Abstract
Newborns are particularly susceptible to bacterial infections due to qualitative and quantitative deficiencies of the neonatal innate immune system. However, the mechanisms underlying these deficiencies are poorly understood. Given that fetuses are exposed to high concentrations of estradiol and progesterone during gestation and at time of delivery, we analyzed the effects of these hormones on the response of neonatal innate immune cells to endotoxin, bacterial lipopeptide, and Escherichia coli and group B Streptococcus, the two most common causes of early-onset neonatal sepsis. Here we show that at concentrations present in umbilical cord blood, estradiol and progesterone are as powerful as hydrocortisone for inhibition of cytokine production by cord blood mononuclear cells (CBMCs) and newborn monocytes. Interestingly, CBMCs and newborn monocytes are more sensitive to the effects of estradiol and progesterone than adult peripheral blood mononuclear cells and monocytes. This increased sensitivity is associated with higher expression levels of estrogen and membrane progesterone receptors but is independent of a downregulation of Toll-like receptor 2 (TLR2), TLR4, and myeloid differentiation primary response gene 88 in newborn cells. Estradiol and progesterone mediate their anti-inflammatory activity through inhibition of the NF-κB pathway but not the mitogen-activated protein kinase pathway in CBMCs. Altogether, these results suggest that elevated umbilical cord blood concentrations of estradiol and progesterone acting on mononuclear cells expressing high levels of steroid receptors contribute to impair innate immune responses in newborns. Therefore, intrauterine exposure to estradiol and progesterone may participate in increasing susceptibility to infection during the neonatal period.
INTRODUCTION
Newborns are at high risk of severe bacterial infections. Early-onset neonatal sepsis occurs during the first week of life in 1 to 5 per 1,000 births (14, 24, 25). Group B Streptococcus (GBS) and Escherichia coli are involved in more than 50% of episodes of early-onset neonatal sepsis. Despite antimicrobial therapy and improvements in neonatal intensive care, the mortality due to neonatal sepsis remains high (5 to 15%) and survivors are at risk of permanent neurologic damage (14, 24, 25). The particularly high susceptibility to infection during the neonatal period has been associated with quantitative and qualitative deficiencies of innate immunity (such as reduced levels of opsonins, decreased cytokine production, and impaired function of phagocytes) and adaptive immunity (such as impaired function of antigen-presenting cells and T cells and decreased production of immunoglobulins) (1, 19, 36). Although the differences between the neonatal and adult immune responses are relatively well described, the underlying mechanisms that account for the decreased ability of newborns to mount efficient immune responses remain largely unknown.
Fetuses are chronically exposed to high levels of steroid hormones due to placental production. Concentrations of estradiol in umbilical cord blood range from 2 to 150 nM, which are higher than peak concentrations of estradiol during the menstrual cycle (22, 41). Progesterone circulates in umbilical cord blood at 1 to 5 μM, which is higher than maternal blood levels during pregnancy (8, 42). The vast majority of newborns who develop sepsis become symptomatic during the first 24 h after birth (5), when blood levels of estradiol and progesterone are still elevated. Moreover, animal studies and in vitro experiments on immortalized cell lines suggest that estradiol and progesterone are important modulators of the immune response (13, 21, 38). Therefore, we hypothesized that the high levels of estradiol and progesterone present in umbilical cord blood contribute to decrease neonatal innate immune responses. Supporting this hypothesis, we show that estradiol and progesterone at relevant physiologic concentrations for newborns strongly inhibit NF-κB signal transduction and cytokine production in cord blood mononuclear cells (CBMCs) and monocytes exposed to microbial products. Altogether, our results highlight intrauterine exposure to estradiol and progesterone as a potential basis for the relative immunodeficiency and increased susceptibility to infection observed during the neonatal period.
MATERIALS AND METHODS
Cells and reagents.
Umbilical cord blood from 34 healthy term newborns and peripheral blood from 12 healthy adult volunteers were collected according to a protocol approved by the Ethics Committee of the University Hospital of Lausanne (Lausanne, Switzerland). Clinical characteristics of the newborns are provided in Table 1. The median ages of adult volunteers were 31 and 37 years for females and males, respectively (range, 25 to 35 and 27 to 46 years, respectively). Blood from female adult volunteers was collected during the follicular stage of the menstrual cycle. None of the subjects included in the study had any sign of infection or history of intra-amniotic infection. Blood was anticoagulated with heparin (10 U/ml). Mononuclear cells were extracted by Ficoll Hypaque (GE Healthcare, Uppsala, Sweden) gradient density centrifugation. Viability, determined by trypan blue exclusion, was >98%. Monocytes were isolated from CBMCs and peripheral blood mononuclear cells (PBMCs) by positive selection using magnetic microbeads coupled to an anti-CD14 antibody (Miltenyi Biotec, Auburn, CA) with a purity of >95%. The percentages of CD14 positive cells were 11.35 ± 8.59 in CBMCs and 12.45 ± 1.90 in adult PBMCs, as determined by fluorescent-activated cell sorter analysis. Cells were cultured in RPMI 1640 medium (Invitrogen, Basel, Switzerland) supplemented with 5% charcoal-stripped fetal calf serum. MCF-7 cells were obtained from the American Type Culture Collection (Manassas, VA).
Table 1.
Clinical characteristics of the newborns enrolled in the study
| Clinical characteristic | Result |
|---|---|
| No. (%) of male gender | 16 (47) |
| No. (%) of newborns delivered by cesarean section | |
| 19 (56) | |
| Median (range) gestational age (wk) | 39.1 (37–40.5) |
| Median (range) birth wt (g) | 3,065 (2,850–3,760) |
| Median (range) Apgar score at: | |
| 1 min | 9 (7–10) |
| 5 min | 10 (9–10) |
| 10 min | 10 (9–10) |
| Median (range) cord blood arterial pH | 7.27 (7.13–7.36) |
| Median (range) age of mother (yr) | 28 (20–39) |
| Median (range) parity of mother | 1 (1–3) |
Reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless indicated otherwise. E. coli O18 and GBS were obtained from blood cultures of septic newborns. E. coli O11:B4 lipopolysaccharide (LPS) was purchased from List Biological Laboratories (Campbell, CA). Pam3CSK4 lipopeptide was purchased from EMC Microcollections (Tuebingen, Germany). The estrogen receptor α (ER-α) agonist propylpyrazole triol (PPT) and the ER-β agonist diarylpropionitrile (DPN) were purchased from Tocris Bioscience (Bristol, United Kingdom), and 6-amino-4-(4-phenoxyphenylethylamino)quinazoline was purchased from Calbiochem (San Diego, CA).
Cytokine measurements.
CBMCs, PBMCs, and monocytes were preincubated as indicated in the figure legends with 17β-estradiol, progesterone, hydrocortisone, PPT, DPN, or norgestrel before stimulation with LPS (100 ng/ml), Pam3CSK4 (1 μg/ml), heat-killed E. coli (107 bacteria/ml) or GBS (108 bacteria/ml), and phorbol-12-myristate-13-acetate (PMA; 10 ng/ml) plus ionomycin (100 ng/ml). Concentrations of tumor necrosis factor (TNF) and interleukin-6 (IL-6) in cell culture supernatants collected after 6 h (TNF) and 24 h (IL-6) of stimulation were measured by bioassay using WEHI 164 clone 13 mouse fibrosarcoma cells (TNF) and 7TD-1 mouse hybridoma cells (IL-6) as described previously (27). Concentrations of IL-8 in cell culture supernatants collected after 24 h were measured by enzyme-linked immunosorbent assay (ELISA; BD Biosciences, San Diego, CA).
Western blot analyses.
CBMCs (6 × 106 cells/ml in 6-well culture plates) were cultured for 16 h with steroid hormones and then stimulated with E. coli, as indicated in the figure legends. At the end of the incubation, cells were washed with ice-cold phosphate-buffered saline. Whole-cell extracts were obtained by incubating CBMCs for 30 min at 4°C in a buffer consisting of 10 mM Tris, 150 mM NaCl, 10 mM NaF, 1 mM EDTA, 1 mM Na-orthovanadate, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 0.5% NP-40, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 μg/ml pepstatin (28). Cytoplasmic and nuclear extracts were prepared as described previously (31). For Western blot analysis of Toll-like receptor 2 (TLR2) and TLR4, protein extracts were obtained by lysing cells in a buffer containing 10 mM Tris, 100 mM NaCl, 1 mM EDTA, 1 mM NaF, 0.1% SDS, 0.5% Na-deoxycholate, 1% Triton X-100, 1% glycerol, 1 mM PMSF, 2 mM Na-orthovanadate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 μg/ml pepstatin. Protein concentration was measured using the bicinchoninic acid protein assay (Pierce Biotechnology Inc., Rockford, IL). Protein extracts were fractioned through 12% (wt/vol) SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Membranes were incubated with antibodies directed against TLR2, TLR4 (Hycult Biotechnology, Uden, Netherlands), ER-α, ER-β, myeloid differentiation primary response gene 88 (MYD88), NF-κB p65, total and phosphorylated IκBα, c-Jun (Santa Cruz Biotechnology, CA), total and phosphorylated p38, extracellular signal-regulated kinase 1 (ERK1)/ERK2 mitogen-activated protein kinases (MAPKs), and β-actin (Cell Signaling Technology, Danvers, TX). After membranes were washed, they were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Pierce Biotechnology Inc.). Signals were revealed using enhanced chemiluminescence detection (GE Healthcare).
RNA analyses by quantitative real-time PCR.
Total RNA was isolated from CBMCs and PBMCs using an RNeasy kit (Qiagen, Hombrechtikon, Switzerland). Reverse transcription of 1 μg of RNA was performed using an ImProm II RT system kit (Promega, Dübendorf, Switzerland). Real-time PCR was performed with a 7500 Fast real-time PCR system (Applied Biosystems, Rotkreuz, Switzerland) using the Power SYBR green PCR master mix (Applied Biosystems) as described previously (29). The following sense and antisense primers (5′-3′ sequences) were used for amplification: TNF, CAGAGGGCCTGTACCTCATC and GGAAGACCCCTCCCAGATAG; IL-6, GGAAGACCCCTCCCAGATAG and TTTTCTGCCAGTGCCTCTTT; ER-α, AGCACCCTGAAGTCTCTGGA and GATGTGGGAGAGGATGAGGA; ER-β, GATGTGGGAGAGGATGAGGA and TCTACGCATTTCCCCTCATC; progesterone receptor (PR), AAATCATTGCCAGGTTTTCG and TGCCACATGGTAAGGCATAA; progesterone receptor membrane component 1 (PGRMC1), TATGGGGTCTTTGCTGGAAG and GCCCACGTGATGATACTTGA; membrane progesterone receptor α (mPR-α), TCAGACCTGGCGCTTCTATT and AGGTGAAAGAGGCAAGGACA; mPR-β, CACGAAGGACCCACAAAACT and CAATCCCAAGCACCACCTAT; mPR-γm, ACTATGGTGCCGTCAACCTC and CAGAGTCTGGGCTTCTGGAC; TLR2, GCCTCTCCAAGGAAGAATCC and TCCTGTTGTTGGACAGGTCA; TLR4, ACCTGGCTGGTTTACACGTC and CTGCCAGAGACATTGCAGAA; MYD88, GGATGGTGGTGGTTGTCTCT and AGGATGCTGGGGAACTCTTT; IκBα, GCAAAATCCTGACCTGGTGT and GCTCGTCCTCTGTGAACTCC; and hypoxanthine phosphoribosyltransferase (HPRT), GAACGTCTTGCTCGAGATGTG and CCAGCAGGTCAGCAAAGAATT. Gene-specific expression of the target transcripts was normalized to the expression of HPRT and was expressed in arbitrary units (A.U.). HPRT expression was not influenced by cell treatment with steroids or stimuli (threshold cycle value = 25.6 ± 0.18, n = 33).
Statistical analyses.
Data are expressed as means ± standard errors of the means (SEMs). Comparisons between the different groups were performed by analysis of variance followed by the Fisher protected least significant difference test. Findings were considered statistically significant when P was <0.05.
RESULTS
Estradiol and progesterone inhibit cytokine production by CBMCs stimulated with microbial products.
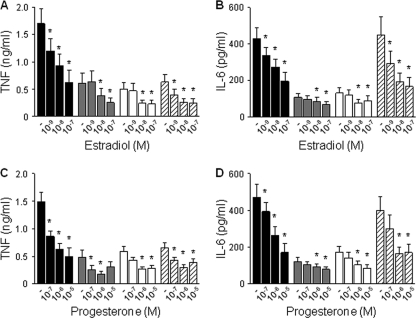
CBMCs exposed to E. coli, GBS, LPS, or Pam3CSK4 lipopeptide produced TNF and IL-6. Preincubation of CBMCs for 16 h with estradiol (10−7 to 10−9 M; Fig. 1 A and B) or progesterone (10−5 to 10−7 M; Fig. 1C and D) at concentrations circulating in umbilical cord blood inhibited TNF and IL-6 production in a dose-dependent manner. Indeed, 10−8 M estradiol and 10−6 M progesterone reduced TNF production by 38 to 58% and 54 to 64%, respectively, and IL-6 production by 20 to 57% and 24 to 59%, respectively. Of note, estradiol and progesterone inhibited TNF and IL-6 production by CBMCs from males and females in a similar fashion (n = 4 in each group). To analyze whether steroids affected TNF and IL-6 gene expression, we quantified by real-time PCR TNF and IL-6 mRNA levels in CBMCs preincubated with estradiol or progesterone prior to stimulation with E. coli for 2 h. Estradiol (10−8 M) and progesterone (10−6 M) reduced TNF gene expression by 27 and 19%, respectively, and IL-6 gene expression by 33 and 16%, respectively (n = 5 in each group, P < 0.05 versus no steroids). Thus, estradiol and progesterone are potent inhibitors of microbial product-induced cytokine mRNA and protein production in CBMCs.
Fig. 1.
Estradiol and progesterone inhibit TNF and IL-6 production by CBMCs. CBMCs were incubated for 16 h with estradiol (10−9 to 10−7 M) (A and B) or progesterone (10−7 to 10−5 M) (C and D) prior to stimulation with E. coli (black bars), GBS (gray bars), LPS (white bars), or Pam3CSK4 (hatched bars). TNF (A and C) and IL-6 (B and D) concentrations in cell culture supernatants were measured by bioassay. Data represent means ± SEMs of 8 independent experiments performed in triplicate. *, P < 0.05 versus no steroids.
Estradiol and progesterone inhibit cytokine production as efficiently as hydrocortisone but have no additive inhibitory effect.
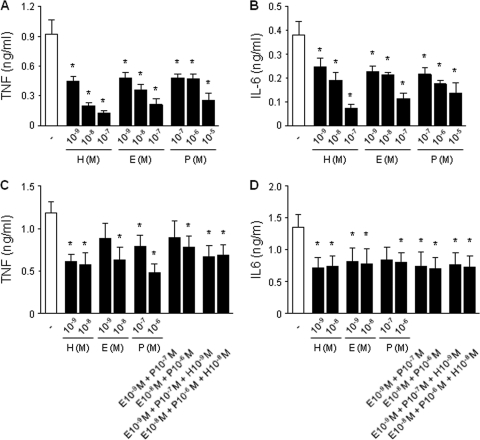
Considering that glucocorticoids are potent inhibitors of cytokine production by newborn immune cells (4), we compared the effects of estradiol (10−7 to 10−9 M) and progesterone (10−5 to 10−7 M) with those of physiologic concentrations of hydrocortisone (10−7 to 10−9 M). Equimolar concentrations of hydrocortisone and estradiol and 100-fold higher concentrations of progesterone inhibited E. coli-induced TNF and IL-6 production to the same extent (Fig. 2 A and B). Then, we compared the effects of steroids added either alone or in combination. Combined treatment with estradiol (10−9 or 10−8 M), progesterone (10−7 or 10−6 M), and hydrocortisone (10−9 or 10−8 M) did not provide stronger inhibition over treatment with a single hormone (Fig. 2C and D). Therefore, estradiol and progesterone inhibit cytokine production as efficiently as hydrocortisone but have no additive inhibitory effect.
Fig. 2.
Estradiol and progesterone inhibit TNF and IL-6 production by CBMCs as efficiently as hydrocortisone but have no additive inhibitory effect. CBMCs were incubated for 16 h with hydrocortisone (H; 10−9 to 10−7 M), estradiol (E; 10−9 to 10−7 M), or progesterone (P; 10−7 to 10−5 M) either alone (A and B) or in combination (C and D) prior to stimulation with E. coli. TNF (A and C) and IL-6 (B and D) concentrations in cell culture supernatants were measured by bioassay. Data represent means ± SEMs of 5 to 6 independent experiments performed in triplicate. *, P < 0.05 versus no steroids.
CBMCs and newborn monocytes are more susceptible to the anti-inflammatory effect of estradiol and progesterone than adult PBMCs and monocytes.
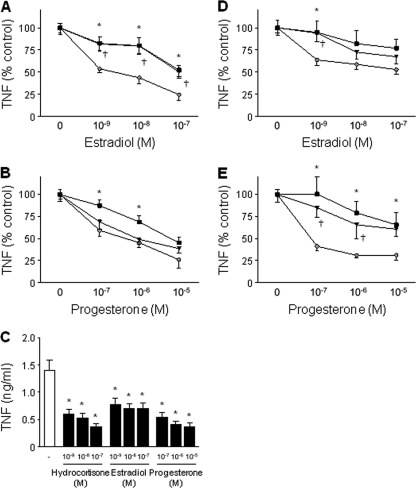
After birth, estradiol and progesterone blood levels decrease within less than 1 week to 0.1 nM and 5 nM, respectively, which corresponds to levels observed in adult males. Whereas estradiol has been shown to inhibit cytokine production by adult PBMCs, the effect of progesterone on adult immune cells is less clear (3, 13, 47). To evaluate the developmental response of immune cells to steroids, PBMCs from adult males and premenopausal females were preincubated for 16 h with estradiol (10−7 to 10−9 M) or progesterone (10−5 to 10−7 M) prior to stimulation with E. coli (Fig. 3 A and B). Estradiol and progesterone inhibited TNF production in PBMCs from adult males and premenopausal females. Comparative studies revealed that estradiol (10−7 to 10−9 M) more powerfully inhibited TNF production in CBMCs than in adult PBMCs from both genders. Progesterone (10−6 to 10−7 M) more powerfully inhibited TNF production in CBMCs than in PBMCs from adult males.
Fig. 3.
CBMCs and newborn monocytes are more susceptible to the anti-inflammatory effect of estradiol and progesterone than adult PBMCs and monocytes. (A and B) PBMCs from adult males (black squares) and premenopausal females (black inverted triangles) and CBMCs (gray circles) were incubated for 16 h with estradiol (A) or progesterone (B) prior to stimulation with E. coli. TNF concentrations in cell culture supernatants were measured by bioassay. Results were expressed in percent TNF release versus that for cells stimulated without steroids. Data represent means ± SEMs of 5 to 6 independent experiments performed in triplicate. *, P < 0.05 between PBMCs from adult males and CBMCs; †, P < 0.05 between PBMCs from adult females and CBMCs. (C) Monocytes from newborns were preincubated for 16 h with hydrocortisone, estradiol, or progesterone prior to stimulation with E. coli. Data represent means ± SEMs of 5 to 6 independent experiments performed in triplicate. *, P < 0.05 versus no steroids. (D and E) Monocytes from adult males (black squares), premenopausal females (black triangles), and newborns (gray circles) were incubated for 16 h with estradiol or progesterone prior to stimulation with E. coli. TNF concentrations in cell culture supernatants were measured by bioassay. Data represent means ± SEMs of 5 to 6 independent experiments performed in triplicate. *, P < 0.05 between monocytes from adult males and newborns; †, P < 0.05 between monocytes from adult females and newborns.
Monocytes are the main source of TNF and IL-6 upon microbial stimulation in whole blood (46). Therefore, we determined the impact of steroids on E. coli-induced TNF production in purified monocytes from newborns, adult males, and premenopausal females. Similar to what observed with CBMCs and adult PBMCs, estradiol (10−9 M) and progesterone (10−5 to 10−7 M) more potently inhibited TNF production in newborn monocytes than in adult monocytes (Fig. 3C to E). Furthermore, as described in Materials and Methods, flow cytometry analyses revealed that CBMCs and adult PBMCs contain similar proportions of monocytes. Thus, innate immune cells of newborns are more susceptible than those of adults to the anti-inflammatory effect of estradiol and progesterone.
Estrogen and progesterone receptors are expressed at higher levels in CBMCs than in adult PBMCs.
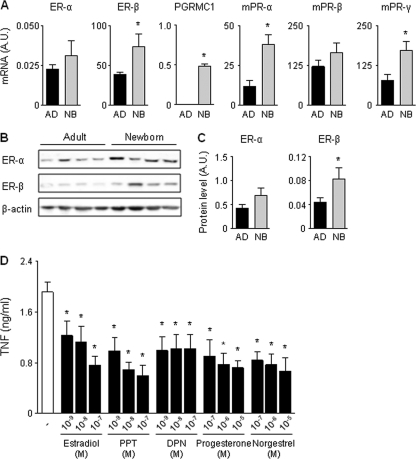
Estradiol and progesterone mediate their biological effects through binding to the nuclear receptors ER-α and ER-β and PR, respectively. Additionally, progesterone may act through membrane-associated receptors, such as PGRMC1, mPR-α, mPR-β, and mPR-γ (10, 40). ER-α, ER-β, mPR-α, mPR-β, and mPR-γ mRNAs were expressed in CBMCs and adult PBMCs (Fig. 4 A). Interestingly, ER-β, mPR-α, and mPR-γ mRNAs were present at higher levels in CBMCs than in adult PBMCs, whereas PGRMC1 was expressed only in CBMCs. PR mRNA was not detected in CBMCs or adult PBMCs (data not shown), suggesting that progesterone acts through membrane PRs in these cells. In line with mRNA analyses, ER-α and ER-β proteins were expressed in CBMCs and adult PBMCs, with higher levels (1.9-fold) of ER-β in CBMCs (Fig. 4B and C). To confirm the expression of functional steroid receptors in CBMCs, we analyzed the effect of ER-α (PPT)-, ER-β (DPN)-, and PR (norgestrel)-specific agonists on E. coli-induced TNF production in CBMCs (Fig. 4D). PPT and norgestrel dose dependently inhibited E. coli-induced TNF production as efficiently as estradiol and progesterone at equimolar concentrations. DPN also inhibited E. coli-induced TNF production, but the effect was not dose dependent. Overall, these data indicate that CBMCs express higher levels of ERs and mPRs than adult PBMCs, which may contribute to their increased sensitivity to estradiol and progesterone.
Fig. 4.
CBMCs express higher levels of estrogen and progesterone receptors than adult PBMCs and respond to specific receptor agonists. (A) Real-time PCR analysis of ER-α, ER-β, PR, PGRMC1, mPR-α, mPR-β, and mPR-γ mRNA levels in PBMCs from adult males (AD) and CBMCs (NB) from males. Gene-specific expression was normalized to the expression of HPRT. Data (means ± SEMs; n = 5 to 6) are expressed in A.U. relative to expression of the corresponding gene in MCF-7 breast cancer cells used as a positive control. *, P < 0.05 versus adult PBMCs. (B and C) Western blot (B) and densitometric (C) analyses of ER-α and ER-β in adult PBMCs (AD) and CBMCs (NB). Data (means ± SEMs; n = 6) are expressed in A.U. relative to expression of the corresponding protein in MCF-7 cells. (D) TNF concentrations in cell culture supernatants of CBMCs incubated with estradiol, progesterone, or agonists specific for ER-α (PPT), ER-β (DPN), and PR (norgestrel) for 16 h prior to stimulation with E. coli. Data represent means ± SEMs of 6 independent experiments performed in triplicate. *, P < 0.05 versus no steroids.
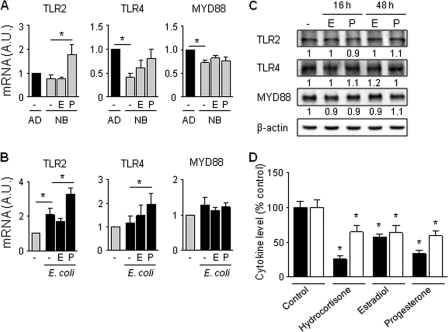
Estradiol and progesterone inhibit cytokine production in CBMCs by acting downstream of TLRs and MYD88.
The impaired production of proinflammatory cytokines by newborn innate immune cells exposed to microbial products has been associated with a reduced expression of TLRs and MYD88 (32, 45). We therefore questioned whether estradiol and progesterone (10−7 and 10−5 M, respectively, for 16 h) modulate the expression of MYD88, TLR2 (involved in the recognition of Pam3CSK4 and Gram-positive bacteria), and TLR4 (required for the sensing of LPS and Gram-negative bacteria) in unstimulated and E. coli-stimulated CBMCs. Confirming previous observations (32, 45), TLR4 and MYD88 were expressed at lower mRNA levels in CBMCs than adult PBMCs (Fig. 5 A). Progesterone increased TLR2 mRNA expression in unstimulated CBMCs and TLR2 and TLR4 mRNA expression in E. coli-stimulated CBMCs (Fig. 5B). However, incubation of CBMCs with estradiol or progesterone for 16 or 48 h did not alter TLR2, TLR4, and MYD88 protein levels in CBMCs (Fig. 5C). Thus, estradiol and progesterone do not mediate their anti-inflammatory activity in CBMCs by downregulating TLR2, TLR4, or MYD88 expression. These data suggest that estradiol and progesterone decrease the innate immune response of CBMCs by acting downstream of TLR/MYD88. Reinforcing this assumption, hydrocortisone, estradiol, and progesterone inhibited TNF and IL-8 production in CBMCs activated by PMA plus ionomycin, which activate protein kinase C intracellular signaling independently of TLRs (Fig. 5D).
Fig. 5.
The anti-inflammatory effect of estradiol and progesterone on CBMCs does not depend on modulation of TLR2, TLR4, and MYD88 expression. (A and B) Adult PBMCs (AD) and CBMCs (NB) (n = 5 to 6) were incubated for 16 h with estradiol (E; 10−7 M) or progesterone (P; 10−5 M) (A) prior to stimulation for 2 h with E. coli (B). TLR2, TLR4, and MYD88 mRNA expression was analyzed by real-time PCR. Data are expressed in A.U. relative to the expression of the corresponding gene in adult PBMCs (A) or in untreated CBMCs (B) and represent means ± SEMs. *, P < 0.05. (C) Western blot analysis of TLR2, TLR4, and MYD88 expression in CBMCs incubated for 16 h or 48 h with estradiol (10−7 M) or progesterone (10−5 M). Densitometric values (mean of 5 to 6 independent experiments) are expressed relative to expression in untreated CBMCs (set at 1). (D) CBMCs were incubated for 16 h with hydrocortisone (10−7 M), estradiol (10−7 M), or progesterone (10−5 M) prior to stimulation with PMA and ionomycin. TNF (black bars) and IL-8 (white bars) concentrations in cell culture supernatants were measured by bioassay and ELISA. Data represent means ± SEMs of 4 independent experiments performed in triplicate. *, P < 0.05 versus no steroids.
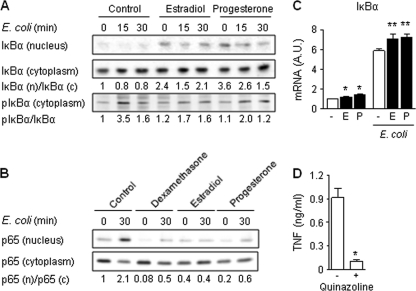
Estradiol and progesterone inhibit the NF-κB signaling pathway.
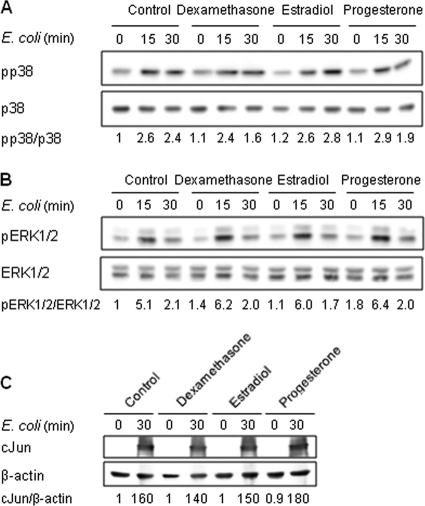
Interaction of microbial products with TLRs stimulates the NF-κB and MAPK pathways which regulate the transcriptional activation of cytokine genes. Considering that estradiol and progesterone inhibited E. coli-induced TNF and IL-6 mRNA upregulation, we analyzed the impact of steroids on the activation of NF-κB and MAPKs in CBMCs. Stimulation of CBMCs with E. coli increased the phosphorylation of IκBα (Fig. 6 A), the NF-κB inhibitor, and the translocation of NF-κB p65 into the nucleus (Fig. 6B). Pretreatment with estradiol or progesterone increased mRNA levels (Fig. 6C) and nuclear content (Fig. 6A) of IκBα. In line with these results, estrogen and progesterone strongly inhibited E. coli-induced IκBα phosphorylation (Fig. 6A) and NF-κB p65 nuclear translocation (Fig. 6B). The relevance of these findings was demonstrated by showing that quinazoline, an inhibitor of NF-κB activity, inhibited E. coli-induced TNF production by CBMCs (Fig. 6D). E. coli stimulation also activated the p38, ERK1/ERK2, and Jun N-terminal protein kinase (JNK) MAPK pathways in CBMCs, as deduced from the increased levels of phosphorylated p38 and ERK1/ERK2 and increased levels of nuclear c-Jun (Fig. 7 A to C). Neither estradiol nor progesterone altered E. coli-induced p38, ERK1/ERK2, and c-Jun activation. Thus, estradiol and progesterone interfere with the NF-κB pathway but not the MAPK signaling pathway in CBMCs.
Fig. 6.
Estradiol and progesterone inhibit E. coli-induced NF-κB activation. CBMCs were incubated for 16 h with dexamethasone (10−7 M), estradiol (E; 10−7 M), or progesterone (P; 10−5 M) prior to stimulation with E. coli for 15 or 30 min (A and B) or 2 h (C). (A and B) Total and phosphorylated (p) IκBα (A) and NF-κB p65 (p65) (B) in nuclear (n) and cytosolic (c) extracts were detected by Western blotting. Densitometric values (means of 3 to 6 independent experiments) are expressed relative to expression in untreated CBMCs (set at 1). (C) IκBα mRNA expression was quantified by real-time PCR and normalized to the expression of HPRT. Data represent means ± SEMs of 6 independent determinations. * and **, P < 0.05 versus untreated and E. coli-stimulated CBMCs, respectively. (D) CBMCs were preincubated for 1 h with 10−5 M quinazoline prior to incubation with E. coli for 6 h. TNF concentrations in cell culture supernatants were measured by bioassay. Data represent means ± SEMs of 4 independent experiments performed in triplicate. *, P < 0.05.
Fig. 7.
Estradiol and progesterone do not inhibit E. coli-induced MAPK activation. CBMCs were incubated for 16 h with dexamethasone (10−7 M), estradiol (10−7 M), or progesterone (10−5 M) prior to stimulation for 15 or 30 min with E. coli. Phosphorylated (p) and total p38 (A) and ERK1/ERK2 (B) in cytosolic extracts and nuclear c-Jun (C) were detected by Western blotting. Densitometric values (mean of 3 to 6 independent experiments) are expressed relative to expression in untreated CBMCs (set at 1).
DISCUSSION
We report for the first time that estradiol and progesterone strongly inhibit activation of the NF-κB pathway and production of proinflammatory cytokines by CBMCs exposed to microbial products. Thus, the high concentrations of estradiol and progesterone to which fetuses are chronically exposed (8, 22, 41, 42) may well participate to limit the capacity of newborns to mount innate immune responses to microbial infection.
Several lines of evidence support the contention that elevated levels of steroids have profound effects on the fetal and neonatal immune system. First, estradiol and progesterone strongly inhibited TNF and IL-6 production induced by purified microbial products as well as E. coli and GBS in CBMCs and/or purified newborn monocytes. Second, estradiol and progesterone at relevant physiologic concentrations were as potent inhibitors of cytokine production as hydrocortisone, an endogenous glucocorticoid with well-known anti-inflammatory properties (4, 44). Third, CBMCs and newborn monocytes were more sensitive to the anti-inflammatory effects of estradiol and progesterone than adult PBMCs and monocytes. Fifth, CBMCs expressed higher levels of a panel of estrogen and progesterone receptors than adult PBMCs. Finally, estradiol and progesterone inhibited the NF-κB pathway, which plays a seminal role in the control of inflammatory and innate immune responses. Blood levels of estradiol and progesterone are particularly elevated at birth and decrease 10- to 100-fold within 48 h (12, 17, 43). Considering that most of the newborns developing sepsis become symptomatic within the first 24 h of life (5), the potent anti-inflammatory effect of estradiol on cord blood monocytes may have clinically relevant implications in early-onset neonatal sepsis.
Steroid hormones mediate their effects by binding to intracellular receptors, leading to the translocation of hormone-receptor complexes into the nucleus, where they modulate gene transcription through binding to hormone-responsive elements (44). Our results point toward a role for both ER-α and ER-β in mediating the inhibitory effects of estradiol in CBMCs. Progesterone regulates numerous biological processes by activating nuclear PR (20). However, experimental data suggest that progesterone can also act through the glucocorticoid receptor (GR) (18). In CBMCs, progesterone may act independently of GR or PR since PR was not expressed in these cells (33, 34; our unpublished data); and norgestrel, which does not bind to GR, inhibited E. coli-induced TNF production by CBMCs. Additionally, the PR/GR antagonist RU486 did not attenuate progesterone-mediated immune suppression in CBMCs (34). Recent studies indicate that some effects of progesterone result from the activation of membrane receptors. Several candidate receptors such as the progestin and adipoQ receptors and members of the membrane-associated progestin receptor family have been identified (10, 16, 23, 40). We found that the progestin and adipoQ receptors mPR-α and mPR-γ are expressed at higher levels in CBMCs than in adult PBMCs. PGRMC1, a member of the membrane-associated progestin receptor family, was expressed only in CBMCs. Therefore, CBMCs express a panel of receptors which could mediate the anti-inflammatory effects of progesterone. Yet, the implication of these progesterone receptor candidates in the innate immune response remains to be determined.
The limited ability of newborn cells to mount an immune response has been associated with lower expression levels of TLR2, TLR4, and MYD88 (32, 45). Our results confirm a decreased expression of TLR4 and MYD88 and a trend toward a reduced expression of TLR2 in CBMCs than in adult PBMCs. Yet, neither estradiol nor progesterone reduced baseline and E. coli-stimulated expression of TLR2, TLR4, and MYD88 in CBMCs. Similarly, steroids did not reduce TLR2 and TLR4 expression in immune and nonimmune cells (6, 9, 15, 26, 30, 35). Given that estradiol and progesterone inhibited TNF and IL-8 production induced by PMA and ionomcyin, our findings suggest that these hormones act downstream of TLRs and MYD88 in CBMCs.
It is well recognized that glucocorticoids reduce proinflammatory cytokine production primarily through inhibition of the NF-κB and MAPK signaling pathways (44). In CBMCs stimulated with E. coli, estradiol and progesterone inhibited NF-κB signaling. Neither estradiol nor progesterone significantly increased the inhibitory effect of quinazoline on TNF production (data not shown), suggesting that NF-κB is the major pathway by which estradiol and progesterone act to decrease cytokine production. Estradiol and progesterone had no effect on ERK1/2, p38, and JNK MAPK activity. These results contrast with those of previous studies in RAW 264.7 mouse macrophages showing that progesterone but not estradiol increased IκBα expression and nuclear localization and that estradiol decreased JNK activation and nuclear levels of c-Jun and JunD (21, 37). In line with reduced expression of IL-6 in CBMCs treated with estradiol and progesterone, both steroids inhibited E. coli-induced phosphorylation of signal transducer and activator of transcription 3 (STAT3; n = 4, P < 0.05), a downstream target of IL-6.
Previous reports have identified estradiol and progesterone to be important immune modulators. Yet, estradiol and progesterone can have either immunostimulant or immunosuppressive effects (6, 7, 21, 37, 47). Possible explanations for the observed differences include factors such as the concentration, timing and duration of hormone treatment, and expression of specific receptor subtypes and receptor coactivators in distinct cell types of different species (2, 6, 13, 38, 39). Therefore, the impact of physiologic exposure of newborn immune cells to estradiol and progesterone cannot be extrapolated from data gathered in the literature.
The present study performed in a clinically relevant in vitro model highlights the important role of estradiol and progesterone as physiologic regulators of the fetal and neonatal immune response. Although additional studies will be required to investigate whether gestational age influences susceptibility of newborn innate immune cells to estradiol and progesterone, our data suggest that high blood levels of estradiol and progesterone may contribute to the relative immunodeficiency and increased susceptibility to infection observed in newborns. We speculate that our findings are particularly relevant in the context of chorioamnionitis. Indeed, upon intrauterine infection, high concentrations of estradiol and progesterone may, on the one hand, be beneficial to avoid the generation of deleterious proinflammatory responses that can lead to preterm delivery and/or damage to developing organs (11). On the other hand, the anti-inflammatory activity of estradiol and progesterone may put newborns at risk for early-onset sepsis. Thus, interfering with estradiol and progesterone signaling may be an attractive strategy to prevent or treat neonatal sepsis.
ACKNOWLEDGMENTS
This work was supported by the Wyeth Foundation for the health of children and adolescents, Abbott International, and the ProTechno Foundation (E.G.); the Santos-Suarez Foundation for Medical Research (T.C.); and the Swiss National Science Foundation (310030-118266 to T.C. and 310030-132744 to T.R.).
Footnotes
Published ahead of print on 25 April 2011.
REFERENCES
- 1. Adkins B., Leclerc C., Marshall-Clarke S. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553–564 [DOI] [PubMed] [Google Scholar]
- 2. An J., et al. 1999. Estradiol repression of tumor necrosis factor-alpha transcription requires estrogen receptor activation function-2 and is enhanced by coactivators. Proc. Natl. Acad. Sci. U. S. A. 96:15161–15166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asai K., et al. 2001. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock 16:340–343 [DOI] [PubMed] [Google Scholar]
- 4. Bessler H., et al. 1999. Effects of dexamethasone on IL-1beta, IL-6, and TNF-alpha production by mononuclear cells of newborns and adults. Biol. Neonate 75:225–233 [DOI] [PubMed] [Google Scholar]
- 5. Bromberger P., et al. 2000. The influence of intrapartum antibiotics on the clinical spectrum of early-onset group B streptococcal infection in term infants. Pediatrics 106:244–250 [DOI] [PubMed] [Google Scholar]
- 6. Calippe B., et al. 2010. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J. Immunol. 185:1169–1176 [DOI] [PubMed] [Google Scholar]
- 7. Carruba G., et al. 2003. Estrogen regulates cytokine production and apoptosis in PMA-differentiated, macrophage-like U937 cells. J. Cell. Biochem. 90:187–196 [DOI] [PubMed] [Google Scholar]
- 8. Dawood M. Y., Helmkamp F. 1977. Human umbilical arterial and venous progesterone concentrations: effect of fetal sex, weight, and mode of delivery. Obstet. Gynecol. 50:450–453 [PubMed] [Google Scholar]
- 9. Galon J., et al. 2002. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 16:61–71 [DOI] [PubMed] [Google Scholar]
- 10. Gellersen B., Fernandes M. S., Brosens J. J. 2009. Non-genomic progesterone actions in female reproduction. Hum. Reprod. Update 15:119–138 [DOI] [PubMed] [Google Scholar]
- 11. Gotsch F., et al. 2007. The fetal inflammatory response syndrome. Clin. Obstet. Gynecol. 50:652–683 [DOI] [PubMed] [Google Scholar]
- 12. Hughes I. A., Riad-Fahmy D., Griffiths K. 1979. Plasma 17OH-progesterone concentrations in newborn infants. Arch. Dis. Child. 54:347–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hunt J. S., et al. 1997. Female steroid hormones regulate production of pro-inflammatory molecules in uterine leukocytes. J. Reprod. Immunol. 35:87–99 [DOI] [PubMed] [Google Scholar]
- 14. Hyde T. B., et al. 2002. Trends in incidence and antimicrobial resistance of early-onset sepsis: population-based surveillance in San Francisco and Atlanta. Pediatrics 110:690–695 [DOI] [PubMed] [Google Scholar]
- 15. Imasato A., et al. 2002. Inhibition of p38 MAPK by glucocorticoids via induction of MAPK phosphatase-1 enhances nontypeable Haemophilus influenzae-induced expression of Toll-like receptor 2. J. Biol. Chem. 277:47444–47450 [DOI] [PubMed] [Google Scholar]
- 16. Karteris E., et al. 2006. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol. Endocrinol. 20:1519–1534 [DOI] [PubMed] [Google Scholar]
- 17. Kenny F. M., Angsusingha K., Stinson D., Hotchkiss J. 1973. Unconjugated estrogens in the perinatal period. Pediatr. Res. 7:826–831 [DOI] [PubMed] [Google Scholar]
- 18. Kontula K., Paavonen T., Luukkainen T., Andersson L. C. 1983. Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochem. Pharmacol. 32:1511–1518 [DOI] [PubMed] [Google Scholar]
- 19. Levy O. 2007. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 7:379–390 [DOI] [PubMed] [Google Scholar]
- 20. Li X., Lonard D. M., O'Malley B. W. 2004. A contemporary understanding of progesterone receptor function. Mech. Ageing Dev. 125:669–678 [DOI] [PubMed] [Google Scholar]
- 21. Miller L., Hunt J. S. 1998. Regulation of TNF-alpha production in activated mouse macrophages by progesterone. J. Immunol. 160:5098–5104 [PubMed] [Google Scholar]
- 22. Nagata C., Iwasa S., Shiraki M., Sahashi Y., Shimizu H. 2007. Association of maternal fat and alcohol intake with maternal and umbilical hormone levels and birth weight. Cancer Sci. 98:869–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peluso J. J. 2003. Progesterone as a regulator of granulosa cell viability. J. Steroid Biochem. Mol. Biol. 85:167–173 [DOI] [PubMed] [Google Scholar]
- 24. Persson E., Trollfors B., Brandberg L. L., Tessin I. 2002. Septicaemia and meningitis in neonates and during early infancy in the Goteborg area of Sweden. Acta Paediatr. 91:1087–1092 [DOI] [PubMed] [Google Scholar]
- 25. Remington J. S., Klein J. O., Baker C., Wilson C. B. (ed.). 2006. Infectious diseases of the fetus and the newborn infant, 6th ed. Elsevier, Philadelphia, PA [Google Scholar]
- 26. Roger T., Chanson A. L., Knaup-Reymond M., Calandra T. 2005. Macrophage migration inhibitory factor promotes innate immune responses by suppressing glucocorticoid-induced expression of mitogen-activated protein kinase phosphatase-1. Eur. J. Immunol. 35:3405–3413 [DOI] [PubMed] [Google Scholar]
- 27. Roger T., David J., Glauser M. P., Calandra T. 2001. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 414:920–924 [DOI] [PubMed] [Google Scholar]
- 28. Roger T., et al. 2009. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc. Natl. Acad. Sci. U. S. A. 106:2348–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roger T., et al. 2010. Histone deacetylase inhibitors impair innate immune responses to Toll-like receptor agonists and to infection. Blood 117:1205–1217 [DOI] [PubMed] [Google Scholar]
- 30. Roger T., et al. 2005. Critical role for Ets, AP-1 and GATA-like transcription factors in regulating mouse Toll-like receptor 4 (Tlr4) gene expression. Biochem. J. 387:355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roger T., et al. 1998. Enhanced AP-1 and NF-kappaB activities and stability of interleukin 8 (IL-8) transcripts are implicated in IL-8 mRNA superinduction in lung epithelial H292 cells. Biochem. J. 330(Pt 1):429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sadeghi K., et al. 2007. Immaturity of infection control in preterm and term newborns is associated with impaired Toll-like receptor signaling. J. Infect. Dis. 195:296–302 [DOI] [PubMed] [Google Scholar]
- 33. Schust D. J., Anderson D. J., Hill J. A. 1996. Progesterone-induced immunosuppression is not mediated through the progesterone receptor. Hum. Reprod. 11:980–985 [DOI] [PubMed] [Google Scholar]
- 34. Schwartz N., Xue X., Elovitz M. A., Dowling O., Metz C. N. 2009. Progesterone suppresses the fetal inflammatory response ex vivo. Am. J. Obstet. Gynecol. 201:e211–e219 [DOI] [PubMed] [Google Scholar]
- 35. Shuto T., et al. 2002. Glucocorticoids synergistically enhance nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via a negative cross-talk with p38 MAP kinase. J. Biol. Chem. 277:17263–17270 [DOI] [PubMed] [Google Scholar]
- 36. Siegrist C. A., Aspinall R. 2009. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 9:185–194 [DOI] [PubMed] [Google Scholar]
- 37. Srivastava S., et al. 1999. Estrogen decreases TNF gene expression by blocking JNK activity and the resulting production of c-Jun and JunD. J. Clin. Invest. 104:503–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Straub R. H. 2007. The complex role of estrogens in inflammation. Endocr. Rev. 28:521–574 [DOI] [PubMed] [Google Scholar]
- 39. Suzuki T., et al. 2007. Estrogen receptor-alpha predominantly mediates the salutary effects of 17beta-estradiol on splenic macrophages following trauma-hemorrhage. Am. J. Physiol. Cell. Physiol. 293:C978–C984 [DOI] [PubMed] [Google Scholar]
- 40. Thomas P. 2008. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front. Neuroendocrinol. 29:292–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Troisi R., et al. 2003. Estrogen and androgen concentrations are not lower in the umbilical cord serum of pre-eclamptic pregnancies. Cancer Epidemiol. Biomarkers Prev. 12:1268–1270 [PubMed] [Google Scholar]
- 42. Trotter A., et al. 1999. Effects of postnatal estradiol and progesterone replacement in extremely preterm infants. J. Clin. Endocrinol. Metab. 84:4531–4535 [DOI] [PubMed] [Google Scholar]
- 43. Trotter A., Maier L., Kron M., Pohlandt F. 2007. Effect of oestradiol and progesterone replacement on bronchopulmonary dysplasia in extremely preterm infants. Arch. Dis. Child. Fetal Neonatal ed. 92:F94–F98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Webster J. I., Tonelli L., Sternberg E. M. 2002. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 20:125–163 [DOI] [PubMed] [Google Scholar]
- 45. Yan S. R., et al. 2004. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect. Immun. 72:1223–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yerkovich S. T., et al. 2007. Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatr. Res. 62:547–552 [DOI] [PubMed] [Google Scholar]
- 47. Yuan Y., et al. 2008. Effects of estradiol and progesterone on the proinflammatory cytokine production by mononuclear cells from patients with chronic hepatitis C. World J. Gastroenterol. 14:2200–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]