) were incubated with 2 × 104 naïve CD4+ T cells that were purified from peripheral blood lymphocytes (PBLs) using MACS isolation (Miltenyi Biotec, CA). At day 5 and day 9, human recombinant IL-2 (rIL-2) (10 U/ml; Cetus, Emeryville, CA) was added to the culture. At day 14, resting T helper cells were restimulated with phorbol myristate acetate (PMA) (30 ng/ml; Sigma-Aldrich, MO) and ionomycin (1 μg/ml; Sigma-Aldrich) for 6 h. During the last 5 h, brefeldin A (10 μg/ml; Sigma-Aldrich) was present to enable detection of the intracellular production of IL-4 and IFN-γ (BD Biosciences) by flow cytometry (FACSCalibur; BD Biosciences, CA).
Statistics.
Significant differences in cytokine expression by DCs activated by GB11 or GB16 LOS were evaluated by Mann-Whitney U tests, and differences in secretion of cytokines by DCs were evaluated by unpaired t tests (GraphPad Prism 4.02; GraphPad Software, San Diego, CA). A P value of <0.05 was considered significant.
RESULTS
Differential binding of Sn and siglec-7 to sialylated LOS structures.
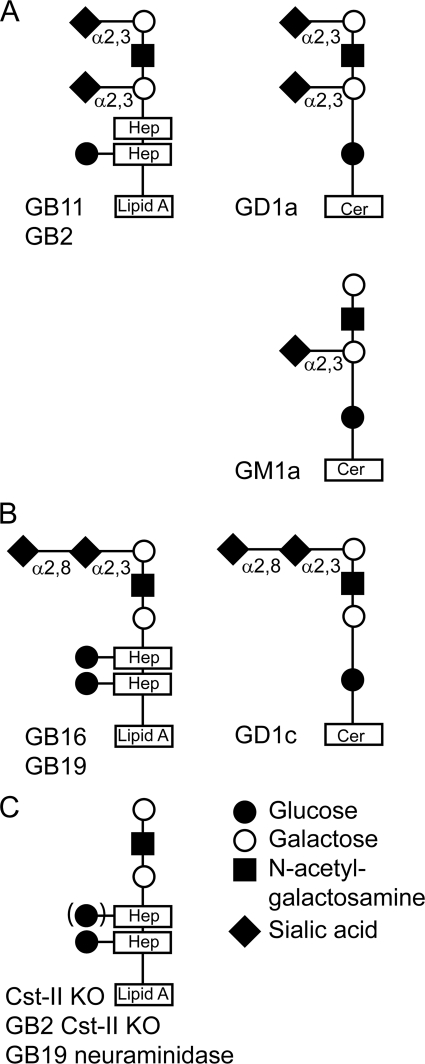
Recent publications have demonstrated that LOS with terminal α2,3-linked sialic acid (GB11) could bind to Sn (21), whereas LOS with terminal α2,8-linked sialic acid interacted with siglec-7 (6); however, the functional consequences of siglec binding were not evaluated on a cellular level. In this study, we used GB11, the LOS structure that mimics the gangliosides GD1a/GM1a containing terminal α2,3-linked sialic acid (Fig. 1A), and GB16, the LOS structure that mimics the ganglioside GD1c, exposing a terminal α2,8-linked sialic acid (Fig. 1B) (18). As a control, we used the same LOS glycan structure from a C. jejuni mutant derived from GB11, which lacks the terminal sialic acid through deletion of the cst-II gene (Cst-II KO), coding for the sialyltransferase (17) (Fig. 1C).
Fig. 1.
Schematic representation of glycan structures from isolates from GBS patients and the cst-II KO mutant. (A) GB11 and GB2, containing terminal α2,3-linked sialic acid and the corresponding ganglioside GD1a mimic. (B) GB16 and GB19, containing terminal α2,8-linked sialic acid and the corresponding ganglioside GD1c mimic. (C) Cst-II KO, GB2 Cst-II KO, and GB19 neuraminidase, lacking sialic acid.
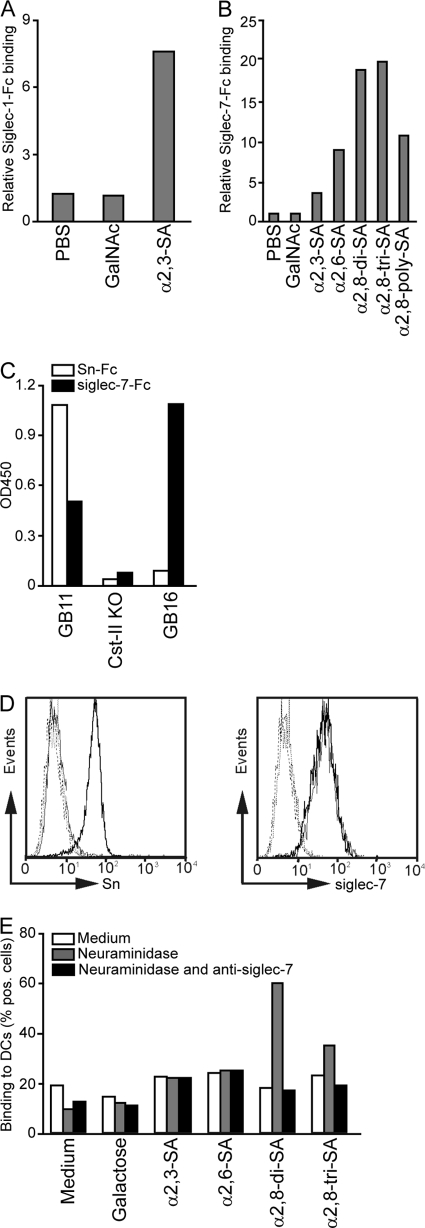
First, the sialic acid binding specificities of the Sn-Fc and siglec-7-Fc proteins were evaluated. Therefore, we analyzed binding of Sn-Fc and siglec-7-Fc to sialylated or N-acetylgalactosylated glycan PAA probes. Sn-Fc interacted with α2,3-linked sialic acids (Fig. 2 A), whereas siglec-7-Fc bound with the highest avidity to α2,8-linked di- and trisialic acids (Fig. 2B) and no siglec-Fc binding to the negative control N-acetylgalactosamine-conjugated PAA was observed (Fig. 2A and B), confirming reported Sn and siglec-7 carbohydrate specificities (36).
Fig. 2.
Sn and siglec-7 have different binding specificities toward sialylated LOS structures. (A and B) Sn-Fc (A) and siglec-7-Fc (B) proteins were precomplexed with anti-human-Fc PO and added to coated PAA-conjugated glycan structures. As a control for the sialic acid specificity of the siglec-Fc proteins, uncoated wells or GalNAc-PAA-coated wells were used. Depicted are the relative bindings of precomplexed siglec-Fc proteins compared to the binding of secondary antibody alone. (C) Sn-Fc and siglec-7-Fc precomplexed with anti-human-Fc PO binding to coated GB11 (α2,3-linked sialic acid), the Cst-II mutant (without sialic acid), and GB16 (α2,8-linked sialic acid) LOS. (D) Fluorescence-activated cell sorter (FACS) analysis of immature DCs (thin lines) and mature DCs (bold lines) stained with MAb 7D2 (anti-Sn, left) or MAb S7.5A (anti-siglec-7, right). Dotted lines are for the corresponding negative controls. (E) Binding of biotinylated glycosylated PAA-conjugated probes to immature DCs as measured by flow cytometry. Binding to nontreated DCs (medium), neuraminidase-pretreated DCs (gray bars), or neuraminidase- and anti-siglec-7-pretreated DCs (black bars) of one representative donor is depicted. The percentage of cells binding the PAA-glycoconjugates is depicted. For all experiments, results from one out of five representative experiments are shown.
Next, we determined the potency of Sn and siglec-7 to interact with the sialylated GB11 and GB16 LOS structures. Sn-Fc displayed binding only to GB11 carrying α2,3-linked sialic acid. In contrast, siglec-7-Fc interacted both with GB16 carrying α2,8-linked sialic acid and with GB11, due to low-affinity interactions with α2,3-linked sialic acids. To assess the sialic acid requirement for this siglec-glycan interaction, binding of siglec-Fc to LOS structures without terminal sialic acids (Cst-II KO) was investigated (29). Neither siglec-Fc protein bound to the mutant without sialic acid (Fig. 2C). Thus, we demonstrate that GB11 and GB16 interact specifically with Sn-Fc or siglec-7-Fc in a sialic acid- and linkage-dependent manner.
Interaction of C. jejuni sialylated LOS structures with DC-expressed siglecs.
To evaluate whether the differential siglec binding of GB11 and GB16 could influence immune responses, we first analyzed the siglec expression pattern of human DCs, which are important cells for induction and differentiation of immune responses. In concordance with the literature, human immature DCs expressed only siglec-7, whereas Sn was not expressed by immature DCs (Fig. 2D) (12, 32).
To test the ability of immature DC-expressed siglec-7 to bind sialylated glycans, the interaction of PAA conjugated with α2,3-linked sialyllactose, α2,6-linked sialyllactose, and α2,8-linked sialic acids (di- and trisialic acids) with DCs was analyzed by flow cytometry. N-Acetylgalactosamine (Fig. 2A and B) was not suitable as a negative control in these cellular assays because of its ability to bind to the DC-expressed lactin C-type macrophage galactose-type lectin (MGL). Instead we used galactose as a negative control since both galactose and N-acetylgalactosamine are known substrates of sialyltransferases. It is well accepted that siglecs expressed on immune cells often have endogenous cis ligands, resulting in masking of siglecs by recognition of sialic acids present on the same cell membrane. Masking of siglecs by endogenous sialic acid ligands generally results in low binding to sialylated trans-ligands (40). Siglecs can be unmasked and released from their cis ligands by neuraminidase treatment. To release DC-expressed siglecs from potential cis ligands, DCs were pretreated with neuraminidase before measurement of sialic acid binding. α2,3-linked sialic acid and α2,6-linked sialic acid displayed low binding to DCs, whereas α2,8-linked di- and trisialic acids bound with high affinity to DCs, suggesting interaction with siglec-7. The binding of sialylated glycans was clearly augmented when siglecs were unmasked by neuraminidase treatment. To determine the contribution of siglec-7 in binding of α2,8-sialylated glycans, as also present on GB16, siglec-7 on DCs was blocked with a monoclonal antibody prior to PAA-conjugated glycan incubation. As expected, binding of α2,8-sialylated glycans was mediated exclusively by DC-expressed siglec-7 (Fig. 2E). In addition, neuraminidase treatment or maturation of DCs with LPS could not improve the low binding of α2,3-linked sialylated structures (data not shown).
TLR4 activation and DC maturation by LOS.
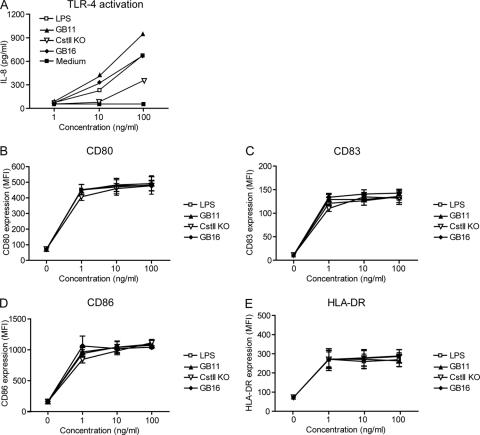
DC activation following pathogen recognition is mediated by TLRs (41). TLR4 recognizes the lipid A moiety of LPS, a glycolipid expressed on the cell surfaces of Gram-negative bacteria (19) (45). To investigate the potency of LOS to trigger TLR4, HEK cells transfected with TLR4 and MD2 were used. As siglecs are expressed exclusively on leukocytes and in the nervous system, it is generally accepted that HEK cells do not express siglecs. Furthermore, HEK cells are not able to bind sialylated glycans (23). After overnight incubation with LOS, IL-8 levels, corresponding to TLR4 activation, were analyzed. Both GB11 and GB16 showed high TLR4 activation, comparable to that with the control LPS of E. coli. Compared to GB11, the lowered capacity of GB16 to induce IL-8 production after TLR4 engagement may indicate that the sialic acid linkage affects the ability to trigger TLR4 activation. The Cst-II KO sialic acid mutant, lacking sialic acids, clearly showed a reduced capacity to trigger TLR4 (Fig. 3A). This corresponds with our previous results showing that TLR4-mediated sensing of C. jejuni by DCs is determined by sialylation (30). Differential effects on TLR4 triggering could not be due to variations in the lipid A moiety, since no apparent differences in the lipid A compositions of the C. jejuni strains used in this study were found by mass spectrometry. TLR4 activation can be modulated by glycan binding to lectins expressed by immature DCs (47). To test if maturation induction of immature DCs is influenced by differently linked sialic acids expressed on LOS, we measured the expression levels of maturation markers CD80, CD83, CD86, and MHCII after overnight incubation of immature DCs with LOS. E. coli LPS was used as a positive control. For all LOS concentrations, GB11 and GB16 induced similar expression levels of markers associated with DC maturation, indicating that the type of linkage of sialic acid, i.e., α2,3 or α2,8 linked, did not influence DC maturation (Fig. 3B to E). These data suggest that the presence of sialic acid as well as the linkage could modulate TLR4 activation; however, both sialylated strains induced DC maturation to similar extents. These results indicate that sialylated LOS-induced maturation is partly independent of TLR4.
Fig. 3.
LOS induces DC maturation and TLR4 activation. (A) TLR4- and MD2-transfected HEK cells were triggered with 1, 10, or 100 ng/ml LOS or E. coli LPS for 16 h. TLR4 activation was determined by analyzing the IL-8 concentration in the supernatant by ELISA. DCs were incubated overnight with indicated concentrations of LOS or E. coli LPS. (B to E) The levels of the costimulatory molecules CD80 (B), CD83 (C), CD86 (D), and MHCII (E) expressed on DCs were measured by FACS analysis. The average mean fluorescence intensity for four donors is shown.
Sialic acid linkage of LOS influences the cytokine profile of DCs.
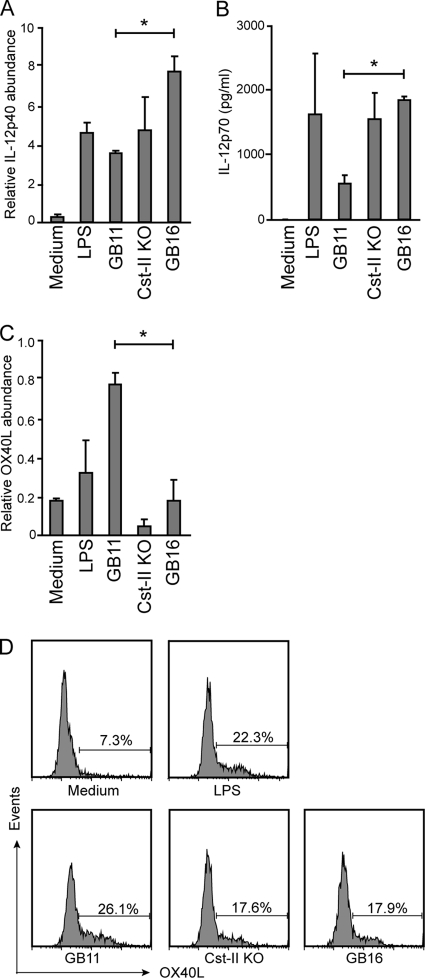
To obtain more information about the functional consequences of DC interactions with differentially sialylated LOS structures, we analyzed expression levels of IL-12p40/70 and OX40L, which are molecules linked to functional T helper 1 and T helper 2 cell polarization, respectively (14). DCs were incubated with E. coli LPS or LOS structures of GB11, the Cst-II mutant, and GB16 for 6 h, and mRNA levels of IL-12p40 were analyzed. The proinflammatory Th1-skewing cytokine IL-12p40 was strongly upregulated in DCs incubated with GB16 (Fig. 4A). Interestingly, the strong TLR4-activating GB11 displayed the lowest IL-12p40 response. These results were confirmed by ELISA, in which DCs incubated with GB16 secreted larger amounts of the Th1-skewing cytokine IL-12p70 than DCs incubated with GB11 (Fig. 4B). In contrast to this, the Th2-polarizing molecule OX40L was expressed at the highest level on DCs incubated with GB11 and was expressed at low levels on DCs incubated with GB16, as shown by RT-PCR (Fig. 4C) and flow cytometry (Fig. 4D). Together, these results predict that sialic acids present on GB11 and GB16 have differential effects on key molecules involved in DC-mediated T helper cell differentiation.
Fig. 4.
IL-12 and OX40L expression levels of DCs are dependent on sialic acid linkage of LOS. (A and C) To analyze mRNA expression levels, DCs were incubated with 100 ng/ml LPS, GB11, the Cst-II mutant, or GB16 for 6 h. Cells were lysed, and the expression of the Th1-related cytokine IL-12p40 (A) or the Th2-related molecule OX40L (C) were analyzed by real-time PCR. *, P < 0.05. (B and D) To measure IL-12p70 and OX40L expression at the protein level, DCs were incubated for 24 h with LPS, GB11, the Cst-II mutant, or GB16. (B) The secretion of Th1-related cytokine IL-12p70 was analyzed by ELISA. *, P < 0.05. (D) The expression of the Th2-related molecule OX40L on DCs was analyzed by flow cytometry (n = 4).
T helper cell differentiation by LOS-activated DCs is dependent on sialic acid linkage.
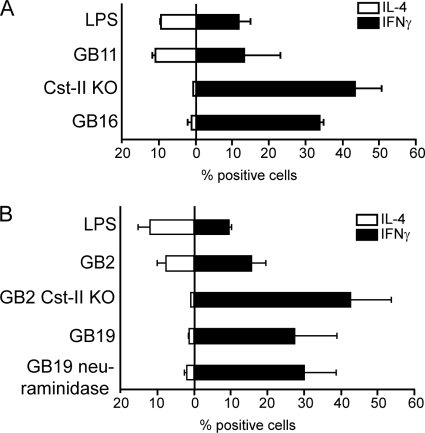
The clearly regulated expression of OX40L and IL-12p40/70 by DCs incubated with LOS suggests that the LOS structures carrying different linkages of the sialic acids which target specific siglecs would distinctly affect T helper differentiation. We therefore performed a DC-T cell coculture assay and measured by flow cytometry at day 14 the production of IFN-γ (Th1) and IL-4 (Th2) cytokines by CD4-positive T cells. As expected by the upregulation of OX40L, the α2,3-linked sialylated LOS structure GB11 induced a more pronounced Th2 response, which shifted toward Th1 differentiation when sialic acids were not present due to the mutation in the sialyltransferase gene (Cst-II KO). Surprisingly, not only the mutant but the siglec-7 binding α2,8-linked sialylated LOS structure GB16 induced a Th1 response as well, indicating that the differently linked sialic acid structures indeed induced opposite DC T helper cell differentiation, likely mediated via DC-expressed siglecs (Fig. 5A). We observed identical T helper profiles with related LOS structures from GB2 (similar to GB11 with terminal α2,3-linked sialic acid), GB19 (similar to GB16 with terminal α2,8-linked sialic acid) (18), and their corresponding structures without sialic acids (GB2 Cst-II KO and GB19 neuraminidase) (Fig. 5B). The T helper differentiation is in concordance with the cytokine expression data. In contrast, T helper polarization did not reflect the TLR4 activation, in which at the concentration of 10 ng/ml as used in the T helper differentiation assay, GB11 and GB16 showed higher TLR4 activation than LPS and Cst-II KO. In conclusion, GB16 induced a distinct T helper differentiation program compared to GB11, which is displayed by the fact that these distinct LOS structures differentially affect OX40L upregulation and IL12p70 production by DCs.
Fig. 5.
T cell polarization by LOS-triggered DCs. LOS-matured DCs with 10 ng/ml of LPS, GB11, the Cst-II mutant, and GB16 (A) or DCs matured with 10 ng/ml LPS, GB2, GB2 Cst-II KO, GB19, and GB19 sialidase (B) were incubated with naïve T cells. After 14 days, the intracellular IL-4 and IFN-γ production by T cells was measured by FACS analysis. Average percentages of positive cells for two donors are shown.
DISCUSSION
GBS is a postinfectious immune-mediated neuropathology which is probably induced by carbohydrate mimicry between C. jejuni LOS and host gangliosides, resulting in a cross-reactivity antibody response to peripheral nerves. Although GB11 and GB16 were both isolated from patients with GBS, the clinical presentations are dissimilar. The specificity of the cross-reactive antibodies found in these patients is highly associated with the type of neurological deficits, probably explained by the distribution of the gangliosides and reachability by antibodies in the human peripheral nervous system.
A recent report was the first to describe the interaction between human monocyte-derived DCs and C. jejuni (22). In agreement with our data, this study showed that interaction with C. jejuni induced upregulation of DC cell surface maturation markers and triggering of NF-κB to stimulate the production of cytokines, including IL-12. Furthermore, this study showed that purified C. jejuni LOS was the major stimulant for the increased production of cytokines by DCs (22). Similarly, murine DCs also undergo activation and induce Th1 effector cell responses against C. jejuni (39). A subsequent study showed that C. jejuni-induced activation of DCs involved cooperative signaling through the TLR4-MyD88 and TLR4-TRIF axis (38). We demonstrated that sialic acid expression by LOS is required for the induction of an efficient immune response by DCs (30). This correlates with earlier studies showing that sialic acids play an important role in DC biology (8, 9). In addition, it has been shown that LOS with terminal α2,3-linked sialic acids bind to Sn (21), whereas LOS with terminal α2,8-linked sialic acid bind siglec-7 (6). In this study we have extended these results by investigating whether the sialylated LOS structures differentially affect DC maturation and subsequent T cell polarization. Dendritic cells express various siglecs on their surface, including siglec-3, siglec-7, siglec-9, siglec-10, and siglec-15 (36). Because we were interested in siglec-mediated DC activation by α2,3- and α2,8-sialylated LOS structures, which have been demonstrated before to bind to Sn and siglec-7, respectively (6, 21), we measured Sn and siglec-7 expression on our human monocyte-derived DCs. On immature DCs we detected siglec-7 expression, whereas Sn was detected on mature DCs only, as demonstrated previously (28). With anti-siglec-7 antibodies we could completely block the binding of α2,8-linked sialylated structures to DCs (Fig. 2E). We could not detect α2,3-linked sialylated PAA binding to DCs even after neuraminidase treatment or LPS maturation followed by neuraminidase treatment. The effect of GB11 on DCs could be explained by a higher affinity of Sn to GB11 due to composition of the underlying glycan structure, which is different in GB11 compared to the PAA probe. Furthermore, the lipid A tail of GB11 is likely required for DC modulation. In addition to our data (Fig. 2A and C), a recent study confirmed Sn binding to α2,3-sialylated LOS structures (21). However, we were not able to block GB11-induced DC maturation with anti-Sn antibodies (data not shown), suggesting that GB11 in addition to Sn may bind other DC-expressed lectins. Since DCs express more lectins with a binding preference for α2,3-linked sialylated structures, including siglec-9 (36), we cannot exclude that other DC-expressed lectins are responsible for the GB11-induced DC activation. Siglec-7 carries an inhibitory ITIM motif in its cytoplasmic tail. The possibility that LOS binds to siglec-7 and modulates subsequent cellular signaling needs to be further determined in future experiments in which phosphorylation of the ITIM motif as well as activation of signaling proteins downstream of siglec-7 could be analyzed. Sn has no internalization motif, although Sn can mediate endocytosis of bacteria, possibly in synergy with other receptors (26).
As described previously, the sialic acid binding sites of siglecs expressed on several cell types are masked by endogenous ligands (40). Likewise, we found low binding of sialylated PAA structures to untreated DCs, whereas upon neuraminidase treatment, high binding of sialylated PAA structures was observed. When non-neuraminidase-treated DCs were incubated with GB16, we did find a Th1-skewed phenotype of DCs and a subsequent Th1 response, suggesting abolishment of the cis interactions of siglec-7 with its ligand. The incubation of α2,8-sialylated PAA glycans could not abolish this cis interaction (Fig. 2E), indicating that the conformation of the sialic acid with its underlying glycan and protein is not strong enough to compete for sialylated cis ligands. In contrast, the underlying glycan structure in combination with the lipid A part of GB16 LOS could be a better competitor and thus induce trans interactions with siglec-7. In vivo, unmasking of DC-expressed siglecs can occur when sialic acids on bacteria outcompete cis interactions to mediate trans recognition, depending on avidity effects. In addition, other mechanisms could be involved in disrupting the cis interactions of siglec-7, such as binding of the LOS lipid A moiety to TLR4 to serve as a scaffold to recruit siglec-7. In a wide range of bacteria, intrinsic sialidase activity has been described, especially in strains isolated from various infections (11). Likewise, C. jejuni might possess sialidase activity and thereby unmask DC-expressed siglecs to create their own binding sites and thus modulate DC function.
Although C. jejuni binding to siglecs has been observed in previous studies, the effects of these interactions on immune responses are poorly understood. As DCs are siglec-expressing cells which can be programmed to promote Th1 responses or Th2 responses, we investigated the interaction of C. jejuni-expressed LOS with DCs for subsequent T cell polarization. Interestingly, we found that activation of DCs with GB11, targeting lectins that bind α2,3-linked sialic acids, induced Th2 skewing. In contrast, targeting α2,8-linked sialic acid binding siglecs or lectins capable of binding galactose with unsialylated LOS induced Th1 skewing. Unfortunately, we were not able to inhibit cytokine and T helper responses by adding siglec-7 blocking antibody in our assays. The antibody was suitable only for blocking binding assays (Fig. 2E), as it worked in an agonistic manner in cellular assays, causing siglec-7 signaling. The T helper skewing could help to explain the differences in pathological conditions observed between patients after infection with LOS structures mimicking GD1a/GM1a gangliosides (GB11) or with LOS structures mimicking GD1c gangliosides (GB16), as the nature of the T helper response determines the class switching in mature B cells and thus the isotype of the antibodies produced. We used highly purified LOS, so it is unlikely that our LOS preparations contain contaminants that could trigger other pattern recognition receptors (PRRs) on DCs. In addition, we found that TLR2 (data not shown) and TLR4 (Fig. 3A) activation by the sialylated LOS structures did not reflect the T cell polarization data. This finding was in agreement with a study by Al-Sayeqh et al. in which they showed that C. jejuni activates NF-κB independently of TLR4 (1).
In conclusion, we found that the molecules IL-12 and OX40L, which are involved in DC-mediated Th differentiation, displayed clear opposite expression levels when α2,3- and α2,8-linked sialylated LOS structures were compared. Accordingly, GB11 with α2,3-linked sialic acid promoted Th2 responses, whereas the LOS structure GB16, with α2,8-linked terminal sialic acid, induced a Th1 response. Together, these data indicate that besides the presence of cross-reactive antibodies, the glycan structure of LOS and especially the linkage of the terminal sialic acid may affect the functional properties of DCs in T cell polarization and could thus contribute to the different mechanisms of disease involved in GBS.
ACKNOWLEDGMENTS
We thank A. J. van Beelen for helpful suggestions.
This work was supported by V-ICI Ph.D. student grant 2005, by an Mrace grant from Erasmus MC, by the Netherlands Organization for Health Research and Development (NWO) (TOP-grant 9120.6150, NWO-VENI 863.08.020), and by MS Research Stichting 05-598.
Footnotes
Published ahead of print on 18 April 2011.
REFERENCES
- 1. Al-Sayeqh A. F., Loughlin M. F., Dillon E., Mellits K. H., Connerton I. F. 2010. Campylobacter jejuni activates NF-kappaB independently of TLR2, TLR4, Nod1 and Nod2 receptors. Microb. Pathog. 49:294–304 [DOI] [PubMed] [Google Scholar]
- 2. Ang C. W., Jacobs B. C., Laman J. D. 2004. The Guillain-Barre syndrome: a true case of molecular mimicry. Trends Immunol. 25:61–66 [DOI] [PubMed] [Google Scholar]
- 3. Angata T., Brinkman-Van der Linden E. 2002. I-type lectins. Biochim. Biophys. Acta 1572:294–316 [DOI] [PubMed] [Google Scholar]
- 4. Angata T., Varki A. 2002. Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem. Rev. 102:439–469 [DOI] [PubMed] [Google Scholar]
- 5. Avril T., Floyd H., Lopez F., Vivier E., Crocker P. R. 2004. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J. Immunol. 173:6841–6849 [DOI] [PubMed] [Google Scholar]
- 6. Avril T., Wagner E. R., Willison H. J., Crocker P. R. 2006. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect. Immun. 74:4133–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banchereau J., Steinman R. M. 1998. Dendritic cells and the control of immunity. Nature 392:245–252 [DOI] [PubMed] [Google Scholar]
- 8. Bax M., et al. 2007. Dendritic cell maturation results in pronounced changes in glycan expression affecting recognition by siglecs and galectins. J. Immunol. 179:8216–8224 [DOI] [PubMed] [Google Scholar]
- 9. Bax M., van Vliet S. J., Litjens M., Garcia-Vallejo J. J., van Kooyk Y. 2009. Interaction of polysialic acid with CCL21 regulates the migratory capacity of human dendritic cells. PLoS One 4:e6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carlin A. F., Lewis A. L., Varki A., Nizet V. 2007. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J. Bacteriol. 189:1231–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corfield T. 1992. Bacterial sialidases—roles in pathogenicity and nutrition. Glycobiology 2:509–521 [DOI] [PubMed] [Google Scholar]
- 12. Crocker P. R., Paulson J. C., Varki A. 2007. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7:255–266 [DOI] [PubMed] [Google Scholar]
- 13. Crocker P. R., Redelinghuys P. 2008. Siglecs as positive and negative regulators of the immune system. Biochem. Soc. Trans. 36:1467–1471 [DOI] [PubMed] [Google Scholar]
- 14. de Jong E. C., et al. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J. Immunol. 168:1704–1709 [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Vallejo J. J., et al. 2004. Approach for defining endogenous reference genes in gene expression experiments. Anal. Biochem. 329:293–299 [DOI] [PubMed] [Google Scholar]
- 16. Gilbert M., et al. 2000. Biosynthesis of ganglioside mimics in Campylobacter jejuni OH4384. Identification of the glycosyltransferase genes, enzymatic synthesis of model compounds, and characterization of nanomole amounts by 600-mhz (1)h and (13)c NMR analysis. J. Biol. Chem. 275:3896–3906 [DOI] [PubMed] [Google Scholar]
- 17. Godschalk P. C., et al. 2004. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barre syndrome. J. Clin. Invest. 114:1659–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Godschalk P. C., et al. 2007. Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barre and Miller Fisher syndromes. Infect. Immun. 75:1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hargreaves D. C., Medzhitov R. 2005. Innate sensors of microbial infection. J. Clin. Immunol. 25:503–510 [DOI] [PubMed] [Google Scholar]
- 20. Hartnell A., et al. 2001. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 97:288–296 [DOI] [PubMed] [Google Scholar]
- 21. Heikema A. P., et al. 2010. Characterization of the specific interaction between sialoadhesin and sialylated Campylobacter jejuni lipooligosaccharides. Infect. Immun. 78:3237–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu L., Bray M. D., Osorio M., Kopecko D. J. 2006. Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect. Immun. 74:2697–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hudson S. A., Bovin N. V., Schnaar R. L., Crocker P. R., Bochner B. S. 2009. Eosinophil-selective binding and proapoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6′-sulfated sialyl Lewis x. J. Pharmacol. Exp. Ther. 330:608–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacobs B. C., Hazenberg M. P., van Doorn P. A., Endtz H. P., van der Meche F. G. 1997. Cross-reactive antibodies against gangliosides and Campylobacter jejuni lipopolysaccharides in patients with Guillain-Barre or Miller Fisher syndrome. J. Infect. Dis. 175:729–733 [DOI] [PubMed] [Google Scholar]
- 25. Jacobs B. C., et al. 1996. Campylobacter jejuni infections and anti-GM1 antibodies in Guillain-Barre syndrome. Ann. Neurol. 40:181–187 [DOI] [PubMed] [Google Scholar]
- 26. Jones C., Virji M., Crocker P. R. 2003. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol. Microbiol. 49:1213–1225 [DOI] [PubMed] [Google Scholar]
- 27. Kelm S., Schauer R. 1997. Sialic acids in molecular and cellular interactions. Int. Rev. Cytol. 175:137–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirchberger S., et al. 2005. Human rhinoviruses inhibit the accessory function of dendritic cells by inducing sialoadhesin and B7-H1 expression. J. Immunol. 175:1145–1152 [DOI] [PubMed] [Google Scholar]
- 29. Koga M., Takahashi M., Masuda M., Hirata K., Yuki N. 2005. Campylobacter gene polymorphism as a determinant of clinical features of Guillain-Barre syndrome. Neurology 65:1376–1381 [DOI] [PubMed] [Google Scholar]
- 30. Kuijf M. L., et al. 2010. TLR4-mediated sensing of Campylobacter jejuni by dendritic cells is determined by sialylation. J. Immunol. 185:748–755 [DOI] [PubMed] [Google Scholar]
- 31. Latz E., et al. 2002. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the Toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J. Biol. Chem. 277:47834–47843 [DOI] [PubMed] [Google Scholar]
- 32. Lock K., Zhang J., Lu J., Lee S. H., Crocker P. R. 2004. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology. 209:199–207 [DOI] [PubMed] [Google Scholar]
- 33. McMillan S. J., Crocker P. R. 2008. CD33-related sialic-acid-binding immunoglobulin-like lectins in health and disease. Carbohydr. Res. 343:2050–2056 [DOI] [PubMed] [Google Scholar]
- 34. Mosmann T. R., Coffman R. L. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145–173 [DOI] [PubMed] [Google Scholar]
- 35. Nicoll G., et al. 1999. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem. 274:34089–34095 [DOI] [PubMed] [Google Scholar]
- 36. O'Reilly M. K., Paulson J. C. 2009. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol. Sci. 30:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rapoport E., Mikhalyov I., Zhang J., Crocker P., Bovin N. 2003. Ganglioside binding pattern of CD33-related siglecs. Bioorg. Med. Chem. Lett. 13:675–678 [DOI] [PubMed] [Google Scholar]
- 38. Rathinam V. A., Appledorn D. M., Hoag K. A., Amalfitano A., Mansfield L. S. 2009. Campylobacter jejuni-induced activation of dendritic cells involves cooperative signaling through Toll-like receptor 4 (TLR4)-MyD88 and TLR4-TRIF axes. Infect. Immun. 77:2499–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rathinam V. A., Hoag K. A., Mansfield L. S. 2008. Dendritic cells from C57BL/6 mice undergo activation and induce Th1-effector cell responses against Campylobacter jejuni. Microbes Infect. 10:1316–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Razi N., Varki A. 1999. Cryptic sialic acid binding lectins on human blood leukocytes can be unmasked by sialidase treatment or cellular activation. Glycobiology 9:1225–1234 [DOI] [PubMed] [Google Scholar]
- 41. Reis e Sousa C. 2004. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin. Immunol. 16:27–34 [DOI] [PubMed] [Google Scholar]
- 42. Reis e Sousa C. 2006. Dendritic cells in a mature age. Nat. Rev. Immunol. 6:476–483 [DOI] [PubMed] [Google Scholar]
- 43. Sallusto F., Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Snijders A., et al. 1996. Regulation of bioactive IL-12 production in lipopolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. J. Immunol. 156:1207–1212 [PubMed] [Google Scholar]
- 45. Trent M. S., Stead C. M., Tran A. X., Hankins J. V. 2006. Diversity of endotoxin and its impact on pathogenesis. J. Endotoxin Res. 12:205–223 [DOI] [PubMed] [Google Scholar]
- 46. Van Belkum A., et al. 2001. A Campylobacter jejuni gene associated with immune-mediated neuropathy. Nat. Med. 7:752–753 [DOI] [PubMed] [Google Scholar]
- 47. van Vliet S. J., Garcia-Vallejo J. J., van Kooyk Y. 2008. Dendritic cells and C-type lectin receptors: coupling innate to adaptive immune responses. Immunol. Cell Biol. 86:580–587 [DOI] [PubMed] [Google Scholar]
- 48. Varki A., Angata T. 2006. Siglecs—the major subfamily of I-type lectins. Glycobiology 16:1R–27R [DOI] [PubMed] [Google Scholar]
- 49. Yamaji T., Teranishi T., Alphey M. S., Crocker P. R., Hashimoto Y. 2002. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha 2,8-disialyl and branched alpha 2,6-sialyl residues. A comparison with Siglec-9. J. Biol. Chem. 277:6324–6332 [DOI] [PubMed] [Google Scholar]
- 50. York M. R., et al. 2007. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and Toll-like receptor agonists. Arthritis Rheum. 56:1010–1020 [DOI] [PubMed] [Google Scholar]
- 51. Zhu J., Paul W. E. 2008. CD4 T cells: fates, functions, and faults. Blood 112:1557–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]