Abstract
Natural killer (NK) cells are important components of a protective immune response against intracellular pathogens such as Leishmania parasites, which reside within myeloid cells. Previous in vivo studies in murine cutaneous or visceral leishmaniasis showed that NK cells are activated by conventional dendritic cells in a Toll-like receptor 9-, interleukin-12 (IL-12)-, and IL-18-dependent manner during the early phase of infection and help to restrict the tissue parasite burden by unknown mechanisms. Here, we tested whether NK cells contribute to the control of Leishmania infections by lysing or by activating infected host cells. Coculture experiments revealed that activated NK cells from poly(I:C)-treated mice readily killed tumor target cells, whereas Leishmania infantum- or L. major-infected macrophages or dendritic cells remained viable. Infection with Leishmania did not significantly alter the expression of NK cell-activating molecules (retinoic acid early transcript alpha [Rae-1α], mouse UL16-binding protein-like transcript 1 [MULT-1], CD48) or inhibitory molecules (major histocompatibility complex [MHC] class I, nonclassical MHC class 1b molecule Qa-1) on the surface of myeloid cells, which offers an explanation for their protection from NK cell cytotoxicity. Consistent with these in vitro data, in vivo cytotoxicity assays revealed poor cytolytic activity of NK cells against adoptively transferred infected wild-type macrophages, whereas MHC class I-deficient macrophages were efficiently eliminated. NK cells activated by IL-12 and IL-18 stimulated macrophages to kill intracellular Leishmania in a cell contact-independent but gamma interferon-, tumor necrosis factor-, and inducible nitric oxide synthase-dependent manner. We conclude that Leishmania parasites, unlike viruses, do not render infected myeloid cells susceptible to the cytotoxicity of NK cells. Instead, soluble products of NK cells trigger the leishmanicidal activity of macrophages.
INTRODUCTION
Natural killer (NK) cells participate not only in the control of tumors and the rejection of transplants but also in the development of protective immunity against intracellular pathogens (31, 51, 77). One of their central effects is the lysis of infected host cells. This has been best demonstrated for infections with viruses that up- or downregulate activating or inhibitory NK cell receptors or encode NK cell-activating ligands in their genome (2, 37, 38, 40, 71). Several studies suggested that NK cells also exert cytolytic effects against host cells infected with nonviral pathogens (1, 47, 53, 55, 74). On the other hand, both viral and nonviral infections exist in which host cells acquire resistance to NK cell lysis (10, 20, 21, 40). However, even without host cell lysis, NK cells might still operate as cytotoxic effectors, either by direct killing of extracellular microbes or by inducing the death of intracellular pathogens via the transfer of perforin or granulysin (1, 25, 42, 45, 52, 68, 75).
In addition to their cytotoxic activity, NK cells fulfill several regulatory functions, which also contribute to the control of infectious pathogens. NK cell-mediated host cell lysis itself facilitates T cell responses (30). Further enhancement results from NK cell-derived cytokines. Gamma interferon (IFN-γ) and tumor necrosis factor (TNF) pave the way for development of Th1 cells (32) and activate macrophages for the expression of antimicrobial effector mechanisms, such as inducible nitric oxide synthase (iNOS) (5, 6). Recently, human NK cells were found to produce IL-22, which promoted phagolysosomal fusion and impaired the growth of mycobacteria within macrophages (16). Finally, during viral infections that trigger a strong expansion of NK cells or during later phases of infections, NK cells might also acquire memory-like protective functions (70) or negative regulatory activities such as those seen, for example, in murine visceral leishmaniasis (44).
Infections with different species of the protozoan parasite Leishmania lead to cutaneous (e.g., Leishmania major) or visceral (e.g., L. infantum) disease. A series of findings argues for a protective role of NK cells in murine leishmaniasis, at least during the early phase of infection. These include (i) the aggravated course of Leishmania infections in NK cell-deficient bg/bg (beige) mice (28) or in NK cell-depleted mice (34, 36, 57), (ii) the disease-ameliorating effect of the activation or transfer of NK cells in Leishmania-infected mice (34, 43, 56), (iii) the expression of IFN-γ by NK cells early after infection in vivo (3, 17, 24, 39, 57, 58, 61), and (iv) the involvement of NK cells in the protection elicited by dendritic cell (DC)-based vaccines (54). However, it should be noted that the NK cell depletions were performed only transiently prior to and early during infection using reagents (e.g., anti-NK1.1 antibody) that were not entirely selective for NK cells.
Various cytokines and cell types participate in NK cell activation in cutaneous or visceral leishmaniasis. The key players are (i) interleukin-12 (IL-12), which is essential for NK cell activation (58) and released by myeloid DCs in response to Leishmania in a Toll-like receptor 9 (TLR9)- and vascular cell adhesion molecule-1/very late antigen-4-dependent manner in vitro and in vivo (22, 39, 61, 65); (ii) IFN-α/β, which accounts for the early expression of iNOS and thereby facilitates IL-12 responsiveness in L. major-infected mice (17, 18); (iii) IL-18, which supports NK cell activation in L. infantum-infected mice (24); and (iv) antigen-primed CD4+ T cells that activate NK cells via IL-2 in the L. major mouse model (4, 57). In situ, NK cells form part of the early inflammatory infiltrate after Leishmania infection at the site of infection and colocalize with myeloid cells in vivo (3, 72).
To date, we have only a partial understanding of the mechanisms by which NK cells might contribute to the control of Leishmania parasites in vivo. During L. major infection, NK cell-derived IFN-γ was shown to drive the Th1 differentiation of CD4+ T cells (57) and to restrict early parasite dissemination (17, 33). On the basis of in vitro experiments, NK cells might also lyse infected macrophages (1, 55). There are conflicting data whether NK cells directly kill extracellular Leishmania promastigotes (1, 43), an activity which could result from their constitutive expression of leishmanicidal granulysin or related molecules (68). More popular is the thought that NK cell-derived IFN-γ activates macrophages for the expression of iNOS (17); however, until now there has been no direct in vitro or in vivo evidence for this notion.
In the present study, we tested NK cells for their ability to lyse or activate Leishmania-infected macrophages. Unexpectedly, Leishmania-infected macrophages were entirely resistant to the cytotoxic activity of NK cells in vitro and in vivo. Instead, activated NK cells efficiently stimulated macrophages for the destruction of intracellular Leishmania in an IFN-γ-, TNF-, and iNOS-dependent manner.
MATERIALS AND METHODS
Mice.
Female or male C57BL/6 (B6) mice were purchased from Charles River, Sulzfeld, Germany. Breeding pairs of B6 protein tyrosine phosphatase (PTPRC; CD45.1+) congenic mice and of mice with a disrupted iNOS gene (35) (iNOS−/−; 11th generation backcross to B6), IFN-γ gene (14) (IFN-γ−/−; 10th generation backcross to B6), or IFN-γ receptor (IFN-γR) gene (26) (IFN-γR−/−; 10th generation backcross to B6) were from the Jackson Laboratory (Bar Harbor, ME). Bone marrow from β2-microglobulin-deficient (β2m−/−) mice (29) (11th generation backcross to B6; Jackson) was a kind gift of D. Vöhringer (Institute for Immunology, LMU Munich, Germany). Breeding pairs of recombination activating gene 2 (RAG2)/common γ-chain double mutant (RAG2/γc−/−) mice, which were generated by intercrossing RAG2−/− mice (62) (10th generation backcross to B6) with γc−/− mice (19) (12th generation backcross to B6), were obtained from J. Kirberg (MPI, Freiburg, Germany). Breeding pairs of RAG1−/− mice (48) (Jackson; 10th generation backcross to B6) were provided by T. Winkler (Department of Genetics, FAU Erlangen, Erlangen, Germany).
All mice were bred and kept under specific-pathogen-free conditions at the Franz Penzoldt Center for Animal Research, Universitätsklinikum Erlangen, and at the animal facility of the institute following the animal welfare protocol approved by the government of Middle Franconia (registration no. 54-2532.1-24/08). For experiments, sex-matched mice at the age of 6 to 12 weeks were used.
Parasites and infection of mice.
The origin, propagation, and preparation of L. infantum promastigotes (strain MHOM/00/98/LUB1) (8) and of L. major amastigotes (strain MHOM/IL/81/FEBNI) (64) were described before (61, 69). For infection of mice with L. infantum, 107 promastigotes were injected intravenously (i.v.) via the tail vein or intraperitoneally (i.p.).
Myeloid cells and in vitro infection.
Bone marrow-derived macrophages (BM-Mφ) were generated by culturing 6 × 106 nucleated bone marrow cells in 50 ml conditioned Dulbecco modified Eagle medium (supplemented with 10% [vol/vol] fetal calf serum [FCS], 50 μM 2-mercaptoethanol, 1% [vol/vol] 100× nonessential amino acids, 5% [vol/vol] horse serum, and 15% [vol/vol] supernatant [SN] from L929 fibroblast cultures [ATCC clone CCL-1] as a source of macrophage colony-stimulating factor) in Teflon bags for 8 days (59). Peritoneal exudate cells (PECs) were harvested from the peritoneal cavities of mice 4 days after i.p. injection of 3 ml of 4% (wt/vol) Brewer's thioglycolate broth (Difco, Detroit, MI) and contained >90% CD11b+ F4/80+ peritoneal exudate macrophages (PE-Mφ) (60). Bone marrow-derived dendritic cells (BM-DCs) were differentiated by culturing 6 × 106 bone marrow cells in RPMI 1640 medium (catalog no. 21875; Invitrogen, Karlsruhe, Germany) supplemented with 10% (vol/vol) FCS, 10 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin (termed “complete RPMI 1640 medium”), to which 10% (vol/vol) SN from the recombinant mouse-granulocyte macrophage colony-stimulating factor-transfected Ag8653 myeloma cell line (78) was added. The cells were cultured for 8 days in 60-cm2 culture dishes (Nalge Nunc International, Rochester, NY) with 10 ml of medium initially, before fresh medium was added on days 3 and 5 (41).
For infection, BM-Mφ or PE-Mφ were cocultured with L. infantum promastigotes or skin lesion-derived L. major amastigotes at ratios from 1:4 to 1:10 (see figure legends) for 14 to 16 h or 4 h, respectively, in Teflon bags using complete RPMI 1640 medium. Infection of BM-DCs was carried out similarly, except for using petri dishes. After infection, the cells were harvested and washed three times with complete RPMI 1640 medium to remove free parasites. The percentage of infected macrophages (60 to 90%) was determined by Diff-Quik (Medion Diagnostics AG, Switzerland) staining.
In some experiments involving the transfer of infected macrophages in vivo, the parasites used for infection were labeled with the cell proliferation dye eFluor 670 (eBioscience, Frankfurt, Germany). Briefly, L. infantum promastigotes were resuspended in 5 ml of phosphate-buffered saline (PBS) to which 1 μl of a 5 mM dye solution was added, and mixture was vortexed and incubated at 37°C for 10 min. To stop the reaction, ice-cold complete RPMI 1640 medium was added, followed by 5 min incubation on ice. Additional washes with ice-cold PBS were performed to remove excess dye. All cell culture reagents and media contained less than 10 pg/ml endotoxin as determined by a colorimetric Limulus amebocyte lysate assay (QCL-1000; Lonza, Cologne, Germany).
Antibodies and flow cytometry.
For surface phenotyping and cell sorting, fluorochrome (fluorescein isothiocyanate, phycoerythrin, or allophycocyanin [APC])-labeled or biotinylated monoclonal antibodies against the following mouse antigens were used: NK1.1 (PK136), CD3ε (145-2C11), CD11b (M1/70), CD11c (HL3), CD48 (HM48-1), and mouse UL-16-binding protein-like transcript 1 (MULT-1; 5D10) (all from eBioscience); Qa-1 (6A8.6F10.1A6) and H-2kb (AF6-88.5; BD Biosciences, Heidelberg, Germany); F4/80 (CI:A3-1; AbD Serotec, Düsseldorf, Germany); and pan-retinoic acid early transcript 1 (pan-Rae-1) (186107; R&D Systems, Wiesbaden, Germany). The specificity of the stainings was verified by the use of appropriate isotype control antibodies. Propidium iodide (PI) was included at 1 μg/ml in the final wash to detect dead cells.
Purification of NK cells.
Spleens were aseptically harvested from B6 wild-type (WT) or IFN-γ−/− mice, and single-cell suspensions were prepared in complete RPMI 1640 medium. After passage through a 100-μm-mesh-size cell strainer (BD Falcon), red blood cells were lysed by NH4Cl treatment. NK cells were purified by MACS technology using anti-DX5 MicroBeads (Miltenyi, Bergisch-Gladbach, Germany) and by subsequent MoFlo sorting gating on NK1.1+ CD3− cells (purity > 99%).
In vitro NK cell cytotoxicity assay.
After determination of the percentage of NK1.1+ CD3− NK cells within the splenocyte population by fluorescence-activated cell sorter (FACS) analysis, splenocytes were added to target cells at different NK cell/target cell ratios and incubated for a period of 4 h (YAC-1 cells) or 4 to 12 h (BM-Mφ, PE-Mφ, DCs) in complete RPMI 1640 medium at 37°C in 5% CO2, 95% humidified air. The target cells were previously labeled with ∼150 μCi 51Cr (specific activity, 400 to 1,200 Ci/g; Perkin-Elmer, Rodgau, Germany) for 90 min; and the spontaneous (target cells alone), maximal (51Cr-labeled cells directly added to LUMA plate), and coculture-elicited 51Cr release into the SNs was measured using a TopCount NXT microplate gamma counter (Perkin-Elmer).
In vivo NK cell cytotoxicity assay.
WT or major histocompatibility complex (MHC) class I-deficient (β2m−/−) BM-Mφ were infected with L. infantum promastigotes at a parasite/macrophage ratio of 7:1 and incubated in Teflon bags for 18 h. Splenocytes (from uninfected B6 CD45.1+ mice) or BM-Mφ (from uninfected or infected B6 CD45.1+ or β2m−/− CD45.1− mice) were resuspended in 10 ml of PBS containing 0.1% bovine serum albumin and were labeled with 0.5 or 5 μM 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen), respectively, for 10 min at 37°C. Labeling of cells was stopped by the addition of ice-cold complete RPMI 1640 medium and by two washes with ice-cold PBS. After the cells were counted, WT BM-Mφ (CD45.1+, CFSEhi), β2m−/− BM-Mφ (CD45.1−, CFSEhi), and WT splenocytes (CD45.1+, CFSElow) were mixed in equal ratios and injected i.p. into WT B6 recipient mice (2.5 × 105 cells per population in a total volume of 0.5 ml PBS/mouse) that had been i.p. treated with PBS, poly(I:C) (50 μg), or L. infantum promastigotes (107) 18 h prior to cell transfer. At 16 h after cell transfer, the mice were killed, the peritoneal exudates were collected, and the percentage of CFSE-labeled cells recovered from the peritoneal cavity was determined by FACS analysis. The cytotoxicity for each mouse group was measured using the following formula (12): percent specific lysis = [1 − (rnaïve/rprimed)] × 100, where r is the number of CD45.1+ CFSElo splenocytes/number of CFSEhi Mφ (CD45.1+ WT or CD45.1− β2m−/−) from naïve (i.e., PBS-treated) or primed [i.e., poly(I:C)-treated or L. infantum-infected] mice. In the case of PBS-treated mice, the calculated cytotoxicity value was 0% lysis, as rprimed equals rnaïve. Any alteration in rprimed (CD45.1+ CFSElo splenocytes/CFSEhi Mφ) observed in poly(I:C)- or L. infantum-treated mice was regarded as specific cytotoxicity.
Intracellular cytokine staining of NK cells.
Splenocytes from naïve B6 mice were cultured for 8 or 24 h with medium alone, with recombinant mouse IL-12 (rmIL-12) plus rmIL-18 (10 ng/ml each; R&D Systems), or with 50 ng/ml phorbol myristate acetate (Sigma-Aldrich) plus 750 ng/ml ionomycin (Sigma-Aldrich) in the presence of 10 μg/ml brefeldin A. After the cells were stained for NK cell surface markers (CD3− NK1.1+ or CD3− DX5+), the cells were fixed with Cytoperm Cytofix fixative (BD Biosciences) for 20 min and incubated with APC-conjugated rat anti-mouse IFN-γ (XMG1.2; eBioscience) or rat anti-mouse TNF (MP6-XT22; BD Biosciences) (61).
Coculture of NK cells and macrophages.
BM-Mφ generated from B6 WT, IFN-γR−/−, or iNOS−/− mice were plated at 1.5 × 105/400 μl in Lab-Tek eight-well Permanox chamber slides (catalog no. 177445; Nalge Nunc International) using complete RPMI 1640 medium and were allowed to adhere for 2 h in air with 5% CO2 and 95% humidity. After removal of nonadherent cells by three washes with PBS, macrophage monolayers were infected for 18 h with stationary-phase L. infantum promastigotes in a 10:1 parasite/cell ratio (1.5 × 106/400 μl), which led to an infection rate of 40 to 80% (Diff-Quik staining). Cells were washed three times with PBS to remove extracellular parasites and were cocultured with purified NK cells at a NK cell/macrophage ratio of 6:1 in the presence or absence of cytokines (rmIL-12, 10 ng/ml; rmIL-18, 10 ng/ml; R&D Systems). In some experiments the macrophages were treated with rmIFN-γ (20 ng/ml; kindly provided by G. Adolf, Boehringer Ingelheim, Vienna, Austria) and/or rmTNF (10 ng/ml; R&D Systems), and in others they were treated with a rat anti-TNF neutralizing antibody (clone MP6-XT22; R&D Systems) or a control rat IgG (Jackson ImmunoResearch, Dianova, Hamburg) at a final concentration of 5 μg/ml. The final culture volume was adjusted to 200 μl per well. Cells received 50 μl fresh medium (containing the respective stimuli and reagents) every 24 h. After 72 h, SNs were collected for cytokine and NO2− determinations and the monolayers were washed three times with PBS and stained with Diff-Quik for analysis of intracellular Leishmania. The parasite load in macrophages was calculated by determining the mean number of parasites per 100 macrophages of the culture (percent infection rate × average number of parasites per infected cell) in five randomly selected microscopic fields at ×1,000 magnification. The number of parasites in the nonstimulated macrophages (medium only) was set equal to 100%.
For transwell experiments, macrophages were plated at 0.5 × 106/500 μl on 12-mm-diameter round coverslips (catalog no. CB00120RA1; Thermo Scientific) placed in a 24-well cell culture dish and infected with L. infantum promastigotes in a 5:1 parasite/cell ratio. Cytokines were added as described above, and NK cells were seeded in a NK cell/macrophage ratio of 2:1 either directly to the macrophage monolayer or in Costar transwell inserts (0.4-μm pore size; Corning Life Sciences, Wiesbaden, Germany) placed above the coverslip. The final volume in the macrophage compartment was adjusted to 1 ml, and that in the insert was adjusted to 150 μl. After 72 h of coculture, the intracellular parasite load was determined as described above.
Assays for cytokine and nitrite determination.
TNF and IFN-γ in SNs of coculture experiments were determined using commercial enzyme-linked immunosorbent assay systems from eBioscience and BD Biosciences, respectively. The NO2− content was measured with the Griess assay using 50 μl of culture SN and an equal volume of Griess reagent (9).
Statistical analysis.
Standard errors of the mean (SEMs) were determined, and statistical significance was analyzed using either the nonparametric Mann-Whitney test or the unpaired Student t test. A P value of <0.05 was considered significant.
RESULTS
Activated NK cells exhibit cytolytic properties against tumor targets but do not lyse Leishmania-infected myeloid cells in vitro.
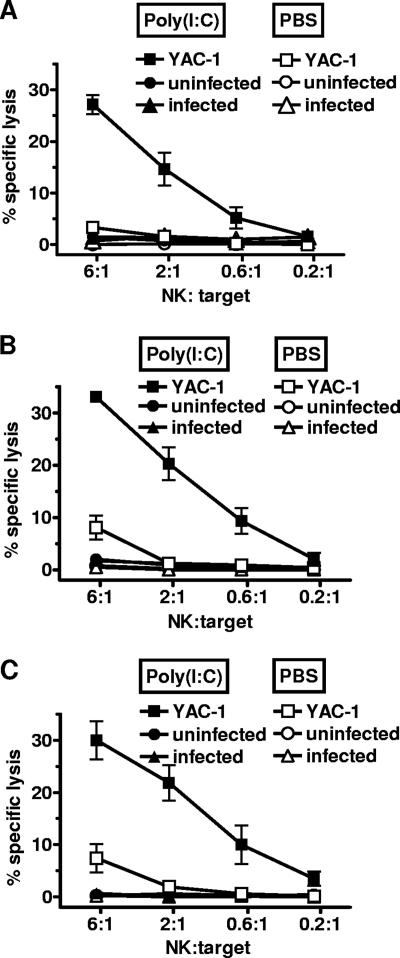
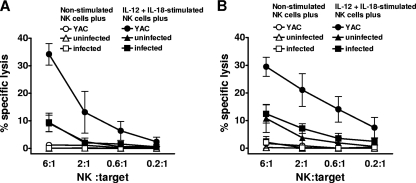
We investigated whether activated NK cells exerted cytotoxic effects against infected host cells in vitro. To this end, different types of myeloid cells, all of which function as host cells for Leishmania, were cocultured with splenocytes isolated from PBS or poly(I:C)-treated mice, and chromium (51Cr) release assays were performed. As expected, in vivo-activated NK cells efficiently lysed YAC-1 tumor targets, which lack expression of MHC class I, one of the strongest NK cell-inhibitory molecules, on the cell surface. In contrast, BM-Mφ, PE-Mφ, and BM-DCs were resistant to NK cell cytotoxicity in vitro, regardless of whether they were uninfected or infected with L. infantum promastigotes or L. major amastigotes and whether the coculture period lasted 4 or 12 h (Fig. 1 and data not shown). Importantly, infection with Leishmania did not increase the susceptibility of myeloid cells to NK cell-mediated lysis. The same observation was made when we used NK cells that were purified from naïve RAG1−/− mice and activated in vitro by IL-12 and IL-18, two key stimulators for the activation of NK cells during L. major and L. infantum infection (Fig. 2). Thus, neither in vivo- nor in vitro-activated NK cells show the capacity to specifically lyse Leishmania-infected myeloid cells.
Fig. 1.
In vivo-activated NK cells do not lyse Leishmania-infected host cells in vitro. B6 RAG1−/− mice (2 to 3 mice per group) were treated i.p. with 50 μg of poly(I:C) or with PBS. Splenocytes from these mice were incubated with 51Cr-labeled BM-Mφ (A), PE-Mφ (B), or BM-DCs (C) at the indicated NK/target cell ratio for 12 h. The myeloid target cells were either uninfected or infected with L. major amastigotes (parasite/cell ratio = 4:1). Splenocytes incubated with YAC-1 tumor cells were used as a positive control for NK cell cytotoxicity. Results shown represent the mean (±SEM) of 2 to 3 independent experiments.
Fig. 2.
In vitro-activated NK cells readily kill YAC-1 tumor targets but are not cytotoxic against uninfected or infected B6 BM-Mφ. NK cells from the spleens of RAG1−/− mice were stimulated for 18 h with rmIL-12 plus rmIL-18 and used as effector cells in 4-h (A) and 18-h (B) chromium-51 release assays. The mean (±SEM) of three independent experiments is shown as percent specific lysis against uninfected and L. infantum-infected BM-Mφ (parasite/cell ratio = 10:1) or YAC-1 tumor cells.
Leishmania infection does not modulate the expression of ligands for activating or inhibitory NK cell receptors on the surface of myeloid cells.
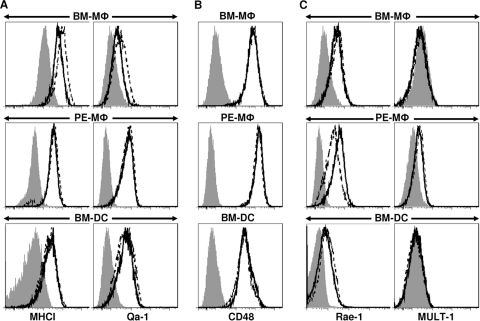
The cytotoxic activity of NK cells is controlled by a set of activating and inhibitory receptors on their surface. NK cells recognize cells that overexpress ligands for activating receptors or underexpress ligands for inhibitory receptors, or both. A shift in the balance from inhibitory to activating signals will result in target cell lysis by NK cells (31). We therefore tested CD11b+ F4/80+ BM-Mφ, CD11b+ F4/80+ PE-Mφ, and CD11b+ CD11c+ BM-DCs for the surface expression of (i) MHC class I antigens (the ligands for the inhibitory members of the Ly49 NK cell receptor family) and Qa-1b (a nonclassical MHC-like molecule that primarily functions as a high-affinity ligand of the inhibitory NK cell receptor NKG2A [13]); (ii) CD48, which interacts with the 2B4 receptor on NK cells and transmits inhibitory or activating signals, depending on the degree of receptor expression and cross-linking (11, 27); and (iii) Rae-1α and MULT-1, which are ligands of the activating NK cell receptor NKG2D (31). In general, infection of the three different myeloid cell populations with L. infantum promastigotes did not alter the expression of these ligands. In two of four experiments, we measured a slight upregulation of MHC class I and Qa-1b selectively in infected BM-Mφ and a downregulation of Rae-1α only in infected PE-Mφ and not in the other two cell populations (Fig. 3). Importantly, in none of the four experiments did we observe an NK cell ligand expression pattern that would promote the activation of NK cells. Comparable results were obtained when the infections were performed in the presence of naïve or activated NK cells or when L. major amastigotes freshly isolated from skin lesions were used (data not shown). From these data we conclude that the resistance of myeloid cells to NK cell lysis in vitro might result from maintaining the balance between activating versus inhibitory NK receptor ligands, despite infection with Leishmania.
Fig. 3.
Leishmania infection does not alter the levels of NK cell regulatory molecules on the surface of macrophages or DCs. BM-Mφ, PE-Mφ, or BM-DCs were infected with L. infantum promastigotes at a parasite/cell ratio of 7:1 for 14 h. CD11b+ F4/80+ Mφ and CD11c+ CD11b+ DCs were stained for the expression of the NK cell-receptor ligands MHC class I and Qa-1b (A; inhibitory), CD48 (B; inhibitory or activating), or Rae-1α and MULT-1 (C; activating). The histograms represent the expression levels of these molecules on the surface of uninfected (solid lines) or infected (dashed lines) cells. Isotype controls (shaded) were included for each staining. The data are representative of 4 independent experiments.
NK cells do not kill Leishmania-infected macrophages in vivo.
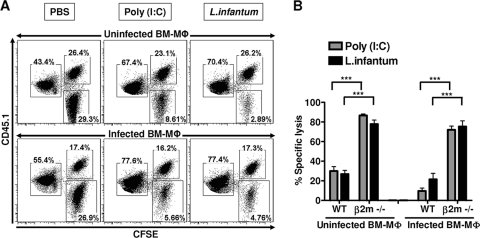
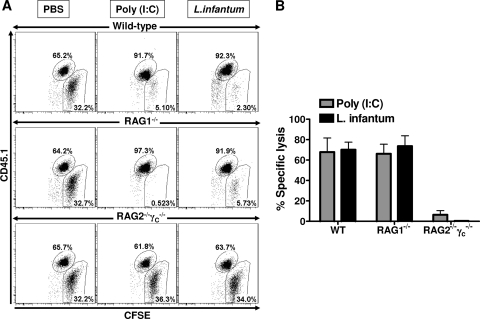
The failure of NK cells to recognize infected macrophages as targets in vitro might reflect the lack of additional NK cell-activating signals such as those delivered by myeloid DCs. Hence, we sought to study the cytotoxicity of NK cells against infected macrophages in vivo. For this purpose, a mixture of CFSE-labeled WT BM-Mφ (uninfected or infected with L. infantum promastigotes), β2m−/− BM-Mφ (uninfected or infected; these served as a positive control due to their MHC class I deficiency), and WT splenocytes (used as a reference population for the transferred cells) were injected into the peritoneal cavity of mice whose NK cells had already been activated by the treatment with poly(I:C) or the infection with L. infantum 18 h prior to the cell transfer. At 16 h after cell transfer the mice were killed and peritoneal exudates were collected and analyzed by flow cytometry. The transferred cells were distinguished from each other and the immune cells of the recipient by their congenic marker (CD45.1+) and by the labeling with different concentrations of CFSE. The infection status of the transferred macrophages was ascertained by using fluorescently labeled parasites (data not shown). The percent decrease in the number of CFSEhi macrophages relative to the number of CFSElo splenocytes was used as a measure of cytotoxicity (see Materials and Methods). As expected, β2m−/− BM-Mφ (CFSEhi CD45.1−) were readily eliminated from the peritoneal cavity of poly(I:C)-treated or L. infantum-infected mice. In contrast, only minimal cytotoxicity was observed against WT macrophages in the very same mice. However, this minimal cytotoxicity was not specific for infected macrophages, as it was also observed with uninfected target cells (Fig. 4). The ultimate proof that both poly(I:C) and L. infantum led to proper activation of NK cells in the peritoneal cavity and that the observed lysis of β2m−/− BM-Mφ resulted from NK cell activity was obtained by comparison of the cytotoxicity against β2m−/− BM-Mφ in WT versus RAG1−/− or RAG2/γc−/− recipient mice. Whereas the cytotoxicity observed in RAG1−/− mice, which contain NK cells but lack T and B cells, was comparable to that in WT mice, there was no target cell lysis detectable in RAG2/γc−/− mice, which are devoid of all lymphocytes, including NK cells (Fig. 5). Together, these results demonstrate that, albeit activated NK cells have the potential to eliminate MHC class I-deficient macrophages, Leishmania-infected macrophages are spared from their cytotoxicity.
Fig. 4.
Leishmania-infected macrophages are resistant to NK cell lysis in vivo. WT B6 mice (CD45.1−) were treated i.p. with PBS, poly(I:C) (50 μg), or L. infantum promastigotes (107 parasites). At 18 to 20 h posttreatment or postinfection, 2.5 × 105 CFSE-labeled uninfected or infected BM-Mφ (CFSEhi CD45.1+, L. infantum promastigote/cell ratio for infection = 7:1) from congenic B6 PTPRC mice were injected i.p. along with an equal number of uninfected or infected MHC class I-deficient (β2m−/−) BM-Mφ (CFSEhi CD45.1−, parasite/cell ratio for infection = 7:1) and uninfected WT splenocytes (CFSElo CD45.1+). (A) The percentage of CFSE-labeled cells recovered from the peritoneal cavity at 14 to 16 h posttransfer was analyzed by flow cytometry. After gating on CFSE-positive cells, the transferred populations were distinguished on the basis of their differential intensity of CFSE fluorescence (high or low) and expression of CD45.1 (positive or negative). (B) The bar graph represents the mean (±SEM) of percent specific lysis obtained from four independent experiments with four mice per group as described in Materials and Methods. ***, P < 0.0001 (Mann-Whitney test).
Fig. 5.
NK cells account for the cytotoxic activity against myeloid cells detected in the peritoneal cavity of infected or poly(I:C)-treated mice. B6 WT, RAG1−/−, and RAG2/γc−/− mice were treated i.p. with PBS, poly(I:C) (50 μg), or L. infantum promastigotes (107 parasites). At 18 to 20 h posttreatment or postinfection, CFSE-labeled uninfected MHC class I-deficient (β2m−/−) BM-Mφ (CFSEhi CD45.1−) and WT splenocytes (CFSElow CD45.1+) were injected into the peritoneal cavity. (A) The percentage of CFSE-labeled cells recovered from the peritoneal cavity at 14 to 16 h posttransfer was analyzed by flow cytometry and used to calculate the percent specific lysis. (B) The bar graph depicts the mean (±SEM) of percent specific lysis of WT, RAG1−/−, or RAG2/γc−/− peritoneal cells against uninfected β2m−/− BM-Mφ targets in two independent experiments with three mice per group.
Activated NK cells stimulate BM-Mφ for the kill of intracellular Leishmania in a cell contact-independent but IFN-γ-, TNF-, and iNOS-dependent manner.
Having excluded target cell lysis as a mechanism by which NK cells confer protection in leishmaniasis, we investigated their ability to trigger leishmanicidal activities within macrophages in a nonlytic fashion. We therefore cocultured L. infantum-infected BM-Mφ with highly purified NK cells from naïve mice in the presence of IL-12 and IL-18, which are required for optimal NK cell activation in mouse cutaneous and visceral leishmaniasis (24, 39, 61). After 72 h of coculture, the total parasite load decreased by 76% to 97% (Fig. 6 A, C, and D). In the nonstimulated 72-h control cultures (Mφ only), the macrophage infection rate varied between 38% and 74% and the absolute number of parasites per 100 Mφ was between 64 and 344, whereas in the presence of NK cells plus IL-12 and IL-18, the infection rate went down to 4% to 24% and the parasite numbers per 100 Mφ ranged from 2 to 43 in this series of experiments. Importantly, there was no correlation between the reduction of the macrophage parasite load induced by activated NK cells and the absolute parasite numbers in the control cultures (data not shown). Thus, NK cell-mediated activation of infected macrophages is not influenced by the parasite burden within the macrophages.
Fig. 6.
Activated NK cells stimulate infected macrophages to kill intracellular Leishmania in a cell contact-independent but IFN-γ-, TNF-, and iNOS-dependent manner. BM-Mφ were infected with L. infantum promastigotes for 18 h in 8-well chamber slides (A, C, and D; parasite/cell ratio = 10:1) or 24-well plates (B; parasite/cell ratio = 5:1) and were subsequently cocultured with the indicated cytokines and/or purified splenic NK cells from naïve mice. After 72 h the intracellular parasite load was determined (mean [±SEM] of 3 to 4 independent experiments). Rhombus-shaped symbols denote significance compared to unstimulated cells (NS). * or #, P < 0.05; ** or ##, P < 0.01; *** or ###, P < 0.001 (Student's t test); n.d., not detectable. (A) BM-Mφ monolayer alone versus NK cells cocultured with BM-Mφ (ratio, 6:1). (B) Coculture of NK cells with BM-Mφ (ratio, 6:1). The NK cells were added directly to the BM-Mφ plated on coverslips in a 24-well plate (direct contact) or seeded into culture inserts placed above the infected BM-Mφ monolayer (transwell). (C) (Left panel) B6 WT NK cells were cocultured with B6 WT, IFN-γR−/−, or iNOS−/− BM-Mφ (ratio, 6:1); (right panel) B6 WT or IFN-γ−/− NK cells were cocultured with B6 WT BM-Mφ (ratio, 6:1). (D) NK cells and BM-Mφ from B6 WT mice were cocultured (ratio, 6:1) with the addition of anti-TNF neutralizing antibody or normal IgG (5 μg/ml).
To further characterize the ability of activated NK cells to trigger antiparasitic effects in macrophages, we titrated the NK cell/BM-Mφ ratio (6:1, 3:1, 1:1, 0.1:1, to 0.01:1) in two independent experiments. Comparable reductions in the parasitic load were observed with NK cell/BM-Mφ ratios from 6:1 down to 0.1:1, indicating that less than one NK cell per macrophage is sufficient to elicit parasite killing by the host cell (data not shown).
The macrophage-activating effect of NK cells was independent of physical interactions between both cell populations, as it was also observed under transwell conditions (Fig. 6B). Furthermore, it was mediated by IFN-γ and TNF and the expression of iNOS. This was revealed by (i) the analysis of IFN-γR−/− BM-Mφ (Fig. 6C, left panel), IFN-γ−/− NK cells (Fig. 6C, right panel), anti-TNF antibodies (Fig. 6D), and iNOS−/− BM-Mφ (Fig. 6C, left panel); (ii) the determination of cytokine and NO2− levels in the respective culture SNs (Table 1); and (iii) the detection of cell-associated IFN-γ, but not of TNF, in activated NK cells using intracellular cytokine staining (data not shown). As controls, BM-Mφ were stimulated with IFN-γ plus TNF, which led to the expected, almost complete elimination of intracellular parasites (7, 69) (Fig. 6A to D), whereas the use of IFN-γ or TNF alone (Fig. 6A) or of IL-12 plus IL-18 in the absence of NK cells (Fig. 6A, C, and D) was largely ineffective. Likewise, NK cells added to the infected BM-Mφ monolayers in the absence of IL-12 plus IL-18 caused no or only very limited (4.1% to 35.7%) killing of intracellular Leishmania, which was not observed when the two cell populations were separated from each other (Fig. 6B, direct contact versus transwell).
Table 1.
Cytokine and NO2− levels in SNs of BM-Mφ cultures and BM-Mφ/NK coculturesa
| Culture no. | BM-Mφ | Cultured with: |
Measured concn (±SEM)b |
||||
|---|---|---|---|---|---|---|---|
| NK | Stimulus | Blocking agent | IFN-γ (ng/ml) | TNF (pg/ml) | NO2− (μM) | ||
| 1 | WT | 0 (±0) | 6 (±3) | 0 (±0) | |||
| 2 | WT | IFN-γ + TNF | 12 (±4) | 1,750 (± 512) | 56 (±3) | ||
| 3 | WT | IL-12 + IL-18 | 0 (±0) | 18 (±3) | 3 (±3) | ||
| 4 | WT | WT | 0 (±0) | 69 (±29) | 0 (±0) | ||
| 5 | WT | WT | IL-12 + IL-18 | 144 (±43) | 274 (±67) | 50 (±4) | |
| 6 | IFN-γR−/− | 0 (±0) | 4 (±1) | 0 (±0) | |||
| 7 | IFN-γR−/− | IFN-γ + TNF | 22 (±4) | 652 (±131) | 0 (±0) | ||
| 8 | IFN-γR−/− | IL-12 + IL-18 | 0 (±0) | 24 (±18) | 0 (±0) | ||
| 9 | IFN-γR−/− | WT | 0 (±0) | 76 (±51) | 0 (±0) | ||
| 10 | IFN-γR−/− | WT | IL-12 + IL-18 | 276 (±43) | 241 (±62) | 0 (±0) | |
| 11 | WT | IgG | 0 (±0) | 5 (±4) | 0 (±0) | ||
| 12 | WT | Anti-TNF | 0 (±0) | 1 (±0) | 0 (±0) | ||
| 13 | WT | IFN-γ + TNF | IgG | 13 (±3) | 801 (±356) | 48 (±3) | |
| 14 | WT | IFN-γ + TNF | Anti-TNF | 6 (±3) | 221 (±140) | 4 (±2) | |
| 15 | WT | IL-12 + IL-18 | IgG | 0 (±0) | 13 (±4) | 0 (±0) | |
| 16 | WT | IL-12 + IL-18 | Anti-TNF | 0 (±0) | 8 (±6) | 0 (±0) | |
| 17 | WT | WT | IgG | 0 (±0) | 110 (±89) | 0 (±0) | |
| 18 | WT | WT | Anti-TNF | 0 (±0) | 50 (±37) | 0 (±0) | |
| 19 | WT | WT | IL-12 + IL-18 | IgG | 106 (±75) | 168 (±81) | 52 (±5) |
| 20 | WT | WT | IL-12 + IL-18 | Anti-TNF | 116 (±72) | 67 (±35) | 49 (±13) |
BM-Mφ from B6 or IFN-γR−/− mice were infected for 18 h with a 10-fold excess of L. infantum promastigotes and subsequently cultured for 72 h with different cytokines with or without addition of splenic NK cells from B6 mice. Under some conditions, anti-TNF blocking antibody or a control IgG was added. SNs were tested for cytokine and NO2− content.
Data are expressed as mean (±SEM) of 3 to 6 independent experiments.
From these data we conclude that IL-12/IL-18-stimulated NK cells activate Leishmania-infected macrophages in a cell contact-independent but IFN-γ- and TNF-dependent manner for the expression of iNOS, which is the key effector mechanism in murine leishmaniasis (17, 50, 67).
DISCUSSION
NK cell cytotoxicity against host cells infected with nonviral pathogens.
Host cell lysis is considered to be a rapidly available and highly efficient effector mechanism of NK cells during infections with intracellular pathogens. Its protective role in viral diseases is well documented and highly plausible, because viruses require host cells for replication. Very little is known whether NK cell-mediated lysis of host cells also contributes to the control of nonviral pathogens. In the present study, we tested the hypothesis that myeloid cells infected with the facultative intracellular parasite Leishmania also become a target of NK cell cytotoxicity in vitro and in vivo. This hypothesis was based on several considerations. First, Leishmania released from lysed myeloid cells is thought to be highly susceptible to humoral defense mechanisms of the host (e.g., complement), so that target cell lysis even without simultaneous parasite destruction could be beneficial to the host organism. Second, NK cells and myeloid cells frequently colocalize during cutaneous or visceral leishmaniasis (3, 72), raising the possibility of functional interactions. Third, previous experiments involving activation, depletion, or transfer of NK cells provided evidence for a protective role of NK cells in cutaneous and visceral leishmaniasis (3, 17, 24, 28, 34, 36, 39, 43, 56–58, 61).
To our knowledge, the present study is the first to address the issue of NK cell-mediated target cell lysis in a nonviral infection model in vivo. The data clearly show that upon infection with Leishmania parasites WT macrophages do not become susceptible to cytolysis by activated NK cells either in vitro or in vivo. Resistance to NK cell lysis was paralleled by an unaltered expression of activating or inhibitory NK cell receptors on the surface of the infected host cells. For the in vivo analysis, we had to resort to a newly established L. infantum peritoneal infection model, because infected macrophages that were transferred i.v. into previously i.v. infected mice became trapped in the lung and did not home to the sites of NK cell activation in the spleen or liver (data not shown). The validity of the peritoneal model was ascertained by verifying the presence of activated NK cells in the peritoneal cavity after i.p. infection, which was unequivocally demonstrated by the expression of IFN-γ in peritoneal NK1.1+ CD3− cells (data not shown) and the prominent lysis of susceptible β2m−/− macrophage targets by these NK cells (Fig. 4).
Several previous studies suggested that NK cells exert cytotoxic effects on host cells infected with various nonviral intracellular pathogens. These include human monocytes harboring Mycobacterium tuberculosis (74), human erythrocytes carrying Plasmodium falciparum schizonts (53), and possibly also human neutrophils after phagocytosis of Haemophilus influenzae (47). There are also two reports that claimed in vitro NK cell cytotoxicity against BALB/c macrophages infected with promastigotes of L. major or L. amazonensis, respectively (1, 55). However, there are several problems with the experimental design of both studies. These include (i) the application of IL-2-activated killer (LAK) cells without further phenotypic characterization (55) or with proven contamination by NKT and CD8+ T cells (1), which are known to lyse and activate Leishmania-infected myeloid cells (10, 66), (ii) the use of extremely high LAK cell-Mφ ratios (≥10:1) and Leishmania-Mφ ratios (≥40:1) (55), and (iii) the nonquantitative analysis of cytotoxicity without including uninfected macrophages as controls (1).
Thus, on the basis of our own in vitro and in vivo results, we argue that highly purified NK cells do not exert a cytolytic effect against infected macrophages in vitro, except for the small degree of lysis already seen with uninfected cells. This notion is further supported by the observation that human immature DCs infected with L. infantum were resistant to NK cell-mediated lysis due to the upregulation of HLA-E (a ligand for the inhibitory NK cell receptor CD94/NKG2A) (10) and that LAK cells also failed to lyse mouse macrophages infected with the related pathogen Trypanosoma cruzi (79).
NK cells and cytokine-mediated macrophage activation.
IFN-γ and TNF are known activators of macrophages, and IFN-γ is the key cytokine for the induction of iNOS in mice. In vitro IFN-γ-mediated induction of iNOS requires endogenous TNF (for a review, see reference 6). As NK cells are an early source of IFN-γ in Leishmania infections (3, 17, 39, 57, 61), it might appear to be trivial to postulate NK cell cytokine-mediated macrophage activation. However, whether this actually occurs upon contact of activated NK cells with infected macrophages has never been tested. In fact, in response to Leishmania, macrophages can release IL-10 (49), which is one of the cytokines that is able to antagonize NK cell functions and to suppress the release of NK cell-activating cytokines (15, 73). In addition, NK cells themselves produce IL-10 (44, 46), which then might block macrophage activation, including the expression of iNOS (5, 6). The results reported in the present study convincingly show that in an NK cell/macrophage coculture system, IL-12 and IL-18 drive the production of sufficiently high levels of IFN-γ and TNF for the subsequent induction of macrophage antileishmanial activities. In accordance with our previous analyses on the origin of IFN-γ in macrophage cultures (60), the comparative measurement of IFN-γ in macrophage cultures versus NK cell/macrophage cocultures and intracellular cytokine staining identified NK cells as the primary source of IFN-γ (Table 1, cultures 19 versus 15, and data not shown). With respect to TNF, macrophages rather than NK cells appear to be the producers, because NK cells were negative for intracellular TNF (data not shown) and IFN-γ is known to activate Leishmania-infected macrophages for the expression of endogenous TNF (23).
Although IFN-γ is an essential component of the macrophage-activating effect of IL-12/IL-18-stimulated NK cells (Fig. 6C), it is possible that, in addition to IFN-γ, other soluble factors secreted by NK cells are involved. This is suggested by the observation that the decrease of the parasite burden in the macrophage-NK cell coculture system was considerably smaller when rmIL-12 plus rmIL-18 was replaced by rmIFN-γ (Fig. 6A). It is also worth noting that the role of TNF is not necessarily restricted to the synergistic induction of iNOS together with IFN-γ, as iNOS-independent antileishmanial effects of TNF have been repeatedly reported (63, 76). This possibility is supported by our observation that anti-TNF treatment of IL-12/IL-18-stimulated BM-Mφ/NK cell cocultures largely blocked the killing of Leishmania parasites, without causing a significant reduction of nitrite accumulation (Fig. 6D; Table 1, cultures 20 versus 19).
In some of our experiments on the interaction of NK cells with infected macrophages, we noticed a small decrease of the macrophage parasite burden even in the presence of nonactivated NK cells (Fig. 6B and C). The effect was IFN-γ and iNOS dependent (Fig. 6C), which argues against the possibility that naïve NK cells act by the release of perforin and other cytotoxins that are taken up by the neighboring infected macrophage and mediate Leishmania killing, as suggested for other pathogens in an NK cell-free in vitro system (75).
In conclusion, despite being potent cytotoxic effectors, NK cells activated by Leishmania infection fail to recognize infected macrophages as targets, most likely due to the lack of proper NK cell stimulatory signals. This finding provides a perfect example of host-pathogen interaction where Leishmania use macrophages as a safe niche for their initial survival, while the host avoids unwanted killing of infected cells, which otherwise could lead to the release and dissemination of viable parasites. However, due to their capacity to respond to IL-12 and IL-18 by releasing IFN-γ, NK cells efficiently assist macrophages in the control of Leishmania parasites.
ACKNOWLEDGMENTS
We are grateful to David Vöhringer and Thomas Winkler (Microbiology Institute and Department of Genetics, Erlangen, Germany) for providing mice, Silke Huber (Institute for Immunology, LMU München) for help with the preparation of bone marrow samples, Andrea Debus for technical assistance, Uwe Appelt (Sorting Core Facility, Erlangen, Germany) for cell sorting, and Evelyn Ullrich (Department of Internal Medicine 5, Erlangen, Germany) for discussions.
This study was supported by grants from the Deutsche Forschungsgemeinschaft to C.B. and U.S. (Bo 996/3-3; SFB643, Project A6).
Footnotes
Published ahead of print on 25 April 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Aranha F. C. S., Ribeiro U., Basse P., Corbett C. E. P., Laurenti M. D. 2005. Interleukin-2 activated natural killer cells may have a direct role in the control of Leishmania amazonensis promastigote and macrophage infection. Scand. J. Immunol. 62:334–341 [DOI] [PubMed] [Google Scholar]
- 2. Babic M., et al. 2010. Cytomegalovirus immunoevasin reveals the physiological role of “missing self” recognition in natural killer cell dependent virus control in vivo. J. Exp. Med. 207:2663–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bajenoff M., et al. 2006. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J. Exp. Med. 203:619–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bihl F. Primed antigen-specific CD4+ T cells are required for NK cell activation in vivo upon Leishmania major infection. J. Immunol. 185:2174–2181 [DOI] [PubMed] [Google Scholar]
- 5. Bogdan C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2:907–916 [DOI] [PubMed] [Google Scholar]
- 6. Bogdan C. 2009. Regulation and antimicrobial function of inducible nitric oxide synthase in macrophages, p. 367–378 In Russell D. G. (ed.), Phagocyte-pathogen interaction. ASM Press, Washington, DC [Google Scholar]
- 7. Bogdan C., Moll H., Solbach W., Röllinghoff M. 1990. Tumor necrosis factor-α in combination with interferon-γ, but not with interleukin 4 activates murine macrophages for elimination of Leishmania major amastigotes. Eur. J. Immunol. 20:1131–1135 [DOI] [PubMed] [Google Scholar]
- 8. Bogdan C., et al. 2001. Visceral leishmaniosis in a German child that had never entered a known endemic area: case report and review of the literature. Clin. Infect. Dis. 32:302–307 [DOI] [PubMed] [Google Scholar]
- 9. Bogdan C., Thüring H., Dlaska M., Röllinghoff M., Weiss G. 1997. Mechanism of suppression of macrophage nitric oxide release by IL-13. J. Immunol. 159:4506–4513 [PubMed] [Google Scholar]
- 10. Campos-Martin Y., et al. 2006. Immature human dendritic cells infected with Leishmania infantum are resistant to NK-mediated cytolysis but are efficiently recognized by NKT cells. J. Immunol. 176:6172–6179 [DOI] [PubMed] [Google Scholar]
- 11. Chlewicki L. K., Velikovsky C. A., Balakrishnan V., Mariuzza R. A., Kumar V. 2008. Molecular basis of the dual functions of 2B4 (CD244). J. Immunol. 180:8159–8167 [DOI] [PubMed] [Google Scholar]
- 12. Coles R. M., Mueller S. N., Heath W. R., Carbone F. R., Brooks A. G. 2002. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J. Immunol. 168:834–838 [DOI] [PubMed] [Google Scholar]
- 13. Colmenero P., et al. 2007. Qa-1(b)-dependent modulation of dendritic cell and NK cell cross-talk in vivo. J. Immunol. 179:4608–4615 [DOI] [PubMed] [Google Scholar]
- 14. Dalton D. K., et al. 1993. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259:1739–1742 [DOI] [PubMed] [Google Scholar]
- 15. D'Andrea A., et al. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon γ-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178:1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dhiman R., et al. 2009. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J. Immunol. 183:6639–6645 [DOI] [PubMed] [Google Scholar]
- 17. Diefenbach A., et al. 1998. Type 1 interferon (IFN-α/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity 8:77–87 [DOI] [PubMed] [Google Scholar]
- 18. Diefenbach A., Schindler H., Röllinghoff M., Yokoyama W., Bogdan C. 1999. Requirement for type 2 NO-synthase for IL-12 signaling in innate immunity. Science 284:951–955 [DOI] [PubMed] [Google Scholar]
- 19. DiSanto J. P., Muller W., Guy-Grand D., Fischer A., Rajewsky K. 1995. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. U. S. A. 92:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferlazzo G., et al. 2003. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur. J. Immunol. 33:306–313 [DOI] [PubMed] [Google Scholar]
- 21. Florido M., Correia-Neves M., Cooper A. M., Appelberg R. 2003. The cytolytic activity of natural killer cells is not involved in the restriction of Mycobacterium avium growth. Int. Immunol. 15:895–901 [DOI] [PubMed] [Google Scholar]
- 22. Gorak P. M. A., Engwerda C. R., Kaye P. M. 1998. Dendritic cells, but not macrophages produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28:687–695 [DOI] [PubMed] [Google Scholar]
- 23. Green S. J., Crawford R. M., Hockmeyer J. T., Meltzer M. S., Nacy C. N. 1990. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-γ stimulated macrophages by induction of tumor necrosis factor-α. J. Immunol. 145:4290–4297 [PubMed] [Google Scholar]
- 24. Haeberlein S., Sebald H., Bogdan C., Schleicher U. 2010. IL-18, but not IL-15, contributes to the IL-12-dependent induction of NK-cell effector functions by Leishmania infantum in vivo. Eur. J. Immunol. 40:1708–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hidore M. R., Nabavi N., Sonleitner F., Murphy J. W. 1991. Murine natural killer cells are fungicidal to Cryptococcus neoformans. Infect. Immun. 59:1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang S., et al. 1993. Immune response in mice that lack the interferon-γ receptor. Science 259:1742–1744 [DOI] [PubMed] [Google Scholar]
- 27. Kim E. O., et al. 2010. Unidirectional signaling triggered through 2B4 (CD244), not CD48, in murine NK cells. J. Leukoc. Biol. 88:707–714 [DOI] [PubMed] [Google Scholar]
- 28. Kirkpatrick C. E., Farrell J. P., Warner J. F., Dennert G. 1985. Participation of natural killer cells in the recovery of mice from visceral leishmaniasis. Cell. Immunol. 92:163–171 [DOI] [PubMed] [Google Scholar]
- 29. Koller B. H., Marrack P., Kappler J. W., Smithies O. 1990. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 248:1227–1230 [DOI] [PubMed] [Google Scholar]
- 30. Krebs P., et al. 2009. NK-cell-mediated killing of target cells triggers robust antigen-specific T-cell-mediated and humoral responses. Blood 113:6593–6602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lanier L. L. 2008. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 9:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laouar Y., Sutterwala F. S., Gorelik L., Flavell R. A. 2005. Transforming growth factor-b controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nat. Immunol. 6:600–607 [DOI] [PubMed] [Google Scholar]
- 33. Laskay T., Diefenbach A., Röllinghoff M., Solbach W. 1995. Early parasite containment is decisive for resistance to Leishmania major infection. Eur. J. Immunol. 25:2220–2227 [DOI] [PubMed] [Google Scholar]
- 34. Laskay T., Röllinghoff M., Solbach W. 1993. Natural killer cells participate in the early defense against Leishmania major infection in mice. Eur. J. Immunol. 23:2237–2241 [DOI] [PubMed] [Google Scholar]
- 35. Laubach V. E., Shesely E. G., Smithies O., Sherman P. A. 1995. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc. Natl. Acad. Sci. U. S. A. 92:10688–10692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laurenti M. D., et al. 1999. The role of natural killer cells in the early period of infection in murine cutaneous leishmaniasis. Braz. J. Med. Biol. Res. 32:323–325 [DOI] [PubMed] [Google Scholar]
- 37. Lee S. H., Kim K. S., Fodil-Cornu N., Vidal S. M., Biron C. A. 2009. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J. Exp. Med. 206:2235–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee S. H., Miyagi T., Biron C. A. 2007. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 28:252–259 [DOI] [PubMed] [Google Scholar]
- 39. Liese J., Schleicher U., Bogdan C. 2007. TLR9 signalling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur. J. Immunol. 37:3424–3434 [DOI] [PubMed] [Google Scholar]
- 40. Lisnic V. J., Krmpotic A., Jonjic S. 2010. Modulation of natural killer cell activity by viruses. Curr. Opin. Microbiol. 13:530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lutz M. B., et al. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77–92 [DOI] [PubMed] [Google Scholar]
- 42. Ma L. L., et al. 2004. NK cells use perforin rather than granulysin for anticryptococcal activity. J. Immunol. 173:3357–3365 [DOI] [PubMed] [Google Scholar]
- 43. Manna P. P., Chakrabarti G., Bandyopadhyay S. 2010. Innate immune defense in visceral leishmaniasis: cytokine mediated protective role by allogeneic effector cell. Vaccine 28:803–810 [DOI] [PubMed] [Google Scholar]
- 44. Maroof A., et al. 2008. Posttranscriptional regulation of II10 gene expression allows natural killer cells to express immunoregulatory function. Immunity 29:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marr K. J., et al. 2009. Cryptococcus neoformans directly stimulates perforin production and rearms NK cells for enhanced anticryptococcal microbicidal activity. Infect. Immun. 77:2436–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mehrotra P. T., et al. 1998. Production of IL-10 by human natural killer cells stimulated with IL-2 and/or IL-12. J. Immunol. 160:2637–2644 [PubMed] [Google Scholar]
- 47. Miyazaki S., et al. 2007. Gr-1high polymorphonuclear leukocytes and NK cells act via IL-15 to clear intracellular Haemophilus influenzae in experimental murine peritonitis and pneumonia. J. Immunol. 179:5407–5414 [DOI] [PubMed] [Google Scholar]
- 48. Mombaerts P., et al. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869–877 [DOI] [PubMed] [Google Scholar]
- 49. Mosser D. M., Zhang X. 2008. Interleukin-10: new perspectives on an old cytokine. Immunol. Rev. 226:205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murray H. W., Nathan C. F. 1999. Macrophage microbicidal mechanisms in vivo: reactive nitrogen vs. oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 189:741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Newman K. C., Riley E. M. 2007. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat. Rev. Immunol. 7:279–291 [DOI] [PubMed] [Google Scholar]
- 52. Obata-Onai A., et al. 2002. Comprehensive gene expression analysis of human NK cells and CD8(+) T lymphocytes. Int. Immunol. 14:1085–1098 [DOI] [PubMed] [Google Scholar]
- 53. Orago A. S., Facer C. A. 1991. Cytotoxicity of human natural killer (NK) cell subsets for Plasmodium falciparum erythrocytic schizonts: stimulation by cytokines and inhibition by neomycin. Clin. Exp. Immunol. 86:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Remer K. A., Roeger B., Hambrecht C., Moll H. 2010. Natural killer cells support the induction of protective immunity during dendritic cell-mediated vaccination against Leishmania major. Immunology 131:570–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Resnick M., et al. 1988. Lysis of murine macrophages infected with intracellular pathogens by interleukin 2-activated killer (LAK) cells in vitro. Cell. Immunol. 113:214–219 [DOI] [PubMed] [Google Scholar]
- 56. Sanabria M. X., Vargas-Inchaustegui D. A., Xin L., Soong L. 2008. Role of natural killer cells in modulating dendritic cell responses to Leishmania amazonensis infection. Infect. Immun. 76:5100–5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scharton T. M., Scott P. 1993. Natural killer cells are a source of IFN-γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 178:567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Scharton-Kersten T., Afonso L. C. C., Wysocka M., Trinchieri G., Scott P. 1995. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J. Immunol. 154:5320–5330 [PubMed] [Google Scholar]
- 59. Schleicher U., Bogdan C. 2009. Generation, culture and flow-cytometric characterization of primary mouse macrophages. Methods Mol. Biol. 531:203–224 [DOI] [PubMed] [Google Scholar]
- 60. Schleicher U., Hesse A., Bogdan C. 2005. Minute numbers of contaminant CD8+ T cells or CD11b+CD11c+ NK cells are the source of IFN-γ in IL-12/IL-18-stimulated mouse macrophage populations. Blood 105:1319–1328 [DOI] [PubMed] [Google Scholar]
- 61. Schleicher U., et al. 2007. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J. Exp. Med. 204:893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shinkai Y., et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855–867 [DOI] [PubMed] [Google Scholar]
- 63. Soares Rocha F. J., Schleicher U., Mattner J., Alber G., Bogdan C. 2007. Cytokines, signaling pathways, and effector molecules required for the control of Leishmania (Viannia) braziliensis in mice. Infect. Immun. 75:3823–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Solbach W., Forberg K., Kammerer E., Bogdan C., Röllinghoff M. 1986. Suppressive effect of cyclosporin A on the development of Leishmania tropica-induced lesions in genetically susceptible BALB/c mice. J. Immunol. 137:702–707 [PubMed] [Google Scholar]
- 65. Stanley A. C., et al. 2008. VCAM-1 and VLA-4 modulate dendritic cell IL-12p40 production in experimental visceral leishmaniasis. PLoS Pathog. 4:e1000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stefani M. M. A., Müller I., Louis J. 1994. Leishmania major-specific CD8+ T cells are inducers and targets of nitric oxide produced by parasitized macrophages. Eur. J. Immunol. 24:746–752 [DOI] [PubMed] [Google Scholar]
- 67. Stenger S., Donhauser N., Thüring H., Röllinghoff M., Bogdan C. 1996. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J. Exp. Med. 183:1501–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stenger S., et al. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121–125 [DOI] [PubMed] [Google Scholar]
- 69. Stenger S., Solbach W., Röllinghoff M., Bogdan C. 1991. Cytokine interactions in experimental cutaneous leishmaniasis. II. Endogenous tumor necrosis factor-α production by macrophages is induced by the synergistic action of interferon (IFN)-γ and interleukin (IL) 4 and accounts for the antiparasitic effect mediated by IFN-γ and IL-4. Eur. J. Immunol. 21:1669–1675 [DOI] [PubMed] [Google Scholar]
- 70. Sun J. C., Beilke J. N., Lanier L. L. 2009. Adaptive immune features of natural killer cells. Nature 457:557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sun J. C., Ma A., Lanier L. L. 2009. IL-15-independent NK cell response to mouse cytomegalovirus infection. J. Immunol. 183:2911–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Thalhofer C. J., Chen Y., Sudan B., Love-Homan L., Wilson M. E. 2011. Leukocytes infiltrate the skin and draining lymph nodes in response to the protozoan Leishmania infantum chagasi. Infect. Immun. 79:108–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tripp C. S., Wolf S. F., Unanue E. R. 1993. Interleukin 12 and tumor necrosis factor α are costimulators of interferon-γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. U. S. A. 90:3725–3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vankayalapati R., et al. 2005. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J. Immunol. 175:4611–4617 [DOI] [PubMed] [Google Scholar]
- 75. Walch M., et al. 2007. Perforin enhances the granulysin-induced lysis of Listeria innocua in human dendritic cells. BMC Immunol. 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wilhelm P., et al. 2001. Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking tumor necrosis factor. J. Immunol. 166:4012–4019 [DOI] [PubMed] [Google Scholar]
- 77. Yokoyama W. M. 2008. Mistaken notions about natural killer cells. Nat. Immunol. 9:481–485 [DOI] [PubMed] [Google Scholar]
- 78. Zal T., Volkmann A., Stockinger B. 1994. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J. Exp. Med. 180:2089–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zychlinsky A., Karim M., Nonacs R., Young J. D. 1990. A homogeneous population of lymphokine-activated killer (LAK) cells is incapable of killing virus-, bacteria-, or parasite-infected macrophages. Cell. Immunol. 125:261–267 [DOI] [PubMed] [Google Scholar]