Abstract
The use of a recombinant bacterial vector vaccine is an attractive vaccination strategy to induce an immune response to a carried protective antigen. The superiorities of live bacterial vectors include mimicry of a natural infection, intrinsic adjuvant properties, and the potential for administration by mucosal routes. Escherichia coli is a simple and efficient vector system for production of exogenous proteins. In addition, many strains are nonpathogenic and avirulent, making it a good candidate for use in recombinant vaccine design. In this study, we screened 23 different iron-regulated promoters in an E. coli BL21(DE3) vector and found one, PviuB, with characteristics suitable for our use. We fused PviuB with lysis gene E, establishing an in vivo inducible lysis circuit. The resulting in vivo lysis circuit was introduced into a strain also carrying an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible PT7-controlled protein synthesis circuit, forming a novel E. coli-based protein delivery system. The recombinant E. coli produced a large amount of antigen in vitro and could deliver the antigen into zebrafish after vaccination via injection. The strain subsequently lysed in response to the iron-limiting signal in vivo, implementing antigen release and biological containment. The gapA gene, encoding the protective antigen GAPDH (glyceraldehyde-3-phosphate dehydrogenase) from the fish pathogen Aeromonas hydrophila LSA34, was introduced into the E. coli-based protein delivery system, and the resultant recombinant vector vaccine was evaluated in turbot (Scophtalmus maximus). Over 80% of the vaccinated fish survived challenge with A. hydrophila LSA34, suggesting that the E. coli-based antigen delivery system has great potential in bacterial vector vaccine applications.
INTRODUCTION
Bacterial delivery of protein antigens has great potential due to the low cost of production and mucosal delivery. Two distinct approaches have been used when identifying specific bacterial delivery vectors, attenuated pathogens and commensals. These approaches are based on particular qualities that are unique to live bacteria. Many attenuated pathogenic bacteria can replicate in the host to some extent, with attributes such as cellular tropism, cell-to-cell spreading, and dissemination, resulting in strong humoral and cellular immunity. Furthermore, they can migrate to immune-inductive cells, carrying with them lipopolysaccharide or unmethylated CpG motifs as adjuvants, which are immune stimulatory macromolecules supporting a strong immune response (36). Representative attenuated pathogens include Salmonella enterica serovars Typhi and Typhimurium (17, 18, 23, 41), Shigella (3), Listeria monocytogenes (31), and Yersinia enterocolitica (33). Given the concern for biosafety, commensals have also been tested as antigen delivery vectors. Nonpathogenic Escherichia coli and food-grade Lactococcus lactis are the best cases, with accepted safety, cost-effectiveness, and minimal side effects (51, 52, 55).
An important quality of a live bacterial vector is its ability to present sufficient foreign antigen to the immune system to initiate the desired protective immune response(s) (16). Several approaches have been used to improve antigen delivery by bacterial vectors, including modification of the bacterial vector for better proficiency in invasion and colonization of deep effector lymphoid tissues and optimization of antigen expression for increased exposure of antigen to the host immune system by antigen secretion or antigen surface display. With respect to bacterial vector modification, to maintain the invasive abilities of attenuated Salmonella, Roy Curtiss III and colleagues have adopted an in vivo regulated delayed-attenuation strategy, such that the vaccine, at the time of immunization, exhibits almost the same abilities as a fully virulent wild-type strain to contend with stresses and successfully reach effector lymphoid tissues before display of attenuation to preclude onset of any disease symptoms (13). In other studies, nonpathogenic E. coli strains were genetically modified to be invasive by expressing the inv gene from Yersinia pseudotuberculosis, which conferred the ability to invade nonprofessional phagocytic cells, and the hly gene from Listeria monocytogenes, which allowed expression of listeriolysin O, a perforin cytolysin able to disrupt phagosomal membranes (8). Using such an invasive E. coli strain, efficient and stable protein transfer was observed after coincubation of bacteria with various macrophage or epithelial cell lines (12). However, a major limitation of these kinds of enhancements is the potential biological security concern regarding invasive bacteria in the animal host and the environment.
With respect to antigen expression optimization, Georgiou et al. (22) developed an efficient surface display system, Lpp-OmpA, which was used successfully to anchor different protein antigens onto the cell surface (27). Alternatively, antigen can be directly introduced into the host cell via the type III secretion system of Yersinia or Salmonella, which elicits strong cellular immune responses (21, 36). Other strategies include the use of type I (21, 36) and type II (28) secretion systems to enhance antigen delivery and immunogenicity. Despite the promise of both surface display and secretion, there are potential problems with both strategies. For instance, antigen surface display is usually hindered by a low display efficiency, which is presumably due to overproduction of the display protein perturbing the integrity of the outer membrane (56). Antigen secretion systems are often limited by the size of the polypeptide exported (16).
Over the past 5 years, we have been working on the design of recombinant bacterial vector vaccines. Our work has been focused on antigen display and secretion systems in the attenuated pathogen Vibrio anguillarum (15, 67). Recently we have concentrated our efforts on establishing a bacterial delivery system in which the vector bacteria could produce a large amount of intracellular antigen in vitro, and after being administrated into a host, the resulting strain would lyse efficiently in response to a specific in vivo signal to implement antigen release and biological containment. Based on this delivery model, two key elements need to be established: an in vivo-inducible regulation circuit and an efficient bacteria lysis factor.
Several in vivo-inducible promoters which respond to environmental stimuli in vivo, such as anaerobic conditions (54), oxidizing agent availability (10), and low magnesium (20) or iron concentrations, have been investigated previously. Although iron is one of the most abundant metals on earth, it is often bound to metal-chelating proteins in vivo, and thus the availability of free iron is extremely limited in hosts (5). Therefore, a low free iron concentration serves as an in vivo environmental signal (50). To adapt to the iron starvation conditions present in the host, bacteria have evolved various iron uptake, storage, and metabolism systems to acquire and utilize iron in this environment. Among them, a typical iron uptake regulon has been well described, in which the ferric uptake regulator protein (Fur) acts as a repressor for a number of genes involved in iron uptake, storage, and metabolism upon interaction with its corepressor, Fe2+, and no longer represses those genes in the absence of Fe2+ (2). Many iron-regulated promoters have been identified, such as Psuf from E. coli (49), PhutA from Vibrio cholerae (24), and PfatD from V. anguillarum (1), but thus far, none of them has been applied to build an in vivo-inducible regulation circuit for use in a bacterial vector vaccine.
Many bacterium-killing proteins have been identified, including porin-inducing protein Gef (30), bacterial toxin E3 (63), and EcoRI restriction endonuclease (62). One of the most widely studied killing factors is the lysis protein E from bacteriophage φX174 which is encoded by gene E. Protein E is a 91-amino-acid membrane protein that fuses the inner and outer membranes of Gram-negative bacteria, forming an E-specific lysis tunnel to expel the cytoplasmic contents. The remaining empty internal lumens of bacteria, largely devoid of nucleic acids, ribosomes, or other constituents, are called bacterial ghosts (38). E-mediated lysis has been shown to be fatal in a wide range of Gram-negative bacteria, such as S. enterica serovar Typhimurium, S. enterica serovar Enteritidis, V. cholerae, Klebsiella pneumoniae, Bordetella bronchiseptica, Helicobacter pylori, Actinobacillus pleuropneumoniae, Pseudomonas aeruginosa, Pseudomonas putida, Ralstonia eutropha, and Erwinia cypripedii (37). Thus, the bacteriophage φX174-originated protein E appears to be an effective and broad-spectrum bacterial lysis factor.
In this work, using E. coli BL21(DE3) as a bacterial vector, a strict iron-regulated promoter, PviuB, was identified and applied to control the expression of lysis gene E, thus establishing an in vivo-inducible lysis system. An IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible PT7-controlled antigen expression circuit was integrated into the lysis system to build a novel E. coli-based antigen delivery system. A protective antigen, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), from the important fish pathogen Aeromonas hydrophila was introduced into the delivery system, and its potential as a recombinant bacterial vector vaccine was confirmed by evaluation of immune protection in turbot.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. E. coli strains were grown at 37°C in lysogeny broth (LB) medium (1% tryptone, 0.5% yeast extract, 0.5% NaCl). V. anguillarum and Vibrio parahaemolyticus strains were cultured at 30°C in LB medium supplemented with 2% NaCl. When required, ampicillin (Amp) (100 μg/ml), kanamycin (Km) (25 μg/ml), isopropyl-β-d-thiogalactoside (IPTG) (0.5 mM), FeSO4 (40 μM), and/or 2,2′-dipyridyl (200 μM) was added.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Bacteria | ||
| E. coli | ||
| Top10F′ | General cloning strain | Invitrogen |
| H1717 | Indicator strain; fhuF::λplacMu | 58 |
| BL21(DE3) | F−ompThsdSgal; expression host, vaccine delivery vector | Invitrogen |
| DH5α | recA1endA1gyrA96thi1hsdR17supE44relA1lacZΔM15; gene source of iron-regulated promoters | Invitrogen |
| V. anguillarum MVM425 | Wile-type, Pacific Ocean prototype (O1 serotype) fish pathogen; gene source of iron-regulated promoters | Our lab |
| V. parahaemolyticus | Wild-type pathogen from fish; gene source of iron-regulated promoters | Our lab |
| A. hydrophila | Wild-type pathogen from fish; gene source of gapA | Our lab |
| Genomes and plasmids | ||
| V. cholerae chromosome I | O1 biovar El Tor strain N1696; gene source of iron-regulated promoters | Biao Kan |
| pEIB1 | Endogenous plasmid of V. anguillarum MVM425; gene source of iron-regulated promoters | Our lab |
| pBV220 | Prokaryotic expression vector; source of rrnBT1T2 terminator and MCS | Zhiqing Zhang |
| mTn5gusA-pgfp12 | Carrying the gfpuv gene; gene source of gfp | Chuanwu Xi |
| φX174 genome | E gene source | NEB |
| pMD19T-simple | Cloning vector; Ampr | Takara |
| pMD19TB | pMD19T-simple derivative; PviuB insertion | This study |
| pUC18 | Cloning vector; Ampr | Takara |
| pET28a | Expression vector; Kmr | Novagen |
| pUT | Promoter-screening vector; pUC18 derivative with lac promoter and MCS deletion and with rrnBT1T2 terminator and MCS from pBV220 insertion | This study |
| pUTG | pUT derivative containing gfp open reading frame | This study |
| pUTBG | pUT derivative containing PviuBgfp TT | This study |
| pUTa | pUT derivative; replacement of pBR322 ori with p15A ori | This study |
| pUTaBE | pUTa derivative containing PviuaBE TT | This study |
| pETGA | pET28a derivative containing PT7gapA TT | This study |
Plasmid construction.
The lac promoter and multiple-cloning site (MCS) of pUC18 were deleted by digestion with PvuII/NdeI and replaced with a 506-bp sequence containing the MCS and the rrnBT1T2 terminator from plasmid pBV220 to yield plasmid pUT. The 720-bp gfpuv1 gene, amplified from plasmid mTn5gusA-pgfp12, was inserted into BamHI/PstI-digested pUT to yield the promoter-screening plasmid pUTG. A series of primers were designed to amplify the candidate promoters from bacterial chromosomes or plasmids (Table 2). The amplified promoter products, possessing the native start codon, Shine-Dalgarno sequence, and −35 and −10 promoter elements plus additional upstream bases, were inserted into pUTG, and the resultant plasmids were transformed into E. coli BL21(DE3) for iron-regulated promoter screening.
Table 2.
Primers used for cloning
| Primer | Sequence (5′→3′)a |
|---|---|
| MCST-for | CAGCTGTGGGGTGTGTGATACGAAACGAAG |
| MCST-rev | CATATGGAGTTTGTAGAAACGCAAAAAGGC |
| GFP-for | CGGAATCCATGGCTAGCAAAGGAGAA |
| GFP-rev | AACTGCAGTTATTTGTACAGTTCATC |
| PfatD-for | TCCCCCGGGAAAGCCTTGAAGAGCACG |
| PfatD-rev | CGGGATCCTTAGAATGCTCTCCAGA |
| PhuvA-for | CGGAATTCGCGCAGCTTTGCTTGC |
| PhuvA-rev | CGGGATCCCTGAGTTTTACCTTA |
| PhuvX-for | CGGAATTCTTGGGCTTGTTGGCTCTC |
| PhuvX-rev | CGGGATCCTTCCAGTTCGTTCTTCGC |
| PtonB-for | CGGAATTCGCCACGAGCCAACTTCCC |
| PtonB-rev | CGGGATCCATAACAACAAAGCGTTC |
| PvabA-for | CGGAATTCCTTTTGATAGTTCGGTGG |
| PvabA-rev | CGGGATCCTTTCCTAACTTTACTC |
| PentC-for | CGGAATTCGACGCTGGTGGAAACAATACGC |
| PentC-rev | CGGGATCCATCATCCTCCACAAAAT |
| PfhuF-for | CGGAATTCCACTAGAATGCGCCTCCGTGGTA |
| PfhuF-rev | CGGGATCCAATCGGGATAGTAATC |
| Pfiu-for | CGGAATTCGACCTACACTTATCAGGCACTACCC |
| Pfiu-rev | CGGGATCCTTTGCAGGTGACTTTTTC |
| PyncE-for | CGGAATTCTGTTGGATGTTTGCCCTTGC |
| PyncE-rev | CGGGATCCTGACGACTCCCTTTGAT |
| Pfes-for | CGGAATTCTTTTGCGGATTTCATCTGCGGT |
| Pfes-rev | CGGGATCCTTCGTCATTCAGACGCT |
| PfecI-for | CGGAATTCCGTGCGCCACAACCGTCCTCCGTAT |
| PfecI-rev | CGGGATCCGCGGAGTGCATCAAAAGTT |
| Psuf-for | CGGAATTCCCAGAGCAATCTTTCACCTGCCCAATV |
| Psuf-rev | CGGGATCCCGATTTACCTCACTTCATC |
| Pfes-for | CGGAATTCCGCACCGAGTTACGGCTGCTTAC |
| Pfes-rev | CGGGATCCTTCGTCATTCAGACGCT |
| PiutA-for | CGGAATTCTTTCAAACATCGCAAACCATCAC |
| PiutA-rev | CGGGATCCTGTATTTATCTTTTTGTTG |
| PpsuA-for | CGGAATTCCGTGACAGACCAATCTTTGGCCGGTAG |
| PpsuA-rev | CGGGATCCTCCATAATTCCGTTTAGTTGT |
| PpvsA-for | CGGAATTCCCGCCAAAGGGGTCAGGGTCAAT |
| PpvsA-rev | CGGGATCCGAGCTTTCCTAATTTTCTAAC |
| PfhuA-for | CGGAATTCGACCGACAGATCGACCAATTTCA |
| PfhuA-rev | CGGGATCCGATAAAACCTCTGCTATAGA |
| PhutW-for | CGGAATTCCTTCGCTACCGATTTGGATGGGA |
| PhutW-rev | CGGGATCCTTCTCAAGCCAATCCCCATACGACA |
| PhutA-for | TCCCCCGGGAATTTTTCGGTCGCAGCCAAGA |
| PhutA-rev | CGGGATCCTTGAACTACCTGCAATTTCCA |
| PvibF-for | CGGAATTCTGCTGTGCCTGCGATCAGTGCCA |
| PvibF-rev | CGGGATCCCTTTAAACCCACAGATTCA |
| PviuA-for | CGGAATTCCGACTGAGCGATGTAAAACCTTA |
| PviuA-rev | CGGGATCCTTGAATTTCTCCTTAACTC |
| PviuB-for | CGGAATTCCACGTTGGTATGCGACCTCTTCA |
| PviuB-rev | CGGGATCCTCTTTCACCTAATTTATCTTA |
| PtonB1-for | CGGAATTCCGATACCGCCTGCATGGGCTGAA |
| PtonB1-rev | CGGGATCCGATGATCAGCGATATTGCTCCTG |
| PirgB-for | CGGAATTCATCCGAGCCGTACAGCGTAGACA |
| PirgB-rev | CGGGATCCAGGTATTTGACCCTTAAAG |
| E-for | CGGAATCCATGG TACGCTGGACTTTGTGGGAT |
| E-rev | AACTGCAGTCACTCCTTCCGCACGTAAT |
| GapA-for | CGGGATCCATGACTATCAAAGTAGGTATTAAC |
| GapA-rev | CCCAAGCTTTTACTTAGAGATGTGAGCGATC |
Restriction enzyme sites used for cloning of PCR products are underlined.
pUTa was constructed by replacement of the pBR322 replication origin of pUT with the p15A origin from pACYC184. The 276-bp E gene, amplified from the φX174 genome, was inserted into BamHI/PstI-digested pUTa to yield pUTaE. PviuB was then ligated into EcoRI/BamHI-digested pUTaE, resulting in the iron-regulated lysis plasmid pUTaBE. The 996-bp gapA gene amplified from the A. hydrophila LSA34 chromosome was inserted into BamHI/HindIII sites of pET28a under the control of the T7 promoter, generating pETGA. Both pUTaBE and pETGA were transformed into E. coli BL21(DE3) for E lysis and antigen GAPDH expression.
Detection of GFP synthesis in iron-limiting medium.
Overnight cell cultures were inoculated (1:100, vol/vol) into fresh LB medium containing appropriate antibiotics and cultured in a shaker at 200 rpm and 37°C. At early log phase, typically an optical density at 600 nm (OD600) of 0.8 to 1.0, 2,2′-dipyridyl was added to induce the expression of green fluorescent protein (GFP). After 20 h of iron-limiting induction, a 1-ml cell culture sample was taken, centrifuged at 12,000 × g for 3 min, washed, and resuspended in phosphate-buffered saline (PBS) (pH 7.2) to the same OD600 value (OD600 = 1.0). For each sample, 100 μl of cell suspension was added to a 96-well flat-bottom polystyrene plate (Costar) and measured with a fluorescence plate reader (Tecan, GENios Pro, Austria). The excitation wavelength was set at 485 nm, and emission was detected at 535 nm.
Fur titration assay.
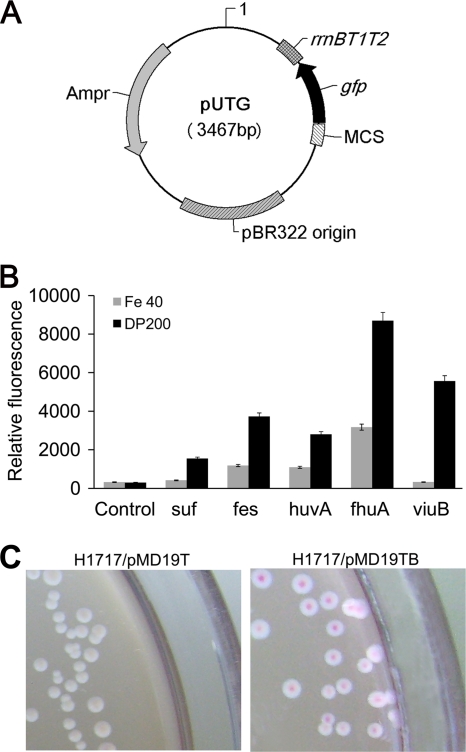
The Fur-regulated promoter carried on a multicopy plasmid can be identified by transformation into E. coli strain H1717. This strain carries a Fur-regulated fhuF::lacZ gene fusion which is particularly sensitive to changes in the concentration of the Fur repressor (58). The Fur-regulated promoter with Fur boxes introduced on a multicopy plasmid in E. coli H1717 will compete with Fur proteins, which can dimerize and repress the fhuF::lacZ gene fusion in the presence of iron, and thus lower the amount of Fur molecules in cells. The decrease of Fur molecules can derepresses the transcription of the lacZ gene and lead to a Lac+ phenotype. This can be used as a selectable marker for cloned Fur-regulated promoters, since Lac+ colonies are red on MacConkey plates supplemented with Fe, while Lac− colonies remain white.
In this experiment, the viuB promoter was inserted into the pMD19T-simple vector, and the resultant recombinant plasmid, pMD19TB, was transferred into E. coli indicator strain H1717 competent cells, producing strain E. coli H1717/pMD19TB. At the same time, E. coli H1717 with pMD19T-simple vector (E. coli H1717/pMD19T) was used as a negative control. The overnight cell cultures were diluted to spread on MacConkey plates with 25 μM FeSO4 for colony color detection. The experiment was performed in triplicate.
Detection of GFP synthesis in vivo in zebrafish.
At the beginning, an experiment was performed to test whether GFP could be produced during the process of immersion. BL21(DE3)/pUTG or BL21(DE3)/pUTBG was incubated in the immersion solution, and samples were taken from the immersion solution at regular times and adjusted to identical cell density for fluorescence detection. BL21(DE3)/pUTBG was also cultured in iron-limited LB medium to induce GFP synthesis, and the bacterial sample, adjusted to the identical cell density, was taken as the positive control for analysis.
Zebrafish were cultured in a laboratory breeding system, and their care and feeding followed established protocols (http://zfin.org/zf_info/zfbook/zfbk.html). The transparent zebrafish larvae were bred as previously described (29). Overnight cell cultures of recombinant E. coli were inoculated (1:100, vol/vol) into fresh LB medium containing antibiotics and cultured in a shaker at 200 rpm and 37°C for 5 h, and then the cells were harvested and resuspended in phosphate-buffered saline (PBS) (pH 7.2). Zebrafish at the age of 6 to 8 days were selected and immersed in cell suspensions of E. coli BL21(DE3) and E. coli BL21(DE3)/pUTBG at a concentration of 108 viable bacteria per ml for 1 h, and after being washed with PBS at least four times, the infected fish were bred in fresh water. The GFP expression in vivo in zebrafish was analyzed with an inverted fluorescence microscope and by confocal microscopy at 9 h postinfection.
For microscopy analysis, all the fish were anesthetized with MS-222 (Tricaine methanesulfonate; Sigma). An inverted fluorescence microscope (Olympus IX71 with a ×20 zoom magnification) was used. GFP fluorescence was detected by exposure of the fish to UV light in the excitation range of 450 to 490 nm. Images were captured using a Hamamatsu OrcaIIIm charge-coupled-device (CCD) camera. Fish were further examined with an inverted Leica TCS SP5 confocal microscope. The samples were detected using Plan Apo 20.0×/0.70 water-corrected lenses. Differential interference contrast (DIC) images of the fish were first captured with the DC300 camera using Leica IM50 software, and the GFP fluorescence was then detected by scanning samples using the 488-nm argon laser line for excitation.
Assay for E. coli lysis in vitro.
E. coli strains BL21(DE3)/pUTaBE and BL21(DE3)/pUTa were grown at 37°C overnight with 40 μM FeSO4 to ensure tight repression of lysis gene E. To induce E expression, the cultures were diluted to an OD600 of 0.1 and cultured in a shaker at 200 rpm and 37°C. At early log phase (OD600 = 0.3 to 0.4), 2,2′-dipyridyl was added to cultures to create iron-limiting conditions. Cell samples were taken at 30 min, 60 min, 90 min, and 120 min postinduction to measure both the OD600 and the CFU to determine the growth and lysis of the bacteria in iron-limiting medium.
The cell samples taken at 30 min and 120 min postinduction were harvested by centrifugation at 4,000 × g for 15 min, washed twice in PBS (pH 7.2), and incubated with glutardialdehyde (2.5% in PBS) at 4°C for 2 h. Cells were rinsed 3 times with the same buffer and then dehydrated with a graded series of ethyl alcohol and isoamyl acetate solutions. Following the final dehydration, cells were dried with liquid CO2, mounted on the holder with silver paint, and sputtered with gold-palladium using a Polaron high-resolution sputter coater. All scanning electron micrographs were taken with a Hitachi S-4800 scanning electron microscope.
Assay for antigen release in E. coli in vitro.
The recombinant E. coli strains BL21(DE3)/pETGA+pUTaBE and BL21(DE3)/pETGA+pUTa were grown in LB medium at 37°C with ampicillin, kanamycin, and FeSO4. At early log phase, 0.5 mM IPTG was added to induce gapA expression and the culture incubated for an additional 5 h. The cultures were then subcultured in a shaker to obtain an initial OD600 of 0.1, and 2,2′-dipyridyl was added to cultures at early log phase (OD600 = 0.3 to 0.4) to induce cell lysis. After 2 h of induction for cell lysis, the supernatants and cell debris were separated by centrifugation at 4,000 × g for 15 min and the two fractions were analyzed by enzyme-linked immunosorbent assay (ELISA) to quantify the antigen GAPDH. ELISA was performed in 96-well flat-bottom polyvinyl microtiter plates (Costar). The wells were coated with 100 μl of each supernatant fraction and intracellular fraction by overnight incubation at 4°C. Unbound proteins were removed by washing with PBS containing 0.05% Tween 20 (PBST), and the wells were blocked with 200 μl of PBST containing 1% bovine serum albumin (BSA) for 1 h at 37°C. After removal of the blocking solution and washing three times with PBST, the plate was incubated for 2 h with rabbit anti-GAPDH antibody (YingJi Technology, Shanghai, China) at a dilution of 1:50,000 (vol/vol). After three washes, the plate was incubated for another 1 h at 37°C with horseradish peroxidase-conjugated goat anti-rabbit antibody (Jackson Immuno Research Laboratories) at a dilution of 1:5,000 (vol/vol). Finally, the wells were washed three times with PBST, and tetramethylbenzidine (TMB) solution (Tiangen Biotech, Beijing, China) was added for color development. After addition of 2 M H2SO4 for termination of the reaction, the OD450 in each well was measured with a microplate reader (model 550; Bio-Rad, Hercules, CA).
Analysis of bacterial lysis and antigen release in zebrafish.
After 5 h of IPTG induction for GAPDH synthesis in the recombinant E. coli BL21(DE3)/pETGA+pUTaBE and BL21(DE3)/pETGA+pUTa strains as described above, the cultures were washed and resuspended in PBS. A total of 180 healthy zebrafish with average weight of 2 g were selected and divided randomly into three groups. The fish in groups 1 to 3 were injected intraperitoneally (i.p.) with 5 μl of PBS, E. coli BL21(DE3)/pETGA+pUTa suspension (107 CFU per fish), or E. coli BL21(DE3)/pETGA+pUTaBE suspension (107 CFU per fish). At 4, 9, and 24 h postadministration, 20 fish with similar weights were randomly taken from each experimental group and the tissues surrounding and including the abdominal cavities with similar size were collected; the weight of each tissue sample was kept at between 6 and 7 g. The tissue samples were then homogenized in 15 ml PBS. In order to determine the growth and lysis of E. coli in zebrafish, 1 ml of each homogenate sample was diluted serially in PBS and plated in triplicate on LB agar containing kanamycin and ampicillin to count the CFU. In addition, the remaining homogenate of each sample was centrifuged at 4,000 × g for 15 min. The resulting supernatant was further concentrated to 200 μl by using an Amicon Ultra-15 centrifugal filter unit (10-kDa cutoff) (Millipore). The enriched supernatant was analyzed by Western blotting, performed as described previously (67), to assay antigen release in vivo.
Vaccination and challenge.
E. coli BL21(DE3)/pETGA+pUTaBE, E. coli BL21(DE3)/pETGA+pUTa, and purified protein GAPDH were used as immunogens. All the vaccination and challenge experiments were independently repeated three times.
Turbot (Scophtalmus maximus) weighing approximately 10 g each were obtained from an aquaculture farm in Shandong Province, China, and were reared and acclimated for 30 days before the experiment. The recombinant E. coli BL21(DE3)/pETGA+pUTaBE and BL21(DE3)/pETGA+pUTa were induced to produce large amounts of GAPDH cytoplasmically as described above (see “Assay for antigen release in E. coli in vitro”), and the cell cultures were washed and resuspended in PBS for vaccination. Four groups (30 fish in each) were vaccinated by intraperitoneal (i.p.) injection with E. coli BL21(DE3)/pETGA+pUTaBE (107 CFU per fish), E. coli BL21(DE3)/pETGA+pUTa (107 CFU per fish), purified GAPDH antigen with an equal volume of Freund's complete adjuvant (Sigma) (20 μg per fish), or PBS (0.1 ml per fish) as a control. The fish were fed twice daily with commercial turbot feed and reared in aquaria supplied with a continuous flow of recycling water at 16 to 18°C.
Four weeks after immunization, the four groups were injected i.p. with wild-type A. hydrophila LSA34 (5.0 × 107 CFU per fish). Mortality was recorded for 12 days after challenge, and the observation of surviving fish was extended to 4 weeks. The significant difference and the relative percent survival (RPS) were calculated by using Fisher's exact test and the formula (46) RPS = [1 − (% mortality in vaccinated fish/% mortality in control fish)] × 100, respectively.
RESULTS
Screening for iron-regulated promoters.
To screen iron-regulated promoters for our system, a screening plasmid, pUTG, was constructed from pUC18 as shown in Fig. 1A. The GFP gene was taken as the reporter, the multicloning site adjacent to the GFP gene was used to insert the promoter sequence, and a strong rrnBT1T2 terminator derived from the rrnB rRNA operon of E. coli was placed downstream of the GFP gene to protect against read-through transcription. It was not necessary to introduce the fur gene (encoding the regulatory protein Fur, which is essential for iron-dependent promoters) into the screening plasmid pUTG, since the Fur protein is abundant, at 5,000 to 10,000 copies per E. coli cell, and is highly conserved in many Gram-negative and Gram-positive bacteria (66). Thus, the chromosomal fur gene in the E. coli host was adequate to direct the synthesis of sufficient Fur repressor to repress the plasmid-located iron-regulated promoters.
Fig. 1.
Iron-regulated promoter screening. (A) Plasmid map of the reporter vector pUTG. Gene coding regions are represented on the vector map by arrows. rrnBT1T2, ribosomal terminators T1 and T2; gfp, the green fluorescence protein reporter gene; MCS, multiple-cloning site; Ampr, ampicillin resistance gene. (B) GFP expression levels in E. coli carrying Psuf, Pfes, PfhuA, PviuA, and PviuB when grown in LB medium containing 40 μM FeSO4 (Fe 40) as an iron-rich condition or 200 μM 2,2′-dipyridyl (DP200) as an iron-limiting condition. The error bars represent the standard deviations (SD) for three independent experiments performed in triplicate. (C) Fur titration assay for PviuB. E. coli H1717/pMD19T and H1717/pMD19TB were plated separately on MacConkey medium with 25 μM FeSO4.
Iron-dependent gene regulation mechanisms have been thoroughly studied in many different bacteria (39, 40, 45, 59). Based on published reports, 23 candidate promoters were selected from the iron uptake, storage, and metabolism systems of four different bacteria, V. parahaemolyticus, V. cholerae, E. coli, and V. anguillarum; detailed information is shown in Table 3. Each promoter was fused in frame with the GFP gene, and its transcription in LB medium supplemented with 40 μM FeSO4 (iron-rich medium) or 200 μM 2,2′-dipyridyl (iron-limiting medium) was investigated. Samples were taken at defined times and adjusted to an OD600 of 1 to measure the green fluorescence emitted by GFP, and the promoter strength and regulation could be simply correlated with the GFP fluorescence. Among all 23 candidate promoters, Psuf, Pfes, PhuvA, PfhuA, and PviuB showed relatively high transcription activities, with relative fluorescence (RF) values of over 1,500 in iron-limiting medium (Fig. 1B and data not shown), and varied transcription strengths were demonstrated in iron-rich medium. Since strict regulation is a critical feature of an expression system when the recombinant protein is toxic to the host, it is crucial to limit target gene expression to basal levels until an inducer is added. The induction ratio is often used to evaluate the degrees of strict transcription control. Based on the data given in Fig. 1B, the induction ratios of Psuf, Pfes, PhuvA, PfhuA, and PviuB were calculated as 11, 4, 3, 3, 11, and 195, respectively. Promoter PviuB, which showed both high transcription efficiency and tight regulatory control, was determined to be the best choice for iron-regulated expression of lysis gene E in E. coli. The PviuB origin gene viuB encodes a cytoplasmic protein necessary for ferric vibriobactin utilization in V. cholerae. By bioinformatics analysis, besides basic promoter elements of the transcriptional start site, −10 region, −35 region, and Shine-Dalgarno sequence, a potential Fur binding region was found around the −10 region in the promoter sequence, and the Fur box was believed to be the Fur-Fe2+ complex binding site for repressing transcription under iron-rich conditions (6). To further confirm the existence of the Fur box in the PviuB sequence, a Fur titration assay was performed. As shown in Fig. 1C, the colonies of the negative-control strain E. coli H1717/pMD19T were colorless; however, E. coli H1717/pMD19TB showed a red colony phenotype when grown on MacConkey medium with 25 μM FeSO4. This means that the PviuB sequence contains a Fur binding region, which could compete the Fur-iron complex with the fhuF promoter to derepress transcription of the lacZ gene.
Table 3.
Iron-regulated promoter candidates used in this work
| Promoter | Origin gene | Biological function | Origin bacterium | Reference(s) |
|---|---|---|---|---|
| PfatD | fatDCBA operon | Iron transport operon in plasmid pJM1 | V. anguillarum | 9, 59 |
| PvabA | vabABC operon | Encodes 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase | V. anguillarum | 1 |
| PhuvA | huvA operon | Encodes outer membrane heme receptor protein | V. anguillarum | 43 |
| PhuvX | huvXZ operon | Uncharacterized function | V. anguillarum | 43 |
| PtonB | tonB1exbB1D-huvBCD operon | Encodes heme-transporting protein | V. anguillarum | 43 |
| PhutA | hutA operon | Encodes outer member protein required for heme iron utilization | V. cholerae | 24 |
| PhutW | hutWXZ operon | Unknown gene, linked to hutZ | V. cholerae | 65 |
| PfhuA | fhuA operon | Encodes outer membrane receptor for ferrichrome | V. cholerae | 53 |
| PtonB1 | tonB1exbB1D1 operon | Transport of heme across the inner membrane | V. parahaemolyticus | 47 |
| PvibF | vibF operon | Encodes protein involved in ferric vibriobactin biosynthesis | V. cholerae | 7 |
| PviuA | viuA operon | Encodes vibriobactin outer membrane receptor | V. cholerae | 35 |
| PviuB | viuB operon | Vibriobactin utilization gene | V. cholerae | 6 |
| PirgB | irgB operon | Encodes iron-regulated transcriptional activator | V. cholerae | 41 |
| PiutA | iutA operon | Encodes ferric aerobactin receptor precursor | V. parahaemolyticus | 15 |
| PpvuA | pvuBCDE operon | Encodes ferric siderophore receptor homolog | V. parahaemolyticus | 60 |
| PpvsA | pvsABCDE operon | Encodes ferric vibrioferrin receptor | V. parahaemolyticus | 60 |
| PentC | entCEBA operon | Enterobactin biosynthesis | E. coli | 11 |
| PfecI | fecIR operon | Encodes Fe-citrate transport regulator | E. coli | 57 |
| Pfes | fes operon | Fe-enterobactin utilization | E. coli | 26 |
| Pfiu | ybiXL operon | Encodes TonB-dependent outer membrane receptor | E. coli | 4 |
| PfhuF | fhuF operon | Reduction of Fe(III) in ferrioxamine B | E. coli | 44 |
| Psuf | suf operon | Fe-S formation | E. coli | 48 |
| PyncE | yncE operon | Encodes pyrroloquinoline quinone-containing periplasmic oxidase | E. coli | 49 |
PviuB transcription in zebrafish.
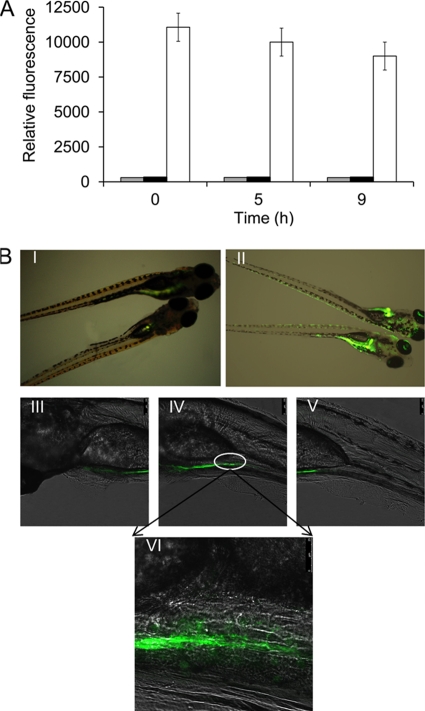
Since free iron is limited in vivo, we wanted to determine whether PviuB could sense the iron limitation signal in an animal host and initiate the target gene transcription in an in vivo-responsive manner. First, whether GFP could be produced in the immersion solution before entry into the animal host was identified by culturing E. coli BL21(DE3)/pUTBG in immersion solution. Samples were taken from the immersion solution at regular times and adjusted to the identical cell density for fluorescence detection. Compared with the positive control, basically no positive GFP signal was detected in both E. coli BL21(DE3)/pUTG and E. coli BL21(DE3)/pUTBG during the process of in vitro immersion, indicating that GFP synthesis could not be induced in the immersion solution (Fig. 2A).
Fig. 2.
GFP expression controlled by PviuB in vivo in zebrafish. (A) GFP expression levels of BL21(DE3)/pUTG (gray bars) and BL21(DE3)/pUTBG (black bars) incubated in immersion solution. BL21(DE3)/pUTBG (white bars) was induced to synthesize GFP as a positive control. Immersion solution was prepared as phosphate-buffered saline (PBS) with 1% bovine serum albumin. (B) Visualization of GFP synthesis under the control of PviuB in zebrafish by fluorescence microscopy. Fish were immersed in bacterial suspensions (108 CFU/ml) of BL21(DE3)/pUTG (panel I) and BL21(DE3)/pUTBG (panel II) for 1 h and detected at 9 h postinfection. For further analysis, zebrafish infected by BL21(DE3)/pUTBG were observed by confocal microscopy. Panels III, IV, and V, different parts of the fish from head to tail, respectively (magnification, ×400); panel VI, details of the site marked by a box in panel IV (magnification, ×1,000).
For testing the in vivo-responsive transcription of PviuB, transparent zebrafish larvae were used as the animal model. The fish were immersed in a cell suspension containing 108 CFU/ml of recombinant E. coli BL21(DE3)/pUTBG or E. coli BL21(DE3)/pUTG and examined at regular intervals using an inverted fluorescence microscope at a magnification of ×20. As shown in Fig. 2B, compared with the control E. coli BL21(DE3)/pUTG, which showed only a negative background fluorescence in zebrafish (Fig. 2B, panel I), E. coli BL21(DE3)/pUTGB resulted in strong fluorescence signal in the fish gastrointestinal tract and also weaker fluorescence signals around the skin mucosa and the gill sites at 9 h postinfection (Fig. 2B, panel II). These results suggested that PviuB could respond to the iron-limiting signal in vivo in zebrafish to initiate the GFP expression. Under this condition, it is most possible that the entry of E. coli into the gastrointestinal tract depends on host activities such as drinking of the water or ingestion. In addition, the gills and the skin are also the sites for bacterial entrance into the fish host (48). For further observation, the fish sample was transferred to a confocal microscope, and the front, middle, and rear parts of the fish are displayed in Fig. 2B, panels III to V. Under a magnification of ×1,000, an obvious fluorescence signal was detected in the gastrointestinal tract (Fig. 2B, panel VI). Taken together, these results indicate that PviuB is an efficient and strictly regulated promoter that is responsive to iron signals both in vitro and in vivo, indicating potential in establishing an in vivo-inducible bacterial lysis system.
Iron-regulated E. coli lysis in vitro.
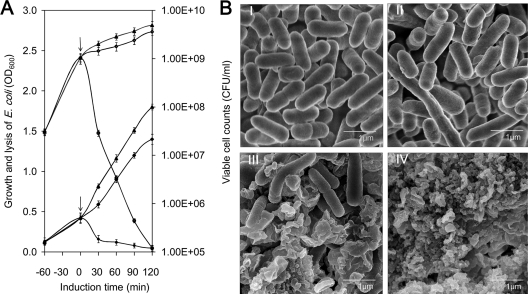
For achieving iron-regulated E. coli lysis, the φX174 gene E, as an E. coli-sensitive lysis factor, was placed under transcriptional control of PviuB and inserted into the medium-copy-number number plasmid pUTa to yield pUTaBE. The lysis behavior of BL21(DE3)/pUTaBE was analyzed in a 30-ml liquid culture. As shown in Fig. 3A, during the 2-h induction, the OD600 value of BL21(DE3)/pUTaBE cell cultures rapidly declined nearly to zero, and correspondingly the viable cell number dropped from 109 CFU/ml to 105 CFU/ml, with a calculated lysis ratio of 99.99% ± 0.01%. The control strain BL21(DE3)/pUTa grown with 2,2′-dipyridyl and strain BL21(DE3)/pUTaBE grown without 2,2′-dipyridyl displayed normal cell growth. Further, electron microscopy observation showed a regular cellular morphology of BL21(DE3)/pUTa (Fig. 3B, panel I) in the presence of 2,2′-dipyridyl, indicating that the inducer 2,2′-dipyridyl itself did not influence cell growth or cause cell lysis. In the absence of 2,2′-dipyridyl, a few BL21(DE3)/pUTaBE cells were longer than normal under electron microscopy (Fig. 3B, panel II). The cause for this phenomenon might be that the trace amount of E protein from leaky expression might inhibit cell division to some extent, since E-mediated lysis depends on cell division activities of E. coli (64). In contrast, many of the BL21(DE3)/pUTaBE cells had lysed by 30 min after induction (Fig. 3B, panel III), and nearly all the E. coli cells collapsed into cell debris by 2 h postinduction (Fig. 3B, panel IV). All the results confirmed that the promoter PviuB could control the transcription of lysis gene E and efficiently lyse the E. coli host in an iron-responsive manner.
Fig. 3.
Growth and lysis of BL21(DE3)/pUTaBE induced under iron-limiting conditions. (A) Growth and lysis (solid symbols) and viable cell counts (open symbols) of E. coli grown in LB. BL2(DE3)/pUTa with 2,2′-dipyridyl induction (diamonds), BL21(DE3)/pUTaBE with 2,2′-dipyridyl induction (circles), and BL21(DE3)/pUTaBE without 2,2′-dipyridyl induction (triangles) are shown. At 0 min, 2,2′-dipyridyl was added (↓). Standard deviations were calculated from the results from three independent experiments. (B) Scanning electron micrographs of E. coli BL21(DE3)/pUTa (without gene E) with 2,2′-dipyridyl addition (panel I) and of E. coli BL21(DE3)/pUTaBE (with gene E) without 2,2′-dipyridyl addition (panel II) and at 30 min (panel III) and 2 h (panel IV) after 2,2′-dipyridyl addition.
Antigen release based on iron-regulated E. coli lysis in vitro.
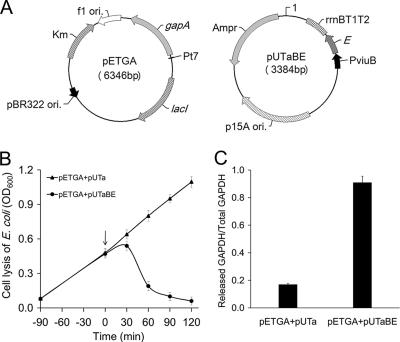
A. hydrophila is a well-known fish pathogen all over the world (61), and the GAPDH protein of A. hydrophila has been shown to be a protective antigen in fish (67). The gapA gene, encoding A. hydrophila GAPDH, was cloned into the T7-based pET28a expression plasmid to construct a highly efficient antigen expression plasmid, pETGA. E. coli host BL21(DE3) was transformed with both pETGA and pUTaBE to result in a recombinant two-plasmid system which possessed both an iron-regulated lysis circuit and an IPTG-inducible high-level antigen expression cassette, located in pUTaBE and pETGA, respectively (Fig. 4A). These two plasmids have different origins of replication, p15A ori and pBR322 ori, which are compatible origins and could coexist in one cell (32).
Fig. 4.
Cell lysis and antigen release in vitro of BL21(DE3)/pETGA+pUTaBE. (A) Plasmid maps for pETGA, the antigen expression vector, and pUTaBE, the iron-regulated lysis vector. gapA, gene encoding A. hydrophila GAPDH; E, lysis gene E from bacteriophage φX174; PT7, T7 promoter; PviuB, promoter of the viuB gene from V. cholerae. (B) Growth and lysis curves of BL21(DE3)/pETGA+pUTa and BL21(DE3)/pETGA+pUTaBE with 2,2′-dipyridyl induction. At 0 min, 2,2′-dipyridyl was added (↓). (C) The relative amount of GAPDH in the culture supernatant and whole cells were roughly evaluated by ELISA, and the ratio of the released GAPDH in the supernatant to the total GAPDH in the whole-cell lysate was calculated for each strain. Error bars indicate standard deviations from three independent experiments.
The recombinant E. coli strains BL21(DE3)/pETGA+pUTaBE and BL21(DE3)/pETGA+pUTa were induced to synthesize GAPDH for 5 h, and then the cultures were subcultured in a shaker to obtain an initial OD600 of 0.1. At early log phase (OD600 = 0.3 to 0.4), 2,2′-dipyridyl was added to cultures to induce cell lysis. Under identical culture and induction conditions, similar amounts of GPADH were produced by the two strains. However, the control strain BL21(DE3)/pETGA+pUTa grew normally (Fig. 4B) in iron-limiting medium, and 17% of the total GAPDH was detected in the cell supernatant fraction (Fig. 4C), while the lysis strain BL21(DE3)/pETGA+pUTaBE responded to the signal of iron limitation in medium and rapidly lysed (Fig. 4B), indicating that expression of the E gene had occurred. After 2 h of induction, over 90% of the total GAPDH was released to the supernatant (Fig. 4C). Here the total GAPDH was calculated as the sum of the GAPDH from the supernatant and the pellet for each culture. This result revealed that the integration of the iron-regulated lysis circuit and the IPTG-inducible high-level antigen expression cassette in E. coli BL21(DE3) could achieve mass production and controllable release of antigen.
Bacterial lysis and antigen release in zebrafish.
To evaluate bacterial lysis and controlled antigen release in vivo, adult zebrafish were used as the animal model. After 5 h of IPTG induction of gapA expression, the recombinant E. coli BL21(DE3)/pETGA+pUTaBE and BL21(DE3)/pETGA+pUTa were injected i.p. into zebrafish. Fish tissues were sampled at 4, 9, and 24 h postadministration and prepared for subsequent assay.
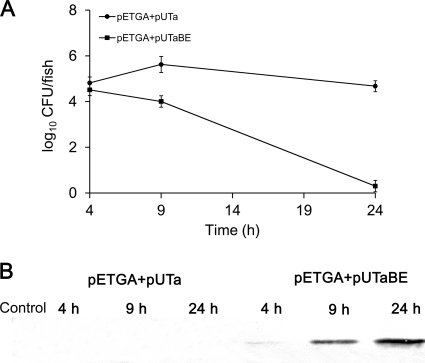
To determine the growth and lysis of E. coli in vivo, the fish samples were plated on LB screening agar for viable cell counts. As shown in Fig. 5A, at 4 h after injection, the numbers of BL21(DE3)/pETGA+pUTaBE and BL21(DE3)/pETGA+pUTa cells were roughly equivalent. At later times, the numbers of BL21(DE3)/pETGA+pUTa cells increased and then remained at between 104 CFU/fish and 105 CFU/fish, while the numbers of BL21(DE3)/pETGA+pUTaBE cells sharply decreased from 9 h and no survivors were recovered after 24 h. This result suggested that the PviuB-controlled E gene was transcribed in vivo and the E protein lysed the E. coli to achieve biological containment.
Fig. 5.
Cell lysis and antigen release in vivo in zebrafish. After intraperitoneal injection with 5 μl of PBS, E. coli BL21(DE3)/pETGA+pUTa (107 CFU per fish), or E. coli BL21(DE3)/pETGA+pUTaBE (107 CFU/fish), 20 fish with similar weights from each group were randomly taken at 4, 9, and 24 h postinjection. The tissues surrounding and including the abdominal cavities were collected, and the weight of each sample was kept at between 6 and 7 g. The tissue samples were then homogenized in 15 ml PBS. (A) Bacterial survival in zebrafish. Each homogenate sample was diluted serially in PBS and plated in triplicate on LB agar containing kanamycin and ampicillin to count the CFU. (B) Antigen release by bacterial cells in zebrafish. Each homogenate sample, collected at defined time intervals, was centrifuged to harvest the supernatant, and the concentrated supernatant was analyzed by Western blotting to detect the antigen GAPDH.
Fish tissue samples were further analyzed by Western blotting to detect GAPDH released from the E. coli cells. As shown in Fig. 5B, at 4, 9, and 24 h, no GAPDH from BL21(DE3)/pETGA+pUTa (no protein E) was detected in homogenized samples, which indicated that the expressed GAPDH was retained mainly in the cytoplasm, since the conditions used to homogenize the fish tissues do not lyse the bacterial cells. In contrast, GAPDH from BL21(DE3)/pETGA+pUTaBE (protein E synthesized) was detected in homogenized samples and increased with time. The time-responsive pattern of antigen release in fish injected with BL21(DE3)/pETGA+pUTaBE coincided with that of bacterial lysis as described above (Fig. 5B), indicating that E-mediated E. coli lysis resulted in antigen release in vivo.
Immune protection evaluation in turbot.
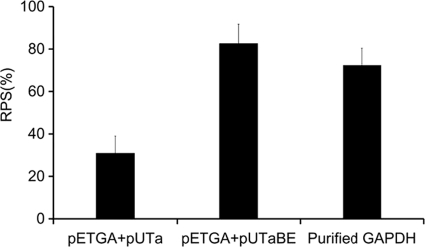
To investigate the potential of the E-mediated antigen delivery and release system in a live bacterial vectored vaccine, the recombinant strain BL21(DE3)/pUTaBE+pETGA was used as the vaccine candidate for further evaluation of immune protection efficacy in turbot. The fish were injected i.p. with the vaccine strains, purified GAPDH, or PBS and challenged with pathogenic A. hydrophila LSA34 at 30 days postvaccination. Most of the fish that died displayed typical abdominal distension, internal hemorrhages, and skin ulcerations at the injection site. No external lesions were observed in the surviving fish. Challenge of the PBS-injected control group resulted in 96.7% mortality. As shown in Fig. 6, candidate vaccine BL21(DE3)/pETGA+pUTaBE and purified GAPDH provided similar levels of protection, with RPSs of 82% and 72%, respectively, which were significantly greater than that of BL21(DE3)/pETGA+pUTa (RPS of 31%; P < 0.005).
Fig. 6.
Protection by vaccine candidate BL21(DE3)/pETGA+pUTaBE in turbot against A. hydrophila challenge. The fish (30 fish in each group, divided into three parallel groups) were vaccinated i.p. with E. coli BL21(DE3)/pETGA+pUTaBE (107 CFU per fish), E. coli BL21(DE3)/pETGA+pUTa (107 CFU per fish), purified GAPDH antigen with an equal volume of Freund's complete adjuvant (Sigma) (20 μg per fish), or PBS as a control (0.1 ml per fish). Four weeks after immunization, the four groups were injected i.p. with wild-type A. hydrophila LSA34 (5.0 × 107 CFU per fish). The significant difference and the relative percent survival (RPS) were calculated by using Fisher's exact test and the formula RPS = [1 − (% mortality in vaccinated fish/% mortality in control fish)] × 100, respectively.
Through live E. coli vector-based antigen delivery and release in animal host, the passenger antigen could confer a protective immune response equivalent to that of purified antigen with adjuvant. In the strain without the lysis-based antigen release system pUTaBE, the recombinant E. coli expressing gapA could not evoke effective immune protection (RPS = 31%; P < 0.005), indicating that the controllable mass release of antigen, initially produced by the bacterial vector into the host in the form of a cytoplasmic protein, would facilitate access of antigen to the immune-related cells and in turn promote a protective immune response in the animal host.
DISCUSSION
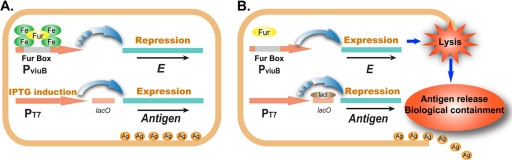
In this work, a novel live bacterial vector system for antigen delivery was established. As illustrated in Fig. 7, nonpathogenic E. coli was applied as the live vector, in which heterologous protective antigen was produced under the control of the strong promoter PT7 and synthesis of the E coli-sensitive lysis protein E was tightly regulated by the iron-responsive promoter PviuB. When cultured in vitro in an iron-rich medium, the recombinant E. coli could grow normally, with the cytoplasmic synthesis of antigen and the repression of lysis gene E by the Fur-iron complex (Fig. 7A); when administrated to the host, the recombinant E. coli could initiate the synthesis of lysis protein E in response to the iron-limiting signal in vivo and lyse to release the antigen in the host, simultaneously conferring biological containment (Fig. 7B). As a live bacterial delivery system, the E. coli-based antigen delivery system combines features of highly efficient antigen production, controlled antigen release, and active biological containment.
Fig. 7.
A novel antigen delivery and release system in E. coli. (A) Iron-rich conditions. In iron-rich medium, the iron-Fur complex binds to the Fur box of PviuB and represses the transcription of the E gene, while the antigen gene is expressed under control of the IPTG-inducible T7 promoter. (B) Iron-limiting conditions. In the host organism, antigen gene expression is repressed by LacI in the absence of IPTG. Meanwhile the iron-Fur complex is dissociated and the transcription of the E gene initiated. Bacterial cells are rapidly lysed by protein E to achieve antigen release and biological containment.
Based on iron-dependent gene regulation mechanisms in different bacteria, several novel iron-regulated cassettes have been constructed. The fhuCDB operon of V. cholerae is involved in ferrichrome iron utilization and is part of the Fur regulon. The promoter of the fhuCDB operon, PfhuC showed transcription activity in V. cholerae vaccine strain CVD and was identified to be an in vivo-inducible promoter in a mouse model by real-time bioluminescent imaging (42). The promoter of the fecA2 gene, encoding an 88-kDa iron(III) dicitrate transport protein in Helicobacter pylori, was identified to regulate iron-responsive transcription of the reporter gene of luciferase (25). The PentC promoter of the entCEBA operon, encoding enzymes for enterobactin biosynthesis in E. coli, was applied to an iron chelator-inducible expression system (34). In this work, an iron-regulated promoter, PviuB, with strict regulation and efficient transcription was successfully identified, and more importantly, the PviuB controlled iron-regulated cassette was shown to function well not only in vitro but also in vivo in two animal hosts, indicating that this promoter can function as part of an in vivo-inducible expression system. In addition, the PviuB-type promoter may have other applications as an iron availability biosensor in the food industry and for environmental and industrial monitoring.
Another key factor in our E. coli-based antigen delivery system is the lysis gene E. According to previous reports, under the control of either the thermosensitive λpL/pR-cI857 promoter or chemical-inducible promoter repressor systems such as lacPO or the tol expression system, protein E typically lyses E. coli by forming transmembrane tunnels specifically at the areas of potential division sites and expelling the inner contents to produce intact cell envelopes, called bacterial ghosts (38). However, the PviuB-controlled E lysis in this work lysed the bacterial cells into debris, which may be more conducive for antigen release, and this may be attributed to the dual roles of E protein and iron-limiting conditions in cells. Since the iron-limiting condition is a specific signal not only in vivo but also in the environment, the PviuB-controlled E lysis system has the potential to be developed into an active biological containment system for genetically engineered E. coli or other bacteria in agriculture, waste treatment, and the food industry, where large quantities of cells may need to be introduced into the environment or biological hosts.
The promoter PT7-controlled expression systems based on bacteriophage T7 RNA polymerase are often the optimal choice for the high-level production of recombinant proteins (19). In this work, the PT7-controlled antigen expression was integrated with PviuB-regulated bacterial lysis into a recombinant system which actualized high-level antigen expression in vitro and mass antigen release in a vaccinated host to elicit efficient protective immune responses against lethal challenge. In addition, the integrated antigen delivery system is believed to be superior to general bacterium-based antigen surface display or secretion systems in which most of the expressed antigen is retained in the cell cytoplasm. Furthermore, this recombinant system could be modified to suit a number of different needs for antigen or other functional protein delivery in organisms.
Via i.p. injection vaccination, the E. coli-based recombinant system could effectively deliver and release antigen into the fish host and thus activate immune protection in turbot against lethal challenge. However, by immersion vaccination, only trace amounts of antigen were detected in the homogenized samples of fish gill, intestine, and skin mucus (data not shown), which coincided with the poor immune protection (RPS of <10%) observed in turbot. The reason that the recombinant E. coli vector vaccine failed in mucosal vaccination might lie in the fact that E. coli BL21(DE3) is a nonpathogenic and noninvasive bacterium for turbot and therefore was unable to efficiently reach the immune-inductive sites of fish via mucosal routes. Therefore, to extend the application of this antigen delivery and cell lysis system as an effective mucosal vaccine, bacterial hosts with more proficiency in invasion and colonization need to be selected or established in the future.
In this work, we constructed a novel platform for a recombinant vaccine and evaluated its feasibility as a fish vaccine. If this platform is expanded to be used as a mammalian vaccine in the future, the two-plasmid system needs to be further optimized in many aspects, such as using a single plasmid to express the antigen and lysis gene cassettes, replacing the antibiotic marker with the balanced-lethal vector system, or integrating the expression cassettes into the chromosome of the bacterial host. Besides that, the protective antigens from mammalian pathogens need to be screened and applied in this platform for a mammalian vaccine; simultaneously some attenuated bacteria derived from mammalian pathogens can be tested to replace E. coli as the bacterial vector, and the vector bacteria need to be further modified to reduce the putative inflammatory response evoked in the mammalian host. The route of administration of a bacterial vector vaccine in a mammalian host depends on the features of the vector bacteria. Moreover, we believe that this technology could also be applied to many other Gram-negative bacteria which are sensitive to the E gene.
ACKNOWLEDGMENTS
We thank Kenneth Roland of Arizona State University for his critical reading of the manuscript.
This work was supported by the joint project National Natural Science Foundation of China-Austrian Science Fund (30811130545).
Footnotes
Published ahead of print on 2 May 2011.
REFERENCES
- 1. Alice A., López C., Crosa J. 2005. Plasmid- and chromosome-encoded redundant and specific functions are involved in biosynthesis of the siderophore anguibactin in Vibrio anguillarum 775: a case of chance and necessity? J. Bacteriol. 187:2209–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrews S., Robinson A., Rodríguez-Quiones F. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215–237 [DOI] [PubMed] [Google Scholar]
- 3. Barry E., Altboum Z., Losonsky G., Levine M. 2003. Immune responses elicited against multiple enterotoxigenic Escherichia coli fimbriae and mutant LT expressed in attenuated Shigella vaccine strains. Vaccine 21:333–340 [DOI] [PubMed] [Google Scholar]
- 4. Braun V. 2003. Iron uptake by Escherichia coli. Front. Biosci. 8:1409–1421 [DOI] [PubMed] [Google Scholar]
- 5. Bury N., Grosell M. 2003. Waterborne iron acquisition by a freshwater teleost fish, zebrafish Danio rerio. J. Exp. Biol. 206:3529–3535 [DOI] [PubMed] [Google Scholar]
- 6. Butterton J., Calderwood S. 1994. Identification, cloning, and sequencing of a gene required for ferric vibriobactin utilization by Vibrio cholerae. J. Bacteriol. 176:5631–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butterton J., Choi M., Watnick P., Carroll P., Calderwood S. 2000. Vibrio cholerae VibF is required for vibriobactin synthesis and is a member of the family of nonribosomal peptide synthetases. J. Bacteriol. 182:1731–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castagliuolo I., et al. 2005. Engineered E. coli delivers therapeutic genes to the colonic mucosa. Gene Ther. 12:1070–1078 [DOI] [PubMed] [Google Scholar]
- 9. Chai S., Welch T., Crosa J. 1998. Characterization of the interaction between Fur and the iron transport promoter of the virulence plasmid in Vibrio anguillarum. J. Biol. Chem. 273:33841–33847 [DOI] [PubMed] [Google Scholar]
- 10. Christman M., Morgan R., Jacobson F., Ames B. 1985. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell 41:753–762 [DOI] [PubMed] [Google Scholar]
- 11. Christoffersen C., Brickman T., Hook-Barnard I., McIntosh M. 2001. Regulatory architecture of the iron-regulated fepD-ybdA bidirectional promoter region in Escherichia coli. J. Bacteriol. 183:2059–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Critchley R., et al. 2004. Potential therapeutic applications of recombinant, invasive E. coli. Gene Ther. 11:1224–1233 [DOI] [PubMed] [Google Scholar]
- 13. Curtiss R., III, et al. 2010. New technologies in using recombinant attenuated Salmonella vaccine vectors. Crit. Rev. Immunol. 30:255–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fluri D. A., Kemmer C., Daoud-El Baba M., Fussenegger M. 2008. A novel system for trigger-controlled drug release from polymer capsules. J. Control Release 131:211–219 [DOI] [PubMed] [Google Scholar]
- 15. Funahashi T., et al. 2003. An iron-regulated gene required for utilization of aerobactin as an exogenous siderophore in Vibrio parahaemolyticus. Microbiology 149:1217–1225 [DOI] [PubMed] [Google Scholar]
- 16. Galen J., Levine M. 2001. Can a ‘flawless’ live vector vaccine strain be engineered? Trends Microbiol. 9:372–376 [DOI] [PubMed] [Google Scholar]
- 17. Galen J., et al. 2009. Salmonella enterica serovar Typhi live vector vaccines finally come of age. Immunol. Cell Biol. 87:400–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galen J., et al. 2010. A new generation of stable, nonantibiotic, low-copy-number plasmids improves immune responses to foreign antigens in Salmonella enterica serovar Typhi live vectors. Infect. Immun. 78:337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gamer M., Frode D., Biedendieck R., Stammen S., Jahn D. 2009. A T7 RNA polymerase-dependent gene expression system for Bacillus megaterium. Appl. Microbiol. Biotechnol. 82:1195–1203 [DOI] [PubMed] [Google Scholar]
- 20. García Véscovi E., Soncini F., Groisman E. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165–174 [DOI] [PubMed] [Google Scholar]
- 21. Gentschev I., et al. 1996. Development of antigen-delivery systems, based on the Escherichia coli hemolysin secretion pathway. Gene 179:133–140 [DOI] [PubMed] [Google Scholar]
- 22. Georgiou G., et al. 1996. Display of beta-lactamase on the Escherichia coli surface: outer membrane phenotypes conferred by Lpp′-OmpA'-beta-lactamase fusions. Protein Eng. Des. Sel. 9:239–247 [DOI] [PubMed] [Google Scholar]
- 23. Gunn B., Wanda S., Burshell D., Wang C., Curtiss R., III 2010. Construction of recombinant attenuated Salmonella enterica serovar Typhimurium vaccine vector strains for safety in newborn and infant mice. Clin. Vaccine Immunol. 17:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henderson D., Payne S. 1994. Characterization of the Vibrio cholerae outer membrane heme transport protein HutA: sequence of the gene, regulation of expression, and homology to the family of TonB-dependent proteins. J. Bacteriol. 176:3269–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoffelner H., Rieder G., Haas R. 2008. Helicobacter pylori vaccine development: optimisation of strategies and importance of challenging strain and animal model. Int. J. Med. Microbiol. 298:151–159 [DOI] [PubMed] [Google Scholar]
- 26. Hunt M., Pettis G., McIntosh M. 1994. Promoter and operator determinants for fur-mediated iron regulation in the bidirectional fepA-fes control region of the Escherichia coli enterobactin gene system. J. Bacteriol. 176:3944–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Isoda R., Simanski S., Pathangey L., Stone A., Brown T. 2007. Expression of a Porphyromonas gingivalis hemagglutinin on the surface of a Salmonella vaccine vector. Vaccine 25:117–126 [DOI] [PubMed] [Google Scholar]
- 28. Kang H., Curtiss R., III 2003. Immune responses dependent on antigen location in recombinant attenuated Salmonella typhimurium vaccines following oral immunization. FEMS Immunol. Med. Microbiol. 37:99–104 [DOI] [PubMed] [Google Scholar]
- 29. Karlsson J., von Hofsten J., Olsson P. 2001. Generating transparent zebrafish: a refined method to improve detection of gene expression during embryonic development. Mar. Biotechnol. 3:522–527 [DOI] [PubMed] [Google Scholar]
- 30. Knudsen S., Karlstrom O. 1991. Development of efficient suicide mechanisms for biological containment of bacteria. Appl. Environ. Microbiol. 57:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuo C., Sinha S., Jazayeri J., Pouton C. 2009. A stably engineered, suicidal strain of Listeria monocytogenes delivers protein and/or DNA to fully differentiated intestinal epithelial monolayers. Mol. Pharm. 6:1052–1061 [DOI] [PubMed] [Google Scholar]
- 32. Kur M., Piatek R., Kur J. 2007. A two-plasmid Escherichia coli system for expression of Dr adhesins. Protein expression and purification. 55:361–367 [DOI] [PubMed] [Google Scholar]
- 33. Leibiger R., Niedung K., Geginat G., Heesemann J., Trülzsch K. 2008. Yersinia enterocolitica Yop mutants as oral live carrier vaccines. Vaccine 26:6664–6670 [DOI] [PubMed] [Google Scholar]
- 34. Lim J., Hyun S., Kwon A. 2008. Iron chelator-inducible expression system for Escherichia coli. J. Microbiol. Biotechnol. 18:1357–1363 [PubMed] [Google Scholar]
- 35. Litwin C., Boyko S., Calderwood S. 1992. Cloning, sequencing, and transcriptional regulation of the Vibrio cholerae fur gene. J. Bacteriol. 174:1897–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loessner H., et al. 2008. Improving live attenuated bacterial carriers for vaccination and therapy. Int. J. Med. Microbiol. 298:21–26 [DOI] [PubMed] [Google Scholar]
- 37. Marchart J., et al. 2003. Pasteurella multocida- and Pasteurella haemolytica-ghosts: new vaccine candidates. Vaccine 21:3988–3997 [DOI] [PubMed] [Google Scholar]
- 38. Mayr U., et al. 2005. Bacterial ghosts as antigen delivery vehicles. Adv. Drug Deliv. Rev. 57:1381–1391 [DOI] [PubMed] [Google Scholar]
- 39. McHugh J., et al. 2003. Global iron-dependent gene regulation in Escherichia coli: a new mechanism for iron homeostasis. J. Biol. Chem. 278:29478–29486 [DOI] [PubMed] [Google Scholar]
- 40. Mey A., Wyckoff E., Kanukurthy V., Fisher C., Payne S. 2005. Iron and fur regulation in Vibrio cholerae and the role of fur in virulence. Infect. Immun. 73:8167–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moreno M., Kramer M., Yim L., Chabalgoity J. 2010. Salmonella as live Trojan horse for vaccine development and cancer gene therapy. Curr. Gene Ther. 10:56–76 [DOI] [PubMed] [Google Scholar]
- 42. Morin C., Kaper J. 2009. Use of stabilized luciferase-expressing plasmids to examine in vivo-induced promoters in the Vibrio cholerae vaccine strain CVD 103-HgR. FEMS Immunol. Med. Microbiol. 57:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mourino S., Osorio C., Lemos M., Crosa J. 2006. Transcriptional organization and regulation of the Vibrio anguillarum heme uptake gene cluster. Gene 374:68–76 [DOI] [PubMed] [Google Scholar]
- 44. Müller K., Matzanke B., Schünemann V., Trautwein A., Hantke K. 1998. FhuF, an iron-regulated protein of Escherichia coli with a new type of [2Fe-2S] center. Eur. J. Biochem. 258:1001–1008 [DOI] [PubMed] [Google Scholar]
- 45. Nakano M., et al. 2008. Hfq regulates the expression of the thermostable direct hemolysin gene in Vibrio parahaemolyticus. BMC Microbiol. 8:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Newman S. G. 1993. Bacterial vaccines for fish. Annu. Rev. Fish Dis. 3:145–185 [Google Scholar]
- 47. Occhino D., Wyckoff E., Henderson D., Wrona T., Payne S. 1998. Vibrio cholerae iron transport: haem transport genes are linked to one of two sets of tonB, exbB, exbD genes. Mol. Microbiol. 29:1493–1507 [DOI] [PubMed] [Google Scholar]
- 48. O'Toole R., von Hofsten J., Rosqvist R., Olsson P.-E., Wolf-Watz H. 2004. Visualisation of zebrafish infection by GFP-labelled Vibrio anguillarum. Microb. Pathog. 37:41–46 [DOI] [PubMed] [Google Scholar]
- 49. Outten F., Djaman O., Storz G. 2004. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol. Microbiol. 52:861–872 [DOI] [PubMed] [Google Scholar]
- 50. Payne S. 1993. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1:66–69 [DOI] [PubMed] [Google Scholar]
- 51. Radford K., et al. 2002. A recombinant E. coli vaccine to promote MHC class I-dependent antigen presentation application to cancer immunotherapy. Gene Ther. 9:1455–1463 [DOI] [PubMed] [Google Scholar]
- 52. Radford K., Jackson A., Wang J., Vassaux G., Lemoine N. 2003. Recombinant E. coli efficiently delivers antigen and maturation signals to human dendritic cells: presentation of MART1 to CD8+ T cells. Int. J. Cancer 105:811–819 [DOI] [PubMed] [Google Scholar]
- 53. Rogers M., Sexton J., DeCastro G., Calderwood S. 2000. Identification of an operon required for ferrichrome iron utilization in Vibrio cholerae. J. Bacteriol. 182:2350–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salam M., Katz J., Zhang P., Hajishengallis G., Michalek S. 2006. Immunogenicity of Salmonella vector vaccines expressing SBR of Streptococcus mutans under the control of a T7-nirB (dual) promoter system. Vaccine 24:5003–5015 [DOI] [PubMed] [Google Scholar]
- 55. Seegers J. 2002. Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends Biotechnol. 20:508–515 [DOI] [PubMed] [Google Scholar]
- 56. Shi H., Wen Su W. 2001. Display of green fluorescent protein on Escherichia coli cell surface. Enzyme Microb. Technol. 28:25–34 [DOI] [PubMed] [Google Scholar]
- 57. Stiefel A., et al. 2001. Control of the ferric citrate transport system of Escherichia coli: mutations in region 2.1 of the FecI extracytoplasmic-function sigma factor suppress mutations in the FecR transmembrane regulatory protein. J. Bacteriol. 183:162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stojiljkovic I., Baëumler A. J., Hantke K. 1994. Fur regulon in gram-negative bacteria: identification and characterization of new iron-regulated Escherichia coli genes by a fur titration assay. J. Mol. Biol. 236:531–545 [DOI] [PubMed] [Google Scholar]
- 59. Stork M., Di Lorenzo M., Welch T., Crosa L., Crosa J. 2002. Plasmid-mediated iron uptake and virulence in Vibrio anguillarum. Plasmid 48:222–228 [DOI] [PubMed] [Google Scholar]
- 60. Tanabe T., et al. 2003. Identification and characterization of genes required for biosynthesis and transport of the siderophore vibrioferrin in Vibrio parahaemolyticus. J. Bacteriol. 185:6938–6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thune R., Stanley L., Cooper R. 1993. Pathogenesis of gram-negative bacterial infections in warmwater fish. Annu. Rev. Fish Dis. 3:37–68 [Google Scholar]
- 62. Torres B., Jaenecke S., Timmis K., Garcia J., Diaz E. 2000. A gene containment strategy based on a restriction modification system. Environ. Microbiol. 2:555–563 [DOI] [PubMed] [Google Scholar]
- 63. Torres B., Jaenecke S., Timmis K. N., Garcia J. L., Diaz E. 2003. A dual lethal system to enhance containment of recombinant micro-organisms. Microbiology 149:3595–3601 [DOI] [PubMed] [Google Scholar]
- 64. Witte A., Brand E., Mayrhofer P., Narendja F., Lubitz W. 1998. Mutations in cell division proteins FtsZ and FtsA inhibit PhiX174 protein-E-mediated lysis of Escherichia coli. Arch. Microbiol. 170:259–268 [DOI] [PubMed] [Google Scholar]
- 65. Wyckoff E., Schmitt M., Wilks A., Payne S. 2004. HutZ is required for efficient heme utilization in Vibrio cholerae. J. Bacteriol. 186:4142–4151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zheng M., Doan B., Schneider T., Storz G. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhou L., et al. 2010. A novel multivalent vaccine based on secretory antigen-delivery induces protective immunity against Vibrio anguillarum and Aeromonas hydrophila. J. Biotechnol. 146:25–30 [DOI] [PubMed] [Google Scholar]