Abstract
Toll-like receptors (TLRs) play a central role in macrophage activation and control of parasitic infections. Their contribution to the outcome of Leishmania infection is just beginning to be deciphered. We examined the interaction of Leishmania panamensis with TLRs in the activation of host macrophages. L. panamensis infection resulted in upregulation of TLR1, TLR2, TLR3, and TLR4 expression and induced tumor necrosis factor alpha (TNF-α) secretion by human primary macrophages at comparable levels and kinetics to those of specific TLR ligands. The TLR dependence of the host cell response was substantiated by the absence of TNF-α production in MyD88/TRIF−/− murine bone marrow-derived macrophages and mouse macrophage cell lines in response to promastigotes and amastigotes. Systematic screening of TLR-deficient macrophages revealed that TNF-α production was completely abrogated in TLR4−/− macrophages, consistent with the increased intracellular parasite survival at early time points of infection. TNF-α secretion was significantly reduced in macrophages lacking endosomal TLRs but was unaltered by a lack of TLR2 or MD-2. Together, these findings support the participation of TLR4 and endosomal TLRs in the activation of host macrophages by L. panamensis and in the early control of infection.
INTRODUCTION
Protozoan parasites of the genus Leishmania are obligate intracellular pathogens that infect and impact the lives of millions of people worldwide. Leishmania species induce a broad spectrum of pathologies in humans, ranging from cutaneous lesions to progressive fatal visceral disease (43). Although the determinants of pathogenesis remain unclear, the host immune response and the infecting Leishmania species contribute to the diverse clinical manifestations (57).
Macrophages act as the principal host cells for Leishmania, playing a central role in the development of the immune response to these parasites via antigen presentation, secretion of microbicidal products, and production of inflammatory mediators (cytokines and chemokines). These early events during host cell-parasite interaction can ultimately determine the outcome of infection (9). Previous studies of naturally infected humans have provided evidence of differences in the innate and acquired immune responses to Leishmania (Viannia) subspecies associated with the clinical outcome of infection. Macrophages from naturally exposed individuals presenting recurrent or chronic disease (clinical susceptibility) were more permissive to in vitro infection than cells from individuals with subclinical infection (clinical resistance) (9, 52).
Experimental studies have revealed that a diverse repertoire of receptors is involved in Leishmania-macrophage interactions (5, 11, 37, 39); nevertheless, the relationships between these receptors, host cell activation, and outcome of infection remain largely unknown. Toll-like receptors (TLRs) provide a link between innate and adaptive immunity, allowing recognition of a wide range of microbial products, including those of protozoan pathogens (22, 24, 35). TLR-ligand interaction initiates signal transduction through the adaptor proteins MyD88 and/or TRIF, resulting in downstream activation of NF-κB and interferon (IFN) regulatory factors and the subsequent transcription of inflammatory mediators such as tumor necrosis factor alpha (TNF-α), type I interferons, and interleukin-12 (IL-12) and of the costimulatory molecules CD80 and CD86 in monocytes/macrophages and dendritic cells (DCs).
Gene knockout studies with mice have revealed a critical role for TLR signaling in the immune response against Leishmania parasites (32, 60). MyD88−/− and TLR4−/− C57BL/6 mice were more susceptible to disease following Leishmania major infection (17, 19, 30, 31, 42), and depletion of TLR9 impaired NK cell activation in murine models of both visceral leishmaniasis caused by L. infantum and cutaneous leishmaniasis produced by L. major (33, 55). A link between TLRs and microbicidal capacity was evidenced by the requirement of TLR2 and TLR3 for intracellular killing of L. donovani organisms in IFN-γ-primed macrophages (21). Notably, the absence of TLR2 was recently shown to promote IL-12 production by DCs during L. braziliensis infection, suggesting that this receptor may also play a negative regulatory role (61).
Although knowledge of the nature and identity of Leishmania molecules that interact with individual TLRs is limited, some parasite ligands and cognate TLR receptors on different cells participating in the immune response have been described. L. major lipophosphoglycan (LPG) was shown to activate human NK cells and murine monocytes through TLR2 (3, 19) and has been implicated in the induction of nitric oxide (NO) production by human peripheral blood mononuclear cells (PBMCs) via TLR2 (27). Additionally, the P8 proteoglyco- lipid complex (P8 PGLC) expressed by L. pifanoi was recently shown to promote macrophage activation through TLR4 (62), and L. major DNA was shown to activate DCs through TLR9 and thereby to promote IFN-γ production by CD4+ T cells (1). The implications of these findings and the participation of TLR signaling in the onset of the immune response during human infections with species of the Leishmania (Viannia) subgenus have yet to be determined.
Th1/Th2 polarization does not explain the clinical outcome of human infections with Leishmania (Viannia) species, which have a propensity to produce latent infection and relapsing (2) and chronic disease. Early in vitro responses are characterized by a mixed profile of proinflammatory (IFN-γ and TNF-α) and anti-inflammatory (IL-10 and IL-13) cytokine production (20). Refractory disease presentations are often accompanied by exuberant Th1 responses, including increased TNF-α production, which has been implicated in pathogenesis (54). In this study, we present evidence that TLRs are involved in both activation and secretion of TNF-α by human and murine macrophages infected by L. panamensis and participate in the early control of infection.
MATERIALS AND METHODS
Parasites.
L. panamensis (MHOM/CO/86/1166) promastigotes were cultured in Schneider's Drosophila medium (Sigma) supplemented with 20% heat-inactivated fetal bovine serum (HIFBS) (Gibco) at 25°C. Luciferase- and green fluorescent protein (GFP)-expressing promastigotes (L.p-Luc and L.p-GFP) were grown in medium supplemented with 50 mg/ml of G418 (Gibco). Promastigotes were harvested during stationary phase and opsonized for 1 h at 34°C in RPMI 1640 (Gibco) supplemented with 10% heat-inactivated human AB serum when indicated. Live intracellular amastigotes were obtained by infection of differentiated adherent U937 cells with opsonized promastigotes (50:1 parasite-to-cell ratio) for 5 days at 34°C and 5% CO2. After infection, cells were lysed by passage through a 27G needle, and amastigotes were recovered by centrifugation over a Percoll density gradient (Amersham Biosciences).
Macrophages.
Human promonocytic U-937 cells were cultured as previously described (52). Cell differentiation was induced by phorbol 12-myristate 13-acetate (PMA) stimulation (100 ng/ml) (Sigma) 4 days prior to infection. Immortalized macrophage cell lines generated from wild-type (WT), MyD88/TRIF−/−, MyD88−/−, TRIF−/−, TLR2−/−, TLR4−/−, and UNC93b mutant C57BL/6 mice were cultured as previously described (23). Clonal lines were tested for the induction of TNF-α and IL-6 in response to a panel of TLR ligands, including synthetic lipopeptide, polyinosinic-poly(C) [poly(I:C)], lipopolysaccharide (LPS), resiquimod, and CpG DNA. In all cases, the predicted responses, based on the known responses of native bone marrow-derived macrophages (BMMs), were observed (e.g., the TLR4 knockout responded to all TLR ligands except for LPS). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Cellgro) supplemented with 10% HIFBS and 10 μg of ciprofloxacin/ml (Bayer). Cells were plated and stimulated with TLR ligands or infected with L. panamensis parasites as described in the text.
All mice were housed and bred under pathogen-free conditions. Bone marrow was harvested from mice as previously described (62), in accordance with the guidelines set forth by the University of Massachusetts Medical School and approved by its Institutional Animal Care and Use Committee. BMM precursors from WT (C57BL/6), MD-2−/−, MyD88/TRIF−/−, MyD88−/−, TRIF−/−, or TLR4−/− mice were differentiated in culture for 9 to 10 days in the presence of conditioned medium (DMEM supplemented with 20% L929 cell culture supernatant, 10% HIFBS, and 10 μg/ml ciprofloxacin) prior to use.
Isolation and culture of human monocyte-derived macrophages.
Sixty milliliters of peripheral blood was obtained from healthy adult volunteers in accordance with the protocol and with informed consent forms approved by the Institutional Review Board of the Centro Internacional de Entrenamiento e Investigaciones Medicas (CIDEIM). PBMCs were obtained from peripheral blood by Ficoll-Hypaque 1077 (Sigma) density gradient centrifugation. Monocytes were allowed to differentiate into macrophages by culture for 7 days at 37°C and 5% CO2 in complete RPMI (Sigma) following adherence to plates coated with 2% sterile endotoxin-free gelatin and autologous human serum. All plasticware, glassware, and water used for cell culture were endotoxin free.
L. panamensis infection and TLR ligand stimulation.
Primary human macrophages were stimulated for 8 h with LPS (1 μg/ml), zymosan (25 μg/ml), Pam2CSK4 (10 μg/ml), poly(I:C) (20 μg/ml), or CpG ODN 2216 (5 μM) (Invivogen) or infected with opsonized L. panamensis promastigotes at a 5:1 parasite-to-cell ratio. Infection was allowed to proceed for 1 h in FBS-free medium at 34°C and 5% CO2. Extracellular parasites were removed by washing with Dulbecco's phosphate-buffered saline (DPBS), and cells were incubated for 8 h at 34°C and 5% CO2.
Mouse macrophage cell lines and BMMs were infected with L. panamensis promastigotes and amastigotes at 50:1 to 200:1 parasite-to-cell ratios for 1 h, washed with DPBS, and incubated at 34°C and 5% CO2. These parasite-to-cell ratios, using serum-opsonized as well as nonopsonized parasites, were chosen to optimize infection and readout based on prior dose-response experiments. Supernatants were collected and TNF-α was evaluated at 24 h postinfection. Stimulations with Pam2CSK4 (4 μM), poly(I:C) (100 μg/ml), CpG (5 μM), and LPS (1 μg/ml) or with the non-TLR ligand Sendai virus (500 U/ml) were used as positive and negative controls, as indicated. The TLR4/MD-2 antagonist Eritoran (10 μg/ml) (Eisai Research Institute) was used to discriminate activation attributable to endotoxin contamination. Infection was evaluated in Giemsa-stained cells by microscopy.
WT and TLR4−/− macrophages were seeded on glass coverslips and infected with L.p-GFP promastigotes at a 20:1 parasite-to-cell ratio to visualize parasite internalization by confocal microscopy (Leica SP2 AOBS confocal microscope). Cell membranes were stained with CellMask Deep Red stain (Invitrogen).
Fluorescence-activated cell sorter (FACS) analysis.
Human macrophages were harvested, incubated with 10 μg of nonimmune human IgG (BD Biosciences) to block nonspecific binding to FcγR, and then incubated with phycoerythrin (PE)-labeled monoclonal anti-TLR1 (clone GD2.F4), anti-TLR2 (clone TL2.1), or anti-TLR4 (clone HTA125) antibody. Fluorescein isothiocyanate (FITC)-labeled anti-CD14 was used as a monocyte population marker. For intracellular staining, cells were fixed and permeabilized with Cytofix/Cytoperm solution, washed with PermWash solution (BD Biosciences), and incubated with monoclonal anti-TLR3 (clone TLR3.7) or anti-TLR9 (eB72-1665). Cells were fixed with 2% paraformaldehyde. PE-IgG1κ, PE-mouse IgG2a, and PE-mouse IgG1 were used as isotype controls. All antibodies were purchased from eBiosciences. Cells were evaluated using a FACSCalibur flow cytometer (BD Biosciences) and were analyzed with WinMDI 2.9 software (Scripps Research Institute).
Quantification of TNF-α.
An enzyme-linked immunosorbent assay (ELISA) to detect human TNF-α was performed using antibodies from BD Pharmingen following the manufacturer's instructions. Mouse TNF-α was quantified by ELISA with a TNF-α DuoSet ELISA kit (R&D) following the manufacturer's instructions. All samples were evaluated in duplicate.
Luciferase assay.
L.p-Luc promastigotes were used to infect macrophages for 1 h. Noninternalized parasites were removed by washing, and macrophages were further incubated for 4, 16, 24, and 36 h. Cells were lysed and analyzed using a dual-luciferase reporter assay system (Promega) according to the manufacturer's instructions.
Statistical analysis.
Differences in paired groups were evaluated by Student's t test or the Wilcoxon rank test. For group comparisons, the Kruskal-Wallis test was used with Dunn's adjustment. Analyses were performed with GraphPad Prism 5 software (GraphPad Inc., San Diego, CA), and P values of <0.05 were considered significant.
RESULTS
L. panamensis promastigotes induce TNF-α production and increased expression of TLR1, TLR2, TLR3, and TLR4 by human macrophages.
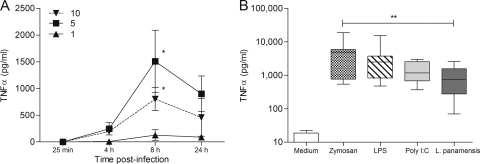
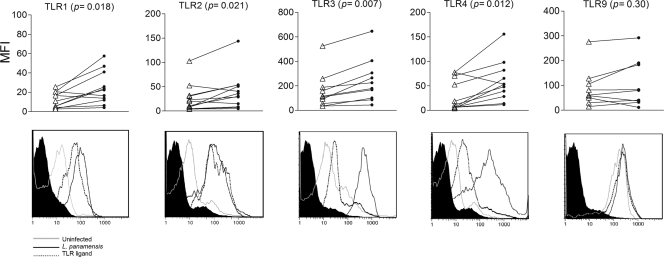
TNF-α production was used as an early readout of TLR-dependent macrophage activation (reviewed in reference 59). TNF-α secretion following exposure to live promastigotes peaked at 8 h and decreased by 24 h (Fig. 1A). The kinetics and levels of production of TNF-α by human macrophages in response to L. panamensis and specific TLR ligands were comparable (Fig. 1B), consistent with TLR-dependent activation of this host cell by L. panamensis. Stimulation of human macrophages with TLR ligands induced increased expression of the corresponding TLRs (Fig. 2, bottom panels, and data not shown). Similarly, coculture with live L. panamensis significantly increased the cell surface expression of TLR1, TLR2, and TLR4 and the intracellular expression of TLR3, but not TLR9, compared to that by unexposed macrophages (Fig. 2).
Fig. 1.
L. panamensis infection induces TNF-α production at comparable levels and kinetics to those with specific TLR ligands. (A) Kinetics of TNF-α production in human primary macrophages exposed to different parasite-to-cell ratios, with maximum cytokine secretion at 8 h postinfection. Data are representative of cytokine production by macrophages of 10 individuals and are expressed as means ± standard errors of the means (SEM). (B) Production of TNF-α in human primary macrophages from 10 different donors was measured by ELISA. Cells were stimulated with zymosan, LPS, or poly(I:C), infected for 8 h with promastigotes at a 5:1 parasite-to-macrophage ratio, or left untreated as a control. Data are expressed as means ± standard deviations (SD). *, P < 0.05; **, P < 0.01.
Fig. 2.
L. panamensis induces increased expression of TLRs 1, 2, 3, and 4 in human macrophages. Human macrophages were cultured without treatment, infected with L. panamensis promastigotes at a 5:1 parasite-to-macrophage ratio, or stimulated with zymosan (TLR1), Pam2CSK4 (TLR2), poly(I:C) (TLR3), LPS (TLR4), or CpG (TLR9). TLR expression was evaluated by flow cytometry after 8 h of exposure to ligands or promastigotes. (Top) Mean fluorescence intensities (MFI) of TLR expression from uninfected (empty triangles) and infected (filled circles) macrophages from 12 different individuals. P values are indicated. (Bottom) Representative histograms of TLR expression of uninfected macrophages and macrophages exposed to specific TLR ligands or L. panamensis promastigotes. Cells were gated on the CD14+ population.
L. panamensis promastigotes and amastigotes activate TNF-α production through TLRs.
Based on the results supporting the involvement of TLRs in the L. panamensis-human macrophage interaction, we investigated the participation of specific TLRs and adaptor molecules in cell activation. Human HEK293 cells stably transfected with individual human TLRs and an NF-κB-luciferase reporter construct were used as a surrogate model of infection and cell activation. However, HEK293 cells deteriorated during coculture with L. panamensis promastigotes, leading us to examine specific TLR activation in C57BL/6-derived macrophage cell lines.
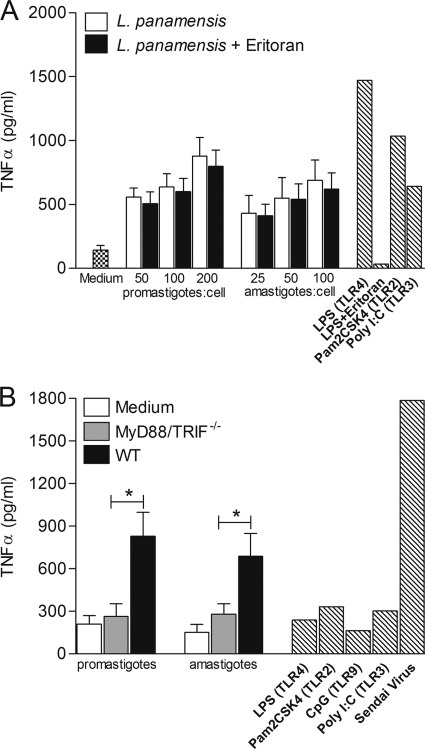
TNF-α production was induced in a dose-dependent manner in the WT C57BL/6 macrophage cell line by both promastigotes and amastigotes of L. panamensis over a wide dose range of parasites (Fig. 3 A). In contrast, neither parasite stage induced TNF-α production in the MyD88/TRIF−/− double-knockout macrophage cell line, in which all TLRs are nonfunctional (Fig. 3B), supporting the involvement of TLR-dependent signaling during macrophage activation by L. panamensis. TNF-α secretion levels induced by opsonized and nonopsonized parasites were indistinguishable (Fig. 3A and data not shown), ruling out cell activation attributable to interactions with serum opsonins in this experimental system.
Fig. 3.
L. panamensis promastigotes and amastigotes induce TNF-α production in a dose-dependent manner and activate macrophages through TLR-dependent signaling. (A) TNF-α production by WT macrophages exposed to increasing parasite-to-cell ratios, using opsonized promastigotes or amastigotes, specific TLR ligands (hatched bars), or no treatment. (B) MyD88/TRIF−/− cells were infected at a 100:1 parasite-to-macrophage ratio. Data are representative of three independent experiments and are expressed as means ± SEM. *, P < 0.05.
MD-2, a small protein that together with TLR4 forms the LPS signaling complex, is an absolute requirement for cells to respond to LPS (56). Eritoran (previously known as B1287) binds to the lipid A binding site of MD-2 and is a potent inhibitor of LPS-mediated activation. Eritoran did not impair induction of TNF-α by L. panamensis, whereas induction by LPS was blocked (Fig. 3A), thereby establishing that the macrophage response to L. panamensis could not be attributable to LPS contamination.
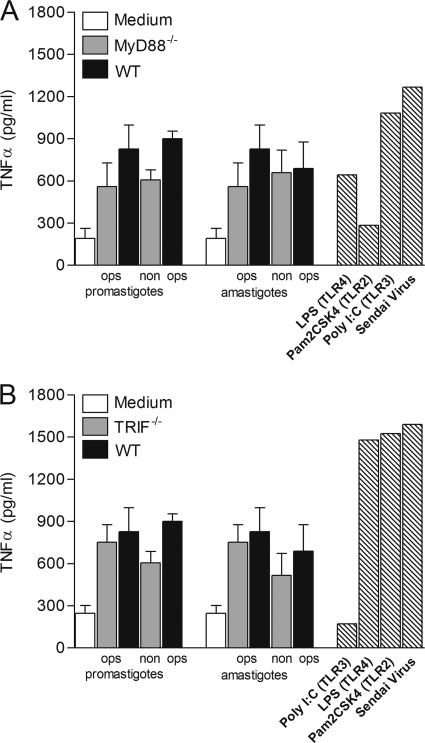
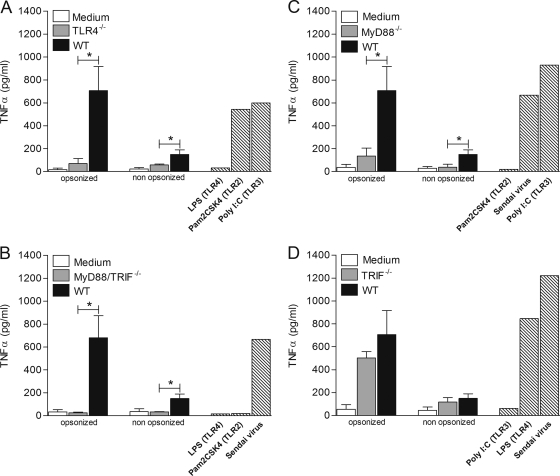
To dissect the TLR signaling pathway activated by L. panamensis in macrophages, we examined the responses of MyD88−/− and TRIF−/− single-knockout macrophages to opsonized and nonopsonized parasites. In contrast to the complete abrogation of TNF-α secretion in MyD88/TRIF−/− double-knockout macrophages cocultured with L. panamensis, specific ablation of either MyD88 or TRIF alone did not significantly alter TNF-α production (Fig. 4 A and B). Similar results were obtained with opsonized and nonopsonized parasites. These results indicate that both MyD88 and TRIF are required to fully induce TNF-α production by L. panamensis in this experimental system.
Fig. 4.
MyD88 or TRIF molecules are required for induction of TNF-α production during L. panamensis infection. TNF-α production was measured in MyD88−/− (A) and TRIF−/− (B) macrophages cocultured with opsonized or nonopsonized L. panamensis promastigotes and amastigotes at a 100:1 parasite-to-macrophage ratio, stimulated with specific TLR ligands or Sendai virus (hatched bars), or left untreated. Data are from three independent experiments and are expressed as means ± SEM.
Endosomal TLRs participate in macrophage activation by L. panamensis infection.
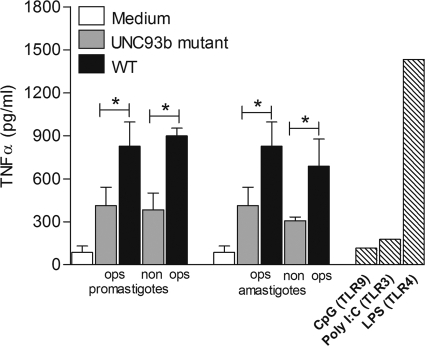
UNC93b is an endoplasmic reticulum (ER) membrane protein required for signaling through endolysosomal TLRs (10) by controlling the trafficking of TLRs 3, 7, 8, and 9 from the ER to the endolysosomal compartment. Hence, UNC93b mutant cells do not respond to the corresponding TLR ligands (28). To investigate the role of the endolysosomal TLRs during L. panamensis infection, we examined the activation of macrophages expressing a nonfunctional form of UNC93b (58). Induction of TNF-α production by both L. panamensis promastigotes and amastigotes was significantly reduced, though not completely abrogated, compared to that by WT cells (Fig. 5), suggesting that signaling through the endolysosomal TLRs also contributes to macrophage activation during L. panamensis infection.
Fig. 5.
Endolysosomal TLRs play a role in the induction of TNF-α production during L. panamensis infection. TNF-α production was measured in UNC93b-deficient macrophages cocultured with L. panamensis promastigotes or amastigotes at a 100:1 parasite-to-macrophage ratio, stimulated with specific intracellular TLR ligands or LPS (hatched bars), or left untreated (medium). Data are from three independent experiments and are expressed as means ± SEM. *, P < 0.05.
TLR4 mediates L. panamensis recognition by macrophages.
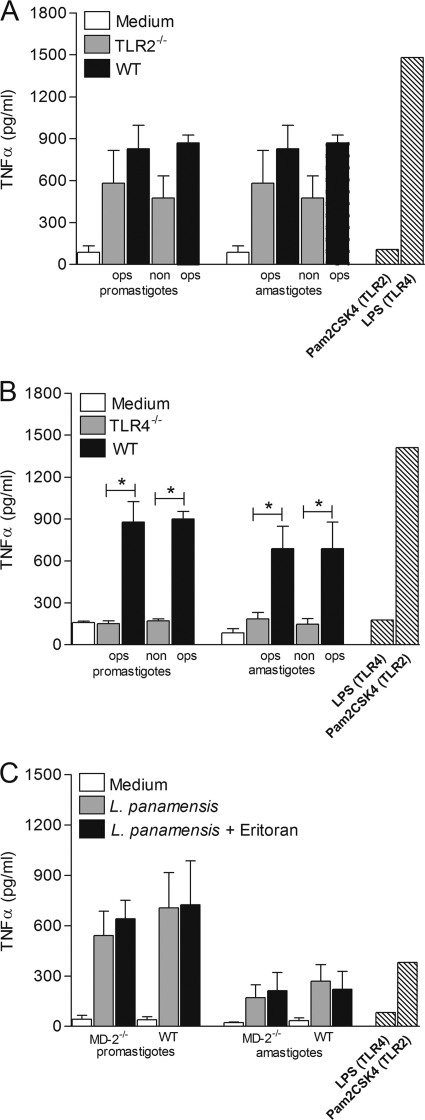
We next examined the induction of TNF-α production in TLR2−/− and TLR4−/− macrophage cell lines. As shown in Fig. 6 A, no significant change in TNF-α secretion was observed in TLR2−/− macrophages compared to WT cells, suggesting that TLR2 is not critical for L. panamensis-mediated macrophage activation. In contrast, TNF-α production was abrogated in TLR4−/− macrophages following infection with either opsonized or nonopsonized promastigotes or amastigotes (Fig. 6B). These results indicate that TLR4, but not TLR2, has an important role in the induction of proinflammatory cytokine secretion by macrophages during infection by L. panamensis.
Fig. 6.
L. panamensis induces TNF-α production by macrophages through TLR4 but not TLR2. TNF-α production was measured in TLR2−/− (A) and TLR4−/− (B) macrophage cell lines and in differentiated MD-2−/− BMMs (from C57BL/6 mice) (C). BMMs were left untreated (medium) or cocultured with L. panamensis promastigotes and amastigotes at a 100:1 parasite-to-macrophage ratio or were stimulated with specific TLR ligands or LPS alone or in combination with Eritoran (hatched bars). Figures summarize three independent experiments and are expressed as means ± SEM. *, P < 0.05.
BMMs from MD-2-deficient C57BL/6 mice were used to confirm the specificity of L. panamensis-mediated macrophage activation via TLR4 and to rule out unequivocally any activation attributable to endotoxin contamination. Both promastigotes and amastigotes induced similar and significant levels of TNF-α production in MD-2−/− and WT BMMs, confirming that macrophage activation through TLR4 was induced by L. panamensis infection (Fig. 6C), not by LPS contamination.
TLR4 is required for induction of leishmanicidal activity of macrophages.
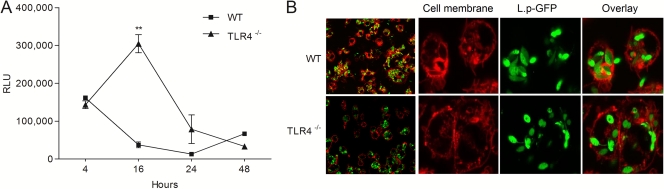
Evaluation of intracellular killing of L. panamensis over the time course of infection from 4 to 48 h showed that by 16 h postinfection, intracellular parasite survival in WT macrophages was reduced by up to 70%. In contrast, L. panamensis not only survived but also replicated during the first 16 h in TLR4−/− macrophages (Fig. 7A). The parasite burdens at the initial time point (4 h) of infection were comparable between WT and TLR4−/− cells (165,380 and 143,500 relative light units [RLU], respectively), confirming similar infection rates in both cell lines. Confocal microscopy of WT and TLR4−/− cells infected with GFP-transfected parasites corroborated equivalent parasite infectivity and internalization (Fig. 7B). By 24 h postinfection, parasite survival decreased in TLR4−/− macrophages, to levels comparable to those for WT host cells, supporting the participation of TLR4 during the very early stages of parasite-macrophage interaction.
Fig. 7.
TLR4 mediates early macrophage leishmanicidal activity. (A) Parasite infection and survival in WT (C57BL/6) and TLR4−/− macrophages infected with L.p-Luc promastigotes. The figure shows the RLU for three independent experiments performed in duplicate. **, P < 0.01 versus WT cells. (B) Confocal microscopy images of WT and TLR4−/− mouse macrophages infected with L.p-GFP promastigotes. Cell membranes were stained with CellMask Deep Red stain. Images show parasites internalized after 30 min of infection. Images of entire slides (right side) show a representative overview of WT and TLR4−/− macrophage infections.
Other MyD88-dependent receptors may participate in macrophage activation in response to L. panamensis.
To extend the findings obtained with macrophage cell lines to an infection approximating natural host cell interaction, we systematically evaluated the responses of primary C57BL/6 WT BMMs and knockout BMMs lacking specific TLRs and adaptor molecules. As observed in murine cell lines, TNF-α production was significantly reduced in TLR4−/− and MyD88/TRIF−/− BMMs exposed to L. panamensis (Fig. 8 A and B). However, in contrast to the results for knockout cell lines, in which deficiency of either MyD88 or TRIF alone did not significantly alter TNF-α production, MyD88−/− BMMs cocultured with L. panamensis produced significantly less TNF-α upon exposure to L. panamensis than did WT cells (Fig. 8C and D). Deficiency of TRIF did not significantly alter TNF production by BMMs. In addition, a striking and consistently greater production of TNF-α was induced in BMMs by opsonized versus nonopsonized parasites. This increased TNF-α response coincided with the higher percentage of infection observed for BMMs exposed to opsonized parasites (data not shown). The enhancement of TNF-α secretion by opsonized parasites in this experimental model is consistent with the participation of receptors other than TLR4 in L. panamensis-mediated BMM activation.
Fig. 8.
Activation of primary bone marrow macrophages by L. panamensis is TLR4 dependent, and signaling is preferentially mediated by MyD88. TNF-α production was measured in differentiated TLR4−/− (A), MyD88−/− (B), TRIF−/− (C), and MyD88/TRIF−/− (D) BMMs (from C57BL/6 mice) left untreated (medium), cocultured with L. panamensis promastigotes at a 100:1 parasite-to-macrophage ratio, or stimulated with specific TLR ligands or Sendai virus (hatched bars). Figures summarize data from three independent experiments and are expressed as means ± SEM. *, P < 0.05.
DISCUSSION
Macrophages are central to host defense, from pathogen detection and clearance to the induction of adaptive immune responses (40). The outcome of Leishmania infection is determined largely by the ability of macrophages to recognize and respond to the parasite (4, 6, 8, 9, 37, 41, 52). Although several macrophage receptors evidently participate in the Leishmania-host cell interaction (5, 11, 37, 39, 41), their role in the modulation of host immune responses and clinical outcomes of infection is not yet clear. This study sought to determine the participation of TLRs in L. panamensis-mediated modulation of host cell responses.
Contrary to the negative regulation of host cell functions reported for L. donovani infection, strong pro- and anti-inflammatory cytokine production is a hallmark of infection by parasites of the Leishmania (Viannia) subgenus (43). TNF-α is thought to play an important role during L. braziliensis infection, contributing to disease control in the early stages of infection and to exacerbation of disease at later stages of infection (2, 16, 50, 53). Mucosal leishmaniasis is characterized by high levels of TNF-α production, and genetic polymorphisms in the promoter region of TNF genes have been linked to susceptibility to mucosal disease (4, 13). The early induction of TNF-α production in isolated human macrophages by L. panamensis promastigotes provided initial evidence of putative receptor-mediated cell activation. The concurrence of the kinetics of TNF-α production induced by L. panamensis and by specific TLR ligands and the upregulation of TLR1, TLR2, TLR3, and TLR4 expression in human macrophages exposed to L. panamensis promastigotes (Fig. 2) further supported the possibility of TLR-mediated activation and TNF-α production.
Deletion of both MyD88 and TRIF, essential adaptor molecules in signal transduction initiated by TLR engagement, resulted in the abrogation of TNF-α secretion by mouse macrophages. This finding demonstrated that host cell activation by both L. panamensis amastigotes and promastigotes is TLR dependent. Although differences in macrophage responses to promastigotes and amastigotes of other Leishmania species are well documented (29), including decreased superoxide and increased anti-inflammatory cytokines in response to infection with amastigotes (15, 46, 47), the induction of TNF-α by parasites of these life stages was indistinguishable in this experimental infection model. This finding suggests that L. panamensis TLR ligands are expressed similarly by both amastigotes and promastigotes or, alternatively, that different stage-specific molecules can redundantly stimulate TLR signaling.
The most compelling evidence to date for the participation of TLRs in Leishmania infection and pathogenesis is the enhanced susceptibility of MyD88-deficient mice to L. major, accompanied by impaired proinflammatory cytokine synthesis and diminished Th1 T-cell activation (17, 42). However, this enhanced susceptibility to L. major has not been attributed to any specific TLR family member. Using MyD88−/− and TRIF−/− macrophage cell lines, we found that the absence of either of these adaptor molecules did not significantly alter TNF-α production induced by L. panamensis promastigotes or amastigotes. These findings suggest that signaling in response to L. panamensis occurs through both pathways and that in the absence of either, the other can compensate, sustaining TLR signaling and host cell activation.
Studies addressing the role of MyD88 in macrophage activation during infection with the closely related species L. braziliensis have yielded conflicting results. Recently, Carvalho et al. showed that TNF-α secretion by DCs during L. braziliensis infection was MyD88 independent (14). In contrast, Vargas-Inchaustegui and collaborators reported that L. braziliensis-infected MyD88−/− DCs exhibited reduced activation and decreased production of IL-12p40, which is fundamental to the generation of protective immunity (61). Notwithstanding differences in experimental models, these results raise the issue of intra- as well as interspecies variation in TLR-mediated activation. Despite the similarity of L. braziliensis and L. panamensis as related members of the Viannia subgenus, parasites of these species may trigger different mechanisms of cell activation. Therefore, generalization of results obtained with one species may not be appropriate or accurately portray the interaction with other Leishmania species, even within the same taxonomic grouping.
The partial abrogation of TNF-α production in UNC93b-deficient mutant macrophages in response to L. panamensis infection supports the involvement of signaling through endolysosomal TLRs and concurs with evidence of endolysosomal TLR participation in the immune response to other Leishmania species. TLR3 has been implicated in the recognition of L. donovani by IFN-γ-primed macrophages (21), and TLR9 has been implicated in DC-mediated NK cell activation and in T-cell responses following L. infantum and L. major infection, respectively (33, 55). L. major DNA has been shown to promote IFN-γ production by CD4+ T cells through TLR9-dependent activation of DCs (1). The potential therapeutic applicability of activation through endolysosomal TLRs has been demonstrated by the enhanced resolution of experimental L. donovani infection and human cutaneous leishmaniasis (12, 36), using the TLR7/8 ligand imiquimod. Although our results support the participation of endolysosomal TLR signaling during L. panamensis infection, the specific TLR(s) involved remains to be identified. Since all of the endolysosomal TLRs recognize nucleotide ligands, it is reasonable to hypothesize that Leishmania nucleic acids are involved in the innate immune response to infection.
TLR2 has been reported to be involved in the host response to L. major and L. donovani through the recognition of LPG, which activates human NK cells and murine macrophages through this receptor (3, 19). Inhibition of TLR2 expression impaired NO production and TNF-α secretion in IFN-γ-primed mouse macrophages (21). Our results showed that although L. panamensis induced increased TLR2 expression in human macrophages, the absence of TLR2 in murine macrophage lines did not affect TNF-α induction by promastigotes or amastigotes. This finding and the observation that parasites of the Viannia subgenus express 10-fold less LPG than L. donovani (44) suggest that TLR2 may not be a crucial determinant of the host cell response to L. panamensis.
The importance of TLR4 signaling in Leishmania infection is supported by the increased susceptibility of TLR4−/− C57BL/6 and BALB/c mice to L. major and L. pifanoi infections, respectively (30, 31). Furthermore, L. pifanoi P8 PGLC has been shown to induce TLR4-dependent macrophage cytokine and chemokine production (62). Our data provide evidence that TLR4 is also important during the early L. panamensis-macrophage interaction, since reduced TNF-α production and increased parasite survival occurred in TLR4−/− infected mouse macrophages. The results were further supported by ruling out any possibility of attributing activation to endotoxin contamination by use of MD-2−/− BMMs (Fig. 6C), thereby providing unequivocal evidence that the TLR4 response was induced by L. panamensis parasites. The significant decrease in TNF-α production in MyD88−/− BMMs is consistent with the ability of TLR4 to signal preferentially through MyD88 (25), as previously reported for other Leishmania species (18, 19, 42). These findings encourage identification of the nature of the ligand(s) involved in the TLR4-dependent response to L. panamensis.
A noteworthy difference between the activation of immortalized macrophage cell lines and BMMs was the enhanced TNF-α secretion induced by opsonized parasites in BMMs. Under physiological conditions, opsonins (including complement components and antibodies) interact rapidly with the cell surfaces of promastigotes after their inoculation into the dermis of mammals or with amastigotes when they leave their host cells (48). Opsonization promotes the uptake of parasites by phagocytic cells (63), principally mediated by Fc and complement receptors, and also mediates the release of anti-inflammatory cytokines (26, 38, 39). Opsonization could also favor the synergistic recognition of Leishmania parasites through various receptors (7), eliciting enhanced or diminished activating signals and cytokine production (49, 51). Although not evaluated in this study, differences in expression levels of TLRs or other receptors that interact with Leishmania, the maturation stage (34), and the functional integrity of permanent cell lines (45) may account for differences observed in the responses to parasite opsonization elicited in primary macrophages and macrophage cell lines. Interpretation and extrapolation of results obtained with cell lines to primary cell populations or either of these to whole organisms and vice versa merit consideration of the characteristics and limitations of each model system. Although the generalization of observations in these models to natural human infection is uncertain, exploitation of these experimental models allowed the discrimination of TLRs involved in the L. panamensis-macrophage interaction. Findings with human macrophages also support the involvement of multiple TLRs, including TLR4, in the host cell interaction with L. panamensis.
In conclusion, our results demonstrate TLR4-mediated macrophage activation and cooperation with other TLRs during infection by L. panamensis. These findings provide insight into the mechanism of parasite recognition and give evidence that early induction of TNF-α production mediated by ligation of TLRs contributes to control of infection. An understanding of the participation of TLRs in the outcome of Leishmania infection may allow the exploitation of these receptors or their ligands in the development of coadjuvant immunotherapy.
ACKNOWLEDGMENTS
We thank Liliana Valderrama and Diane McMahon-Pratt for their insights during the development of this investigation and for critical reviews of the manuscript. The support of Mauricio Perez with statistical analysis, Adriana Navas with cytometric analysis, Anna Cerny with animal husbandry, and Kristen Halmen with technical assistance is gratefully acknowledged.
This work was supported by an NIH/NIAID grant (AI065866), an NIH/John E. Fogarty International Center GID training grant (TW006589), a COLCIENCIAS doctoral training fellowship (041-2005) to C.G., and an NIH/NIAID grant (AI079293) to D.G.
The authors have no financial or other conflict of interest.
Footnotes
Published ahead of print on 25 April 2011.
REFERENCES
- 1. Abou Fakher F. H., Rachinel N., Klimczak M., Louis J., Doyen N. 2009. TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions. J. Immunol. 182:1386–1396 [DOI] [PubMed] [Google Scholar]
- 2. Bacellar O., et al. 2002. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect. Immun. 70:6734–6740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Becker I., et al. 2003. Leishmania lipophosphoglycan (LPG) activates NK cells through Toll-like receptor-2. Mol. Biochem. Parasitol. 130:65–74 [DOI] [PubMed] [Google Scholar]
- 4. Blackwell J. M. 1996. Genetic susceptibility to leishmanial infections: studies in mice and man. Parasitology 112(Suppl.):S67–S74 [PubMed] [Google Scholar]
- 5. Blackwell J. M., et al. 1985. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J. Exp. Med. 162:324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bogdan C., Gessner A., Solbach W., Rollinghoff M. 1996. Invasion, control and persistence of Leishmania parasites. Curr. Opin. Immunol. 8:517–525 [DOI] [PubMed] [Google Scholar]
- 7. Bosetto M. C., Giorgio S. 2007. Leishmania amazonensis: multiple receptor-ligand interactions are involved in amastigote infection of human dendritic cells. Exp. Parasitol. 116:306–310 [DOI] [PubMed] [Google Scholar]
- 8. Bosque F., Milon G., Valderrama L., Saravia N. G. 1998. Permissiveness of human monocytes and monocyte-derived macrophages to infection by promastigotes of Leishmania (Viannia) panamensis. J. Parasitol. 84:1250–1256 [PubMed] [Google Scholar]
- 9. Bosque F., Saravia N. G., Valderrama L., Milon G. 2000. Distinct innate and acquired immune responses to Leishmania in putative susceptible and resistant human populations endemically exposed to L. (Viannia) panamensis infection. Scand. J. Immunol. 51:533–541 [DOI] [PubMed] [Google Scholar]
- 10. Brinkmann M. M., et al. 2007. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J. Cell Biol. 177:265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brittingham A., Mosser D. M. 1996. Exploitation of the complement system by Leishmania promastigotes. Parasitol. Today 12:444–447 [DOI] [PubMed] [Google Scholar]
- 12. Buates S., Matlashewski G. 1999. Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: efficacy and mode of action. J. Infect. Dis. 179:1485–1494 [DOI] [PubMed] [Google Scholar]
- 13. Cabrera M., et al. 1995. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J. Exp. Med. 182:1259–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carvalho L. P., Pearce E. J., Scott P. 2008. Functional dichotomy of dendritic cells following interaction with Leishmania braziliensis: infected cells produce high levels of TNF-alpha, whereas bystander dendritic cells are activated to promote T cell responses. J. Immunol. 181:6473–6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Channon J. Y., Roberts M. B., Blackwell J. M. 1984. A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunology 53:345–355 [PMC free article] [PubMed] [Google Scholar]
- 16. Da-Cruz A. M., de Oliveira M. P., De Luca P. M., Mendonca S. C., Coutinho S. G. 1996. Tumor necrosis factor-alpha in human American tegumentary leishmaniasis. Mem. Inst. Oswaldo Cruz 91:225–229 [DOI] [PubMed] [Google Scholar]
- 17. Debus A., Glasner J., Rollinghoff M., Gessner A. 2003. High levels of susceptibility and T helper 2 response in MyD88-deficient mice infected with Leishmania major are interleukin-4 dependent. Infect. Immun. 71:7215–7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Trez C., et al. 2004. Myd88-dependent in vivo maturation of splenic dendritic cells induced by Leishmania donovani and other Leishmania species. Infect. Immun. 72:824–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Veer M. J., et al. 2003. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 33:2822–2831 [DOI] [PubMed] [Google Scholar]
- 20. Díaz Y. R., Rojas R., Valderrama L., Saravia N. G. 2010. T-bet, GATA-3, and Foxp3 expression and Th1/Th2 cytokine production in the clinical outcome of human infection with Leishmania (Viannia) species. J. Infect. Dis. 202:406–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flandin J. F., Chano F., Descoteaux A. 2006. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-gamma-primed macrophages. Eur. J. Immunol. 36:411–420 [DOI] [PubMed] [Google Scholar]
- 22. Gazzinelli R. T., Denkers E. Y. 2006. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat. Rev. Immunol. 6:895–906 [DOI] [PubMed] [Google Scholar]
- 23. Halle A., et al. 2008. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 9:857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janeway C. A., Jr., Medzhitov R. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216 [DOI] [PubMed] [Google Scholar]
- 25. Kagan J. C., et al. 2008. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat. Immunol. 9:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kane M. M., Mosser D. M. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141–1147 [DOI] [PubMed] [Google Scholar]
- 27. Kavoosi G., Ardestani S. K., Kariminia A. 2009. The involvement of TLR2 in cytokine and reactive oxygen species (ROS) production by PBMCs in response to Leishmania major phosphoglycans (PGs). Parasitology 136:1193–1199 [DOI] [PubMed] [Google Scholar]
- 28. Kim Y. M., Brinkmann M. M., Paquet M. E., Ploegh H. L. 2008. UNC93B1 delivers nucleotide-sensing Toll-like receptors to endolysosomes. Nature 452:234–238 [DOI] [PubMed] [Google Scholar]
- 29. Kima P. E. 2007. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int. J. Parasitol. 37:1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kropf P., et al. 2004. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect. Immun. 72:1920–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kropf P., et al. 2004. Infection of C57BL/10ScCr and C57BL/10ScNCr mice with Leishmania major reveals a role for Toll-like receptor 4 in the control of parasite replication. J. Leukoc. Biol. 76:48–57 [DOI] [PubMed] [Google Scholar]
- 32. Liese J., Schleicher U., Bogdan C. 2008. The innate immune response against Leishmania parasites. Immunobiology 213:377–387 [DOI] [PubMed] [Google Scholar]
- 33. Liese J., Schleicher U., Bogdan C. 2007. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur. J. Immunol. 37:3424–3434 [DOI] [PubMed] [Google Scholar]
- 34. Martinez F. O., Gordon S., Locati M., Mantovani A. 2006. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177:7303–7311 [DOI] [PubMed] [Google Scholar]
- 35. Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature 449:819–826 [DOI] [PubMed] [Google Scholar]
- 36. Miranda-Verastegui C., et al. 2009. First-line therapy for human cutaneous leishmaniasis in Peru using the TLR7 agonist imiquimod in combination with pentavalent antimony. PLoS Negl. Trop. Dis. 3:e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mosser D. M., Brittingham A. 1997. Leishmania, macrophages and complement: a tale of subversion and exploitation. Parasitology 115(Suppl.):S9–S23 [DOI] [PubMed] [Google Scholar]
- 38. Mosser D. M., Edelson P. J. 1984. Activation of the alternative complement pathway by Leishmania promastigotes: parasite lysis and attachment to macrophages. J. Immunol. 132:1501–1505 [PubMed] [Google Scholar]
- 39. Mosser D. M., Edelson P. J. 1985. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J. Immunol. 135:2785–2789 [PubMed] [Google Scholar]
- 40. Mosser D. M., Edwards J. P. 2008. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8:958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mosser D. M., Rosenthal L. A. 1993. Leishmania-macrophage interactions: multiple receptors, multiple ligands and diverse cellular responses. Semin. Cell Biol. 4:315–322 [DOI] [PubMed] [Google Scholar]
- 42. Muraille E., et al. 2003. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol. 170:4237–4241 [DOI] [PubMed] [Google Scholar]
- 43. Murray H. W., Berman J. D., Davies C. R., Saravia N. G. 2005. Advances in leishmaniasis. Lancet 366:1561–1577 [DOI] [PubMed] [Google Scholar]
- 44. Muskus C., et al. 1997. Carbohydrate and LPG expression in Leishmania viannia subgenus. J. Parasitol. 83:671–678 [PubMed] [Google Scholar]
- 45. Pan C., Kumar C., Bohl S., Klingmueller U., Mann M. 2009. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol. Cell. Proteomics 8:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pearson R. D., Harcus J. L., Roberts D., Donowitz G. R. 1983. Differential survival of Leishmania donovani amastigotes in human monocytes. J. Immunol. 131:1994–1999 [PubMed] [Google Scholar]
- 47. Pham N. K., Mouriz J., Kima P. E. 2005. Leishmania pifanoi amastigotes avoid macrophage production of superoxide by inducing heme degradation. Infect. Immun. 73:8322–8333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prina E., et al. 2004. Dendritic cells as host cells for the promastigote and amastigote stages of Leishmania amazonensis: the role of opsonins in parasite uptake and dendritic cell maturation. J. Cell Sci. 117:315–325 [DOI] [PubMed] [Google Scholar]
- 49. Qiao H., Andrade M. V., Lisboa F. A., Morgan K., Beaven M. A. 2006. FcepsilonR1 and Toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood 107:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ribeiro-de-Jesus A., Almeida R. P., Lessa H., Bacellar O., Carvalho E. M. 1998. Cytokine profile and pathology in human leishmaniasis. Braz. J. Med. Biol. Res. 31:143–148 [DOI] [PubMed] [Google Scholar]
- 51. Rittirsch D., et al. 2009. Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways. PLoS Pathog. 5:e1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Robledo S., Wozencraft A., Valencia A. Z., Saravia N. 1994. Human monocyte infection by Leishmania (Viannia) panamensis. Role of complement receptors and correlation of susceptibility in vitro with clinical phenotype. J. Immunol. 152:1265–1276 [PubMed] [Google Scholar]
- 53. Rocha F. J., Schleicher U., Mattner J., Alber G., Bogdan C. 2007. Cytokines, signaling pathways, and effector molecules required for the control of Leishmania (Viannia) braziliensis in mice. Infect. Immun. 75:3823–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Saravia N. G., et al. 1990. Recurrent lesions in human Leishmania braziliensis infection—reactivation or reinfection? Lancet 336:398–402 [DOI] [PubMed] [Google Scholar]
- 55. Schleicher U., et al. 2007. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J. Exp. Med. 204:893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shimazu R., et al. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Soong L. 2008. Modulation of dendritic cell function by Leishmania parasites. J. Immunol. 180:4355–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tabeta K., et al. 2006. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 7:156–164 [DOI] [PubMed] [Google Scholar]
- 59. Takeda K., Kaisho T., Akira S. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376 [DOI] [PubMed] [Google Scholar]
- 60. Tuon F. F., et al. 2008. Toll-like receptors and leishmaniasis. Infect. Immun. 76:866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vargas-Inchaustegui D. A., et al. 2009. Distinct roles for MyD88 and Toll-like receptor 2 during Leishmania braziliensis infection in mice. Infect. Immun. 77:2948–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Whitaker S. M., Colmenares M., Pestana K. G., McMahon-Pratt D. 2008. Leishmania pifanoi proteoglycolipid complex P8 induces macrophage cytokine production through Toll-like receptor 4. Infect. Immun. 76:2149–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Woelbing F., et al. 2006. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J. Exp. Med. 203:177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]